The labeling kinetics of 5 dendritic cell (DC) subtypes within the lymphoid organs of healthy laboratory mice during continuous administration of bromodeoxyuridine (BrdU) was determined to investigate developmental relationships and determine turnover rates. Individual DC subtypes behaved as products of separate developmental streams, at least as far back as their dividing precursors. The rate of labeling varied with the lymphoid organ and the DC subtype. Labeling was faster overall in spleen and mesenteric lymph nodes (LNs) and slower in thymus and skin-draining LNs. The CD8+ DC subtype displayed the most rapid turnover, with a uniformly short (3-day) lifespan in spleen but with distinct short-lived and longer-lived subgroups in thymus. All the skin-derived DCs in LNs showed delayed and slow BrdU labeling, indicating a long overall lifespan; however, this was shown to reflect a long residence time in skin rather than a long-duration presenting antigen in the draining LN. Epidermal-derived Langerhans DCs displayed longer BrdU labeling lag and slower overall turnover than the dermal-derived DCs, and the movement of fluorescent Langerhans DC from skin to LN was slower than that of dermal DCs following skin painting with a fluorescent dye. However, once they arrived in lymphoid organs, all DCs present in healthy, uninfected mice displayed a rapid turnover, and this turnover was even faster after antigenic or microbial product stimulation.
Introduction
Dendritic cells (DCs) collect, transport, and process antigens, finally presenting them to T lymphocytes as peptide fragments on major histocompatibility complex (MHC) molecules.1,2 All DC subtypes serve this basic function but differ in antigen processing,3,4 in migratory patterns,1,2,5-8 and in the nature of the response they induce in T cells.9-14 The lifespan of different DC subtypes within lymphoid organs, where they interact with T cells, is therefore one determinant of how long an antigenic stimulus persists and what type of immune response will result. In this study we use continuous bromodeoxyuridine (BrdU) labeling15 16 to determine the lifespan of DC subtypes in the lymph nodes (LNs), thymus, and spleen of steady-state, uninfected adult laboratory mice. The rate of labeling of the DCs, or rather the converse rate of loss of unlabeled cells, serves as a measure of DC lifespan. Because mature DCs are nondividing end-cells, the DNA precursor BrdU initially enters dividing precursors, then flows into mature DCs. Accordingly, the kinetics of labeling also provides information on the developmental history of the DC subtypes and on any precursor-product relationships between them.
We have already demonstrated that all 3 subtypes of mature DCs in spleen have a remarkably short lifespan and are products of independent streams of development.16,17 This limited persistence in the spleen of individual antigen-presenting cells would limit the duration of a splenic T-cell response and has implications for the maintenance of T-cell memory. However, it has been claimed18 that certain skin-derived DCs survive for a long time in LN, allowing persistent T-cell stimulation in LN following an antigen pulse. Therefore, we extended our studies to LNs, where we delineated 2 DC subtypes in addition to those found in spleen; these arrive in LNs through the lymph and include the mature form of skin Langerhans cells.8 We confirm the data of Ruedl et al,18 who found that the skin-derived DCs in LNs display slow labeling with BrdU. This led them to conclude that skin-derived DCs have a long lifespan in LN. However, experiments on the potentially migratory DCs in skin itself led us to a different interpretation. We found the overall long lifespan of skin-derived DCs reflects a long residence time in skin, not in LN. We found that the lifespan of DCs in normal laboratory mice varies, depending on the DC subtype and the lymphoid organ, but in all cases the residence time of mature DCs within the lymphoid organs themselves was short.
Materials and methods
Mice
Mice were bred under specific pathogen-free conditions at the Walter and Eliza Hall Institute (WEHI) and were used at 6 to 9 weeks of age. Most experiments used female mice of the C57BL/6J WEHI strain. The T-cell receptor (TCR)–transgenic mice used were ovalbumin (OVA)–specific, MHC class II–restricted (OT-II) mice19provided by Dr W. Heath (WEHI).
Antigen and adjuvant stimulation of mice
OT-II mice were injected intravenously with either OVA or bovine serum albumin (BSA; Sigma-Aldrich, Castle Hill, Australia) suspended in phosphate-buffered saline (PBS) (50 μg/mouse). Where noted, lipopolysaccharide (LPS) from Escherichia coli (5 μg/mouse, L3137; Sigma-Aldrich) was included in the OVA or BSA solution injected.
Lymphoid organs
Organs used for DC isolation and analysis were spleen, thymus, and LN—the latter were either mesenteric LNs, a pool of skin-draining LNs (axillary, brachial, and inguinal), or auricular LNs that drain ear skin.
Isolation of mature DCs
The procedure was as described recently.16,17 20Briefly, spleen, thymus, or LN fragments were digested for 20 minutes at room temperature with collagenase-DNase, then were treated for 5 minutes with EDTA (ethylenediaminetetraacetic acid). All subsequent procedures were performed at 0°C to 4°C in a divalent metal-free medium. Light-density cells were selected by centrifugation at 4°C in a 310 mOsm Nycodenz medium, with the density optimized for each tissue at 1.082 g/cm3 for LN, 1.077 g/cm3 for spleen, and 1.075g/cm3 for thymus. Cells other than DCs were then depleted by an immunogenic bead procedure. For spleen and LNs, the depleting mAbs were anti-CD3 (KT3), anti-Thy1 (T24/31.7), anti-B220 (RA3-6B2), anti Gr-1 (RB6-8C5), and antierythrocyte (TER-119); for thymus, in which the CD4+CD8− DCs were absent, the depleting mAb also included anti-CD4 (GK1.5), anti-F4/80 (F4/80), and anti-CD11b (M1/70). This DC preparation, approximately 80% pure, was then used for presorting.
Presorting to eliminate autofluorescent cells
DC preparations contained autofluorescent cells (5%-30%) that were not DCs but that could contaminate a DC sample.17Such autofluorescent cells, together with dead cells, were eliminated before immunofluorescent labeling by high-speed presorting. Dead cells were labeled by including propidium iodide (PI; 1 μg/mL) in the suspension medium. Nonfluorescent cells were then selected, as described previously.17 Residual PI was removed by washing before cells were stained and fixed.
DC migration from ear skin in culture
The procedure was modified from that of Schuler and Steinman,21 with the exit of DCs from the skin enhanced by chemokine according to the approach of Kellermann et al.22Ears were removed from 10 to 20 mice, cleared of hair, briefly washed in 70% ethanol, then placed ventral side down and split, removing the dorsal skin from the cartilage. Dorsal skin was placed split side down in 1 mL modified mouse osmolarity RPMI 1640 medium containing 10% fetal calf serum for 2 to 4 hours at 37°C in a 10% CO2in-air incubator to eliminate the non-DCs initially released. The skin was then transferred to another 1 mL culture medium, this time containing 0.1 μg recombinant mouse 6Ckine (R&D Systems, Minneapolis, MN). After 24-hour incubation at 37°C, the cells that had migrated to the culture medium were harvested and kept in cold medium. The skin was transferred to fresh warm medium containing 6Ckine and then was incubated for another 24 hours. Cells that migrated from the skin over both incubations were pooled. The yield was 3 to 6 × 104 DCs per ear.
Immunofluorescence labeling of DCs
Monoclonal antibodies, fluorescence conjugates, and labeling procedures have been specified previously.8,16,17,20 To identify and sort all DCs, the pan-DC markers used were high levels of MHC class II or CD11c. Anti-CD11c (N418) was used as Cy5, phycoerythrin (PE), or Alexa594 conjugates. Anti-MHC class II (N22 or M5/114) was used as Cy5 or Alexa 594 conjugates; conjugation levels were less than maximal to ensure the strong staining for MHC class II did not cause inaccurate color compensation. Markers used to separate the DC subpopulations were CD8α, CD4, and DEC-205 (CD205). Anti-CD8α (YTS169.4), anti-CD4 (GK1.5), and anti–DEC-205 (NLDC-145) were used as PE, Cy5, or Alexa 594 conjugates. The second-stage stain for biotin-conjugated mAbs was Alexa594- or Cy5-streptavidin. Permeabilization and staining of cells for the cytoplasmic domain of langerin using the HD24 mAb was as specified previously.8
Bromodeoxyuridine administration and immunofluorescence labeling
The procedure was similar to that developed for T cells.15 Groups of 4 to 8 mice were initially injected intraperitoneally with 1 mg bromodeoxyuridine (BrdU) (Sigma, St Louis, MO) in saline and then continuously given BrdU (0.8 mg/mL) in sterile drinking water that was changed daily. After various times, the DCs were isolated, presorted to remove autofluorescent cells, and stained as above. Control DCs from mice not given BrdU were isolated in parallel. Surface-stained DCs were washed, resuspended in cold 0.15 M NaCl, and fixed by drop-wise addition of cold 95% ethanol. They were incubated on ice for 30 minutes, washed with PBS, and incubated for 30 minutes at room temperature with PBS containing 1% paraformaldehyde and 0.01% Tween 20. Control DCs were then pelleted, incubated for 10 minutes at room temperature with 50 U DNase I (Boehringer Mannheim, Germany) in 0.15 M NaCl containing 4.2 mM MgCl2, and washed. They were then incubated for 30 minutes at room temperature with fluorescein isothiocyanate (FITC)–conjugated anti-BrdU mAb (Becton Dickinson, San Jose, CA), washed, and resuspended in PBS.
Flow cytometric analysis of bromodeoxyuridine-labeled cells
BrdU-stained and surface marker–stained DCs were analyzed on a FACStar Plus flow cytometer (Becton Dickinson). Data were gated for MHC class II+ DCs, and those gated DCs were divided into subsets based on the expression of CD8α, CD4, and DEC-205, as described elsewhere.8 17 The distinction between the BrdU-positive and -negative FITC fluorescence made use of background control DCs from mice not treated with BrdU but stained with anti-BrdU antibody. A computational technique (described inhttp://www.wehi.edu.au/cytometry/Abstracts/AFCGOOB.html) was used to subtract the background histogram, enumerating the upper percentage of cells in the fluorescence-positive cell population (Figure1).
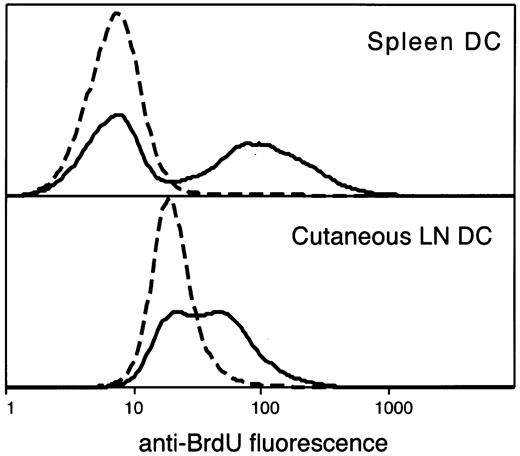
Segregation of BrdU-labeled and unlabeled DCs.
DCs were isolated, stained for DC surface markers and intracellular BrdU, and analyzed by flow cytometry gating on the CD11c+ MHC class IIhi population. Results are typical of those after 2 days of continuous BrdU administration for spleen DCs and 10 days for LN DCs. The continuous line indicates the BrdU-fluorescence staining after BrdU administration, and the broken line indicates the background staining of DCs from mice not administered BrdU but with identical, side-by-side, isolation and staining protocol.
Segregation of BrdU-labeled and unlabeled DCs.
DCs were isolated, stained for DC surface markers and intracellular BrdU, and analyzed by flow cytometry gating on the CD11c+ MHC class IIhi population. Results are typical of those after 2 days of continuous BrdU administration for spleen DCs and 10 days for LN DCs. The continuous line indicates the BrdU-fluorescence staining after BrdU administration, and the broken line indicates the background staining of DCs from mice not administered BrdU but with identical, side-by-side, isolation and staining protocol.
Fluorescence labeling of DCs in ear skin
Results
Separating labeled from unlabeled DCs
In our BrdU administration protocol, BrdU was injected intraperitoneally at the beginning to ensure dividing precursors had immediate access to the label, and continued availability was ensured by the inclusion of BrdU in the drinking water. It was important that all DCs that acquired BrdU-labeled DNA be registered as positive cells. This was readily attained for splenic DCs (Figure 1), in which BrdU-labeled DCs were clearly separated from the background fluorescence of unlabeled DCs; there was nevertheless a little overlap, which resulted in a completely labeled DC population only registering 97% positive. However, with LN DCs (Figure 1) and thymic DCs, the intensity of fluorescence per labeled DC was lower, indicating their immediate dividing precursors differed and had less access to the label. This resulted in more overlap between background and positive cell fluorescence. Rather than increase BrdU dose, which would produce toxic effects, we developed a computational technique that fitted the background histogram to the lower component of the positive sample fluorescence, then subtracted this, leaving the upper component of BrdU-positive cells to be enumerated. This allowed a more precise estimate of the percentage of labeled DCs than by making an arbitrary cutoff between positives and negatives.
Labeling kinetics of different lymphoid organs
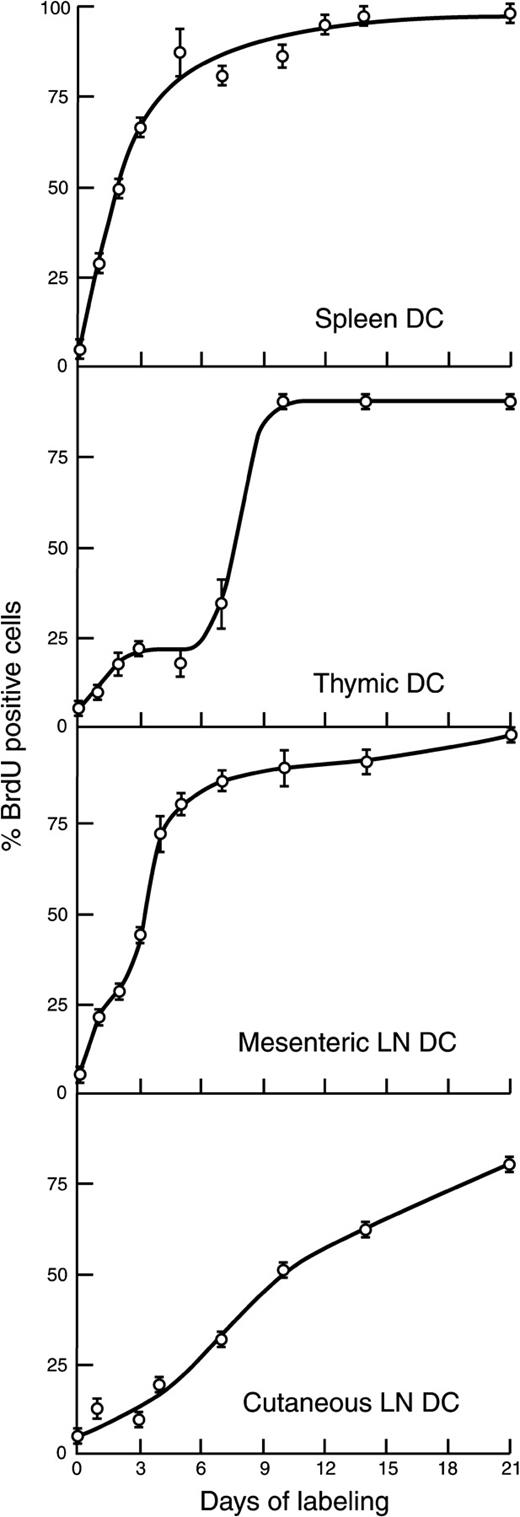
BrdU-labeling kinetics and thus turnover times of the total DCs of different lymphoid organs were compared (Figure2). A short (2-hour) pulse of BrdU gave only marginal labeling, indicating that the DCs were not themselves dividing. Splenic DCs showed the fastest input of labeled cells, without any detectable lag, indicating either immediate generation from dividing precursors in the spleen or rapid replenishment through the bloodstream. Most unlabeled splenic DCs disappeared rapidly, indicating a short half-life within the organ. However, a small proportion of splenic DCs showed a longer lifespan because some unlabeled cells persisted 9 to 12 days.
BrdU-labeling kinetics of the total DC population of different lymphoid organs.
BrdU was administered continually to groups of 4 to 6 mice, and the DCs were isolated at different times from the different pooled lymphoid organs. DCs were surface stained for DC markers, then permeabilized and stained for BrdU incorporated into DNA. DC suspension was then analyzed by flow cytometry, gating for CD11c+MHC class IIhi DCs. Percentages of DCs labeled with BrdU were then determined from profiles similar to those in Figure 1. A parallel group of control mice without BrdU administration provided the DCs for the background control. Results represent pooled data from 3 to 5 separate kinetic experiments. The point near zero time was a 2-hour pulse of BrdU, which should label any DC in cell cycle; later points represent the accumulation of labeled cells from dividing precursors.
BrdU-labeling kinetics of the total DC population of different lymphoid organs.
BrdU was administered continually to groups of 4 to 6 mice, and the DCs were isolated at different times from the different pooled lymphoid organs. DCs were surface stained for DC markers, then permeabilized and stained for BrdU incorporated into DNA. DC suspension was then analyzed by flow cytometry, gating for CD11c+MHC class IIhi DCs. Percentages of DCs labeled with BrdU were then determined from profiles similar to those in Figure 1. A parallel group of control mice without BrdU administration provided the DCs for the background control. Results represent pooled data from 3 to 5 separate kinetic experiments. The point near zero time was a 2-hour pulse of BrdU, which should label any DC in cell cycle; later points represent the accumulation of labeled cells from dividing precursors.
Thymic DCs showed a different labeling pattern (Figure 2). Initially, the accumulation of labeled DCs was as rapid as in spleen, but this reached a plateau and only 20% of the DC population had been replaced by day 3. After an apparent lag, the remaining 80% of the thymic DC population was labeled by day 10. This 2-phase labeling kinetics was consistent over several separate experiments and persisted even when BrdU was repetitively administered by intraperitoneal injection.
Labeling kinetics of LN DCs was particularly interesting because the usual model of DC life history predicted that immature DCs would first spend time as “sentinels” in nonlymphoid tissue before entering the LN. The labeling pattern obtained varied with the LN studied (Figure 2). Mesenteric LN DCs showed rapid labeling with no evidence of a lag. In contrast, the total DCs in the LN draining cutaneous tissue showed some lag in the initial labeling and a more gradual replacement of unlabeled with labeled cells.
Rate of BrdU labeling of different subtypes of thymic DCs
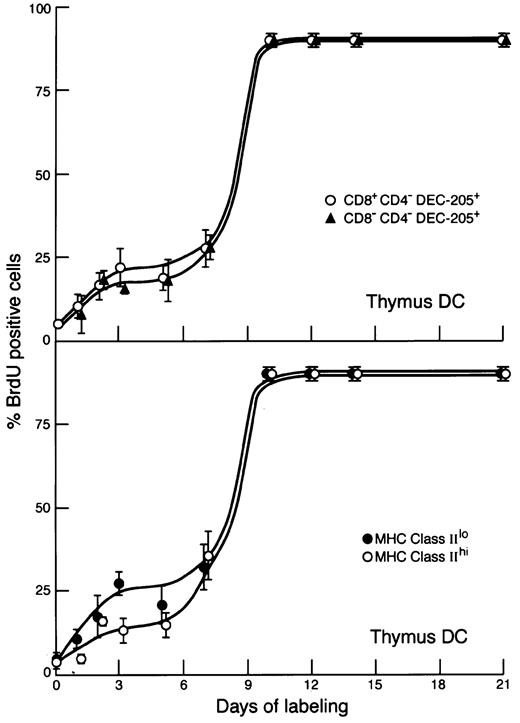
The pronounced break in the thymic DC BrdU-labeling curve suggested heterogeneity in this population. Thymic DCs can be segregated into a major group showing bright staining for CD8α and those showing only moderate staining; the latter do not produce CD8 themselves but pick up CD8αβ from the thymocytes.17Both these DC populations are DEC-205+. On segregating thymic DCs by CD8α staining, little difference was observed in BrdU labeling; both populations showed the 2-phased labeling kinetics (Figure 3). This reinforces the view that they are related cells. Another possible segregation of thymic DCs is into approximately 50% positive and 50% negative for the early B-cell marker BP-1.24 However, at several time points, there was no difference in the BrdU labeling of these 2 subsets.
BrdU-labeling kinetics of subtypes of thymic DCs.
Conditions were similar to those for Figure 2, except that the thymic DCs were subdivided based on surface marker differences. Upper graph: the 60% staining brightest with anti-CD8α (CD8α+) versus the 30% showing the lowest staining (CD8α−). Lower graph: the 50% showing brightest staining with anti-MHC class II(hi) versus the 50% showing less bright staining (lo). In MHC class II staining, the distribution was continuous and the division arbitrary; other cutoff points between low and high MHC class II expression gave similar results. Results are pooled from 4 separate kinetic-labeling experiments.
BrdU-labeling kinetics of subtypes of thymic DCs.
Conditions were similar to those for Figure 2, except that the thymic DCs were subdivided based on surface marker differences. Upper graph: the 60% staining brightest with anti-CD8α (CD8α+) versus the 30% showing the lowest staining (CD8α−). Lower graph: the 50% showing brightest staining with anti-MHC class II(hi) versus the 50% showing less bright staining (lo). In MHC class II staining, the distribution was continuous and the division arbitrary; other cutoff points between low and high MHC class II expression gave similar results. Results are pooled from 4 separate kinetic-labeling experiments.
Another explanation for a discontinuous labeling curve would be discontinuity in the maturation process of a single DC lineage. As label flows into nondividing DCs, the upstream MHC class IIlo DCs should label ahead of the more mature downstream MHC class IIhi DCs. Accordingly, thymic DCs were divided into those showing the lowest level of expression of surface MHC class II versus those showing the highest level of expression. This was an arbitrary division because the fluorescence distribution was continuous. As predicted, label flowed more rapidly into the thymic DCs with the lowest surface MHC class II than into those with the highest. However, this did not explain the biphasic labeling curve because both subgroups showed biphasic labeling (Figure 3). Similar results were obtained regardless of the gate set between high and low MHC class II fluorescence. This labeling of less mature DCs a little ahead of more mature DCs, but without a definite discontinuity between the 2, was also seen for splenic DCs overall and for less mature versus more mature forms of all individual subsets of splenic DCs (data not shown).
Labeling kinetics of individual DC subtypes in spleen
A key issue was whether DC lifespan was dictated by the environment of the individual lymphoid organ or whether DC subtypes showed independent behavior. Spleen DCs can be segregated into 3 subtypes—CD4+8−DEC-205−, CD4−CD8−DEC-205−, and CD4−CD8+DEC-205+.17Of the 3.7 ± 0.5 × 106 DCs recovered per spleen, these subtypes constituted 56% ± 3%, 19% ± 4%, and 23% ± 1%, respectively. As we demonstrated previously16 and confirm in Figure4, the individual DC subtypes in spleen showed distinct labeling patterns. All labeled continuously from the beginning without any evidence of a lag. The CD4−CD8+DEC-205+ DCs showed the fastest turnover in spleen (half-life, 1.5 days) with a linear “first in–first out” labeling pattern. The CD4+CD8− DCs showed the slowest turnover (half-life, 2.9 days), with some DCs persisting longer than others, suggesting a stochastic process. We used these data for comparison with the DC subtypes in other lymphoid organs.
BrdU-labeling kinetics of subtypes of DCs within different peripheral lymphoid organs.
Conditions were similar to those for Figure 2, except that the DCs were subdivided into discrete subtypes as detailed in the text. Symbols coding the subtypes are the same for all graphs. Results are pooled from 3 (mesenteric LNs) to 5 (spleen and cutaneous LNs) separate kinetic experiments.
BrdU-labeling kinetics of subtypes of DCs within different peripheral lymphoid organs.
Conditions were similar to those for Figure 2, except that the DCs were subdivided into discrete subtypes as detailed in the text. Symbols coding the subtypes are the same for all graphs. Results are pooled from 3 (mesenteric LNs) to 5 (spleen and cutaneous LNs) separate kinetic experiments.
Comparison of the labeling kinetics of the common DC subtypes in LN, spleen, and thymus
The 3 populations of DCs found in spleen can also be distinguished in LN, though in LN there is low (less than 5%) representation of the CD4+CD8− DCs.8 Accordingly, for the LN DC-labeling data in Figure 4, this population is omitted. Of the 1.6 ± 0.5 × 105 total DCs recovered from mesenteric LNs, CD8+DEC-205+ DCs constituted 19% ± 7%, and CD8−DEC-205− DCs constituted 41% ± 3%; of the 3.7 ± 0.9 × 105total DCs recovered from pooled cutaneous-draining LN, they constituted 17% ± 6% and 21% ± 2%, respectively. In LN, as in spleen, the CD8+DEC-205+ DCs labeled faster, and hence had a shorter lifespan, than the CD8−DEC-205−DCs. In LN, as in spleen, there was no evidence of an initial labeling lag in either of these DC subtypes. However, the rate of labeling of both DC subtypes varied with the LN studied. It was more rapid in mesenteric LN than in cutaneous-draining LNs. The labeling pattern of the CD8+DEC-205+ DCs in particular differed between the LNs and was almost as rapid as spleen in mesenteric LNs but was similar to thymus, with a biphasic labeling curve, in cutaneous-draining LNs. The total time for most of the CD8+DEC-205+ DCs to be replaced was approximately 3 days in spleen, 4 days in mesenteric LN, and 9 days in thymus and cutaneous-draining LN.
Lifespan of DCs derived from skin
Several groups, using different combinations of markers, have identified in cutaneous-draining LNs the mature forms of the DCs that migrated from the skin.18,23,25 We have segregated in cutaneous-draining LNs 2 skin-derived DC subtypes.8 One is CD4−CD8− but expresses a moderate level of DEC-205, in contrast to the CD4−CD8− DCs in spleen; this we identified as a dermal-derived population, probably similar to other interstitial DCs. It represents 20% ± 5% of the DCs in the cutaneous-draining LNs. A population equivalent to this is found in mesenteric LN,8 where it represents 26% ± 6% of the DCs. A second DC subtype, only found in cutaneous-draining LN, is CD4−CD8loDEC-205hi and expresses the Langerhans cell marker langerin8; we identified this DC as epidermal derived, the mature form of skin Langerhans cells. It represents 33% ± 11% of the DCs in cutaneous-draining LNs. We have found DCs corresponding to both these LN DC subtypes in approximately equal numbers in the cells that migrate out of skin on culture and have shown that they can be separated based on the level of expression of DEC-205 or of langerin.8 The BrdU-labeling kinetics of these LN-restricted DC subtypes is shown in Figure 4.
Both LN-specific DC subtypes in cutaneous-draining LNs showed a pronounced initial lag and a slower labeling rate than the DCs common to LN and spleen. However, the CD4−CD8−DEC-205lo subset in mesenteric LN showed a much faster turnover than their equivalents in the cutaneous-draining LN. The DC population showing the greatest lag and the slowest labeling was the CD4−CD8loDEC-205hi subset restricted to cutaneous LN, the putative mature form of Langerhans cells. The labeling pattern was not continuous even after the initial 4-day lag, and a reduced labeling rate was apparent after 9 days. Even by 21 days, only 40% of the unlabeled CD4−8loDEC-205hi DCs had been replaced by BrdU-labeled cells. This finding of an exceptionally slow turnover agrees with other groups who have used different criteria to identify the Langerhans cell DC lineage.18 25
Comparison of the labeling kinetics of potential migratory skin DCs with the skin-derived DCs in LNs
Does the slow labeling of skin-derived DCs in LN mean these DCs persist longer in the LN after migration? To test this, we compared the BrdU-labeling kinetics of the LN DCs with that of the DCs migrating out of cultured ear skin. Note that the ears were taken from mice that had received BrdU administration for various times before culture, so this procedure simply sampled the pre-existent labeling behavior of potentially migratory DCs within the skin under steady-state conditions. As shown in Figure 5, the DEC-205lo and especially the DEC-205hi DC subsets exiting the skin showed a marked initial lag in labeling, explaining much of the lag in the labeling of their counterparts in cutaneous-draining LN.
Comparison of the BrdU-labeling kinetics of potential skin emigrant DCs with the corresponding DC subtypes in skin-draining LN.
At various times after the administration of BrdU to mice, the incorporation of label into cutaneous LN DCs was determined as in Figure 4. At similar times (and usually from the same mice), the ear skin was removed, then cultured in a medium containing 6Ckine. DCs exiting over a 2-day period were collected and separated into the 2 subtypes, and the incorporation of BrdU was determined. Results for the skin emigrant DCs represent pooled data from 3 separate kinetic experiments.
Comparison of the BrdU-labeling kinetics of potential skin emigrant DCs with the corresponding DC subtypes in skin-draining LN.
At various times after the administration of BrdU to mice, the incorporation of label into cutaneous LN DCs was determined as in Figure 4. At similar times (and usually from the same mice), the ear skin was removed, then cultured in a medium containing 6Ckine. DCs exiting over a 2-day period were collected and separated into the 2 subtypes, and the incorporation of BrdU was determined. Results for the skin emigrant DCs represent pooled data from 3 separate kinetic experiments.
DEC-205int DCs (putative dermal-derived DCs) obtained from the skin by culture showed a moderate rate of labeling after the initial lag, similar to counterpart DEC-205int DCs in cutaneous-draining LNs but preceding them by 2 to 3 days (Figure 5). For more direct comparison, the DCs of the small ear-skin draining auricular LNs were also isolated. These showed 67% labeled DEC-205int DCs at 10 days and 77% ± 1% labeling at 14 days, values close to those of the pooled cutaneous LN DEC-205int DCs. These comparisons suggest the lifespan of the dermal-derived DCs, once having exited the skin and entered the draining LN, is short (approximately 2-3 days).
The DEC-205hi DC (putative epidermal-derived Langerhans cells) obtained from the skin by culture showed exceptionally slow and puzzling labeling kinetics (Figure 5). After an initial 3- to 5-day lag, there was a brief increase in labeling rate, then a distinct slow labeling phase. Only 27% of these potentially migratory DCs were labeled by 14 days, well below the 60% labeling achieved by the corresponding DCs in the pooled cutaneous-draining LN. To check this discrepancy, the DEC-205hiCD8lo in the small ear skin draining auricular LN were isolated, providing more direct comparison. These DCs showed 47% labeling at 10 days and 55% ± 2% labeling at 14 days, values similar to those for equivalent DC subsets in the pooled cutaneous LN. Accordingly, it appears the lifespan of the potentially migratory Langerhans cells in the epidermis itself is long but variable and can last much longer than 2 weeks. Langerhans cells that, under steady state, exit the epidermis to seed the skin-draining LN thus appear to be shorter-lived than most of those that exit cultured ear skin under the influence of 6-Ckine. Because the 2 approaches did not sample the same population, the actual residence time of the DEC-205hiCD8lo DCs within the draining LN could not be determined. However, it appears to be much shorter than the average residence time of Langerhans cells within the skin.
Tracking DCs from skin to LN
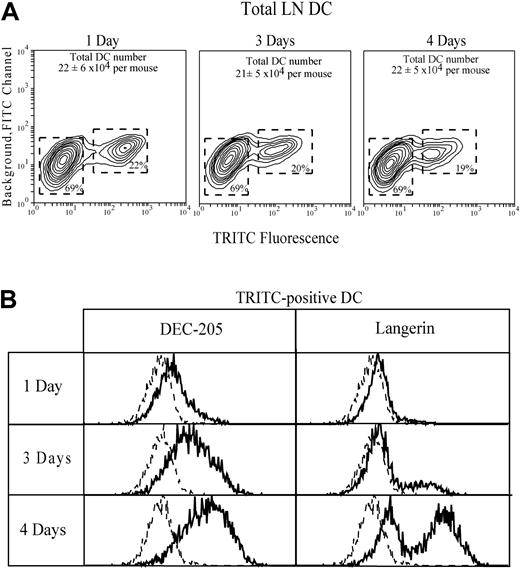
To verify our identification of cutaneous-draining LN CD4−CD8loDEC-205hi and CD4−CD8−DEC-205int as skinderived epidermal Langerhans DCs and dermal DCs, respectively, the ear skin was first painted with TRITC. One to 4 days later, the TRITC-positive cells in the draining auricular LN were then characterized with the use of an intracellular stain for langerin as an additional marker to positively identify progeny of Langerhans cells.8 26 A discrete population of TRITC+DCs, representing approximately 20% of the total DCs, was obtained (Figure 6). Their phenotype was identical whether all TRITC+ cells, or only those with the highest TRITC fluorescence, were characterized. At early time points these cells were DEC-205int langerin−, suggesting a preponderance of dermal DCs. After 3 days, DEC-205hilangerin+ cells appeared in the TRITC+population, and they dominated by 4 days, indicating that the labeled epidermal Langerhans DC were the slowest to migrate to the auricular LN. This delay in movement of TRITC-labeled epidermal Langerhans DCs from skin to LN, compared with dermal DCs, is consistent with their greater lag in the BrdU-labeling curves of Figure 5. The rate of BrdU labeling of the TRITC-marked DCs in the auricular LN was then assessed after 14 days of continuous BrdU administration and 4 days after the ear skin was painted with TRITC. These TRITC+ DCs were 48% ± 10% positive for BrdU, a value close to that of the total CD4−CD8loDEC-205hi putative epidermal-derived Langerhans DC subset in normal steady-state mice not painted with TRITC.
Tracking the movement of DCs from skin to LN by labeling the skin with a fluorescent dye.
The ear skin was painted with TRITC to mark DCs and DC precursors. Identification of skin-derived TRITC-positive gated DCs in the draining auricular LN is shown (A). DC recovery from the auricular LN of control mice before ear painting was 15 × 104 DCs per mouse, a value lower than but not statistically significant different from the recovery from ear-painted mice. The expression of DEC-205 and Langerin by the gated TRITC-positive DC is shown (B); the broken line gives the background, omitting only the specific mAb. Data represent one of 3 experiments giving similar results.
Tracking the movement of DCs from skin to LN by labeling the skin with a fluorescent dye.
The ear skin was painted with TRITC to mark DCs and DC precursors. Identification of skin-derived TRITC-positive gated DCs in the draining auricular LN is shown (A). DC recovery from the auricular LN of control mice before ear painting was 15 × 104 DCs per mouse, a value lower than but not statistically significant different from the recovery from ear-painted mice. The expression of DEC-205 and Langerin by the gated TRITC-positive DC is shown (B); the broken line gives the background, omitting only the specific mAb. Data represent one of 3 experiments giving similar results.
Effect of T-cell, antigen, and microbial stimuli on DC turnover
All the preceding results were with normal mice not subject to deliberate antigenic or microbial stimuli. It was notable that although most DCs turned over rapidly in spleen and mesenteric LN, a proportion appeared to have a longer lifespan because some DCs remained unlabeled beyond 1 week. This might have resulted from a few DCs having received life-prolonging signals during an immune response. The possibility of microbial products or antigen-activated T cells prolonging the lifespan of the DCs already present in the animal was tested in several ways, in each case evaluating changes in splenic DC turnover by measuring the labeling rate around the crucial 2-day time point. In the first experiment, the DCs of RAG-1 null mice were studied because these mice lack T cells. This absence of T cells (and B cells) had only a small effect on the apparent DC turnover rate as measured by BrdU labeling at 2.5 days—for total DCs, it was 60% in the control mice and 69% in the RAG-1 null mice. In the second experiments, antigenic and microbial stimuli were applied to TCR-transgenic, OVA-specific OT-II mice, and the turnover of the splenic DCs was studied. As shown in Table1, injection of the specific antigen OVA into OT-II mice did not prolong the apparent lifespan of any of the DC subtypes; rather, it increased the labeling rate and, hence, the turnover compared to the controls. As we found previously,16 bacterial LPS also increased DC turnover (Table 1). Combined antigen-LPS stimulation gave results similar to that of LPS alone. These conclusions also applied to the individual splenic DC subtypes. Therefore, these aspects of an active immune response to infection seem unlikely to extend the lifespan of the resident DCs but seem likely to increase their rate of turnover.
Effect of a microbial stimulus and antigen-mediated T-cell stimulation on splenic DC lifespan
| Stimulus . | % BrdU-positive cells, day 2 . | |||
|---|---|---|---|---|
| Total DC . | CD4−CD8−DC . | CD4+CD8−DC . | CD4−CD8+DC . | |
| Uninjected | 23 ± 5 | 18 ± 4 | 12 ± 4 | 39 ± 5 |
| OVA | 36 ± 10 | 34 ± 9 | 24 ± 4 | 68 ± 6 |
| OVA and LPS | 48 ± 8 | 35 ± 8 | 50 ± 1 | 69 ± 4 |
| BSA | 27 ± 6 | 29 ± 13 | 21 ± 8 | 39 ± 6 |
| BSA and LPS | 55 ± 4 | 46 ± 5 | 50 ± 1 | 69 ± 1 |
| Stimulus . | % BrdU-positive cells, day 2 . | |||
|---|---|---|---|---|
| Total DC . | CD4−CD8−DC . | CD4+CD8−DC . | CD4−CD8+DC . | |
| Uninjected | 23 ± 5 | 18 ± 4 | 12 ± 4 | 39 ± 5 |
| OVA | 36 ± 10 | 34 ± 9 | 24 ± 4 | 68 ± 6 |
| OVA and LPS | 48 ± 8 | 35 ± 8 | 50 ± 1 | 69 ± 4 |
| BSA | 27 ± 6 | 29 ± 13 | 21 ± 8 | 39 ± 6 |
| BSA and LPS | 55 ± 4 | 46 ± 5 | 50 ± 1 | 69 ± 1 |
OVA-specific, TCR-transgenic TO-II mice were injected intravenously with 50 μg OVA or BSA as a control, with or without 5 μg bacterial LPS. Concurrently, the mice were given 1 mg BrdU intraperitoneally followed by continuous administration of BrdU-treated water for 2 days. DCs were then isolated and BrdU labeling was determined.
Discussion
The kinetics of BrdU labeling by the different DC subtypes provides basic information on 2 aspects of their biologic nature, namely turnover rates and developmental relationships. Regarding possible precursor-product relationships, a slow-labeling subtype cannot be the direct precursor of a rapidly labeling subtype of similar incidence unless division intervenes, and the DCs we isolated did not divide. In addition, provided the subtypes show a clear discontinuity in the expression of markers used to segregate them, an upstream subtype of a single lineage should show an immediate accumulation of labeled cells, whereas a downstream subtype should show a labeling lag. However, as emphasized previously,17 there was no sign of a lag or any precursor-product relationship in the labeling kinetics of the 3 major DC subtypes in spleen, and each behaved as the product of a separate developmental stream. Even though the 2 DC subtypes in cutaneous-draining LNs did show a marked lag in accumulation of BrdU-labeled cells, it did not imply they were the downstream products of the faster labeling DCs within the LN itself. Rather, it reflected their independent origin in the dermis and epidermis of the skin, with their immediate upstream precursors responsible for the labeling lag to be found in these tissues. Thus, the different DC subtypes we have segregated all appear to derive from different developmental streams, at least as far back as their dividing precursors. The stages of hemopoietic development at which these streams diverge remain to be established.
In the spleen the differences in BrdU-labeling kinetics correlates well with the DC subtypes segregated by surface phenotype. However, in the thymus, marked discontinuity in the overall BrdU-labeling kinetics could not be correlated with any DC subsets defined by surface phenotype, at least using our current markers. Even though the thymic DCs showing lower surface levels of MHC class II did label a little ahead of those showing higher levels, as expected, this appeared to be a rapid and continuous transit and did not explain the 2-phase labeling kinetics. One group of approximately 20% thymic DCs showed the same rapid 3-day turnover as splenic DCs, whereas the remainder showed a labeling lag. It took approximately 9 days for these unlabeled thymic DCs to disappear. We have not established the basis of this heterogeneity in turnover rates. However, its persistence with different routes and levels of BrdU administration suggests it is not a technical artifact of the labeling procedure. The more slowly labeled major population might derive from endogenous precursors, with a nondividing immature intermediate accounting for the labeling lag. The more rapidly labeled major population might derive from dividing or rapidly labeled DC precursors entering through the bloodstream, as we propose for spleen. Whatever the explanation, similar labeling discontinuity occurs among the CD8α+ DCs of cutaneous-draining LN.
The DC subtype with the fastest turnover in all organs is the DC expressing high levels of CD8α. These had originally been considered long-lived, lymphoid-tissue resident DCs, but the converse appears to be the case; reasons the earlier studies were misleading have been discussed elsewhere.16 Langerhans cells have been reported to express CD8α after arrival in LN,27 and indeed we find low levels of CD8α on the putative mature forms of this lineage.8 It has been suggested these could transform into CD8hi DCs. However, the labeling kinetics makes it clear that in the normal LN, the slow-labeling CD8loLangerhans-type DC does not transform into the faster labeling CD8hi population. In addition, CD8α+ DCs are not found among either dermal- or epidermal-derived DCs exiting cultured ear skin.8 This suggests the CD8αhiDCs always have a different life history and might arrive in spleen and LN directly from the bloodstream or, alternatively, might be derived from an endogenous precursor population.
In addition to the differences in the rate of turnover between different DC subtypes, it is clear that the environment associated with the individual lymphoid organs influences DC lifespan. DC turnover for all individual DC subtypes is faster in spleen than in mesenteric LN and faster in mesenteric LN than in cutaneous-draining LN. The higher rate of DC turnover in the mesenteric compared to cutaneous-draining LN might reflect a higher level of stimuli in the normal gut compared to the normal skin of a clean, noninfected laboratory mouse. However, our results indicate that in steady state there is a continual transit of DCs into the skin-draining LNs from the dermis and the epidermis, even when there is no obvious stimulus inducing an exit from skin. These results on turnover rate in steady state are generally in accordance with the earlier studies of Salomon et al25 and Ruedl et al,18 in showing that the DCs that migrate from the skin, and particularly the DCs of the Langerhans cell lineage, have an exceptionally long lifespan as measured from the last dividing precursor to loss, presumably by death, from the draining LN.
Despite this agreement on the long overall lifespan of skin-derived DCs, we disagree with the conclusion of Ruedl et al18 that these DCs have a long lifespan in the LN. This is an important issue because this conclusion would imply that mature Langerhans cells and dermal-derived DCs in LN could persist and present antigen for much longer periods than other DC types, thereby prolonging T-cell responses. To check this we compared in some detail the BrdU-labeling kinetics of the DCs within cutaneous-draining LN with that of the potentially migratory DCs in skin, sampling these by inducing their exit from ear skin on culture and segregating the dermal-derived DCs from the epidermal-derived Langerhans cell DCs.
The first point was that the DCs sampled from skin displayed an initial labeling lag similar to that of their corresponding subtypes in the LN, indicating that most of this lag represented the time for the label to transit from the dividing precursors to nondividing precursors to potentially migratory DCs with the skin itself and did not represent developmental changes within the LN. The labeling lag was greater for the epidermal Langerhans cell DCs than for the dermal DCs. This fits well with the studies of DC dynamics following painting the ear skin with fluorescent marker dye. These showed that for the bulk of the marked cells in the skin, which included a range of developmental states, the dermal DCs migrated from skin to LN more rapidly than the epidermal Langerhans DC, which showed a 3-day lag. Particular stimuli might reduce this lag and hasten the exit of Langerhans DCs.
After the initial BrdU-labeling lag, the rate of labeling of potentially migratory dermal and epidermal DCs in ear skin was slow, indicating that most of the overall long lifespan of these DC subtypes was spent within the skin itself. The slow entry of labeled cells into the LN did not reflect a slow turnover of DCs in the LN. Rather, it reflected the fact that the DCs arriving in the LN contained only a small proportion of labeled cells because of slow turnover in the skin of a large pool of immature cells. In the case of the DEC-205int putative dermal DCs, the labeling curve of potentially migratory cells in ear skin was similar in form to that of their counterparts in the LN, but they labeled 2 to 3 days earlier. This suggested that once they entered LNs, their remaining lifespan was short, approximately 2 to 3 days. In the case of DEC-205hiputative epidermal Langerhans DCs, the labeling rate of the potentially migratory cells in the ear skin was, surprisingly, much lower than that of their counterparts in the LN, including the draining auricular LN. Evidently the population of Langerhans cells induced to leave the epidermis in the 6Ckine-containing cultures included a much higher proportion of older, slow-turnover, or sessile cells than the population seeding the LN in steady state. Because of this evident difference in sampling, the lifespan of an epidermal-derived Langerhans cell, once it arrives in the LN, could not be accurately determined. However, given the very slow labeling rate of this DC population overall in the skin, it is clear that most of the slow entry of BrdU cells into the mature LN forms can be attributed to a slow turnover in the epidermis. Their remaining lifespan once they enter the LN is likely to be short, perhaps very short.
Our overall conclusion is that for the steady-state uninfected laboratory mouse, the residence time of a DC once it arrives in a lymphoid organ varies from short (9 days) to very short (1-2 days). The impact of T-cell or microbial stimuli did not prolong the lifespan of such pre-existent DCs but appeared to shorten their residence time in lymphoid organs (Table 1). This should place limits on the duration of a T-cell–mediated immune response, unless there is a continual input of antigen from another source. However, there is one important caveat to this conclusion. We have emphasized elsewhere28that certain DC subtypes are only generated from precursors as a result of microbial stimuli, and such DCs would not have been sampled in this study of uninfected laboratory mice. One such DC subtype is the progeny of the type 1 interferon-producing, plasmacytoid DC precursor, a precursor that transforms into a DC only on viral or bacterial product stimulation.29 Systematic study of the lifespan of the novel DC populations induced by microbial invasion is now needed to determine whether these could provide a long-lived, antigen-presenting, T-cell stimulus.
Supported by the World Health Organization Special Program for Research and Training in Tropical Diseases. The Wellcome Trust provided the funds for the MoFlo cell sorter.
A.T.K. and S.H. contributed equally to the study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ken Shortman, Immunology Division, The Walter and Eliza Hall Institute of Medical Research, Post Office Royal Melbourne Hospital, Victoria, 3050, Australia.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal