Alpha-galactosylceramide (α-GalCer), which is a specific ligand for CD1d-restricted variable-α14 chain (Vα14) natural killer T (NKT) cells, exerts a potent antitumor effect. We recently demonstrated that interferon-γ (IFN-γ) secreted by both NKT cells and NK cells plays a critical role in mediating the antimetastatic effect of α-GalCer; however, the IFN-γ–dependent antitumor mechanisms remain poorly defined. In the present study, we demonstrate IFN-γ–dependent inhibition of tumor angiogenesis by α-GalCer. In α-GalCer–treated mice, subcutaneous tumor growth and tumor-induced angiogenesis were inhibited in an IFN-γ–dependent manner. The α-GalCer–activated splenic or hepatic mononuclear cells inhibited murine endothelial cell proliferation in vitro, and this inhibitory effect was mediated mostly by IFN-γ produced by NKT cells and NK cells. NK cell depletion resulted in significant but partial inhibition of tumor growth and angiogenesis in vivo. These results suggest that the IFN-γ–mediated inhibition of tumor angiogenesis is critically involved in the effector mechanisms of antitumor effects evoked by α-GalCer.
Introduction
Natural killer T (NKT) cells constitute a distinctive subpopulation of mature T cells that coexpress an NK cell marker, NK1.1 antigen (Ag), and a highly restricted T-cell receptor (TCR) repertoire composed of a single invariant variable-α14–joining-α281 (Vα14-Jα281) chain.1,2 Recent studies have revealed that the invariant TCR on Vα14 NKT cells recognizes glycolipid Ags, such as α-galactosylceramide (α-GalCer) presented by the major histocompatibility complex (MHC) class I–like molecule CD1d.1-4 The α-GalCer specifically stimulates Vα14 NKT cells to produce large amounts of interferon-γ (IFN-γ) and interleukin 4 (IL-4), exhibit cytolytic activity, and exert antitumor effect in vivo.4-7 We recently demonstrated that NK cells and IFN-γ, but not perforin-mediated cytotoxicity, critically contributed to the antimetastatic effect of α-GalCer.8,9 NK cells produce IFN-γ secondarily in response to IFN-γ secreted by α-GalCer–activated Vα14 NKT cells.8-11Although IFN-γ was critically important for the antimetastatic effect of α-GalCer, the IFN-γ–mediated antitumor effector mechanisms remain unclear.
Tumor angiogenesis, new vessel formation draining the tumor mass, is critical for tumor progression, outgrowth, and metastatic spread of tumors.12,13 Tumor-induced angiogenesis is up-regulated/down-regulated by proangiogenic and antiangiogenic factors produced by tumor cells and host microenvironments.14-16IFN-γ has been implicated in the regulation of angiogenesis during tumor development.17-21 In the present study, we demonstrated that α-GalCer inhibited tumor-induced angiogenesis in vivo. IFN-γ secreted by α-GalCer–activated NKT cells and secondary activated NK cells was critical for this antiangiogenic effect.
Materials and methods
Mice
Wild-type C57BL/6 (B6) mice, BALB/c mice, and DBA/2 mice were purchased from Japan SLC (Hamamatsu, Japan). IFN-γ–deficient (IFN-γ−/−) B6 mice and CD1-deficient (CD1−/−) B6 mice were generated as previously described.22 23 All mice were maintained under specific pathogen-free conditions and used at 6 to 7 weeks of age according to the institutional guidelines.
Tumor cells
B6-derived melanoma (B16-BL6) and lung carcinoma (3LL) cell lines, BALB/c-derived colon carcinoma (colon 26-L5) cell line, and DBA/2-derived mastocytoma (P815) cell line were maintained in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS). These cells, except for P815, were collected by brief trypsin treatment and resuspended in phosphate-buffered saline (PBS) before inoculation.
Reagents
The α-GalCer [(2S, 3S, 4R)-1-o-(α-D-galactopyranosyl)-2-(N-hexacosanoylamino)-1,3,4-octadecanetiol] was provided by Y. Koezuka and K. Motoki (Kirin Brewery, Gumma, Japan) and prepared as previously described.5,6 For in vivo stimulation of NKT cells, mice were intraperitoneally administered 2 μg/200 μL α-GalCer or 200 μL vehicle.1 To deplete NK cells in vivo, 150 μg antiasialo GM1 (anti-AGM1) antibody (Ab) per mouse (Wako, Osaka, Japan) was intravenously injected 2 days before the α-GalCer treatment.24 To deplete NK cells and NKT cells in vivo, anti-NK1.1 monoclonal Ab (mAb) (PK136; BD Pharmingen, San Diego, CA) was intravenously injected 2 days before the α-GalCer treatment.24 Depletion of respective cells was confirmed by flow cytometric analysis. The number of NKT cells was not affected by the anti-AGM1 Ab treatment (data not shown). Purified mAb (no azide/low endotoxin grade) against mouse IFN-γ (R4-6A2) was purchased from BD Pharmingen. Control rat immunoglobulin G (IgG) was purchased from Sigma Chemical (St Louis, MO).
Angiogenesis assay
Tumor-induced angiogenesis was assessed according to the previously described methods with minor modifications.25Mice were inoculated intradermally with B16-BL6 cells (1 × 105/50 μL), colon 26-L5 cells (8 × 104/50 μL), 3LL cells (1 × 105/50 μL), or P815 cells (2 × 105/50 μL) on the back. At appropriate time periods after tumor inoculation, mice were killed, and the tumor-inoculated skin was separated from the underlying tissues. Angiogenesis was quantified by counting only the vessels directly supplying the tumor under a dissecting microscope. Matrigel angiogenesis assay17 was conducted by intradermally inoculating with B16-BL6 (2 × 106/100 μL) cells containing 10 μg Matrigel (Collaborative Research, Bedford, MA). At 4 days after inoculation, the tumor-inoculated skin was isolated from the underlying tissues, and the vessels drawn into the tumor-containing gel were counted. To evaluate the serum IFN-γ levels, sera were collected from vehicle- or α-GalCer–treated mice at 16 hours after the first administration. IFN-γ levels in the serum were evaluated with the use of specific enzyme-linked immunosorbent assay (ELISA) kits (Endogen, Boston, MA) according to the manufacturer's instructions.
Tumor growth assay
Mice were inoculated intradermally with B16-BL6 cells (1 × 105/50 μL), colon 26-L5 cells (8 × 104/50 μL), 3LL cells (1 × 105/50 μL), or P815 cells (2 × 105/50 μL) on the back and observed every 4 days for tumor growth by measuring with a caliper square along the longer axes (a) and the shorter axes (b). Tumor volumes (mm3) were calculated by the formula: tumor volume (mm3) = ab2/2.
Endothelial cell cultures
Murine hepatic sinusoidal endothelial (HSE) cells were kindly provided by Dr G L Nicolson (The University of Texas, M D Anderson Cancer Center, Houston). HSE cells were maintained on Attachment Factor—coated (Cell Systems, Kirkland, WA) culture flasks in Dulbecco Modified Eagle medium (DMEM)/F12 medium supplemented with 5% FBS and 100 μg/mL endothelial mitogen (Biomedical Technologies, Stoughton, MA). All cultures were maintained at 37°C in a humidified atmosphere of 5% CO2/95% air.
Coculture of HSE cells and mononuclear cells in membrane-separated wells
Coculture experiments were performed in Intercell (equipped with 0.4- μm–pore membrane) (Kurabo, Osaka, Japan), with murine HSE (1 × 104) cells being plated at the bottom of a 24-well culture plate (Costar, Cambridge, MA). Splenic (3 × 106/200 μL) or hepatic (5 × 105/200 μL) mononuclear cells (MNCs) isolated from vehicle- or α-GalCer–treated mice were placed onto the inserts. The splenic or hepatic MNCs were isolated 16 hours after vehicle or α-GalCer administration as described previously.8 In the blocking experiments, anti–IFN-γ mAb (R4-6A2) or control rat IgG was added at 10 μg/mL to the cultures. After 72-hour incubation, proliferation of HSE cells in the bottom wells was measured by evaluating cell number by crystal violet staining. Briefly, cells were washed twice with PBS, fixed with 2.5% glutaraldehyde for 20 minutes at room temperature, and stained with 0.5 mL crystal violet (0.1% in 20% methanol solution). After washes, color was dissolved in 30% acetic acid and read at 590 nm on a microplate reader (Immuno Mini NJ-2300; Nippon Inter-Med, Tokyo, Japan). A calibration curve was set up with known numbers of cells, and a linear correlation between absorbance and cell number was established from 1 × 104cells to 4 × 105 cells. To evaluate cytokine levels in the cultures, cell-free culture supernatants were harvested from the bottom wells. IFN-γ levels in the culture supernatants were evaluated with the use of specific ELISA kits (OptEIA; BD Pharmingen).
Statistical analysis
Data were analyzed by means of the the Mann-Whitney Utest. P < .05 was considered significant.
Results
Effect of α-GalCer on subcutaneous tumor growth and tumor-induced angiogenesis
We first investigated the effect of α-GalCer treatment on the outgrowth of subcutaneously inoculated B16-BL6 melanoma cells in wild-type B6 mice. Administration of α-GalCer significantly inhibited the subcutaneous outgrowth of B16-BL6 cells during the early time points (days 8-16) but not later (Figure1A). A similar inhibition of subcutaneous tumor growth by α-GalCer treatment was also observed with colon 26-L5, 3LL, and P815 cells (data not shown). These results suggested that α-GalCer could inhibit the early formation of solid tumor mass.
Effects of α-GalCer on subcutaneous tumor growth and tumor angiogenesis.
Wild-type B6 mice were intradermally inoculated with B16-BL6 (1 × 105) cells and intraperitoneally administered α-GalCer (2 μg/200 μL) or vehicle (200 μL) from day 0 and then every 4 days for 28 days. (A) Tumor size was measured every 4 days after tumor inoculation. Data are represented as the mean ± SD of 5 mice in each group. Similar results were obtained in 2 independent experiments. *P < .05 compared with vehicle. (B) The tumor-inoculated sites in vehicle- or α-GalCer–treated mice at day 10. (C) Tumor-inoculated sites were isolated from vehicle- or α-GalCer–treated mice when the tumor size reached the indicated volumes and tumor-supplying vessels were counted. The mean tumor sizes of vehicle- and α-GalCer–treated groups were not different within any one range described. (At 100 to 200 mm3, vehicle 153 ± 24, α-GalCer 161 ± 22. At 300 to 400 mm3, vehicle 365 ± 25, α-GalCer 358 ± 34. At greater than 500 mm3, vehicle 622 ± 56, α-GalCer 608 ± 70.) Data are represented as the mean ± SD of 5 mice in each group. Similar results were obtained in 2 independent experiments. *P < .01.
Effects of α-GalCer on subcutaneous tumor growth and tumor angiogenesis.
Wild-type B6 mice were intradermally inoculated with B16-BL6 (1 × 105) cells and intraperitoneally administered α-GalCer (2 μg/200 μL) or vehicle (200 μL) from day 0 and then every 4 days for 28 days. (A) Tumor size was measured every 4 days after tumor inoculation. Data are represented as the mean ± SD of 5 mice in each group. Similar results were obtained in 2 independent experiments. *P < .05 compared with vehicle. (B) The tumor-inoculated sites in vehicle- or α-GalCer–treated mice at day 10. (C) Tumor-inoculated sites were isolated from vehicle- or α-GalCer–treated mice when the tumor size reached the indicated volumes and tumor-supplying vessels were counted. The mean tumor sizes of vehicle- and α-GalCer–treated groups were not different within any one range described. (At 100 to 200 mm3, vehicle 153 ± 24, α-GalCer 161 ± 22. At 300 to 400 mm3, vehicle 365 ± 25, α-GalCer 358 ± 34. At greater than 500 mm3, vehicle 622 ± 56, α-GalCer 608 ± 70.) Data are represented as the mean ± SD of 5 mice in each group. Similar results were obtained in 2 independent experiments. *P < .01.
When the tumor-inoculated sites were inspected, we noticed that a smaller size of tumor mass in the α-GalCer–treated mice was associated with a reduced number of vessels around the tumor mass (tumor-induced angiogenesis) as represented in Figure 1B. To examine whether the reduced angiogenesis was a result or a cause of the tumor growth inhibition, we compared the number of blood vessels supplying tumors of the same volume in vehicle-versus– α-GalCer–treated mice. As shown in Figure 1C, α-GalCer treatment significantly reduced the number of blood vessels when tumor volumes were less than 400 mm3. This suggested that the reduced angiogenesis was not a result, but a cause, of the inhibition of tumor growth in α-GalCer–treated mice.
α-GalCer inhibits tumor growth and tumor-induced angiogenesis in an IFN-γ–dependent manner
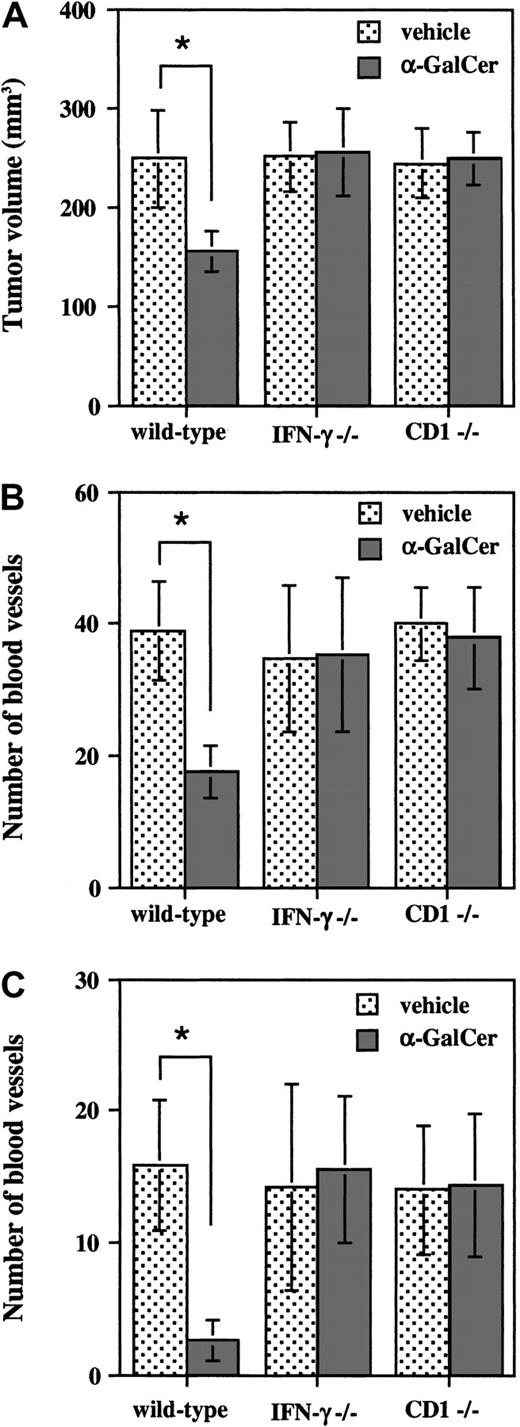
Our previous studies demonstrated that α-GalCer–induced antimetastatic activity was mediated by IFN-γ, but not perforin-mediated cytolysis.8,9 Thus, we next examined the involvement of IFN-γ in the inhibition of subcutaneous tumor growth and angiogenesis by α-GalCer using IFN-γ−/− mice. As shown in Figure 2A-B, α-GalCer treatment in wild-type B6 mice significantly inhibited the growth of subcutaneously inoculated B16-BL6 cells and the tumor angiogenesis as estimated at day 10. In contrast, no significant inhibition of either tumor growth or tumor angiogenesis by α-GalCer treatment was observed in IFN-γ−/− mice or CD1−/− mice lacking Vα14 NKT cells (Figure 2A-B). To further examine the antiangiogenic effect of α-GalCer, we used the Matrigel angiogenesis assay.17 Mice were intradermally inoculated with a larger number (2 × 106) of B16-BL6 cells embedded in Matrigel matrix, and the tumor-induced angiogenesis was assessed after a short period (4 days). As shown in Figure 2C, α-GalCer treatment markedly inhibited the angiogenesis in wild-type mice but not in IFN-γ−/− mice or CD1−/− mice. These results indicated that α-GalCer inhibited subcutaneous tumor growth and angiogenesis in an IFN-γ–dependent manner.
IFN-γ–dependent inhibition of tumor growth and tumor-induced angiogenesis by α-GalCer.
Wild-type, IFN-γ−/−, or CD1−/− B6 mice were intradermally inoculated with B16-BL6 cells (1 × 105) and then intraperitoneally administered α-GalCer (2 μg/200 μL) or vehicle (200 μL) on days 0, 4, and 8. At 10 days after tumor inoculation, mice were killed and the tumor-inoculated skin was isolated. Tumor size was measured (panel A) and tumor-supplying vessels were counted (panel B). (C) Wild-type, IFN-γ−/−, or CD1−/− B6 mice were intradermally inoculated with B16-BL6 (2 × 106) cells embedded in Matrigel (10 μg/100 μL) and then intraperitoneally administered α-GalCer (2 μg/200 μL) or vehicle (200 μL). At 4 days after inoculation, vessels drawn into the gel were counted. All data are represented as the mean ± SD of 5 mice in each group. Similar results were obtained in 2 independent experiments. *P < .01.
IFN-γ–dependent inhibition of tumor growth and tumor-induced angiogenesis by α-GalCer.
Wild-type, IFN-γ−/−, or CD1−/− B6 mice were intradermally inoculated with B16-BL6 cells (1 × 105) and then intraperitoneally administered α-GalCer (2 μg/200 μL) or vehicle (200 μL) on days 0, 4, and 8. At 10 days after tumor inoculation, mice were killed and the tumor-inoculated skin was isolated. Tumor size was measured (panel A) and tumor-supplying vessels were counted (panel B). (C) Wild-type, IFN-γ−/−, or CD1−/− B6 mice were intradermally inoculated with B16-BL6 (2 × 106) cells embedded in Matrigel (10 μg/100 μL) and then intraperitoneally administered α-GalCer (2 μg/200 μL) or vehicle (200 μL). At 4 days after inoculation, vessels drawn into the gel were counted. All data are represented as the mean ± SD of 5 mice in each group. Similar results were obtained in 2 independent experiments. *P < .01.
Splenic and hepatic mononuclear cells from α-GalCer–treated mice inhibit proliferation of murine endothelial cells in vitro
To further explore the mechanisms by which α-GalCer inhibits tumor angiogenesis, we devised an in vitro assay system for estimating the effects of α-GalCer–activated NKT and/or NK cells on proliferation of endothelial cells by a transmembrane coculture. Murine HSE cells were seeded on the bottom wells, and splenic or hepatic MNCs from vehicle- or α-GalCer–treated mice were placed on the upper wells, which were separated from the bottom wells by a 0.4-μm–pore membrane. After 72-hour coculture, proliferation of HSE cells in the bottom wells was estimated by determining the cell numbers by staining with crystal violet. As represented in Figure3, splenic and hepatic MNCs from α-GalCer–treated mice markedly inhibited the proliferation of HSE cells as compared with those from vehicle-treated mice. Direct cell-cell contact was not required to inhibit the HSE cell proliferation by α-GalCer–activated MNCs, because proliferation was observed when these cells were cultured in the membrane-separated wells.
Inhibition of HSE cell proliferation by transmembrane coculture with MNCs from α-GalCer–treated mice.
Wild-type B6 mice were intraperitoneally administered vehicle (200 μL) or α-GalCer (2 μg/200 μL). At 16 hours later, isolated splenic (3 × 106) or hepatic (5 × 105) MNCs were cocultured with murine HSE (1 × 104) cells in membrane-separated wells for 72 hours. The number of HSE cells in the bottom wells was determined by crystal violet staining. Data are indicated as the mean ± SD of triplicate samples. Similar results were obtained in 3 independent experiments. *P < .01.
Inhibition of HSE cell proliferation by transmembrane coculture with MNCs from α-GalCer–treated mice.
Wild-type B6 mice were intraperitoneally administered vehicle (200 μL) or α-GalCer (2 μg/200 μL). At 16 hours later, isolated splenic (3 × 106) or hepatic (5 × 105) MNCs were cocultured with murine HSE (1 × 104) cells in membrane-separated wells for 72 hours. The number of HSE cells in the bottom wells was determined by crystal violet staining. Data are indicated as the mean ± SD of triplicate samples. Similar results were obtained in 3 independent experiments. *P < .01.
Critical contribution of IFN-γ produced by both NKT and NK cells to the inhibition of endothelial cell proliferation in vitro
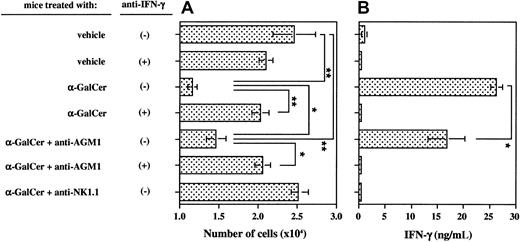
We next examined the involvement of IFN-γ in the inhibition of endothelial cell proliferation by splenic MNCs from α-GalCer–treated mice. As shown in Figure4, α-GalCer–activated splenic MNCs produced a large amount of IFN-γ (Figure 4B) and markedly inhibited the proliferation of HSE cells (Figure 4A). This inhibitory effect on the proliferation of HSE cells was completely abolished by addition of neutralizing antimouse IFN-γ mAb (Figure 4A). These results indicated that the inhibition of HSE cell proliferation by α-GalCer–activated MNCs was totally mediated by IFN-γ. We further examined the relative contributions of NK cells and NKT cells to the inhibition of HSE cell proliferation by α-GalCer–activated MNCs, since we had previously observed a substantial contribution of secondarily activated NK cells to α-GalCer–induced IFN-γ production.8-11 Specific depletion of NK cells by anti-AGM1 Ab before α-GalCer administration partially inhibited the production of IFN-γ (Figure 4B) and partially abolished the inhibition of HSE cell proliferation (Figure 4A). The residual inhibitory effect on HSE cell proliferation was completely abolished by neutralization of IFN-γ (Figure 4A). In contrast, depletion of both NK cells and NKT cells by anti-NK1.1 mAb completely abolished the α-GalCer–induced IFN-γ production (Figure 4B) and the inhibition of HSE cell proliferation (Figure 4A). Similar results were obtained with hepatic MNCs (data not shown). These results indicated that both α-GalCer–activated NKT cells and secondarily activated NK cells contributed to the inhibition of endothelial cell proliferation via their IFN-γ production.
Critical contribution of IFN-γ and both NKT and NK cells to inhibition of HSE cell proliferation by splenic MNCs from α-GalCer–treated mice.
Untreated, anti-AGM1 Ab–treated, or anti-NK1.1 mAb–treated wild-type B6 mice were intraperitoneally administered α-GalCer (2 μg/200 μL) or vehicle (200 μL). Splenic MNCs were isolated 16 hours later and cocultured with HSE cells as in Figure 3 in the presence of 10 μg/mL anti–IFN-γ mAb (+) or control IgG (−). The number of HSE cells was determined by crystal violet staining (panel A) and the production of IFN-γ by splenic MNCs was determined by ELISA (panel B). Data are indicated as the mean ± SD of triplicate samples. Similar results were obtained in 3 independent experiments. *P < .05. **P < .01.
Critical contribution of IFN-γ and both NKT and NK cells to inhibition of HSE cell proliferation by splenic MNCs from α-GalCer–treated mice.
Untreated, anti-AGM1 Ab–treated, or anti-NK1.1 mAb–treated wild-type B6 mice were intraperitoneally administered α-GalCer (2 μg/200 μL) or vehicle (200 μL). Splenic MNCs were isolated 16 hours later and cocultured with HSE cells as in Figure 3 in the presence of 10 μg/mL anti–IFN-γ mAb (+) or control IgG (−). The number of HSE cells was determined by crystal violet staining (panel A) and the production of IFN-γ by splenic MNCs was determined by ELISA (panel B). Data are indicated as the mean ± SD of triplicate samples. Similar results were obtained in 3 independent experiments. *P < .05. **P < .01.
Relative contribution of NKT cells and NK cells to the antiangiogenic effect of α-GalCer in vivo
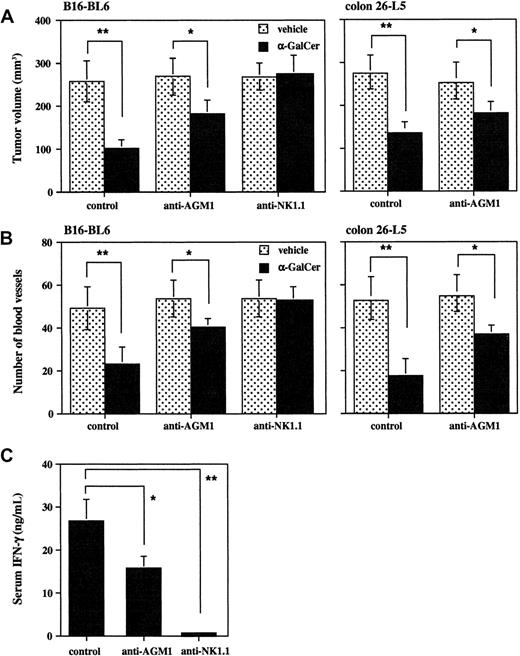
Finally, we determined the relative contribution of NKT cells and NK cells to the antiangiogenic effect of α-GalCer in vivo. Specific depletion of NK cells by anti-AGM1 Ab prior to α-GalCer administration partially reduced the α-GalCer–induced serum IFN-γ elevation (Figure 5C) and also partially reduced the α-GalCer–induced inhibition of B16-BL6 tumor growth (Figure 5A) and tumor angiogenesis (Figure 5B) as compared with control mice. Similar results were obtained with colon 26-L5 (Figure 5A-B, right panels), 3LL, and P815 (data not shown). In contrast, the depletion of both NK cells and NKT cells by anti-NK1.1 mAb completely abolished all these α-GalCer–induced effects (Figure 5A-B, left panels). These results indicated that both primary activated NKT cells and secondary activated NK cells contributed to the antitumor and antiangiogenic effects of α-GalCer in vivo.
Partial contribution of NK cells to inhibition of tumor growth and tumor-induced angiogenesis by α-GalCer.
Untreated, anti-AGM1 Ab–treated, or anti-NK1.1 mAb–treated wild-type B6 or BALB/c mice were intradermally inoculated with B16-BL6 (1 × 105) or colon 26-L5 (8 × 104) cells, and then intraperitoneally administered α-GalCer (2 μg/200 μL) or vehicle (200 μL) on days 0, 4, and 8. At 10 days after tumor inoculation, mice were killed and the tumor-inoculated skin was isolated. Tumor size was measured (panel A) and tumor-supplying vessels were counted (panel B). Serum IFN-γ level at 16 hours after the first administration of α-GalCer in B6 mice was also evaluated by ELISA (panel C). Data are represented as the mean ± SD of 5 mice in each group. Similar results were obtained in 2 independent experiments. *P < .05. **P < .01.
Partial contribution of NK cells to inhibition of tumor growth and tumor-induced angiogenesis by α-GalCer.
Untreated, anti-AGM1 Ab–treated, or anti-NK1.1 mAb–treated wild-type B6 or BALB/c mice were intradermally inoculated with B16-BL6 (1 × 105) or colon 26-L5 (8 × 104) cells, and then intraperitoneally administered α-GalCer (2 μg/200 μL) or vehicle (200 μL) on days 0, 4, and 8. At 10 days after tumor inoculation, mice were killed and the tumor-inoculated skin was isolated. Tumor size was measured (panel A) and tumor-supplying vessels were counted (panel B). Serum IFN-γ level at 16 hours after the first administration of α-GalCer in B6 mice was also evaluated by ELISA (panel C). Data are represented as the mean ± SD of 5 mice in each group. Similar results were obtained in 2 independent experiments. *P < .05. **P < .01.
Discussion
The present study demonstrated that α-GalCer inhibited the early stage of subcutaneous tumor growth and the tumor-induced angiogenesis, and that these inhibitory effects were totally mediated by IFN-γ produced by primary activated NKT cells and secondary activated NK cells. The outgrowth and spread of tumors depend on an adequate blood supply achieved through angiogenesis.12-14,26 27 Our present results suggest that the IFN-γ–dependent antiangiogenic effect may be one mechanism for the antitumor effects of α-GalCer.
It has been reported that the antiangiogenic effect of IFN-γ in vivo was mediated mainly by IFN-γ–inducible antiangiogenic chemokines, such as IFN-γ–inducible protein of 10 kDa (IP-10) and monokine induced by IFN-γ (Mig).28 29 In the present study, the inhibitory effect of α-GalCer on tumor-induced angiogenesis was not observed in IFN-γ−/− mice (Figure 2), and neutralization of IFN-γ completely abrogated the inhibition of endothelial cell proliferation by α-GalCer–activated MNCs in vitro (Figure 4). Moreover, recombinant IFN-γ inhibited the proliferation of HSE cells in a dose-dependent manner in vitro (data not shown). These results suggest that the antiangiogenic effect of α-GalCer might be mediated by IFN-γ–dependent inhibition of endothelial cell proliferation at the tumor site, which was required for tumor angiogenesis. However, it remains to be determined whether the IFN-γ–dependent inhibition of endothelial cell proliferation was a direct effect of IFN-γ or mediated by other inducible cytokines or chemokines produced by IFN-γ–stimulated endothelial cells. It is also possible that IFN-γ might inhibit the tumor-induced angiogenesis by suppressing the production of angiogenic factors by tumor cells or by inducing the production of antiangiogenic factors by tumor cells or host stroma cells in vivo. Further studies are needed to determine the detailed mechanisms of IFN-γ–mediated antiangiogenic effect.
It has recently been reported that NK cells could lyse endothelial cells in a cell contact–dependent manner.30Although a direct contact with α-GalCer–activated MNCs was not required for the inhibition of HSE cell proliferation in vitro (Figure3), NKT and/or NK cell–mediated injury of endothelial cells might contribute to the antiangiogenic effect of α-GalCer in vivo. However, this seems unlikely, because we previously demonstrated that the antitumor effect of α-GalCer was not mediated by perforin- or Fas ligand (FasL)–dependent cytotoxicity.8 Since some members of the tumor necrosis factor (TNF) family other than FasL, including TNF-α and vascular endothelial cell growth inhibitor (VEGI), have been also implicated in the inhibition of tumor angiogenesis,31-33 the possible contribution of these molecules to the IFN-γ–dependent antiangiogenic effect of α-GalCer remains to be explored in further studies.
The present study indicated that NK cells played a partial but substantial role in the inhibition of subcutaneous tumor growth, angiogenesis, and endothelial cell proliferation by α-GalCer. Although we previously reported that the systemic administration of recombinant IFN-γ failed to inhibit tumor-induced angiogenesis,34 it was also reported that local production of IFN-γ by gene transfer inhibited tumor angiogenesis in vivo.19 We recently demonstrated a rapid depletion of primary activated NKT cells but a prolonged production of IFN-γ by secondary activated NK cells after α-GalCer treatment.8Therefore, the prolonged production of IFN-γ by secondary activated NK cells might play a substantial role in sustaining the antiangiogenic effect of α-GalCer. It was previously reported that IL-12 also inhibited tumor-induced angiogenesis and endothelial cell functions in an IFN-γ–dependent manner20,35-37 and that this effect was mediated by NK cells.38 However, it has been also demonstrated that IL-12 preferentially activated NKT cells rather than NK cells,39,40 and that IL-12 played a critical role in α-GalCer–induced NKT cell activation.41 We also observed that IL-12 partially contributed to the α-GalCer–induced IFN-γ production42 and to the inhibition of endothelial cell proliferation by α-GalCer–activated splenic MNCs (data not shown). Therefore, both IL-12 secreted by α-GalCer–presenting dendritic cells and IFN-γ secreted by α-GalCer–activated NKT cells might contribute to the secondary IFN-γ production by NK cells, which augmented the antitumor effect of an NKT cell–specific ligand, α-GalCer.
We thank Yasuhiko Koezuka and Kazuhiro Motoki (Pharmaceutical Research Laboratory, Kirin Brewery) for generously providing α-GalCer, and Mitsuhiro Matsuo and Kazuko Hayashi for technical assistance.
Supported by research grant from Human Frontier Science Program Organization; Y.H. was supported by the Uehara Foundation Research Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yoshihiro Hayakawa, Cancer Immunology, Peter MacCallum Cancer Institute, Locked Bag 1, A'Beckett St, Victoria 8006, Australia; e-mail: y.hayakawa@pmci.unimelb.edu.au.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal