Abstract
An absent platelet glycoprotein (GP) Ib-IX receptor results in the Bernard-Soulier syndrome and is characterized by severe bleeding and the laboratory presentation of macrothrombocytopenia. Although the macrothrombocytopenic phenotype is directly linked to an absent GP Ib-IX complex, the disrupted molecular mechanisms that produce the macrothrombocytopenia are unknown. We have utilized a mouse model of the Bernard-Soulier syndrome to engineer platelets expressing an α-subunit of GP Ib (GP Ibα) in which most of the extracytoplasmic sequence has been replaced by an isolated domain of the α-subunit of the human interleukin-4 receptor (IL-4Rα). The IL-4Rα/GP Ibα fusion is membrane expressed in Chinese hamster ovary (CHO) cells, and its expression is facilitated by the presence of human GP IX and the β-subunit of GP Ib. Transgenic animals expressing a chimeric receptor were generated and bred into the murine Bernard-Soulier syndrome–producing animals devoid of mouse GP Ibα but expressing the IL-4Rα/GP Ibα fusion sequence. The characterization of these mice revealed a 2-fold increase in circulating platelet count and a 50% reduction in platelet size when compared with platelets from the mouse model of the Bernard-Soulier syndrome. Immunoprecipitation confirmed that the IL-4Rα/GP Ibα subunit interacts with filamin-1 and 14-3-3ζ, known binding proteins to the GP Ibα cytoplasmic tail. Mice expressing the chimeric receptor retain a severe bleeding phenotype, confirming a critical role for the GP Ibα extracytoplasmic domain in hemostasis. These results provide in vivo insights into the structural elements of the GP Ibα subunit that contribute to normal megakaryocyte maturation and thrombopoiesis.
Introduction
Giant platelet disorders are a heterogeneous group of hematopoietic defects with structurally abnormal platelets in peripheral blood smears.1,2 Many of these disorders are hereditary and present with a reduced platelet count, leading to their classification as hereditary macrothrombocytopenias. Representative of the group are the Bernard-Soulier syndrome, Epstein syndrome, May-Hegglin anomaly, Fechtner syndrome, and X-linked macrothrombocytopenia.2 The genetic basis for each of these syndromes is different, yet the members of the group are unified in their presentation of macrothrombocytopenia. In each case, the molecular pathology leading to the macrothrombocytopenia has remained obscure but is assumed to be linked to abnormal megakaryocyte maturation and platelet release.
The Bernard-Soulier syndrome is one of the best-characterized syndromes among those presenting with macrothrombocytopenia.3 The disorder is due to mutations within any of the 3 subunits constituting the membrane receptor, glycoprotein (GP) Ib-IX.4 Most commonly, mutations in the GP Ib-IX complex will prevent translocation of the receptor to the megakaryocyte surface, resulting in the Bernard-Soulier syndrome. The bleeding associated with the Bernard-Soulier syndrome is more severe than the macrothrombocytopenia would predict and reflects the critical role for the GP Ib-IX receptor in platelet adhesion during hemostasis.4 Indeed, the role of the GP Ib-IX complex in hemostasis is well established, but the link between an absent GP Ib-IX complex and the generation of a macrothrombocytopenia is less obvious. Speculation on the molecular basis has been fueled by work characterizing an interaction between GP Ibα and proteins of the platelet membrane cytoskeleton, such as filamin-1.5-7 However, an equally plausible explanation might be a GP Ibα link to signal transduction proteins, such as 14-3-3ζ, which would be absent in the Bernard-Soulier syndrome and would potentially, via unknown mechanisms, lead to abnormal megakaryocytopoiesis or thrombopoiesis.8,9 Moreover, a role for GP Ibα extracytoplasmic sequences in platelet formation cannot be excluded, because anti-GP Ibα antibodies can inhibit proplatelet formation in vitro.10
We have recently described a murine model of the human Bernard-Soulier syndrome where the mice exhibit all of the salient features of the human syndrome, including bleeding and macrothrombocytopenia.11 Moreover, the mice display an abnormal megakaryocyte maturation, which may lead to the development of the macrothrombocytopenia.11 12 In the current study we test the hypothesis that the cytoplasmic domain of the α-subunit of GP Ib is necessary for normal megakaryocytopoiesis and platelet release. We present the characterization of transgenically engineered mouse platelets expressing a fusion protein composed of an extracytoplasmic domain of the human interleukin-4 receptor α-subunit (IL-4Rα) fused to the transmembrane and cytoplasmic tail of human GP Ibα. The results demonstrate that the fusion protein is efficiently surface expressed and stabilized by GP Ibβ and GP IX subunits. A chimeric receptor was expressed in the murine model of the Bernard-Soulier syndrome, a model missing the endogenous murine GP Ibα subunit. In vivo, the chimeric complex results in a 2-fold increase in platelet count and in an approximate 50% reduction in platelet size when compared with platelets devoid of mouse GP Ibα. The results provide insights into the structural elements of the GP Ib-IX complex that contribute to normal platelet function, and implications for the role of the GP Ib-IX complex in normal thrombopoiesis are discussed.
Materials and methods
Generation of IL-4Rα/GP Ibα receptor constructs
DNA constructs encoding the ectodomain of the human IL-4 receptor (residues 1-198 of the mature α-subunit of the receptor13) and residues 473 to 610 of the human GP Ibα mature subunit were generated.14 Specifically, aHindIII/BamHI restriction fragment of the IL-4Rα cDNA was generated in a polymerase chain reaction (PCR) using the complete IL-4Rα cDNA provided by Dr Kenji Izuhara (Saga Medical School, Japan). PCR primers for IL-4Rα generated a 5′ product containing a HindIII restriction site (italics), a consensus translation initiation site (underlined), and an initiating Met codon (bold) (forward primer, 5′-GTAAGCTTGCCACCATGGGGTGGCTTTGC-3′). A reverse primer generated 3 Gly codons immediately 3′ to the IL-4Rα Asn198 codon followed by a BamHI restriction site (reverse primer, 5′-CTGGATCCACCGCCGTTGTGCCACTTGGTGC-3′). A second PCR generated a BamHI/EcoRI restriction fragment containing the coding sequence of the mature GP Ibα subunit from residues Ser474 to Leu610 (forward primer, 5′-GGTGGATCCAGAAATGACCCTTTTCTC-3′; reverse primer, 5′-GCGAATTCAGAGGCTGTGGCC-3′). The PCR products were cloned and their DNA sequence confirmed. The cloned PCR products were ligated together using BamHI cohesive ends producing in a 5′ to 3′ direction the coding sequence for the extracytoplasmic domain of the IL4-Rα, 3 Gly residues, and the carboxyl terminus of the GP Ibα subunit. Again, the intact coding sequence was confirmed by DNA sequence analysis.
Once assembled, the IL-4Rα/GP Ibα coding sequence was cloned as aHindIII/EcoRI restriction fragment into the eukaryotic expression vector, pcDNAzeo3.1+ (Invitrogen, Carlsbad, CA). This construct was used for transfection into heterologous cells. For the generation of transgenic animals, a 3-kb GP Ibα promoter cassette was cloned as a HindIII fragment immediately 5′ to the coding sequence. The generation and use of the GP Ibα promoter cassette has been previously described.11 15Microinjection of the transgenic construct into pronuclei was performed by The Scripps Research Institute transgenic core facility.
CHO cells with inducible GP Ibβ and GP IX cDNAs
Chinese hamster ovary (CHO) cells were maintained in 5% CO2 and grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 0.5 mM nonessential amino acids, and 2 mM l-glutamine (Whittaker Bioproducts, Walkersville, MD). Transfection of CHO cells was performed using liposomes (TransFast, Promega, Madison, WI). A CHO cell line expressing the tetracycline-controlled transactivator (CHO-AA8, Tet-Off) was purchased from BD Biosciences Clontech (Palo Alto, CA). The cDNAs for GP Ibβ and GP IX16 17 were cloned into the tetracycline-OFF expression vector, pTRE (Clontech), and transfected into CHO-AA8 cells along with an additional selection plasmid, pTK/Hyg, which facilitated the selection of stable transformants with hygromycin (800 μg/mL, Invitrogen). Incubation of the selected CHO cells in the presence of tetracycline (200 ng/mL) repressed gene expression, and the removal of tetracycline induced both gene products. Stable CHO cells expressing both human GP Ibβ and GP IX were subsequently transfected with a plasmid encoding the IL-4Rα/GP Ibα subunit and selected in the presence of Zeocin (800 μg/mL, Invitrogen). Stable transfectants expressing the fusion protein were identified using a fluorescein isothiocyanate (FITC)–labeled anti–IL-4Rα antibody in flow cytometry.
Immunologic reagents and protein analysis
A monoclonal antibody recognizing the human IL-4 receptor was purchased from R&D Systems (cat. no. MAB230; Minneapolis, MN). The antibody was directly labeled with FITC according to an established protocol.18 Anti-GP Ibβ antibodies were generated by immunizing rabbits with a peptide corresponding to the carboxy-terminal 14 residues of GP Ibβ. An anti-GP IX monoclonal antibody has been previously described.19 A monoclonal antibody labeled with FITC and recognizing the mouse integrin αIIb subunit was obtained from Pharmingen (La Jolla, CA). A purified rabbit polyclonal anti-14-3-3ζ antiserum was purchased from Santa Cruz Technologies (catalog no. sc-1019; Santa Cruz, CA). A rabbit antimouse filamin-1 antisera was kindly provided by Drs Hoffmeister and Stossel (Brigham and Women's Hospital, Boston, MA).
Immunoprecipitation experiments were performed with washed platelets (3.4 × 108 platelets) resuspended in 250 μL modified Tyrode buffer (137 mmol NaCl, 2.7 mM KCl, 2.8 mmol dextrose, 0.4 mmol NaH2PO4, 5 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] [pH 7.4]) and lysed with an equal volume of solubilization buffer (2% Triton X-100, 0.1 mol Tris [tris(hydroxymethyl)aminomethane], 0.01 mol EGTA [ethyleneglycoltetraacetic acid], 0.15 mol NaCl, and 2 mmol Pefabloc SC [Boehringer Mannheim, Indianapolis, IN] [pH 7.4]). The mixture was kept on ice for 45 minutes and centrifuged (13 000g, 10 minutes) to remove the insoluble material. The lysates (500 μL) were mixed with 100 μL (50% vol/vol) protein A beads (IPA-300, Repligen, Cambridge, MA) and 10 μg of the indicated antibody for 90 minutes. The beads were then washed 4 times in an equal volume of modified Tyrode buffer and solubilization buffer. Bound proteins were eluted by boiling in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and analyzed by SDS-PAGE and Western blotting. Immunoreactive proteins were visualized using a chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ) and Kodak BIOMAX MR film.
Screening of transgenic mice
Mouse blood was obtained from a periorbital puncture from anesthetized animals. Blood was collected with heparin-coated capillary tubes and transferred to a tube containing acid-citrate-dextrose (NIH formula A) anticoagulant. Flow cytometric analysis on whole blood was determined using an FITCanti–IL-4R monoclonal antibody. Transgenic animals expressing the IL-4Rα/GP Ibα fusion protein were bred to mice lacking mouse GP Ibα alleles, the mouse model of the human Bernard-Soulier syndrome. Using a breeding strategy as previously described,11 animals deficient in both murine GP Ibα alleles but expressing the IL-4Rα/GP Ibα subunit were identified after 2 generations of breeding. Briefly, GP Ibα−/− mice were bred to mice containing the human transgene (mGP Ibα+/+, IL-4R/IbαTg). Southern blot analysis of the offspring confirmed the presence of heterozygous murine GP Ibα alleles, whereas immunologic screening using anFITCanti–IL-4R monoclonal antibody identified mice also expressing the human transgene product (mGP Ibα+/−, IL-4R/IbαTg). Mice containing heterozygous murine GP Ibα alleles and a functional human transgene were again crossed with GP Ibα–deficient mice ([mGP Ibα+/−, IL-4R/IbαTg] × [mGP Ibα−/−]), and the resultant offspring were genotyped and immunologically screened. This analysis identified mice with a murine GP Ibα−/− locus and a functional human transgene (mGP Ibα−/−, IL-4R/IbαTg). For results comparing mGP Ibα–deficient platelets to mGP Ibα–deficient platelets expressing the IL-4Rα/GP Ibα transgene, littermates were compared from crosses of mGP Ibα−/− × mGP Ibα−/−, IL-4R/IbαTg, following their genotypic classification.
Phenotypic characterizations of mice
Mouse tail bleeding times were determined prior to genotyping analysis by removing 1 to 3 mm of distal mouse tail and immediately immersing the tail in 37°C isotonic saline. A complete cessation of bleeding was defined as the bleeding time. Bleeding time measurements exceeding 10 minutes were stopped by cauterization of the tail. Circulating blood counts were determined using manuals methods (Unopette, Becton Dickinson, Franklin Lakes, NJ) and an automated cell counter (Baker, Allentown, PA). The flow cytometric analysis data were collected, and a determination of the geometric mean for peaks was determined using the software FCSPress (available athttp://www.fcspress.com/).
Results
In vitro characterization of an interleukin-4 receptor/GP Ibα fusion protein
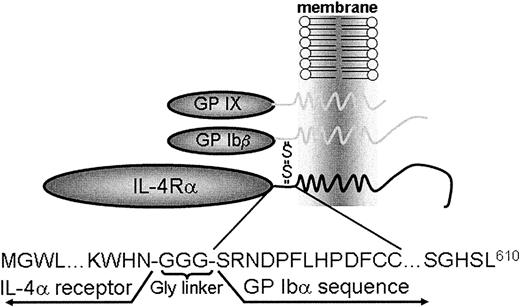
We have previously described a murine model of the human Bernard-Soulier syndrome.11 These mice mimic the human syndrome with a severe bleeding phenotype and the presence of large platelets in peripheral blood smears. The bone marrow of these animals displays abnormal megakaryocyte maturation, which presumably results in an atypical platelet release.11,12 To address the structural requirements within the GP Ibα subunit contributing to thrombopoiesis, we generated a DNA construct encoding the α-subunit of the human interleukin-4 receptor (IL-4Rα)20 fused to the COOH terminus of human GP Ibα (Figure1). The choice of using the human GP Ibα subunit, as opposed to mouse GP Ibα, is based on our previous demonstration that the complete human GP Ibα sequence rescues the mouse Bernard-Soulier syndrome phenotype.11The expressed polypeptide would predictably be composed of the extracytoplasmic domain of the IL-4Rα fused to 3 tandem Gly residues followed by the transmembrane and cytoplasmic sequences of GP Ibα. A short portion of the GP Ibα extractyoplasmic domain was included (13 residues) containing the tandem Cys484/Cys485 of GP Ibα that normally disulfide-link to GP Ibβ just beyond the transmembrane domain (Figure 1).
Schematic representation of an interleukin-4Rα/GP Ib-IX receptor.
The platelet GP Ib-IX receptor complex is composed of the disulfide-linked α- and β-subunits of GP Ib and noncovalently associated GP IX. A coding sequence was generated replacing most of the GP Ibα extracytoplasmic sequence (residues 1-472) with the extracytoplasmic domain (residues 1-198) of the interleukin-4 receptor α chain (IL-4Rα). Studies are presented characterizing the phenotypic consequences of the IL-4Rα/GP Ibα subunit in heterologous cells and in a murine model of the Bernard-Soulier syndrome.
Schematic representation of an interleukin-4Rα/GP Ib-IX receptor.
The platelet GP Ib-IX receptor complex is composed of the disulfide-linked α- and β-subunits of GP Ib and noncovalently associated GP IX. A coding sequence was generated replacing most of the GP Ibα extracytoplasmic sequence (residues 1-472) with the extracytoplasmic domain (residues 1-198) of the interleukin-4 receptor α chain (IL-4Rα). Studies are presented characterizing the phenotypic consequences of the IL-4Rα/GP Ibα subunit in heterologous cells and in a murine model of the Bernard-Soulier syndrome.
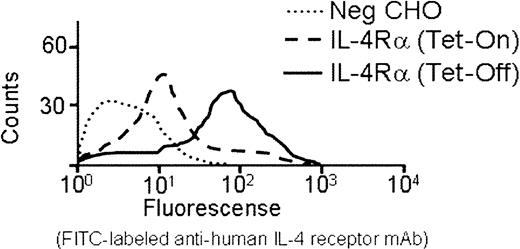
The IL-4Rα/GP Ibα construct was transfected into CHO cells expressing human GP Ibβ and GP IX. Stable CHO cell lines were established expressing all 3 gene products in the same cell: IL-4Rα/GP Ibα, GP Ibβ, and GP IX. Constitutive expression of the IL4-Rα/GP Ibα construct was driven by a cytomegalovirus (CMV) promoter. Expression of both GP Ibβ and GP IX was inducible due to the presence of a tetracycline-inducible promoter (Tet-Off, see “Materials and methods”). As shown in Figure2 for a representative cell line, the induction of GP Ibβ and GP IX caused an approximate 10-fold increase in the surface expression of the IL-4Rα/GP Ibα subunit. This result was corroborated by Western blot analysis of CHO cell lysates using an anti–IL-4R antibody. Upon induction of GP Ibβ and GP IX subunits, a detectable IL-4R antigen was observed (Figure3). In the absence of GP Ibβ and GP IX, no IL-4R antigen was detected (Figure 3). In the same induced samples, an anti-GP Ibβ polyclonal antibody recognized a high molecular weight protein of similar mobility to the IL-4Rα/GP Ibα polypeptide that disappears upon reduction and becomes a single immunoreactive species of about 22 kDa, as predicted for the reduced GP Ibβ subunit of the GP Ib-IX complex.4 Thus, expression of the IL-4Rα/GP Ibα subunit is stabilized by induction of GP Ibβ and GP IX, and the fusion protein associates with GP Ibβ in a manner mimicking the intact human GP Ibα subunit.
Surface expression of an interleukin-4Rα/GP Ib-IX receptor on the surface of Chinese hamster ovary cells.
A stable CHO cell line containing inducible human GP Ibβ and GP IX cDNAs was generated using tetracycline-responsive elements (see “Materials and methods”). Transfection of this cell line with the coding sequence for the IL-4Rα/GP Ibα subunit under the control of a CMV promoter generated a cell line with constitutive expression of IL-4Rα/GP Ibα and inducible expression of GP Ibβ and GP IX. Shown is the fluorescence profile of an anti–IL-4R monoclonal antibody with repressed GP Ibβ and GP IX gene expression (Tet-On) and induced GP Ibβ and GP IX expression (Tet-Off). An approximate 10-fold increase in mean fluorescence produced by the IL-4R monoclonal antibody is generated by the simultaneous expression of GP Ibβ and GP IX. Nontransfected CHOs (Neg CHO) are shown for comparison.
Surface expression of an interleukin-4Rα/GP Ib-IX receptor on the surface of Chinese hamster ovary cells.
A stable CHO cell line containing inducible human GP Ibβ and GP IX cDNAs was generated using tetracycline-responsive elements (see “Materials and methods”). Transfection of this cell line with the coding sequence for the IL-4Rα/GP Ibα subunit under the control of a CMV promoter generated a cell line with constitutive expression of IL-4Rα/GP Ibα and inducible expression of GP Ibβ and GP IX. Shown is the fluorescence profile of an anti–IL-4R monoclonal antibody with repressed GP Ibβ and GP IX gene expression (Tet-On) and induced GP Ibβ and GP IX expression (Tet-Off). An approximate 10-fold increase in mean fluorescence produced by the IL-4R monoclonal antibody is generated by the simultaneous expression of GP Ibβ and GP IX. Nontransfected CHOs (Neg CHO) are shown for comparison.
Synthesis of the interleukin-4Rα/GP Ibα subunit is stabilized by the induction of GP Ibβ and GP IX genes.
Cell lysates of transfected CHO cells induced for the expression of GP Ibβ and GP IX (Tet-Off) were analyzed by Western blot to identify the IL-4Rα/GP Ibα subunit schematically shown in Figure 1. Under nonreducing conditions an anti–IL-4R monoclonal antibody identified a dominant immunoreactive species migrating with an apparent molecular mass of 148 kDa. No IL-4R immunoreactive species was seen in the absence of induced GP Ibβ and GP IX expression (Tet-On). Upon induction (Tet-Off) an anti-GP Ibβ polyclonal antibody identified a dominant immunoreactive species seen under nonreducing conditions with a similar mobility to the major antigen observed with an anti–IL-4R monoclonal antibody (mAb). Upon reduction a single immunoreactive species of approximately 22 kDa is seen, consistent with the reduced single chain molecular weight for the platelet GP Ibβ subunit.
Synthesis of the interleukin-4Rα/GP Ibα subunit is stabilized by the induction of GP Ibβ and GP IX genes.
Cell lysates of transfected CHO cells induced for the expression of GP Ibβ and GP IX (Tet-Off) were analyzed by Western blot to identify the IL-4Rα/GP Ibα subunit schematically shown in Figure 1. Under nonreducing conditions an anti–IL-4R monoclonal antibody identified a dominant immunoreactive species migrating with an apparent molecular mass of 148 kDa. No IL-4R immunoreactive species was seen in the absence of induced GP Ibβ and GP IX expression (Tet-On). Upon induction (Tet-Off) an anti-GP Ibβ polyclonal antibody identified a dominant immunoreactive species seen under nonreducing conditions with a similar mobility to the major antigen observed with an anti–IL-4R monoclonal antibody (mAb). Upon reduction a single immunoreactive species of approximately 22 kDa is seen, consistent with the reduced single chain molecular weight for the platelet GP Ibβ subunit.
Transgenic expression of an interleukin-4R/GP Ibα fusion protein
A megakaryocytic-specific expression promoter cassette functioning in vivo to express the human GP Ibα subunit in megakaryocytes and platelets has been described.21 This promoter cassette was inserted 5′ to the IL-4Rα/GP Ibα coding sequence and was used to generate transgenic mice expressing the fusion. Several different founder animals were identified, but 2 lines expressing surface IL-4Rα antigen were chosen for establishment of mouse colonies. One transgenic line was designated Tg33 and the other Tg51. With the establishment of the IL-4Rα/GP Ibα transgenic lines, we next bred these animals into the mouse model of the Bernard-Soulier syndrome, a colony lacking endogenous mouse GP Ibα alleles (mGP Ibα−/−). First-generation animals were identified with heterozygous mouse GP Ibα alleles (mGP Ib+/−) and an expressed IL-4Rα/GP Ibα transgene. These animals were again bred to animals with a mGP Ibα−/−genotype, and the resultant offspring were screened by Southern blot analysis to identify mice containing the mGP Ibα−/−genotype and flow cytometry to identify the product of the transgene. Having eliminated the endogenous mGP Ibα alleles, these animals were used to characterize the phenotypic consequences of expression of the IL-4Rα/GP Ibα protein in the Bernard-Soulier syndrome model.
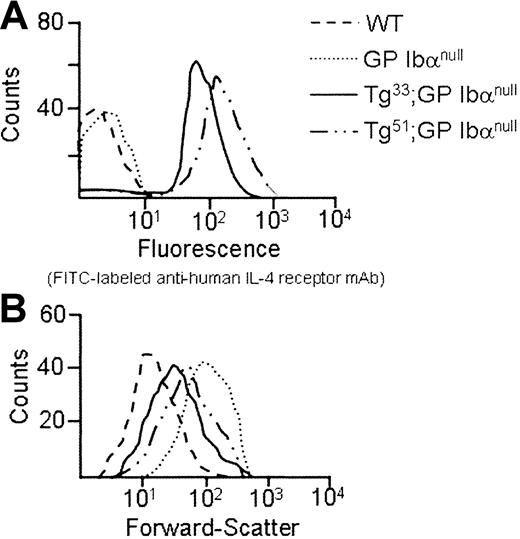
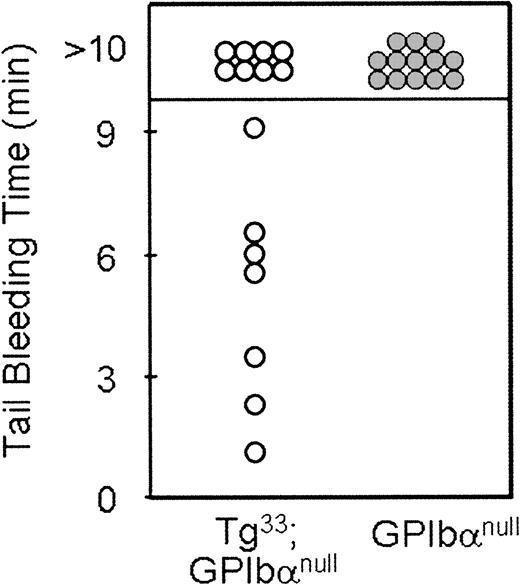
As shown in Figure 4A, platelets from Tg lines 33 and 51 both surface express IL-4Rα antigen. In addition, platelets from Tg51 animals surface express more antigen than platelets from Tg33 animals. Quantitation of the geometric means for each genetic group is presented in Table 1. The phenotypic consequences of transgene expression on platelet size within the circulating platelet population were examined by flow cytometry (Figure 4B). The expression of both IL-4Rα/GP Ibα transgenes, 33 and 51, shifts the forward scatter profile to the left as compared with platelets from mGP Ibα−/− animals, indicating a reduction in particle size. However, the higher levels of transgene expression seen in platelets from Tg51 animals did not produce an additional correction to the population size (Table 1). The geometric mean of the forward scatter profile for platelets from Tg33 animals demonstrated a more than 2-fold decrease in size compared with platelets from mGP Ibα–deficient animals (Table1). A determination of the number of circulating platelets revealed a more than 2-fold increase in platelet number as a consequence of expression of the IL-4Rα/GP Ibα subunit (Table 1). However, the transgene did not correct the platelet count or platelet size to levels observed for platelets from wild-type animals. Tail bleeding times were determined for Tg33 animals and were prolonged with more half of the animals exceeding 10 minutes, indicative of a severe bleeding phenotype (Figure 5). Normal tail bleeding times cluster within a 1- to 3-minute range.11 However, tail bleeding times did suggest that the hemostatic defect in the IL-4Rα/GP Ibα colony is not quite as severe as in the GP Ibα−/− colony (Figure 5). This difference is presumably a direct comparison of the extent of macrothrombocytopenia between the 2 models and its impact on the tail bleeding time assay.
Phenotypic consequences of an interleukin-4Rα/GP Ibα receptor on transgenic mouse platelets.
The IL-4Rα/GP Ibα coding sequence was placed immediately 3′ to a megakaryocytic-specific promoter, and the resultant DNA construct was injected into mice pronuclei to generate transgenic animals. Two different transgenic founders, Tg33 and Tg51, were bred into the murine model of the Bernard-Soulier syndrome. After 2 generations of screening (described in “Materials and methods”), 2 colonies of mice were generated lacking their endogenous murine GP Ibα alleles but still expressing the IL-4Rα/GP Ibα transgene. (A) Shown is the fluorescence profile of platelets obtained from Tg33 and Tg51 transgenic lines screened with an FITCanti–IL-4R mAb. Control platelets are shown from nontransgenic animals and GP Ibα–deficient animals. (B) Forward light scattering profiles from mouse platelets display the entire population of platelet sizes. The platelet population was identified using an antimouse CD41 (integrin αIIb chain) monoclonal antibody. A total of 50 000 recorded events are presented for each genotype. A progressive shift to the left illustrates decreasing particle (platelet) size. The quantitation of this analysis from multiple animals is presented in Table 1.
Phenotypic consequences of an interleukin-4Rα/GP Ibα receptor on transgenic mouse platelets.
The IL-4Rα/GP Ibα coding sequence was placed immediately 3′ to a megakaryocytic-specific promoter, and the resultant DNA construct was injected into mice pronuclei to generate transgenic animals. Two different transgenic founders, Tg33 and Tg51, were bred into the murine model of the Bernard-Soulier syndrome. After 2 generations of screening (described in “Materials and methods”), 2 colonies of mice were generated lacking their endogenous murine GP Ibα alleles but still expressing the IL-4Rα/GP Ibα transgene. (A) Shown is the fluorescence profile of platelets obtained from Tg33 and Tg51 transgenic lines screened with an FITCanti–IL-4R mAb. Control platelets are shown from nontransgenic animals and GP Ibα–deficient animals. (B) Forward light scattering profiles from mouse platelets display the entire population of platelet sizes. The platelet population was identified using an antimouse CD41 (integrin αIIb chain) monoclonal antibody. A total of 50 000 recorded events are presented for each genotype. A progressive shift to the left illustrates decreasing particle (platelet) size. The quantitation of this analysis from multiple animals is presented in Table 1.
Flow cytometry analysis of platelets from mice expressing an IL-4Rα/GP Ibα fusion in a model of murine Bernard-Soulier syndrome
| . | No. of animals tested . | Anti–IL-4R fluorescence/geometric mean (SD) . | Forward scatter/geometric mean (SD) . | Platelet count, × 109/L (SD) . |
|---|---|---|---|---|
| WT | 14 | 2.42 (0.19) | 17.44 (2.10) | 1373 (278) |
| GP Ibα−/− | 56 | 2.64 (0.20) | 61.84 (9.52) | 382 (92) |
| Tg33 (GP Ibα−/−) | 46 | 68.10 (11.17) | 30.52 (4.84) | 905 (178) |
| Tg51 (GP Ibα−/−) | 11 | 109.78 (10.77) | 38.73 (5.39) | 885 (222) |
| . | No. of animals tested . | Anti–IL-4R fluorescence/geometric mean (SD) . | Forward scatter/geometric mean (SD) . | Platelet count, × 109/L (SD) . |
|---|---|---|---|---|
| WT | 14 | 2.42 (0.19) | 17.44 (2.10) | 1373 (278) |
| GP Ibα−/− | 56 | 2.64 (0.20) | 61.84 (9.52) | 382 (92) |
| Tg33 (GP Ibα−/−) | 46 | 68.10 (11.17) | 30.52 (4.84) | 905 (178) |
| Tg51 (GP Ibα−/−) | 11 | 109.78 (10.77) | 38.73 (5.39) | 885 (222) |
Tail bleeding time assays.
Shown are the times obtained for individual GP Ibα−/−(mouse Bernard-Soulier syndrome) and Tg33(Tg33; GP Ibαnull) animals. Normal mouse bleeding times range from 1 to 3 minutes.11 Mice lacking the extracytoplasmic domain of GP Ibα have a severe bleeding phenotype even with an amelioration of the macrothrombocytopenia. These results support the in vivo relevance of the extracytoplasmic GP Ibα ligand-binding domain in normal hemostasis.
Tail bleeding time assays.
Shown are the times obtained for individual GP Ibα−/−(mouse Bernard-Soulier syndrome) and Tg33(Tg33; GP Ibαnull) animals. Normal mouse bleeding times range from 1 to 3 minutes.11 Mice lacking the extracytoplasmic domain of GP Ibα have a severe bleeding phenotype even with an amelioration of the macrothrombocytopenia. These results support the in vivo relevance of the extracytoplasmic GP Ibα ligand-binding domain in normal hemostasis.
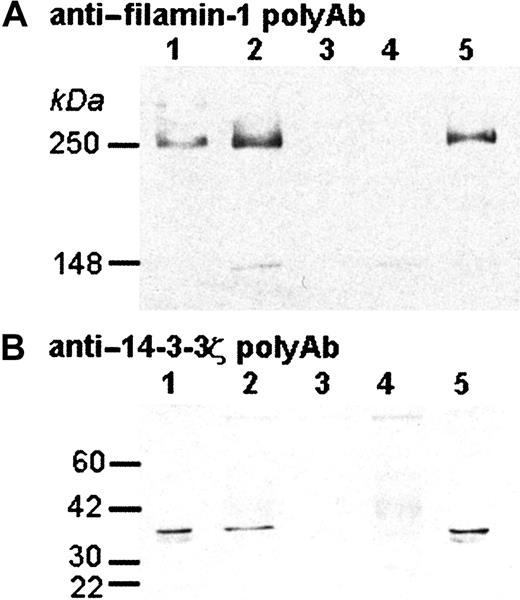
The cytoplasmic tail of GP Ibα has been reported to interact with a number of different platelet proteins.22 Although the protein-protein interactions have been described, the physiologic relevance of the interactions has been more difficult to establish. Given the ability of the chimeric complex to increase platelet count and decrease platelet size, we hypothesized that the cytoplasmic tail has retained the ability to interact with some of these proteins. Therefore, we immunoprecipitated Tg33 platelets with an IL4-R monoclonal antibody and determined if either of the well-characterized interaction partners, filamin-1 and the signal transduction protein 14-3-3ζ, were coimmunoprecipitated. Indeed, immunoprecipitation with the IL-4R monoclonal antibody coprecipitated both filamin-1 and 14-3-3ζ (Figure 6). Thus, with respect to these 2 interaction partners of the native GP Ibα subunit, the fusion protein is mimicking native GP Ibα and provides a molecular explanation for the amelioration of the macrothrombocytopenia.
Coimmunoprecipitation of the interleukin-4Rα/GP Ibα subunit, filamin-1, and 14-3-3ζ.
The ability of the IL-4Rα/GP Ibα subunit to interact with other cytoplasmic proteins was investigated by immunoprecipitation. The immunopurified products were electrophoresed and analyzed by Western blotting using antibodies that recognize filamin-1 (A) or 14-3-3ζ (B). Shown is the resulting autoradiograph produced by the immunoreactive species. Lane 1, normal (wild-type) mouse platelet lysate (no immunoprecipitation); lane 2, immunoprecipiated platelet lysate from animals devoid of mouse GP Ibα but expressing the IL-4Rα transgene (Tg33; GP Ibαnull); lane 3, same lystate as lane 2 but immunoprecipitated with a control IgG; lane 4, normal (wild-type) mouse platelet lysate immunoprecipitated with the anti–IL-4R mAb; lane 5, normal human platelet lysate (no immunoprecipitation).
Coimmunoprecipitation of the interleukin-4Rα/GP Ibα subunit, filamin-1, and 14-3-3ζ.
The ability of the IL-4Rα/GP Ibα subunit to interact with other cytoplasmic proteins was investigated by immunoprecipitation. The immunopurified products were electrophoresed and analyzed by Western blotting using antibodies that recognize filamin-1 (A) or 14-3-3ζ (B). Shown is the resulting autoradiograph produced by the immunoreactive species. Lane 1, normal (wild-type) mouse platelet lysate (no immunoprecipitation); lane 2, immunoprecipiated platelet lysate from animals devoid of mouse GP Ibα but expressing the IL-4Rα transgene (Tg33; GP Ibαnull); lane 3, same lystate as lane 2 but immunoprecipitated with a control IgG; lane 4, normal (wild-type) mouse platelet lysate immunoprecipitated with the anti–IL-4R mAb; lane 5, normal human platelet lysate (no immunoprecipitation).
Discussion
An unexplained yet hallmark feature of the Bernard-Soulier syndrome is macrothrombocytopenia. Numerous studies have characterized the genetic basis for the Bernard-Soulier syndrome, but none have established the molecular basis for the associated macrothrombocytopenia. The present study was undertaken to identify the structural features of the GP Ib-IX complex that contribute to normal platelet biogenesis. Our study focused on the contribution of the α-subunit of the GP Ib-IX complex. The availability of a GP Ibα–deficient mouse facilitated our strategy to produce genetically engineered platelets with an altered GP Ibα subunit. Specifically, the experiment would determine whether a limited portion of GP Ibα could contribute to the normal process of thrombopoesis.
We chose to express a protein composed of the extracytoplasmic domain of the human interleukin-4 receptor (IL-4Rα) fused to the transmembrane and cytoplasmic tail of GP Ibα. The chosen IL-4Rα fragment has been expressed and crystallized as an isolated domain,20 supporting the possibility that the fragment might behave as an isolated domain and, as such, would become a marker for the expression of the fusion protein. Previous studies had established that mouse IL-4 is species specific and does not activate human T cells.23 Thus, the possibility that we might engineer a new stimulatory effect on circulating mouse platelets with a human IL-4 receptor seemed unlikely. We also included a limited coding sequence for the extracytoplasmic domain of GP Ibα because this sequence contains tandem Cys residues that normally disulfide-link to the GP Ibβ subunit (Figure 1). To facilitate the independence of each domain within the polypeptide, a triple glycine repeat was inserted between the 2 sequences (Figure 1). The expression of the fusion protein was initially characterized in heterologous cells (Figures 2and 3). The successful generation of an IL-4Rα/GP Ib-IX complex on the surface of CHO cells provided preliminary evidence that genetically engineered platelets might express a similar complex.
During the course of our preliminary studies, we demonstrated that the extracytoplasmic domain of GP Ibα is not essential for the surface expression of a GP Ib-IX complex. Well-documented motifs within each subunit of the GP Ib-IX complex are the leucine-rich repeats.24 Indeed, the leucine-rich repeats have been proposed to support protein-protein interactions, leading to speculation that the leucine repeats within the extracytoplasmic domains of GP Ibα, GP Ibβ, and GP IX might contribute to surface assembly of the complex.25 Our results suggest that the leucine-rich repeats of GP Ibα have no major role in assembly of the complex. We would propose that residues within the transmembrane domain of each subunit or the formation of the GP Ibα/GP Ibβ disulfide bond just distal to the transmembrane domain (Figure 1) are likely to be key to the assembly and formation of a GP Ib-IX complex.
Relative to our original objective, we demonstrated an amelioration of the macrothrombocytopenia associated with the Bernard-Soulier syndrome. However, the animals retained a severe bleeding phenotype similar to that described for the mouse model of the Bernard-Soulier syndrome. Together, these results highlight the pleiotropic phenotype produced in the absence of a GP Ib-IX complex. Although the expression of the chimeric receptor in the Bernard-Soulier model increases platelet count and decreases platelet size, it is not possible to definitively state that the absence of a GP Ibα cytoplasmic tail is solely responsible for generating the macrothrombocytopenia. First, the fusion protein facilitates the surface expression of the complete complex and, as such, brings GP Ibβ and GP IX back to the platelet surface. Do either of these subunits contribute to the phenotypic corrections observed in these animals? Certainly, a role for GP IX in this regard seems unlikely, because it lacks any appreciable cytoplasmic tail and has no known extracytoplasmic ligand.4 A role for GP Ibβ in the process is still possible because both the α- and β-subunits of GP Ib may share interaction partners, with the best example being interactions with dimeric 14-3-3ζ.26,27 A more definitive answer to this question may come with additional animals generated using a similar approach where specific mutations are introduced to block interactions to the GP Ibα tail. One set of mutations must include those residues that interact with filamin-1.7 Successful generation of these platelets may also define critical intracellular cross talk mechanisms between the GP Ib-IX and αIIbβ3 complexes because the membrane cytoskeleton has been implicated in the GP Ib-IX hemostatic response.28
The results do illustrate the critical importance for expression of the GP Ibα cytoplasmic tail. However, in the context of the chimeric complex we did not observe a correction of platelet number or size to levels observed in normal animals. Indeed, in our previous study, expression of the complete GP Ibα subunit did not correct the Bernard-Soulier syndrome phenotype to normal levels but did significantly improve platelet count, circulating platelet size, and bleeding time.11 One possible explanation is that the fusion protein is not expressed to high enough levels. However, we characterized 2 different transgenic lines with differing levels of surface-expressed IL-4Rα. An increased level of receptor, such as that observed on platelets from Tg51 animals, did not provide an additional correction of the macrothrombocytopenia. Thus, it appears that expression levels are important but higher levels of the fusion protein do not produce a further correction of the phenotype.
The partial correction of the macrothrombocytopenia in this model may also be suggesting that the conformation of the isolated cytoplasmic tail is somewhat dependent upon the extracytoplasmic domain. Indeed, conformation changes produced by ligand binding and a change in the interactions of the cytoplasmic tail with other proteins may be a central issue in normal GP Ib-IX biology.29 Is there any evidence linking the structure of the GP Ibα extracytoplasmic and cytoplasmic domains together? Several years ago a variant GP Ibα molecule (type Bolzano) was described containing a missense mutation within a leucine-rich repeat of the extracytoplasmic domain (A156V).30 This Bernard-Soulier syndrome variant did not preclude surface expression of GP Ibα (although expression was reduced as compared with normal platelets) but did produce a giant platelet phenotype. However, a direct comparison of the type Bolzano platelet size to other Bernard-Soulier syndrome platelets was not done. By a strict definition, the transgenic animals characterized in the current study could be classified as having the Bernard-Soulier syndrome. They have 3 of the hallmark features when compared with platelets from a normal animal: (1) they lack the extracytoplasmic domain of GP Ibα; (2) they have a reduced platelet count; and (3) they have larger-than-normal–sized circulating platelets. Yet, when compared with the homozygous-deficient animal, they illustrate a less severe phenotype. Thus, our results highlight an additional level of heterogeneity that occurs within the designation of a Bernard-Soulier syndrome, and the type Bolzano variant probably represents variation on the phenotypic expression of the human syndrome.
The molecular basis for macrothrombocytopenia is likely to be unique to each of the disorders presenting with the phenotype. However, one unifying theme will most likely be the disruption or perturbation of molecular mechanisms controlling platelet morphogenesis and release. The present study suggests a direct role for the carboxyl terminus of GP Ibα in thrombopoiesis. The utilization of animal models with defined genetic defects presents an opportunity to define fundamental aspects of thrombopoiesis and normal platelet function. Indeed, the cellular environment and cellular characteristics of the megkaryocyte and platelet make in vivo models an excellent experimental framework to study both megakaryocyte and platelet biology. Future studies exploiting these models will define key mechanisms controlling normal platelet biogenesis and function.
The authors acknowledge the Sam and Rose Stein Charitable Trust for the establishment of the DNA Core Facility within the Department of Molecular and Experimental Medicine at The Scripps Research Institute. The administrative assistance of Ms Pamela Fagan is greatly appreciated.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2002-03-0997.
Supported by HL50545 from the Heart, Lung, and Blood Institute of the National Institutes of Health (J.W.) and by the Sumitomo Life Insurance Welfare Service Foundation (2000) and Uehara Memorial Foundation (2001) (T.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jerry Ware, MEM170, The Scripps Research Institute, 10550 N Torrey Pines Rd, La Jolla, CA 92037; e-mail:jware@scripps.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal