Abstract
Fanconi anemia (FA) is a rare autosomal recessive disease, characterized by bone marrow failure and cancer predisposition. So far, 8 complementation groups have been identified, although mutations in FANCA account for the disease in the majority of FA patients. In this study we characterized the hematopoietic phenotype of a Fanca knockout mouse model and corrected the main phenotypic characteristics of the bone marrow (BM) progenitors using retroviral vectors. The hematopoiesis of these animals was characterized by a modest though significant thrombocytopenia, consistent with reduced numbers of BM megakaryocyte progenitors. As observed in other FA models, the hematopoietic progenitors from Fanca−/− mice were highly sensitive to mitomycin C (MMC). In addition, we observed for the first time in a FA mouse model a marked in vitro growth defect ofFanca−/−progenitors, either when total BM or when purified Lin−Sca-1+ cells were subjected to in vitro stimulation. Liquid cultures ofFanca−/−BM that were stimulated with stem cell factor plus interleukin-11 produced low numbers of granulocyte macrophage colony-forming units, contained a high proportion of apoptotic cells, and generated a decreased proportion of granulocyte versus macrophage cells, compared to normal BM cultures. Aiming to correct the phenotype of Fanca−/−progenitors, purified Lin−Sca-1+ cells were transduced with retroviral vectors encoding the enhanced green fluorescent protein (EGFP) gene and human FANCAgenes. Lin−Sca-1+ cells fromFanca−/−mice were transduced with an efficiency similar to that of samples from wild-type mice. More significantly, transductions with FANCA vectors corrected both the MMC hypersensitivity as well as the impaired ex vivo expansion ability that characterized the BM progenitors ofFanca−/−mice.
Introduction
Fanconi anemia (FA) is a rare autosomal recessive disease characterized by developmental abnormalities, bone marrow (BM) failure, and predisposition to cancer, predominantly acute myeloid leukemia.1,2 To date, 8 complementation groups have been identified (FA-A, C, E, D1, D2, E, F, and G), and 6 FA genes have already been cloned: FANCA,3,FANCC,4,FANCD2,5,FANCE,6,FANCF,7 andFANCG.8 Mutations in the FANCA gene account for the disease in about 60% to 70% of all FA patients.1 2
Although the physiological role of FA proteins is still not well understood, protein interaction studies have shown that FANCA, C, E, F, and G form a functional complex.9 Interestingly, recent studies have shown that this complex is involved in the ubiquitination of FANCD2, which then interacts with the breast cancer susceptibility protein BRCA1,10 thus indicating a link between the FA protein complex and the BRCA1 repair machinery.
To understand the pathogenesis of FA and to facilitate the development of therapeutic approaches for FA, knockout mice with a targeted disruption in 3 FA genes (Fancc, Fanca, andFancg) were generated.11-15 These animals reproduced the chromosomal instability to DNA cross-linking agents and compromised gametogenesis observed in human FA patients, but only mild hematopoietic defects were observed in these animal models11-17 (also reviewed in Wong and Buchwald18).
In this study we describe a characteristic FA phenotype in the hematopoietic system of Fanca-deficient mice and show for the first time a genetic correction in the phenotype ofFanca−/− BM progenitors as a result of their transduction with retroviral vectors encoding the human FANCAgene.
Materials and methods
Animals
The generation of mice with a targeted disruption in the Fanconi anemia A gene has been recently described.13 These animals were back-crossed into the C57BL/6J strain at the Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas animal facility (registration number 28079-21 A). Mice were maintained under high-standard conditions (high-efficiency particulate air [HEPA]–filtered air, regulated temperature of 22°C, light/dark cycle of 12 hours, and food and ultraviolet-irradiated water ad libitum) and routinely screened for pathogens. All experimental procedures were carried out according to Spanish and European regulations (Spanish RD 223/88 and OM 13-10-89 of the Ministry of Agriculture, Food and Fisheries, for the protection and use of animals in scientific research; and European convention ETS-123, for the use and protection of vertebrate mammals used in experimentation and other scientific purposes). We used 8- to 10-week-old mice in our studies. Cells from the BM, spleen, and thymus were dispersed in Iscove modified Dulbecco medium (IMDM; Gibco Laboratories, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (Sigma Chemical, St Louis, MO) as described previously.
Flow cytometry
Prior to analysis erythrocytes were lysed for 10 minutes at room temperature in ammonium chloride lysis solution (0.155 mM NH4Cl + 0.01 mM KHCO3 + 10−4 mM EDTA [ethylenediaminetetraacetic acid]) and washed with PBA (phosphate-buffered saline [PBS]−1x + 0.1% bovine serum albumin [BSA] + 0.02% NaN3). For conducting differentiation analyses, BM and spleen cells were stained with B220 conjugated with fluorescein isothiocianate (FITC), CD3-FITC, Mac-FITC, Gr1-FITC, and TER-119–phycoerythrin (PE) monoclonal antibodies (MoAbs) (all from Pharmingen, Palo Alto, CA) to recognize B lymphocytes, T lymphocytes, monocytes, granulocytes, and erythroid cells, respectively. To identify primitive Lin−Sca-1+c-kit+ BM cells, samples were first stained with a cocktail of differentiation markers (Lin) containing CD3-biotinylated, B220-biotinylated, MAC-biotinylated, and GR1-biotinylated MoAbs and washed with PBA. Cells were then stained with Sca1-FITC (Pharmingen), c-kit–PE (Pharmingen), and streptavidin-tricolor (Caltag, Burlingame, CA) for 30 minutes at 4°C and washed again with PBA. Cells from the thymus were stained with CD3-biotinylated, CD4-FITC, and CD8-PE for 30 minutes at 4°C, washed with PBA, and then stained with streptavidin-tricolor for another 30 minutes at 4°C. Finally, cells were washed, resuspended in PBA with 2 μg/mL propidium iodide (PI), and analyzed in an EPICS XL flow cytometer (Coulter Electronics, Hialeah, FL). A minimum number of 104-105 viable cells were acquired. Off-line analysis was done with WinMDI free software package (a kind gift from Dr J. Trotter, The Scripps Research Institute, La Jolla, CA).
Lin−Sca-1+ purification
For the purification of Lin−Sca-1+cells, BM samples were subjected to red blood cell lysis and then sorted by using the MultiSort Kit (Miltenyi Biotec, Gladbach, Germany) following manufacturer's recommendations. Briefly, cells were stained with anti–Sca-1–FITC for 30 minutes at 4°C, washed with purification buffer (PB; PBS− 1x + 0.5% BSA), and stained with anti-FITC MultiSort MicroBeads for 15 minutes at 4°C. Cells were subjected to a positive immunomagnetic selection using an MS column type (Miltenyi Biotec). MultiSort MicroBeads were removed by incubation with the MultiSort release reagent for 10 minutes at 6°C to 12°C and then passed through a second magnetic column to remove unattached magnetic beads. Sca-1+ cells were then stained with a Lin cocktail with biotinylated antibodies against CD3, B220, TER119, GR-1, and Mac-1 for 30 minutes at 4°C, and washed and incubated again with streptavidin MicroBeads for 15 minutes at 6°C to 12°C. A third column was finally applied to remove the Lin+ cells. Lin−Sca-1+ cells were washed with PB and resuspended in IMDM. On average 89%-pure populations of Lin−Sca-1+ were obtained, the recovery being 30% to 60% of the input number of Lin−Sca-1+cells.
Clonogenic assays
To determine the number of granulocyte macrophage colony-forming units (CFU-GMs) progenitors present in total BM or spleen cells and in purified Lin−Sca-1+ cells, samples were plated in MethoCult GF M3534 culture media (StemCell Technologies, Vancouver, BC, Canada) at a concentration of 5 × 104 cells/plate and 1-2 × 103 cells/plate, respectively. Samples were cultured at 37°C in 5% CO2 and fully humidified air, and 7 days later colonies of at least 50 cells were scored under inverted microscope.19 For the determination of megakaryocyte colony-forming unit (CFU-Meg) progenitors, total BM and spleen cells were plated at 1 × 105 cells/plate using the MegaCult C medium (StemCell Technologies). Seven days later, megakaryocytic cells were stained with acetylcholinesterase, and megakaryocytic colonies were defined as aggregates of more than 3 large brownish cells.20 To determine the sensitivity of progenitor cells to mitomycin C (MMC), cells were cultured in MethoCult and MegaCult media containing increasing concentrations of the drug (up to 100 nM MMC).
In vitro expansion of BM cells
Total BM cells were seeded in IMDM supplemented with 20% FBS and 3 different combinations of growth factors: (1) hrIL-11 and mrSCF (kindly provided by Genetics Institute, Cambridge, MA); (2) mrSCF, mIL-3, and hrIL-6 (kindly provided by Immunex); (3) hrIL-11, mrSCF, and hrTPO (kindly provided by Kirin Brewery, Japan); and ProGP (Progenipoietin; dual hFlt3 and hG-CSF receptor agonist; kindly provided by Monsanto, St Louis, MO). Every 7 days, cells were counted, reseeded at the initial cell density, and CFU-GM progenitors evaluated as indicated above. In some experiments purified Lin−Sca-1+ cells were incubated in IMDM with 20% FBS, hrIL-11, and mrSCF at a concentration of 5 × 103 cells/mL. Every 3 days cells were counted and diluted to the initial cell density. All hematopoietic growth factors were used at 100 ng/mL, except thrombopoietin (TPO), which was used at 300 ng/mL. Under our experimental conditions, adherent layers were not observed in these cultures.
Apoptosis analysis
Apoptotic cells were assessed using Annexin-V–FITC (Pharmingen) or tetramethylrhodamine methyl ester (TMRM; Sigma). Annexin-V labeling was performed following manufacturer's instructions. TMRM was used to show loss of mitochondrial inner transmembrane potential associated with the early stages of apoptosis. Cells were incubated for 15 minutes at 37°C in 0.05 μM TMRM in PBS−, washed with cold PBS−, resuspended in PBS−, and kept on ice until analysis in the flow cytometer. Cells with damaged mitochondrial inner transmembrane potential were observed as a population with fluorescence lower than 580 nm.21
Retroviral vectors and packaging cell lines
Two different vectors based on the recombinant vector MSCV2.122 were used in this study. The vector LFAPEG expresses the open reading frame of the human FANCA gene under the control of the retroviral PCC4-cell–pasaged myeloproliferative sarcoma virus (PCMV) long terminal repeat (LTR), and the enhanced green fluorescent protein (EGFP)gene under the phosphoglycerokinase (PGK) promoters. The LPEG vector contains only the PGK promoter/EGFP expression cassette.23 For the generation of the ecotropic vectors, retrovirus-containing supernatants from stable PG13 packaging cells were used to transduce 293T cell–based ecotropic Phoenix cells (kindly provided by Dr Nolan, Stanford University, CA). EGFP+eco-Phoenix cells were sorted in the EPICS ELITE ESP flow cytometer (Coulter) and subsequently grown in IMDM supplemented with 10% FBS. Supernatants were harvested 24 hours after confluency, filtered through 0.45 μm, and used fresh. Titers ranged between 1 and 1.5 × 106 infective particles/mL, as deduced from the infection of NIH-3T3 cells with serial dilutions of retrovirus containing supernatants in the presence of 5 μg/mL polybrene (Sigma).24
Transduction protocol of Lin−Sca-1+cells
Fresh Lin−Sca-1+ cells were prestimulated for 48 hours in IMDM supplemented with 20% FBS, hrIL-11, and mrSCF. Prior to infection, plates were coated for 12 hours with 20 μg/cm2 of CH-296 (Retronectin, Takara Shuzo, Otsu, Japan) and washed with BSA 2% (wt/vol) in PBS.25 Immobilized fibronectin fragments were preloaded with retroviral particles as previously described.26 Finally, Lin−Sca-1+ cells were resuspended at a density of 5 × 104 cells/mL in fresh supernatants supplemented with 20% FBS (final concentration), hrIL-11, mrSCF, 5 μg/mL polybrene (Sigma), and added to the preloaded wells. A total of 4 infections spaced 12 hours apart were conducted. Cells were harvested 4 hours after the last infection cycle to analyze the transduction efficiency and also the MMC sensitivity and ex vivo expansion ability of the transduced cells.
Results
Characterization of the lympho-hematopoietic tissues ofFanca−/−mice
When peripheral blood cells were analyzed, only the platelet numbers were significantly lower inFanca+/+ (1 150 000 ± 320 000 platelets/μL) compared to age-matched Fanca−/−mice (750 000 ± 40 000 platelets/μL;P < .05). Flow cytometry analysis of BM, spleen, and thymus from Fanca+/+ andFanca−/− mice revealed no significant differences in the percentage of mature B220+, GR1+, MAC+, Ter-119+, and CD3+ cells (Table 1). Differences in the content of more primitive BM progenitors (Lin−Sca-1+ or Lin−Sca-1+c-kit+) were not significant when both mouse strains were compared. In the thymus, similar values of CD3+ cells and CD4+CD8+, CD4+CD8−, CD4−CD8+, and CD4−CD8− cells were apparent between both strains. In no instance were significant differences in the absolute number of BM, spleen, and thymus cells observed betweenFanca+/+ and Fanca−/−mice (data not shown).
Phenotypic analysis of the bone marrow, spleen, and thymus of Fanca knockout mice
| Source . | Phenotype . | Fanca+/+ . | Fanca−/− . |
|---|---|---|---|
| BM | B220 | 21.4 ± 2.2 | 23.6 ± 2.8 |
| GR1 | 69.8 ± 10.6 | 59.8 ± 4.5 | |
| MAC | 60.4 ± 10.9 | 58.1 ± 2.9 | |
| CD3 | 1.89 ± 0.38 | 3.46 ± 1.36 | |
| TER-119 | 17.2 ± 1.1 | 15.5 ± 5.1 | |
| Lin−Sca-1+ | 0.74 ± 0.13 | 0.77 ± 0.16 | |
| Lin− c-kit+Sca-1+ | 0.45 ± 0.18 | 0.47 ± 0.16 | |
| Spleen | B220 | 47.1 ± 5.2 | 50.5 ± 2.5 |
| GR1 | 38.5 ± 11.6 | 34.2 ± 2.4 | |
| MAC | 15.1 ± 8.1 | 4.86 ± 0.28 | |
| CD3 | 20.8 ± 4.9 | 34.3 ± 5.0 | |
| TER-119 | 6.6 ± 1.1 | 4.5 ± 0.3 | |
| Thymus | CD3 | 36.3 ± 14.6 | 34.7 ± 12.3 |
| CD4+CD8− | 4.4 ± 1.4 | 3.2 ± 0.98 | |
| CD8+CD4− | 33.4 ± 10.7 | 27.9 ± 11.2 | |
| CD4+CD8+ | 55.3 ± 9.3 | 65.6 ± 10.3 | |
| CD4−CD8− | 5.3 ± 0.7 | 3.1 ± 0.63 |
| Source . | Phenotype . | Fanca+/+ . | Fanca−/− . |
|---|---|---|---|
| BM | B220 | 21.4 ± 2.2 | 23.6 ± 2.8 |
| GR1 | 69.8 ± 10.6 | 59.8 ± 4.5 | |
| MAC | 60.4 ± 10.9 | 58.1 ± 2.9 | |
| CD3 | 1.89 ± 0.38 | 3.46 ± 1.36 | |
| TER-119 | 17.2 ± 1.1 | 15.5 ± 5.1 | |
| Lin−Sca-1+ | 0.74 ± 0.13 | 0.77 ± 0.16 | |
| Lin− c-kit+Sca-1+ | 0.45 ± 0.18 | 0.47 ± 0.16 | |
| Spleen | B220 | 47.1 ± 5.2 | 50.5 ± 2.5 |
| GR1 | 38.5 ± 11.6 | 34.2 ± 2.4 | |
| MAC | 15.1 ± 8.1 | 4.86 ± 0.28 | |
| CD3 | 20.8 ± 4.9 | 34.3 ± 5.0 | |
| TER-119 | 6.6 ± 1.1 | 4.5 ± 0.3 | |
| Thymus | CD3 | 36.3 ± 14.6 | 34.7 ± 12.3 |
| CD4+CD8− | 4.4 ± 1.4 | 3.2 ± 0.98 | |
| CD8+CD4− | 33.4 ± 10.7 | 27.9 ± 11.2 | |
| CD4+CD8+ | 55.3 ± 9.3 | 65.6 ± 10.3 | |
| CD4−CD8− | 5.3 ± 0.7 | 3.1 ± 0.63 |
Data are given as mean ± SEM of at least 4 different animals at age 8 to 10 weeks.
Colony-forming unit content in the hematopoietic organs ofFanca−/−mice
Since the functional properties of hematopoietic progenitors are more directly evaluated by clonogenic assays than by flow cytometry analyses, BM and spleen cells from Fanca+/+ andFanca−/− mice were cultured in methylcellulose media, allowing the growth of CFU-GM and CFU-Meg progenitors, respectively (Table 2). Although BM progenitors from Fanca−/− mice were always below the corresponding values observed inFanca+/+ mice, only in the case of BM CFU-Meg progenitors did differences reach statistical significance (P < .05).
Hematopoietic progenitors in the BM and spleen ofFanca+/+ andFanca−/− mice
| Source . | Cells . | Progenitor . | Fanca+/+ . | Fanca−/− . |
|---|---|---|---|---|
| BM | Total cells | CFU-GM | 157 ± 28 | 145 ± 28 |
| Total cells | CFU-Meg | 59 ± 3 | 45 ± 2* | |
| Lin−Sca-1+ | CFU-GM | 4510 ± 1325 | 3650 ± 850 | |
| Spleen | Total cells | CFU-GM | 138 ± 18 | 98 ± 16 |
| Total cells | CFU-Meg | 102 ± 6 | 88 ± 4 |
| Source . | Cells . | Progenitor . | Fanca+/+ . | Fanca−/− . |
|---|---|---|---|---|
| BM | Total cells | CFU-GM | 157 ± 28 | 145 ± 28 |
| Total cells | CFU-Meg | 59 ± 3 | 45 ± 2* | |
| Lin−Sca-1+ | CFU-GM | 4510 ± 1325 | 3650 ± 850 | |
| Spleen | Total cells | CFU-GM | 138 ± 18 | 98 ± 16 |
| Total cells | CFU-Meg | 102 ± 6 | 88 ± 4 |
Data represent the mean ± SEM values corresponding to 4 experiments involving the culture of unpurified cells and to 3 experiments involving Lin−Sca-1+ cells. In all instances data represent the number of CFUs/5 × 104 cells.
Statistical significance betweenFanca+/+ and Fanca−/−groups at P < .05.
Mitomycin C sensitivity of Fanca−/−progenitors
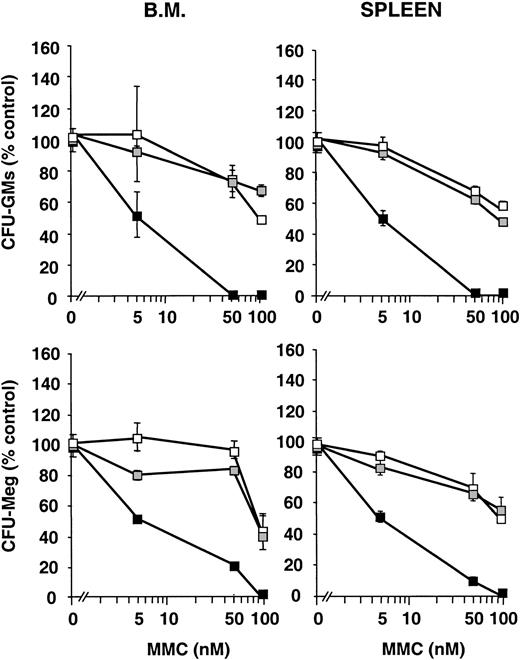
Since one of the characteristic features of FA cells is the hypersensitivity to DNA cross-linking agents, increasing concentrations of MMC were added to myeloid and megakaryocytic cultures fromFanca−/− mice and their respective controls. As shown in Figure 1, doses as high as 50 or 100 nM MMC were only modestly toxic to CFU-GM progenitors obtained from Fanca+/+ orFanca+/− mice, regardless of whether BM or spleen cells were used. In sharp contrast to these observations, a low dose of 5 nM MMC significantly impaired the growth of CFU-GM progenitors from Fanca−/− mice, and 50 nM MMC almost completely abrogated the clonogenic growth of CFU-GM progenitors. Similar conclusions of MMC hypersensitivity were drawn when CFU-Meg progenitors from Fanca−/− mice were assessed (Figure1).
Sensitivity of Fanca-deficient hematopoietic progenitors to mitomycin C.
The figure represents the survival of CFU-GM and CFU-Meg progenitors from Fanca+/+ (■)Fanca+/− (░) and Fanca−/−(▪) to MMC. Each point represents the mean ± SEM corresponding to 4 experiments involving the culture of CFU-GM progenitors and to 3 experiments with CFU-Meg cultures.
Sensitivity of Fanca-deficient hematopoietic progenitors to mitomycin C.
The figure represents the survival of CFU-GM and CFU-Meg progenitors from Fanca+/+ (■)Fanca+/− (░) and Fanca−/−(▪) to MMC. Each point represents the mean ± SEM corresponding to 4 experiments involving the culture of CFU-GM progenitors and to 3 experiments with CFU-Meg cultures.
Ex vivo expansion ability of Fanca−/−bone marrow cells
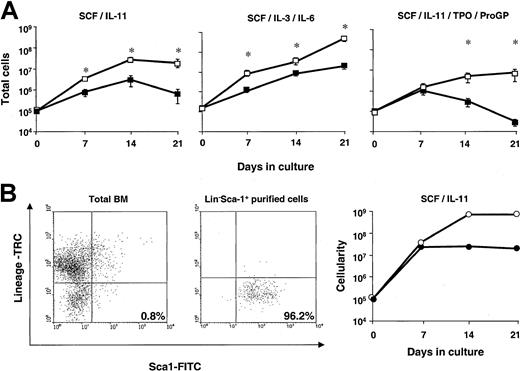
To investigate potential differences in the proliferation ability of Fanca+/+ and Fanca−/−progenitors, ex vivo expansion cultures with BM from these animals were established (see “Materials and methods”). Initially, cultures were stimulated with IL-11/stem cell factor (SCF) on the basis of previous data from our laboratory, showing that this combination of growth factors promotes a significant expansion of hematopoietic progenitors and preserves the repopulating ability of the graft.27 As shown in Figure2A, when BM fromFanca+/+ mice was subjected to ex vivo expansion, an exponential cell growth was observed during a period of 2 weeks, implying a 300-fold amplification in the cellularity of the cultures. As shown in the same figure, the amplification observed in cultures established with Fanca−/− BM was 10 times lower. To investigate whether such a difference was specific to the stimulatory conditions used in these experiments, the proliferation response to 2 other cytokine combinations was analyzed. When IL-3/IL-6/SCF stimulation was used, significant differences between the cellularity of both cultures were also observed. With a more complex combination of growth factors—TPO/ProGP/IL-11/SCF—differences were even more marked: Fanca−/− BM cells were capable of expanding the hematopoietic population for only one week, while the BM of Fanca+/+ mice proliferated for the whole 3-week period, which was the length of the experiment (Figure 2A).
Ex vivo expansion ability of Fanca-deficient bone marrow cells.
(A) Total bone marrow cells from Fanca+/+(■) and Fanca−/−(▪) cells were subjected to ex vivo expansion in the presence of 3 different combinations of growth factors. At weekly intervals, cells were counted and diluted to the input concentration (105 cells/mL). Data represent the total cell number generated by the initial 105 cells. Each point represents the mean ± SEM corresponding to 4 independent experiments. *Statistical significance betweenFanca+/+ and Fanca−/−samples at P < .05. (B) Lin−Sca-1+ cells fromFanca+/+ (○) and Fanca−/−(●) were purified and ex vivo expanded with SCF and IL-11. Cytometry histograms of bone marrow cells before and after purification of the Lin−Sca-1+ population and the ex vivo expansion kinetics following SCF and IL-11 stimulation are shown. The number in the histogram indicates the percentage of cells within the statistics quadrant.
Ex vivo expansion ability of Fanca-deficient bone marrow cells.
(A) Total bone marrow cells from Fanca+/+(■) and Fanca−/−(▪) cells were subjected to ex vivo expansion in the presence of 3 different combinations of growth factors. At weekly intervals, cells were counted and diluted to the input concentration (105 cells/mL). Data represent the total cell number generated by the initial 105 cells. Each point represents the mean ± SEM corresponding to 4 independent experiments. *Statistical significance betweenFanca+/+ and Fanca−/−samples at P < .05. (B) Lin−Sca-1+ cells fromFanca+/+ (○) and Fanca−/−(●) were purified and ex vivo expanded with SCF and IL-11. Cytometry histograms of bone marrow cells before and after purification of the Lin−Sca-1+ population and the ex vivo expansion kinetics following SCF and IL-11 stimulation are shown. The number in the histogram indicates the percentage of cells within the statistics quadrant.
To evaluate whether indirect effects related to cell interactions or secretion of inhibitory or toxic molecules played a role in the differential ex vivo expansions observed in Figure 2A, cultures were now established with purified hematopoietic precursors. To this end, progenitor cells were purified as Lin−Sca-1+cells (LS cells) and then seeded at low cell densities (5 × 103 cells/mL instead of 105 cells/mL). While cultures of total BM were reseeded once weekly, in these experiments cells were diluted with fresh medium to the initial cell concentration twice weekly. As shown in a representative experiment conducted with IL-11/SCF (Figure 2B), the incubation of low concentrations of purified Fanca+/+ LS cells promoted a vast amplification in the cellularity of the cultures (about 104-fold in 2 weeks). However, the growth ofFanca−/− LS cells was again markedly below that observed with Fanca+/+ LS cells, suggesting an intrinsic defect in the hematopoietic progenitors lacking the functional Fanca gene. No appearance of an adherent layer was observed in Fanca−/− BM nor inFanca+/+ BM cultures in any of the culture conditions used.
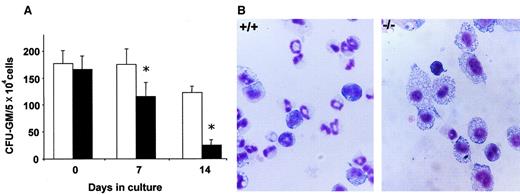
To investigate more deeply the biologic insights involved in the differential growth of Fanca+/+ andFanca−/− progenitors, further studies from cultures stimulated with IL-11/SCF were conducted. As shown in Figure3A, while similar proportions of CFU-GMs were found in fresh BM from Fanca+/+ andFanca−/− mice, a significant decrease in the proportion of progenitors was observed when samples were subjected to ex vivo expansion (1.5- and 5-fold lower in theFanca−/− group at the first and second week, respectively). In addition to this effect, May Grunwald-Giemsa stainings of normal BM cultures consistently showed a large proportion of granulocytic cells, while macrophage differentiating cells constituted the predominant population in Fanca−/−BM cultures (Figure 3B).
Progenitors content and hematopoietic differentiation of bone marrow cells ex vivo expanded with SCF and IL-11.
(A) CFU-GM content of Fanca+/+ (■) andFanca−/−(▪) cultures. *Statistical significance between Fanca+/+ andFanca−/−samples at P < .05. (B) Microphotographs of May-Grünwald/Giemsa staining of bone marrow cytospins from Fanca+/+ andFanca−/−after 14 days in culture. Original magnification, × 600.
Progenitors content and hematopoietic differentiation of bone marrow cells ex vivo expanded with SCF and IL-11.
(A) CFU-GM content of Fanca+/+ (■) andFanca−/−(▪) cultures. *Statistical significance between Fanca+/+ andFanca−/−samples at P < .05. (B) Microphotographs of May-Grünwald/Giemsa staining of bone marrow cytospins from Fanca+/+ andFanca−/−after 14 days in culture. Original magnification, × 600.
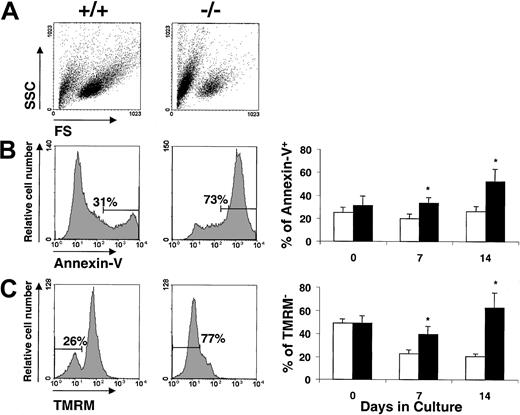
To determine the existence of potential differences in the proportion of cells entering into apoptosis along the culture, ex vivo–expanded samples were tested for apoptotic parameters. Changes in the scatter properties (Figure 4A), changes in the exposure of phosphoserine residues (Annexin-V; Figure 4B), and loss of mithochondrial inner transmembrane potential (TMRM; Figure 4C) were determined in these experiments. As shown in the figure, the proportion of apoptotic cells was markedly increased in cultures fromFanca−/− compared toFanca+/+ mice. Differences were significant at both 7 and 14 days of culture and when determined by both the Annexin-V and TMRM methods.
Analysis of apoptotic cells in bone marrow expanded ex vivo with SCF and IL-11.
The figure shows a representative analysis of the scatter properties (A), annexin-V (B), and TMRM (C) analysis ofFanca+/+ and Fanca−/−BM cultured for 14 days. The kinetics in the proportion of apoptotic cells in the culture is also shown. ■ indicateFanca+/+ cells; ▪,Fanca−/−cells. Data represent the mean ± SE corresponding to 4 independent cultures from 4 different animals. *Statistical significance between Fanca+/+ andFanca−/−samples at P < .05. SSC indicates side scatter; FS, forward scatter.
Analysis of apoptotic cells in bone marrow expanded ex vivo with SCF and IL-11.
The figure shows a representative analysis of the scatter properties (A), annexin-V (B), and TMRM (C) analysis ofFanca+/+ and Fanca−/−BM cultured for 14 days. The kinetics in the proportion of apoptotic cells in the culture is also shown. ■ indicateFanca+/+ cells; ▪,Fanca−/−cells. Data represent the mean ± SE corresponding to 4 independent cultures from 4 different animals. *Statistical significance between Fanca+/+ andFanca−/−samples at P < .05. SSC indicates side scatter; FS, forward scatter.
Genetic correction of the hypersensitivity ofFanca−/−progenitors to mitomycin C
To evaluate whether the MMC hypersensitivity that characterizesFanca−/− progenitors could be reversed by the expression of the human FANCA gene, retroviral-mediated gene transfer experiments were conducted. In these assays, BM fromFanca+/+ and Fanca−/−mice was enriched on LS precursors and then transduced with retroviral vectors encoding the FANCA and/orEGFP cDNAs.
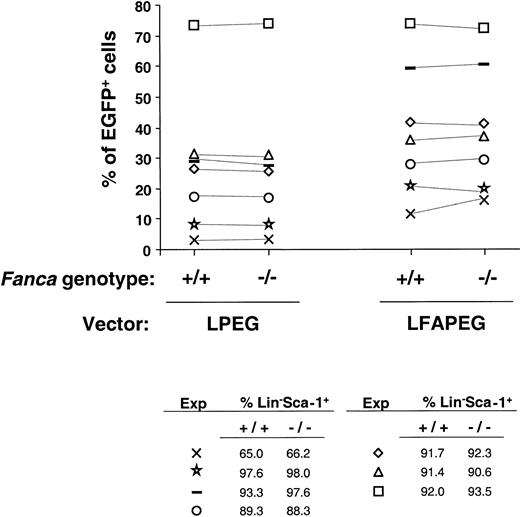
To investigate potential differences in the transduction efficiency of Fanca+/+ andFanca−/− progenitors, the proportion of LS cells expressing EGFP 3 days after infection was evaluated. As shown in Figure 5, neither LPEG (only encoding theEGFP cDNA) nor LFAPEG vectors (also encoding the humanFANCA cDNA) transduced with different efficiencies the LS cells from Fanca+/+ andFanca−/− mice.
Comparison of the transduction efficiency in Lin−Sca-1+ progenitors fromFanca+/+ and Fanca−/−mice.
The figure represents the percentage of EGFP expression after transduction with either LPEG or LFAPEG vectors. Each experiment is represented by a different symbol. The purity of the Lin−Sca-1+ population that was subjected to transduction in each experiment is also shown.
Comparison of the transduction efficiency in Lin−Sca-1+ progenitors fromFanca+/+ and Fanca−/−mice.
The figure represents the percentage of EGFP expression after transduction with either LPEG or LFAPEG vectors. Each experiment is represented by a different symbol. The purity of the Lin−Sca-1+ population that was subjected to transduction in each experiment is also shown.
Data in Figure 6 show the reversion of MMC hypersensitivity of Fanca−/−progenitors as a result of the transfer of the human FANCA.As expected, when the number of colonies generated byFanca+/+ samples was analyzed (Figure 6A), no changes were observed between mock-infected and LFAPEG-infected samples, either at 0, 5, or 50 nM MMC (mean values ranged between 67 and 133 colonies/103 LS cells). When samples fromFanca−/− mice were considered (Figure 6B), a similar number of colonies between the mock-infected and the LFAPEG-infected cells also was observed in cultures grown in the absence of MMC (70 and 73 colonies/103 LS cells, respectively). However, when Fanca−/− cultures were grown with 5 nM MMC, low numbers of colonies were obtained from mock-infected cells (10 colonies/103 LS cells), but not in the LFAPEG group (71 colonies/103 LS cells;P < .05). At 50 nM MMC, the numbers were 2 colonies/103 LS cells for mock-infected samples and 18 colonies/103 LS cells for LFAPEG-transduced cells (P < .05). The scoring of the colonies by fluorescence microscopy showed a progressive increase in the proportion of green colonies in Fanca−/− samples that had been transduced with LFAPEG vectors and then grown with increasing concentrations of MMC, demonstrating a selection for corrected cells by MMC. In contrast to this observation, when samples fromFanca+/+ mice were cultured with increasing concentrations of MMC, no changes in the proportion of green colonies were observed (Figure 6C).
Correction of the mitomycin C sensitivity ofFanca−/− progenitors by retroviral vectors encoding the human FANCA gene.
Purified Lin−Sca-1+ cells fromFanca+/+ and Fanca−/−mice were mock infected or infected with retroviral vectors encoding the FANCA and EGFP genes (LFAPEG vector) and then cultured in the presence of increasing concentrations of MMC. Panels A-C represent the mean ± SEM of data corresponding to 5 independent experiments. Panels D-F show data corresponding to one representative experiment that included an additional group transduced with a vector encoding only the EGFP gene (LPEG vector). In panels C and F, triangles indicate infections with LFPEG; circles, infections with LFAPEG vectors; empty symbols, Lin−Sca-1+ cells obtained fromFanca+/+ mice, and solid symbols, Lin−Sca-1+ cells obtained fromFanca−/−mice.
Correction of the mitomycin C sensitivity ofFanca−/− progenitors by retroviral vectors encoding the human FANCA gene.
Purified Lin−Sca-1+ cells fromFanca+/+ and Fanca−/−mice were mock infected or infected with retroviral vectors encoding the FANCA and EGFP genes (LFAPEG vector) and then cultured in the presence of increasing concentrations of MMC. Panels A-C represent the mean ± SEM of data corresponding to 5 independent experiments. Panels D-F show data corresponding to one representative experiment that included an additional group transduced with a vector encoding only the EGFP gene (LPEG vector). In panels C and F, triangles indicate infections with LFPEG; circles, infections with LFAPEG vectors; empty symbols, Lin−Sca-1+ cells obtained fromFanca+/+ mice, and solid symbols, Lin−Sca-1+ cells obtained fromFanca−/−mice.
To confirm that the functional correction ofFanca−/− progenitors was not due to any molecular event related to the retroviral transduction process, further control samples transduced with vectors encoding only theEGFP gene (LPEG vectors) were considered in 3 additional experiments. Figure 6D-F represents the results corresponding to one of these experiments, which shows that the behavior of the mock-infected and the LPEG-infected cells was essentially identical. The analysis of the green fluorescence in the colonies corresponding to this experiment (Figure 6F) also confirms that only in the case ofFanca−/− samples that had been transduced with the LFAPEG vector; a correlation between the proportion of green colonies and the concentration of MMC used in the cultures could be established.
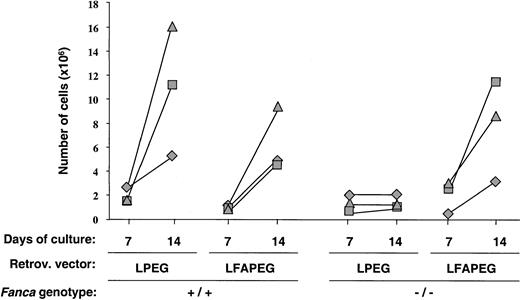
Correction of the impaired ex vivo expansion ability ofFanca−/−progenitors in the absence of pharmacological selection
Figure 7 shows a direct comparison of data corresponding to 3 independent experiments in which LS cells from Fanca+/+ and Fanca−/−mice were transduced with LPEG and LFAPEG vectors and then subjected to ex vivo expansion with IL-11/SCF. As expected, the cellularity of Fanca+/+ LS cultures was markedly increased between days 7 and 14 of incubation, regardless of the vectors used for the transduction. Also as expected, the growth ofFanca−/− LS cells transduced with the LPEG vector was very poor. However, the transduction of LS cells with the LFAPEG vector (35%-75% transduction efficiency in these experiments; not shown) allowed the correction of the limited in vitro growth properties that characterized the Fanca−/− LS progenitor cells.
Correction of the in vitro growth properties of Lin−Sca-1+Fanca−/−progenitors by retroviral vectors encoding the human FANCAgene.
The figure represents the cellularity of cultures initiated with 5 × 103 Lin−Sca-1+ cells that had been transduced with EGFP-encoding (LPEG) orFANCA/EGFP-encoding (LFAPEG) vectors and stimulated with SCF and IL-11. Each symbol represents individual data from 3 independent experiments.
Correction of the in vitro growth properties of Lin−Sca-1+Fanca−/−progenitors by retroviral vectors encoding the human FANCAgene.
The figure represents the cellularity of cultures initiated with 5 × 103 Lin−Sca-1+ cells that had been transduced with EGFP-encoding (LPEG) orFANCA/EGFP-encoding (LFAPEG) vectors and stimulated with SCF and IL-11. Each symbol represents individual data from 3 independent experiments.
Discussion
The progressive cloning of FA genes is facilitating the development of FA experimental models based on the targeted disruption of these genes.18 To date, knockout mice with disruptions in 3 FA genes have been generated. Three strains with disruptions in the Fancc gene,11,12 one of them having disruptions in both the Fancc and Sod1genes,28 have been developed. In addition, mice having a targeted disruption in Fanca13 andFancg/Xrcc914 15 genes also have been generated.
Taking into account that FA constitutes the most frequent genetic cause of BM failure29 and given that mutations in theFANCA gene account for about 60% to 70% of all FA patients, this Fanca knockout mouse model constitutes an invaluable tool for conducting studies on hematopoietic stem cell (HSC) diseases of genetic etiology. Although a number of characteristics of the human FA disease, like pancytopenia and leukemia predisposition, have not been observed in these FA models, other signs associated with FA, including the hypersensitivity to DNA cross-linking agents, have been reported in these animals.11,13-15,30 In the hematopoietic system, only modest reductions of BM progenitors,12 and in some instances mild thrombocytopenia, have been observed in mice lacking the functionalFancc gene.28 A more profound hematopoietic phenotype has been observed in mice having disruptions in both theFancc and the Sod1 genes, suggesting the relevance of an altered redox state in the FA phenotype.28
Regarding the hematological description of Fanca-deficient mice, we observed only modest thrombocytopenia, consistent with data observed in the original description of these animals.13In addition, here we show a significant reduction in BM CFU-Meg progenitors, suggesting that the megakaryocytic lineage is particularly affected in these animals. In contrast to the modest effects observed in the hematopoietic tissues of Fanca−/− mice, both the CFU-GM and CFU-Meg progenitors were highly sensitive to mytomicin C. This result is consistent with observations previously made in the hematopoietic progenitors from Fancc andFancg/Xrcc9 knockouts12,14,15,31 and also in embryonic fibroblasts from Fanca-deficient mice.13
Taking together the above observations, it could be proposed that only after exposure to DNA cross-linking agents could a marked hematopoietic FA be apparent in these knockout models. Nevertheless, data in Figures2-4 reveal the existence of marked in vitro growth defects in hematopoietic progenitors lacking the Fanca gene. As deduced from our experiments in Figure 2, the impaired growth ability ofFanca−/− BM is not restricted to particular conditions of stimulation, since 3 different combinations of growth factors resulted in identical conclusions. At present we cannot formally rule out the possibility that toxic or inhibitory molecules released during the culture play a role in the observed growth defects of Fanca−/− BM.32,33Nevertheless, the observation of a similar growth defect in purifiedFanca−/−Lin−Sca-1+ progenitors—always maintained at low cell densities—strongly suggests the existence of an intrinsic growth defect in BM progenitors lacking a functionalFanca gene. Moreover, because in our culture conditions no stromal cell layer was formed, the impaired growth observed inFanca−/− cells should be related to intrinsic defects of the hematopoietic progenitors instead of defects in the interactions with or function of the BM stroma. Until the description of our data, evidence of the BM growth defect had been observed in theFancc mouse model only by conducting hematopoietic colony growth under suboptimal growth factor concentrations34 and long-term in vivo repopulation assays.17 31 Our in vitro approach extends the BM growth phenotype to other complementation groups and offers a simple model capable of reproducing a characteristic growth defect of FA progenitor cells.
The results in Figures 3 and 4 add further information regarding the cellular mechanisms that could account for the in vitro growth defect observed in Fanca−/− BM. These studies demonstrate that, in contrast to normal BM cells, the response of Fanca−/− BM to in vitro stimulation is characterized by (1) an accelerated depletion in CFU-GM progenitors, (2) an evident granulocyte/macrophage differentiation disbalance, and (3) a marked susceptibility of the expanded population to enter into apoptosis. The relative contribution of these or other processes potentially involved in the growth defect observed inFanca−/− samples (ie, failure to respond to growth signals or deficient cell communications) is currently unknown.
Regarding our observations of increased apoptosis inFanca−/− cells, these results are consistent with previous studies showing the apoptotic predisposition of FA cells.32,35-38 More recent data have clarified the role of the FA proteins—in particular, the group C gene product—in the molecular control of apoptosis.33,34 39-43 Our results add new information about the involvement of Fanca in apoptosis regulation. In addition, this model will be useful for understanding the potential implication of apoptosis in the hematopoeitic dysfunctions associated with Fanca deficiency; in particular, the deficient cell expansion and the differentiation imbalance in the granulocyte/macrophage lineages.
In the context of the gene therapy of FA, we aimed to mimic a clinical approach, in which particular care has to be taken regarding the in vivo pharmacological activation of the HSCs. Therefore, although 5-FU is generally used to promote the proliferation of mouse HSCs,44,45 in our experiments untreated BM cells were first purified for primitive hematopoietic progenitors and HSCs (Lin−Sca-1+; LS cells), then subjected to a short stimulation with SCF/IL-11, and finally transduced with the retroviral vectors. In earlier studies with wild-type mice, we showed that SCF/IL11 stimulation is compatible with the preservation of the long-term repopulating ability of the BM27 and also with the transduction of its long-term repopulating cells (data not shown). The experiments presented in this study with retroviral vectors encoding the therapeutic gene (human FANCA) and/or a reporter gene (EGFP) allowed us to investigate 3 different aspects related to the application of gene therapy in FA.
Initially, and given the limited information about the transduction susceptibility of the hematopoietic progenitors from FA compared to wild-type mice, we investigated the existence of differences in the transduction efficiency of Fanca+/+ andFanca−/− LS cells. Data presented in Figure 5demonstrate comparable transduction efficiencies in the LS progenitors of both animal groups, suggesting a similar susceptibility of transduction in the repopulating cell compartment ofFanca−/− compared to wild-type mice.
A second observation derived from our gene transfer experiments relates to the capacity of retroviral vectors encoding the human FANCAgene to reverse the MMC sensitivity of Fanca-deficient cells. In this respect, data in Figure 6 show for the first time that the transduction of mouse Fanca−/− progenitors with vectors encoding the human FANCA gene reverts their hypersensitivity to MMC. These experiments not only demonstrate the efficacy of retroviral vectors—in particular LFAPEG—for correcting a characteristic FA phenotype, but also demonstrate the applicability ofFanca knockout mice for assessing the efficacy of vectors encoding the human homologous gene.
Finally, the experiments summarized in Figure 7 were conducted to investigate the capacity of FANCA-expressing vectors for normalizing the in vitro growth properties ofFanca−/− BM in the absence of any pharmacological selection. In this respect, the 3 experiments conducted showed an essentially normal growth of Fanca−/−LS cells as a result of their transduction with the humanFANCA cDNA (LFAPEG in Figure 7). These results show the efficacy of our gene therapy approach for correcting the growth impairment that characterized Fanca-deficient BM cells. In addition, our data offer new evidence supporting the hypothesis that FA cells subjected to gene therapy strategies may develop a proliferation advantage in the recipient of a transplant. In this respect the clinical observation that clones with naturally compensatory mutations can propagate and persist in FA patients for long periods of time is significant.46 47
Taken together, our analyses of the hematopoiesis ofFanca−/− mice show novel phenotypic characteristics of the mice BM progenitors that resemble the hematopoietic phenotype of the human FA disease. In addition, our data demonstrate that retroviral vectors encoding the human FANCAcan reverse the phenotype of Fanca−/−progenitors, providing new evidence regarding the efficacy of this animal model for conducting gene therapy studies of FA.
The authors thank I. Ormán for expert assistance with the flow cytometry and cell sorting.
Supported by grants from the Commission of the European Communities; the Comisión Interministerial de Ciencia y Tecnologı́a; the Forschungsverbund Somatische Gentherapie des Bundesministeriums für Bildung und Forschung (beo2103111661), and the Elternitiavtive Kinderkrebsklinik e.V.
P.R. is a recipient of a fellowship from Formación de Personal Investigador (FPI) program of the Ministerio de Ciencia y Tecnologı́a (MCYT).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Juan A. Bueren, Hematopoiesis Project, CIEMAT Avda Complutense, no. 22, 28040 Madrid, Spain; e-mail:juan.bueren@ciemat.es.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal