Abstract
The inefficiency of gene transfer has greatly hindered gene therapy. In vivo selection may increase the frequency of genetically modified cells, thereby circumventing this critical limitation. Here we demonstrate regulated in vivo selection in a large animal. CD34+ cells from 2 dogs were engineered to express a conditional derivative of the thrombopoietin receptor (F36Vmpl). Activation of the receptor through administration of a dimerizing drug, AP20187, produced reversible, drug-dependent rises in genetically modified red cells, white cells, and platelets in both animals, with minimal side effects. Cell growth switches could greatly enhance the efficacy and applicability of gene and cell therapy.
Introduction
Stem cell gene therapy has demonstrated unequivocal efficacy in only a single published trial, for the treatment of X-linked severe combined immunodeficiency,1 2 where replacement of a missing receptor subunit allows pro–T cells to complete development in the thymus. This success is attributable to the tremendous selective advantage conferred upon corrected lymphocytes relative to their unmodified counterparts. However, for most diseases, little or no selective advantage in favor of the genetically corrected cell population can be anticipated.
Several strategies have been devised for generating a selective pressure that favors genetically modified cells and can be applied in vivo. Initial reports in rodent models used genes that confer resistance to cytotoxic drugs. Selectable markers that have been evaluated include dihydrofolate reductase (DHFR), which confers resistance to folate analogues, including trimetrexate3; multidrug-resistance gene 1 (MDR1), which confers resistance to a number of drugs, including taxol4; and 06-methylguanine methyl transferase (MGMT), which confers resistance to alkylating agents, such as BCNU (1,3-bis(2-chloroethyl)-1-nitrosourea).5 Attempts to use drug resistance genes for in vivo selection in large animal models6-8 and in phase 1 clinical trials9-13have been complicated by the resistance of hemopoietic progenitor and stem cells to many cytotoxic agents14,15 and by the considerable side effects associated with cytotoxic drug administration.6 8
We have developed an alternative method for positive selection16,17 that employs a derivative of the thrombopoietin receptor (mpl) to deliver a conditional growth signal to genetically modified cells in response to a nontoxic drug called a chemical inducer of dimerization (CID). The mpl signaling domain is incorporated into a fusion protein (F36Vmpl) that remains functionally inert unless directed to dimerize through administration of the CID. The F36Vmpl fusion therefore acts as a “cell growth switch” that is turned on in the presence of CID and off following withdrawal of CID. In previous studies we have shown that CID-regulated activation of mpl allows transduced murine progenitor cells to be dramatically expanded both in vitro18-20 and in vivo.21
Dogs provide a well-established preclinical model for testing cell and gene therapies,22-25 and results obtained in the dog model can be considered predictive of results in humans. We therefore tested whether CIDs could regulate the growth of F36Vmpl-engineered hemopoietic cells in the dog.
Materials and methods
Animals
The dogs were raised and housed at the Fred Hutchinson Cancer Research Center under conditions approved by the American Association for Accreditation of Laboratory Animal Care. The dogs were provided with commercial chow and chlorinated tap water ad libitum. They were observed for disease for at least 2 months before the study and were immunized for papillomavirus, leptospirosis, distemper, hepatitis, and parvovirus. Marrow draws were performed under general anesthesia. The animals received broad-spectrum antibiotics and recombinant canine granulocyte colony-stimulating factor after transplantation until the absolute neutrophil count exceeded 1000/μL. As preparation for transplantation, the animals received a single myeloablative dose of 920 cGy total body irradiation administered from 2 opposing60Cobalt sources at 7 cGy per minute.
CD34 enrichment of bone marrow cells
Prior to marrow cell harvest, both animals were treated with canine stem cell factor (25 μg/kg body weight subcutaneously, once daily) and canine granulocyte colony-stimulating factor (5 μg/kg body weight subcutaneously, twice daily) for 5 consecutive days.
Bone marrow buffy-coat cells were labeled with biotinylated monoclonal antibody 1H6 (immunoglobulin G1 anti–canine CD34). The cells were washed twice, incubated with streptavidin-conjugated microbeads, washed, and then separated by an immunomagnetic column technique used according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA).
Collection of virally conditioned media (VCM) and determination of viral titer
Retroviral supernatant was collected in Dulbecco modified Eagle medium (DMEM) supplemented with 20% fetal bovine serum (FBS) and 1% Pen/Strep from subconfluent monolayers of F36VmplGFP/PG13 producer cells after incubation for 48 hours at 33°C.
To determine the titer, 5 × 104 HeLa cells were plated in 6-well plates (Corning) in DMEM supplemented with 10% FBS and 1% Pen/Strep. After incubation for 24 hours, the medium was replaced by 1 mL VCM diluted 1:10 in DMEM containing Polybrene at a final concentration of 4 μg/mL. After 6 hours, 1 mL of fresh DMEM was added. After an additional 18 hours, the mixture of VCM and DMEM was exchanged. Cells were cultured for an additional 48 hours, trypsinized, and analyzed by flow cytometry. Titer was determined according to the following formula: 5 × 104 cells plated × 2 (after initial 24 hours of culture before transduction) × 10 (to correct for 1:10 dilution of VCM) × fraction GFP-positive (for the VCM used here, between 0.09 and 0.14). Titer of VCM was determined to be 0.9 × 105 for the first animal and 1.3 × 105 for the second animal.
Transduction of enriched bone marrow cells
CD34+ cells were transduced by established methods26 with the following modifications. Transductions were carried out on a Retronectin-coated surface (Retronectin was generously provided by Takara Shuzo, Kyoto, Japan) twice preloaded with VCM. After 48 hours of prestimulation using canine granulocyte colony-stimulating factor, canine stem cell factor, and human Flt3-ligand (50 ng/mL), the cells were pelleted, resuspended in VCM, and cultured for 4 hours in a 5% CO2 37°C incubator. The cells were then centrifuged and resuspended in human Dexter medium. The procedure was repeated the following day. Immediately following the second exposure to VCM (after a total culture period of approximately 72 hours), cells were reinfused into the irradiated autologous recipient. The absence of replication-competent helper virus was confirmed by PCR for GALV-envelope sequences, using DNA from peripheral blood leukocytes isolated at various time points after transplantation.
AP20187 administration
AP20187 (www.ariad.com/regulationkits) was formulated in 5% solutol at a concentration of 10 mg/mL and stored at 4°C. On the day of injection, an appropriate volume of AP20187 stock solution (1 mL for the first dog and 1.3 mL for the second dog) was diluted to a total volume of 30 mL in 0.9% sodium chloride and injected intravenoulsy over 15 minutes. Each course of AP20187 was administered at a dose of 1 mg/kg twice daily for 30 days.
Flow cytometric analysis
Flow cytometric quantification of at least 20 000 events (gated by forward and right-angle light scatter and excluded for propidium iodide [1 mg/mL] was performed on a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ). Analysis of flow cytometric data was performed with CELLQuest v3.1f software with gating to exclude fewer than 0.1% control cells in the relevant region. Monoclonal antibodies conjugated to phycoerythrin, which had been shown to bind canine CD markers CD3, CD21, and CD14, were used to study canine T cells, B cells, and monocytes, respectively. Antibody DM5 was used to examine dog granulocytes. For intracellular staining, cells were fixed and permeabilized with Intrastain reagents (DAKO, Carpinteria, CA). Antibodies to CD79 (Clone HM57, DAKO) and terminal deoxynucleotidyl transferase (Supertechs, Bethesda, MD) were used.
Results
To test whether the F36Vmpl fusion can generate a proliferative signal in canine hemopoietic cells, we transduced CD34-selected marrow cells with a PG-13 packaged murine stem cell virus (MSCV)–based bicistronic retroviral vector encoding a green fluorescent protein (GFP) reporter and the F36Vmpl fusion (F36VmplGFP).21 Following transduction, cells cultured in the presence of a CID (AP20187, 100 nM) expanded up to 34-fold relative to transduced cells cultured in the absence of CID, and prominent selection of GFP-expressing cells was observed (data not shown). These data confirmed that the F36VmplGFP vector could generate a proliferative signal in canine hemopoietic cells, and we proceeded to studies of CID-mediated in vivo selection.
CID-mediated in vivo selection of genetically modified red blood cells, white blood cells, and platelets
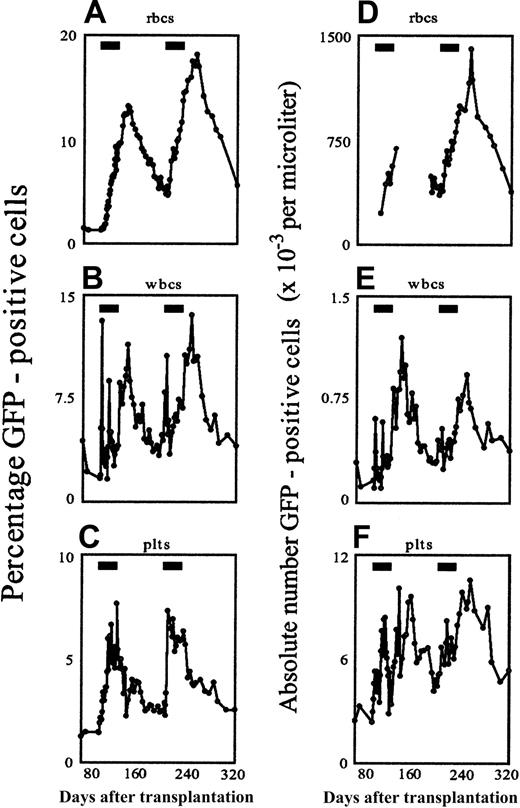
The first dog was a 9-month-old female beagle. Marrow cells were harvested, CD34-selected and transduced using the F36VmplGFP vector, then reinfused into the lethally irradiated autologous recipient. Engraftment was uneventful, and by week 13 after transplantation, stable frequencies of GFP-marked red cells, white cells, and platelets had been achieved in the peripheral blood (all in the range of 1.3%). We then began administration of AP20187 at a dose of 1 mg/kg intravenously twice daily for 30 consecutive days. AP20187 treatment stimulated a striking transient elevation in peripheral hemopoietic cells of multiple lineages, including red cells, white cells, and platelets (Figure1). The most significant responses to AP20187 occurred in GFP+ red cells (Figure 1A), which steadily rose from 1.3% at baseline to 9.5% by the end of drug treatment before peaking at 13.2% more than 2 weeks later. Thereafter, GFP+ red cells gradually declined to 5.2% 76 days after completion of the 30-day course. The earliest indicator of a response to AP20187 occurred among GFP-positive white cells (Figure 1B), which rose to 4.3% by day 2 and 10.5% by day 3 before falling rapidly to 2% to 3% despite continuation of the drug. Standard forward/side scatter parameters indicated that this early spike arose from a wave of GFP-positive granulocytes (data not shown). A similar, albeit less pronounced, spike (to 7.2%) occurred on day 16 of drug treatment, and then GFP-labeled white cells remained in the range of 2% to 4% until the drug treatment was discontinued. Unexpectedly, discontinuation of AP20187 prompted a further rise in GFP-positive white cells, to 6.9% by day 33 (3 days after completion of AP20187 treatment) and 9.2% by day 47, before they gradually fell to the range of 3% to 4% by week 6 after treatment. GFP-labeled platelets also steadily rose in response to AP20187, reaching 7.3%, then promptly fell following discontinuation of AP20187, stabilizing in the range of approximately 2% (Figure 1C). CID administration achieved not only a relative but also an absolute increase in transduced circulating blood cells (Figure 1D-E).
In vivo selection in the peripheral blood in response to CID, depicted as relative (A-C) and absolute (D-F) changes in GFP-positive cells over time.
CD34-selected marrow cells were transduced with the F36VmplGFP vector and infused into the lethally irradiated autologous recipient as described.26 AP20187 administration for 30 consecutive days (indicated by the black rectangles) was begun in weeks 13 and 28 following the infusion of transduced cells, and the percentage of GFP-expressing cells was monitored by flow cytometry. Absolute cell numbers of GFP-positive red cells (D), white cells (E), and platelets (F) are expressed as 10−3 cells/μL blood. In panel D, for technical reasons, some data points are missing before, during, and after the first treatment cycle.
In vivo selection in the peripheral blood in response to CID, depicted as relative (A-C) and absolute (D-F) changes in GFP-positive cells over time.
CD34-selected marrow cells were transduced with the F36VmplGFP vector and infused into the lethally irradiated autologous recipient as described.26 AP20187 administration for 30 consecutive days (indicated by the black rectangles) was begun in weeks 13 and 28 following the infusion of transduced cells, and the percentage of GFP-expressing cells was monitored by flow cytometry. Absolute cell numbers of GFP-positive red cells (D), white cells (E), and platelets (F) are expressed as 10−3 cells/μL blood. In panel D, for technical reasons, some data points are missing before, during, and after the first treatment cycle.
CID-mediated effects in the marrow
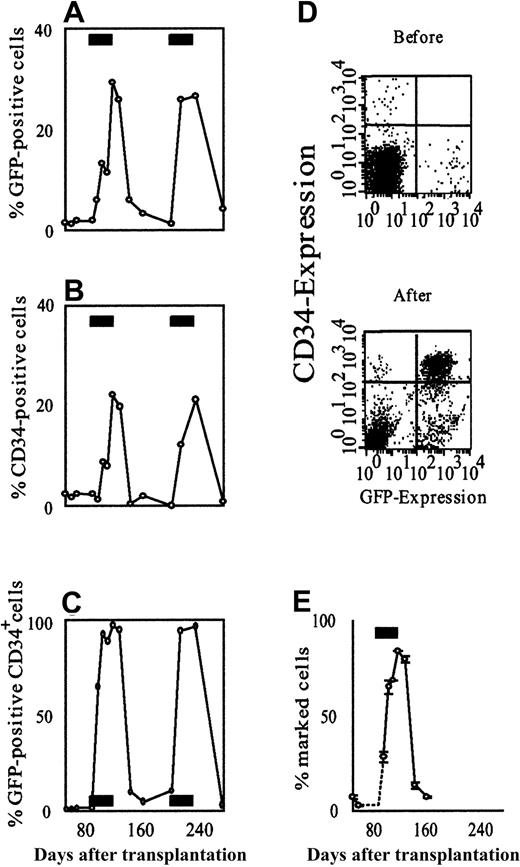
Responses to AP20187 were even more evident in the marrow (Figure2). At baseline, 1.8% of nucleated cells in the marrow were GFP positive, whereas by the end of drug treatment 29.4% of all nucleated cells were GFP labeled. Effects among CD34+ cells were even more prominent. The frequency of CD34+ cells expressing GFP rose from a baseline of 1.4% to 65% after 7 days of drug treatment, reaching a peak of 96.8% by the end of CID treatment. The absolute number of GFP+/CD34+ cells also rose dramatically, such that by the end of the 30-day course of CID they constituted more than 21% of all nucleated cells in the marrow. Five weeks after the drug was stopped, 3.3% of nucleated cells and 4.8% of CD34+cells were GFP positive, and CD34+/GFP+double-positive cells constituted fewer than 0.2% of all nucleated cells in the marrow. We confirmed selection of marked cells in the marrow independently, using quantitative real-time polymerase chain reaction (PCR; Figure 2E) and Southern blot (data not shown). A more detailed analysis of the CD34+/GFP+ double-positive population indicated that it was constituted of pro–B cells, as described further below. A preliminary analysis of integration sites using a highly sensitive linear amplification-mediated (LAM)-PCR method27suggested that multiple clones contributed to the CID response (data not shown).
AP20187 increases the fraction of GFP-positive cells in the bone marrow.
Sequential flow cytometric analysis of GFP-expressing total nucleated cells (A), CD34-expressing cells (B), and GFP-expressing CD34+ cells (C) shows a reversible, CID-dependent increase in the fraction of transduced cells. The effect is most pronounced in the CD34+ cell compartment. (D) Scatter plots of bone marrow cells obtained before and after AP20187 administration. GFP expression is shown on the x-axis, CD34-expression on the y-axis. These plots demonstrate the substantial expansion of CD34/GFP double-positive cells in response to CID. (E) Selection was confirmed on the DNA level by quantitative real-time PCR. Each data point in panel E represents the mean of 2 independent measurements, each carried out in duplicate. Error bars depict the range between the 2 experiments. The black rectangles in panels A-C and E indicate the periods of AP20187 treatment.
AP20187 increases the fraction of GFP-positive cells in the bone marrow.
Sequential flow cytometric analysis of GFP-expressing total nucleated cells (A), CD34-expressing cells (B), and GFP-expressing CD34+ cells (C) shows a reversible, CID-dependent increase in the fraction of transduced cells. The effect is most pronounced in the CD34+ cell compartment. (D) Scatter plots of bone marrow cells obtained before and after AP20187 administration. GFP expression is shown on the x-axis, CD34-expression on the y-axis. These plots demonstrate the substantial expansion of CD34/GFP double-positive cells in response to CID. (E) Selection was confirmed on the DNA level by quantitative real-time PCR. Each data point in panel E represents the mean of 2 independent measurements, each carried out in duplicate. Error bars depict the range between the 2 experiments. The black rectangles in panels A-C and E indicate the periods of AP20187 treatment.
Persistent responsiveness to retreatment with CID
To evaluate the persistence of CID responsiveness, we administered a second identical course of AP20187 treatment to the same animal, beginning 78 days after completion of the first cycle (Figure 1). Repeated treatment reproduced virtually all of the features seen with the first drug cycle, including the initial wave of GFP-positive white cells (mainly neutrophils) peaking on day 4 of drug retreatment and the step-up in GFP-positive white cells following drug discontinuation, reaching a maximal frequency of 10.9%. Similarly, GFP-positive platelets rose to a maximal value of 5.8%, while GFP-positive red cells reached a peak frequency of 18%. The effect of AP20187 retreatment on GFP-positive cells in the marrow also closely mirrored the response to the initial course of treatment (Figure 2). At the end of the second 30-day course of AP20187, 26.6% of nucleated cells in the marrow were GFP positive, while 96.2% of CD34+ cells in the marrow were GFP positive.
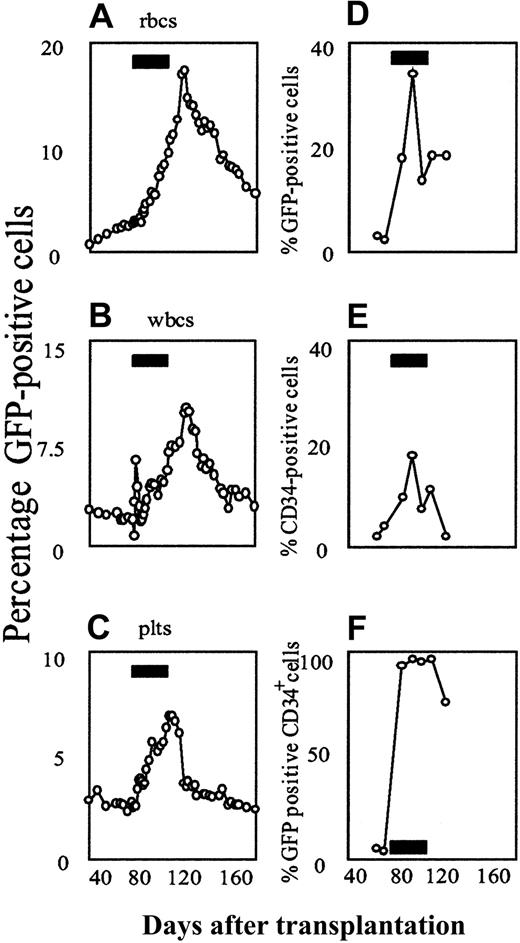
To evaluate the reproducibility of these findings, we treated a 24-month-old male beagle, using an identical protocol. By 10 weeks after transplantation, a stable frequency of GFP-positive cells in the blood and marrow was established and the 30-day course of AP20187 was begun. Results in the second animal closely mirrored those in the first animal, both in the peripheral blood and in the bone marrow (Figure3).
Flow cytometric analysis of AP20187-mediated in vivo selection of F36VmplGFP-transduced cells in the second dog.
(A-C) Peripheral blood. (D-F) Bone marrow. The animal received autologous, retrovirally transduced marrow CD34+ cells, using the same transplantation and drug treatment protocol as described for the first animal. Black rectangles indicate the period of AP20187 treatment.
Flow cytometric analysis of AP20187-mediated in vivo selection of F36VmplGFP-transduced cells in the second dog.
(A-C) Peripheral blood. (D-F) Bone marrow. The animal received autologous, retrovirally transduced marrow CD34+ cells, using the same transplantation and drug treatment protocol as described for the first animal. Black rectangles indicate the period of AP20187 treatment.
CID treatment produces a substantial rise in transduced B cells
A more detailed analysis of the AP20187-expanded CD34+/GFP+ bone marrow cells was carried out (Figure 4). Cytospin preparations of fluorescence-activated cell sorting (FACS)–sorted CD34+/GFP+ bone marrow cells from the first animal revealed a lymphoblastic morphology (Figure 4A). Sorted CD34+/GFP+ cells lacked clonogenicity, with colonies arising from only 0.06% of cells plated in semisolid media. In contrast, sorted CD34+/GFP− from the same dog and CD34+ cells from a normal dog formed colonies at expected frequencies of 10% and 4.8%, respectively. Finally, CD34+/GFP+ bone marrow cells expressed terminal deoxyribonucleotidyl transferase (Tdt; Figure 4B) and CD79 (data not shown), consistent with their identity as pro–B cells. The disappearance of these cells from the marrow at the end of CID treatment was associated with a sustained rise in CD21+/GFP+ leukocytes (ie, B cells) in the peripheral blood (Figure 4C). Subset analysis of peripheral blood leukocytes from the first dog showed peak GFP marking levels of 4.3% among monocytes, 6.4% among granulocytes, and 7.3% among T cells. Peak marking reached 39% after the first drug cycle in the first animal. In the second dog, peak marking in B cells reached even higher levels, of up to more than 50% (Figure 4C).
CD34/GFP double-positive cells emerging in response to CID treatment have a B-lymphoid phenotype.
(A) Cytospin of sorted cells showing a lymphoid morphology (× 1000). (B) Scatter plots of CD34/GFP double-positive cells from the marrow of the second dog (red dots) demonstrate staining with an antibody to terminal deoxynucleotidyl transferase, but no staining with an isotype control antibody. (C) The time course of GFP-positive cells among peripheral blood B cells in response to CID treatment in the second dog. The black rectangle indicates the period of AP20187 treatment. (D) Homing of GFP-positive B cells to a popliteal lymph node. Shown is the deep pole of a germinal center in a popliteal lymph node (100 × objective) stained with a rabbit polyclonal antibody directed against GFP. Black arrows indicate immunoblasts, black arrowheads indicate apoptotic figures, and white arrows indicate mitotic figures, all of which are typical of a germinal center reaction. Brownish stain indicates GFP positivity.
CD34/GFP double-positive cells emerging in response to CID treatment have a B-lymphoid phenotype.
(A) Cytospin of sorted cells showing a lymphoid morphology (× 1000). (B) Scatter plots of CD34/GFP double-positive cells from the marrow of the second dog (red dots) demonstrate staining with an antibody to terminal deoxynucleotidyl transferase, but no staining with an isotype control antibody. (C) The time course of GFP-positive cells among peripheral blood B cells in response to CID treatment in the second dog. The black rectangle indicates the period of AP20187 treatment. (D) Homing of GFP-positive B cells to a popliteal lymph node. Shown is the deep pole of a germinal center in a popliteal lymph node (100 × objective) stained with a rabbit polyclonal antibody directed against GFP. Black arrows indicate immunoblasts, black arrowheads indicate apoptotic figures, and white arrows indicate mitotic figures, all of which are typical of a germinal center reaction. Brownish stain indicates GFP positivity.
To determine whether GFP-positive B cells emerging in response to AP20187 were capable of trafficking to lymphoid tissue, a lymph node biopsy was performed one month after completion of the first drug cycle, when marking in B cells was declining. Flow cytometry showed that among CD21+ B cells, 20% expressed GFP, whereas only 5% of CD3+ T cells were GFP positive. A lymph node biopsy from the second dog also yielded very similar results (data not shown). Immunohistochemistry revealed a substantial number of GFP-positive cells, particularly in the deep pole of germinal centers (Figure 4D). In aggregate, these results indicate that AP20187 induced the expansion of transduced pro–B cells that subsequently expressed CD21, circulated in peripheral blood and finally homed to lymph nodes. This expansion of B cells was unanticipated on the basis of our previous studies in mice.21
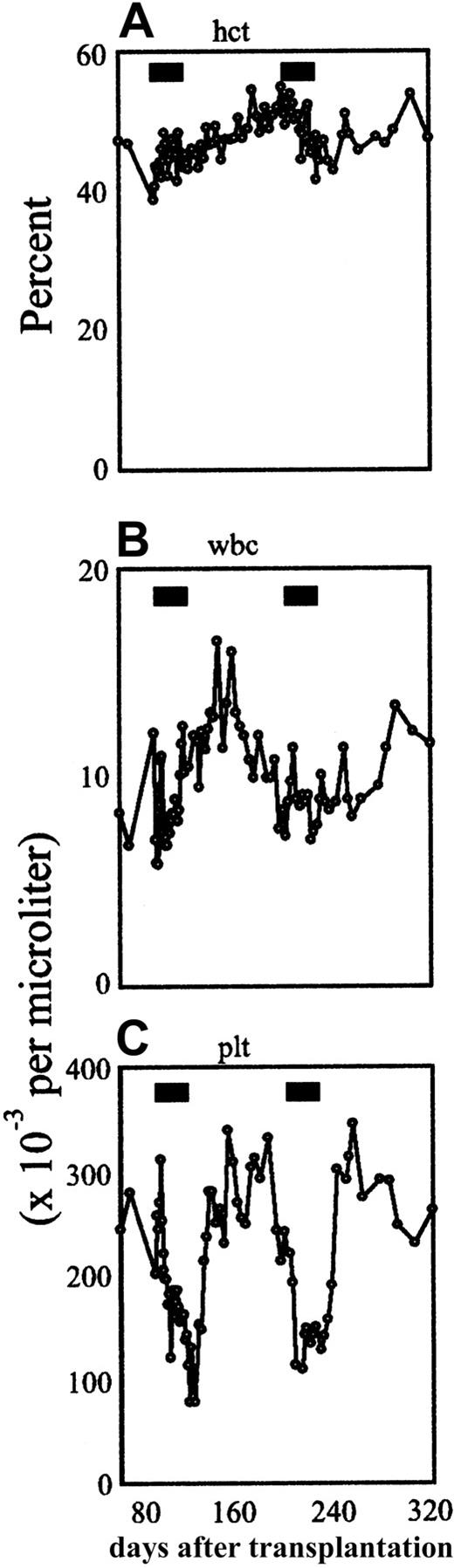
Treatment with AP20187 was associated with minimal side effects. Serial complete blood counts (Figure 5) revealed reversible, mild to moderate thrombocytopenia beginning during the second week of AP20187 treatment, with platelet counts falling to minimal levels of 50 000/μL. No bleeding or other adverse effects were observed. Clinical chemistries and tests of liver and kidney function were performed weekly throughout drug treatment and the results remained normal.
Effects of AP20187 administration on hematological indices.
Time course of hematocrit (A), absolute white cell count (B), and absolute platelet count (C) in the peripheral blood in the first animal. The black bars indicate the treatment periods.
Effects of AP20187 administration on hematological indices.
Time course of hematocrit (A), absolute white cell count (B), and absolute platelet count (C) in the peripheral blood in the first animal. The black bars indicate the treatment periods.
Discussion
These findings provide the first demonstration of CID-regulated in vivo selection of genetically modified cells in a large animal. Selection was most prominent in red blood cells and in B lymphoid cells. The pronounced proliferative effect of mpl signaling on immature B lymphoid cells was unexpected. Experiments in nonhuman primates are ongoing and will be helpful in determining whether this phenomenon, not observed in the mouse, is specific to the canine system. While no serious toxicities were encountered in the present study, long-term observation will be required to evaluate the potential development of malignancy. In this regard, the observed expansion of immature B cells is unnecessary for most applications, with the possible exception of inherited immunodeficiencies. It will be of interest to evaluate the effect of sequences derived from receptors other than mpl on various lineages in the canine system. In vitro data in experiments using primary murine cells suggest that dimerizer-mediated activation of different receptors produces profoundly different outcomes.20 Alternatively, the clinical application of this system may depend on developing vectors that direct expression of the growth switch in a lineage-specific manner.
We chose to demonstrate the feasibility of CID-mediated selection in the setting of myeloablative conditioning. Ultimately, for the treatment of nonmalignant diseases by stem cell gene therapy, it would be desirable to reduce or eliminate cytotoxic conditioning. Conferring a selective proliferative advantage on the transduced cell population may help to achieve this goal. Experiments testing the feasibility of combining reduced conditioning with in vivo selection are warranted.
Recently, substantial progress has been made toward the development of gene therapy vectors for the treatment of hemoglobinopathies such as β-thalassemia and sickle cell disease.28,29 The magnitude and duration of the response in red cells observed in this study would be predicted to have a beneficial effect in patients with β-thalassemia.30These data suggest that incorporating the cell growth switch into a globin vector combined with repeated rounds of CID treatment could lead to clinically relevant levels of genetically corrected red blood cells. In this regard it is important that a related CID, AP1903, has been shown to be safe and is well tolerated in humans.31
In the present study, mild thrombocytopenia was the only side effect of AP20187 administration, and this was promptly reversed on withdrawal from CID treatment (Figure 5). Preliminary investigations suggest that the thrombocytopenia may be a direct side effect of the drug or its solutol-based carrier. It is also possible that the thrombocytopenia arose as an indirect consequence of mpl signaling, either by inducing platelet aggregation (causing artifactual thrombocytopenia) or by inducing the elaboration of cytokines that inhibit platelet production. Of note, preliminary studies using the same AP20187 dose and formulation in nonhuman primates have not produced thrombocytopenia, suggesting that this side effect may be specific to the canine model (data not shown).
The present data demonstrate that the combination of a nontoxic small-molecule drug and a cell growth switch allows for pharmacologically regulated in vivo selection in a clinically relevant large animal model. The use of cell growth switches opens up the possibility for stem cell gene therapy to become feasible for the wide array of diseases for which corrected cells enjoy no selective advantage. Derivatives of this approach have the potential to make gene therapy effective for disorders beyond the hemopoietic system. For example, a recent study has demonstrated the feasibility of using CIDs to regulate the growth of genetically modified myoblasts.32 Finally, cell growth switches might play a fundamental role in unleashing the enormous therapeutic potential of stem cells. A number of recent reports suggest that stem cells from a variety of different tissues retain a surprising degree of plasticity,33 while human embryonic stem cells are capable of generating any tissue.34 In theory, it should be possible to equip stem cells with growth switches that are expressed in a tissue-specific manner. Expression of the growth switch upon elaboration of the desired cell type might allow specific stem cell–derived tissues to be expanded either in vitro or in vivo. The data presented here provide a platform for improving the safety and efficacy of gene therapy. Growth switches may help to fulfill the promise of gene and cell therapy.35
The authors would like to thank David Dalgarno and John Iuliucci for advice; Marilyn Skelly, M. Jane Chladny, David Dalgarno, Katy Dougherty, Melissa C. Richman, Bobbie M. Thomasson, and Kathrin Bernt for technical assistance; Leonard W. Rozamus and Terence Keenan for AP20187; and Eric Bell, Michelle Spector, Alix Joslyn, and the staff of the canine facility of the Fred Hutchinson Cancer Research Center.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2002-03-0792.
Supported by the National Institutes of Health (grants DK 52997, DK 57525, DK 61844, HL 53750, DK 55820, HL 36444, DK 56465, and DK 47754), a fellowship grant from the German Krebshilfe to P.A.H., and a fellowship grant from Cooley's Anemia Foundation to T.N.
Two of the authors (T.C., S.W.) are employed by a company whose technology is featured in the present work.
H.-P.K. and C.A.B. contributed equally to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
C. Anthony Blau, Division of Hematology, Department of Medicine, University of Washington, Box 357710, Seattle, WA 98195; e-mail: tblau@u.washington.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal