Abstract
Hyperhomocysteinemia has been proposed to inhibit the protein C anticoagulant system through 2 mechanisms: decreased generation of activated protein C (APC) by thrombin, and resistance to APC caused by decreased inactivation of factor Va (FVa). We tested the hypotheses that generation of APC by thrombin is impaired in hyperhomocysteinemia in monkeys and that hyperhomocysteinemia produces resistance to APC in monkeys, mice, and humans. In a randomized crossover study, cynomolgus monkeys were fed either a control diet or a hyperhomocysteinemic diet for 4 weeks. Plasma total homocysteine (tHcy) was approximately 2-fold higher when monkeys were on the hyperhomocysteinemic diet than when they were on the control diet (9.8 ± 2.0 μM versus 5.6 ± 1.0 μM; P < .05). After infusion of human thrombin (25 μg/kg of body weight), the peak level of plasma APC was 136 ± 16 U/mL in monkeys fed the control diet and 127 ± 13 U/mL in monkeys fed the hyperhomocysteinemic diet (P > .05). The activated partial thromboplastin time was prolonged to a similar extent by infusion of thrombin in monkeys fed the control diet and in those fed the hyperhomocysteinemic diet. The sensitivity of plasma FV to human APC was identical in monkeys on control diet and those on hyperhomocysteinemic diet. We also did not detect resistance of plasma FV to APC in hyperhomocysteinemic mice deficient in cystathionine β-synthase (plasma tHcy, 93 ± 16 μM) or in human volunteers with acute hyperhomocysteinemia (plasma tHcy, 45 ± 6 μM). Our findings indicate that activation of protein C by thrombin and inactivation of plasma FVa by APC are not impaired during moderate hyperhomocysteinemia in vivo.
Introduction
Hyperhomocysteinemia is a risk factor for stroke, myocardial infarction, peripheral arterial disease, and venous thrombosis.1,2 An association between moderate hyperhomocysteinemia and clinical cardiovascular events has been observed in more than 35 case-control and observational epidemiologic studies, as well as several prospective studies.3 Because elevated levels of plasma total homocysteine (tHcy) can often be lowered by oral administration of folic acid or combinations of B vitamins, treatment of hyperhomocysteinemia has been proposed as a strategy for preventing cardiovascular disease and its complications. This approach is currently being evaluated in several prospective randomized intervention trials.4
Like other cardiovascular risk factors, hyperhomocysteinemia produces endothelial dysfunction, which can be detected on the basis of impaired responses to endothelium-dependent vasodilators.5Hyperhomocysteinemia may also adversely affect the protein C anticoagulant pathway.6 Incubation of cultured endothelial cells with exogenous homocysteine decreased the activity of thrombomodulin, which is the major endothelial cofactor for activation of protein C by thrombin.7-9 In agreement with these findings in vitro, we have observed decreased thrombomodulin activity in the aorta of hyperhomocysteinemic monkeys10 and mice.11 Clinical evidence for impaired activation of protein C in hyperhomocysteinemia has not been convincing,12 however, and the effect of hyperhomocysteinemia on activation of endogenous protein C has not been examined in animal models.
In addition to possibly inhibiting the activation of protein C, hyperhomocysteinemia has been proposed to impair the ability of activated protein C (APC) to inactivate its major substrate, coagulation factor Va (FVa). Undas et al13 reported that homocysteine can react rapidly with free cysteine residues of FV in vitro, resulting in APC resistance similar to that of FV Leiden. This effect of hyperhomocysteinemia could have direct clinical implications because FV Leiden is an established risk factor for venous thrombosis.14
Thus, hyperhomocysteinemia may interfere with the protein C anticoagulant system through 2 mechanisms: decreased activation of protein C by thrombin and thrombomodulin, and resistance to APC caused by decreased inactivation of FVa. In this study, we used a diet that produces hyperhomocysteinemia in monkeys to test the hypothesis that generation of APC by thrombin is impaired during hyperhomocysteinemia in vivo. We also tested the hypothesis that hyperhomocysteinemia alters the APC sensitivity of plasma FV in cynomolgus monkeys, mice deficient in cystathionine–β-synthase (CBS), and humans.
Patients, materials, and methods
Cynomolgus monkeys
In a randomized crossover study, 8 adult cynomolgus monkeys (Macaca fascicularis) were fed a control diet and a hyperhomocysteinemic diet, each for 4 weeks, as described previously.10 The hyperhomocysteinemic diet was enriched in methionine (10 g/kg of body weight), relatively depleted in folic acid (1.5 mg/kg), and free of choline. After 4 weeks on each diet, the monkeys were sedated with ketamine hydrochloride (20 mg/kg given intramuscularly) and anesthetized with sodium pentobarbital (20 mg/kg given intravenously). A nonobstructive catheter was inserted into an axillary artery for blood sampling, and the axillary vein was cannulated for administration of thrombin and supplemental anesthesia (sodium pentobarbital [5 mg/kg per hour]). Arterial pressure was monitored continuously. Human α-thrombin (Enzyme Research Laboratories, South Bend, IN; 25 μg/kg over 10 minutes) in 10 mL saline was infused through the axillary vein catheter as described previously.15 16 At various times (0-120 minutes), blood was collected from the axillary artery catheter directly into a 1/10 volume of 3.8% sodium citrate with 0.3 M benzamidine (for determination of APC) or 3.8% sodium citrate without benzamidine (for other hemostasis assays). Additional blood samples were collected into 3.4 mM EDTA (ethylenediaminetetraacetic acid) for determination of tHcy. Blood samples were placed on ice immediately, and plasma was isolated by centrifugation at 2500g for 30 minutes at 4°C. The protocol was approved by the University of Iowa and Veterans Affairs Animal Care and Use Committees.
CBS-deficient mice
Heterozygous CBS-deficient mice17 were crossbred to C57BL/6J mice for at least 8 generations, and genotyping for the targeted CBS allele was performed by using polymerase chain reaction analysis.17 At weaning, heterozygous CBS-deficient (CBS+/−) mice and wild-type (CBS+/+) litter mates were fed one of 3 diets: (1) a control diet (LM485 chow, Harlan Teklad, Madison, WI) that contained 4.0 g/kg l-methionine and 7.5 mg/kg folic acid; (2) a high-methionine diet (LM485 chow with drinking water supplemented with 0.5%l-methionine);11 or (3) a high-methionine/low-folate diet (TD00205, Harlan Teklad) that contained 8.2 g/kg l-methionine, 0.2 mg/kg folic acid, and 50 g/kg succinyl sulfathiazole. After 5 weeks on the experimental diet, mice were anesthetized and blood was collected by cardiac puncture into a 1/10 volume of 3.8% sodium citrate for hemostasis assays and measurement of plasma tHcy. The protocol was approved by the University of Iowa and Veterans Affairs Animal Care and Use Committees.
Human volunteers
Ten healthy volunteers (6 men and 4 women) without risk factors or clinical evidence of atherosclerosis were recruited by advertisement. Written informed consent was obtained from each subject, and the study protocol was approved by the University of Iowa Institutional Review Board. In a randomized, double-blind crossover study, each subject received oral l-methionine (100 mg/kg) dissolved in cranberry juice on one study day and cranberry juice alone (placebo) on another study day, as described previously.18The 2 study days were separated by at least 2 weeks. Blood samples for measurement of plasma tHcy were collected into chilled EDTA tubes immediately before and 6 and 8 hours after oral administration of methionine or placebo. Blood samples for hemostasis assays were obtained 6 hours after oral administration of methionine or placebo and collected into sodium citrate tubes. All blood samples were placed on ice immediately, and plasma was isolated by centrifugation at 2500g for 30 minutes at 4°C.
Hemostasis assays
Plasma APC was measured by enzyme-capture assay using the antihuman protein C light- chain monoclonal antibody C319and chromogenic substrate S-2366 as described previously.15 One unit of APC was defined as the amount present in 1.0 mL pooled monkey plasma. Activated partial thromboplastin time (APTT) was measured with an ACL-300+ coagulometer (Instrumentation Laboratory, Lexington, MA) by using the Platelin L reagent (Organon Teknika, France). To measure the APC sensitivity of FV, plasma was diluted 1:10 into human FV–deficient plasma (George King Bio-Medical, Overland Park, KS).20 APTT assessment was then performed in the presence of 0 to 20 nM human APC (Enzyme Research Laboratories) as described previously.15
Plasma tHcy
Plasma tHcy, defined as the total concentration of homocysteine after quantitative reductive cleavage of all disulfide bonds,21 was measured by high-performance liquid chromatography. Samples from monkeys were analyzed in Dr Malinow's laboratory,22,23 samples from mice were analyzed in Dr Bottiglieri's laboratory,24 and samples from humans were analyzed in the University of Iowa General Clinical Research Center.25
Other assays
Serum creatinine, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides were measured in the clinical laboratories of the University of Iowa Hospitals.
Statistical analysis
APC-sensitivity results and changes in APTT and APC in response to thrombin were analyzed by using a 2-way repeated-measures analysis of variance with Bonferroni multiple-comparison analysis. Paired 2-tailed Student t tests were used to compare values obtained on separate study days in monkeys or human volunteers. Unpaired Student t tests were used to compare values in CBS+/− or CBS−/− mice fed different diets. AP value of less than .05 was considered to represent statistical significance. Values are reported as mean ± SE.
Results
Anticoagulant responses to thrombin in monkeys
Monkeys were fed a control diet and a hyperhomocysteinemic diet, each for 4 weeks, in a randomized crossover study. The monkeys' body weights and serum levels of creatinine and cholesterol were not affected by the diets (Table 1). Plasma tHcy was approximately 2-fold higher when monkeys were fed the hyperhomocysteinemic diet (P < .05).
Effect of diet on body weight, creatinine, fasting lipid profile, and plasma tHcy in monkeys
| Characteristic . | Control diet (n = 8) . | Hyperhomocysteinemic diet (n = 8) . |
|---|---|---|
| Body weight (kg) | 7.9 ± 0.6 | 7.8 ± 0.6 |
| Creatinine (μmol/L) | 106 ± 0.88 | 115 ± 0.88 |
| Total cholesterol (mmol/L) | 3.47 ± 0.18 | 3.59 ± 0.18 |
| LDL cholesterol (mmol/L) | 1.47 ± 0.10 | 1.42 ± 0.13 |
| HDL cholesterol (mmol/L) | 1.89 ± 0.21 | 2.07 ± 0.16 |
| Triglycerides (mmol/L) | 0.35 ± 0.14 | 0.25 ± 0.05 |
| tHcy (μM) | 5.6 ± 1.0 | 9.8 ± 2.0* |
| Characteristic . | Control diet (n = 8) . | Hyperhomocysteinemic diet (n = 8) . |
|---|---|---|
| Body weight (kg) | 7.9 ± 0.6 | 7.8 ± 0.6 |
| Creatinine (μmol/L) | 106 ± 0.88 | 115 ± 0.88 |
| Total cholesterol (mmol/L) | 3.47 ± 0.18 | 3.59 ± 0.18 |
| LDL cholesterol (mmol/L) | 1.47 ± 0.10 | 1.42 ± 0.13 |
| HDL cholesterol (mmol/L) | 1.89 ± 0.21 | 2.07 ± 0.16 |
| Triglycerides (mmol/L) | 0.35 ± 0.14 | 0.25 ± 0.05 |
| tHcy (μM) | 5.6 ± 1.0 | 9.8 ± 2.0* |
Values are mean ± SE.
P < .05 compared with control diet.
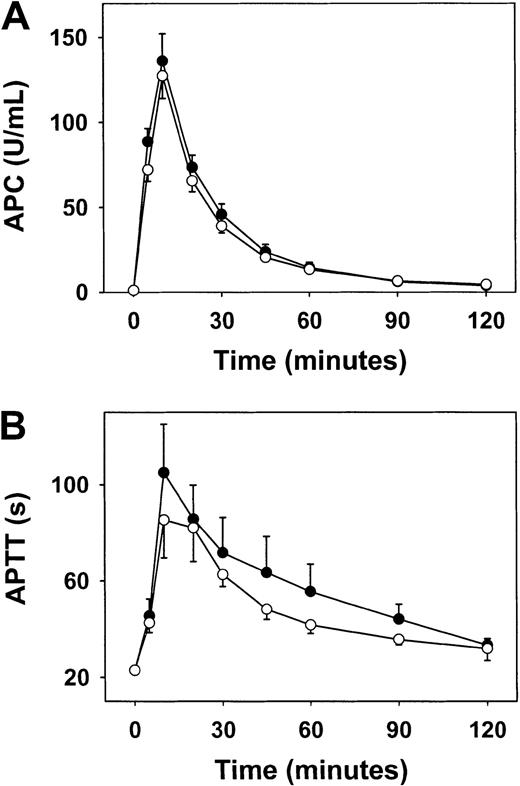
Infusion of thrombin produces activation of protein C and APC-dependent prolongation of APTT in monkeys.15 26 To determine whether these anticoagulant responses are impaired in hyperhomocysteinemia, we measured plasma APC and APTT in response to infusion of human thrombin (25 μg/kg given intravenously over 10 minutes) in monkeys after they had been on each diet for 4 weeks. Baseline values for APC and APTT before infusion of thrombin did not differ significantly between the 2 diets (Figure 1). After infusion of thrombin, the peak level of plasma APC was 136 ± 16 U/mL when monkeys were fed the control diet and 127 ± 13 U/mL when monkeys were fed the hyperhomocysteinemic diet (P > .05; Figure1A). APTT was prolonged to a similar extent by infusion of thrombin during the 2 diets, although there was a trend toward less prolongation when monkeys were fed the hyperhomocysteinemic diet (maximal prolongation, 62 ± 16 seconds versus 82 ± 20 seconds for the control diet; P = .07; Figure 1B). These findings indicate that the hyperhomocysteinemic diet did not produce significant impairment of thrombin-induced activation of protein C in vivo, but do not exclude a possible effect of hyperhomocysteinemia on the ability of APC to prolong APTT in monkey plasma.
Anticoagulant response to infusion of thrombin in monkeys.
Circulating APC (A) and APTT (B) were measured before, during, and after a 10-minute infusion of human thrombin in monkeys after 4 weeks on a control diet (solid circles) or a hyperhomocysteinemic diet (open circles). Values are mean ± SE.
Anticoagulant response to infusion of thrombin in monkeys.
Circulating APC (A) and APTT (B) were measured before, during, and after a 10-minute infusion of human thrombin in monkeys after 4 weeks on a control diet (solid circles) or a hyperhomocysteinemic diet (open circles). Values are mean ± SE.
APC sensitivity of monkey FV
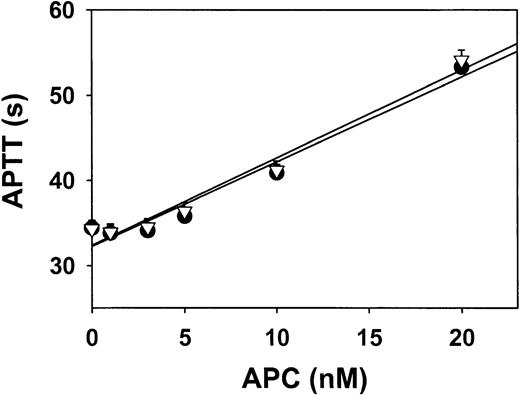
To determine whether hyperhomocysteinemia in monkeys produces resistance to APC by impairing APC-mediated inactivation of FV, the sensitivity of monkey FV to human APC was measured after monkey plasma was diluted 1:10 into human FV–deficient plasma. The sensitivity to APC when monkeys were fed control diets was identical to that when they were fed hyperhomocysteinemic diets (Figure2). This finding suggests that FV from hyperhomocysteinemic monkeys is not resistant to inactivation by APC.
Sensitivity of plasma FV to APC in monkeys.
Plasma collected from monkeys after 4 weeks on a control diet (solid circles) or a hyperhomocysteinemic diet (open triangles) was diluted 1:10 in human FV–deficient plasma, and APTT was measured in the presence of the indicated concentrations of human APC. Values are mean ± SE.
Sensitivity of plasma FV to APC in monkeys.
Plasma collected from monkeys after 4 weeks on a control diet (solid circles) or a hyperhomocysteinemic diet (open triangles) was diluted 1:10 in human FV–deficient plasma, and APTT was measured in the presence of the indicated concentrations of human APC. Values are mean ± SE.
APC sensitivity of FV in hyperhomocysteinemic mice
Beginning at weaning, CBS+/− and CBS+/+mice were fed either a control diet, a high-methionine diet, or a high-methionine/low-folate diet. At 5 weeks of age, all mice appeared to be healthy, and body weight did not differ between CBS+/+ and CBS+/− mice or between mice fed control and experimental diets (data not shown). Compared with the control diet, the high-methionine and high-methionine/low-folate diets produced graded elevations of plasma tHcy in both CBS+/+and CBS+/− mice, and plasma tHcy was higher in CBS+/− mice than in CBS+/+ mice for each diet (Table 2).
Effect of diet and CBS genotype on plasma tHcy in mice
| Type of diet . | CBS+/+ mice . | CBS+/− mice . |
|---|---|---|
| Control | 2.9 ± 0.3 (n = 4) | 6.2 ± 1.0 (n = 6)* |
| High methionine | 12.6 ± 3.0 (n = 12)† | 30.2 ± 6.4 (n = 10)*,† |
| High methionine/low folate | 36.4 ± 5.6 (n = 10)† | 92.8 ± 15.9 (n = 6)*,† |
| Type of diet . | CBS+/+ mice . | CBS+/− mice . |
|---|---|---|
| Control | 2.9 ± 0.3 (n = 4) | 6.2 ± 1.0 (n = 6)* |
| High methionine | 12.6 ± 3.0 (n = 12)† | 30.2 ± 6.4 (n = 10)*,† |
| High methionine/low folate | 36.4 ± 5.6 (n = 10)† | 92.8 ± 15.9 (n = 6)*,† |
Values are mean ± SE micromoles plasma tHcy (no. of mice in group).
P < .05 compared with CBS+/+mice.
P < .05 compared with control diet.
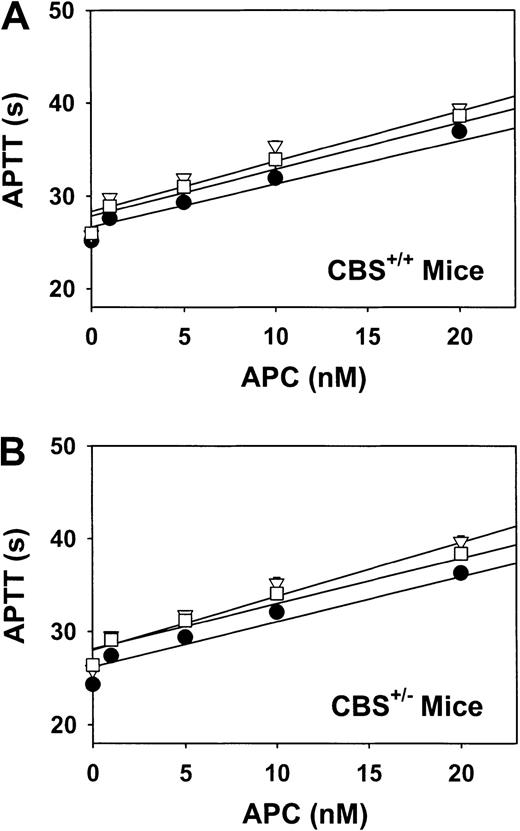
The sensitivity of murine FV to human APC was measured after plasma from CBS+/+ or CBS+/− mice was diluted 1:10 into human FV–deficient plasma. Despite a wide range of plasma tHcy concentrations (3-36 μM) in CBS+/+ mice, the sensitivity of FV to APC did not differ significantly between diets (Figure3A). Similarly, no diet-induced differences in APC sensitivity were observed in CBS+/−mice, despite an even broader range of plasma tHcy concentrations (6-93 μM; Figure 3B).
Sensitivity of plasma FV to APC in mice.
Plasma was collected from CBS+/+ mice (A) or CBS+/− mice (B) fed a control diet (solid circles), a high-methionine diet (open triangles), or a high-methionine/low-folate diet (open squares). Samples were diluted 1:10 in human FV–deficient plasma, and APTT was measured in the presence of the indicated concentrations of human APC. Values are mean ± SE.
Sensitivity of plasma FV to APC in mice.
Plasma was collected from CBS+/+ mice (A) or CBS+/− mice (B) fed a control diet (solid circles), a high-methionine diet (open triangles), or a high-methionine/low-folate diet (open squares). Samples were diluted 1:10 in human FV–deficient plasma, and APTT was measured in the presence of the indicated concentrations of human APC. Values are mean ± SE.
Effect of methionine loading on APC sensitivity in human volunteers
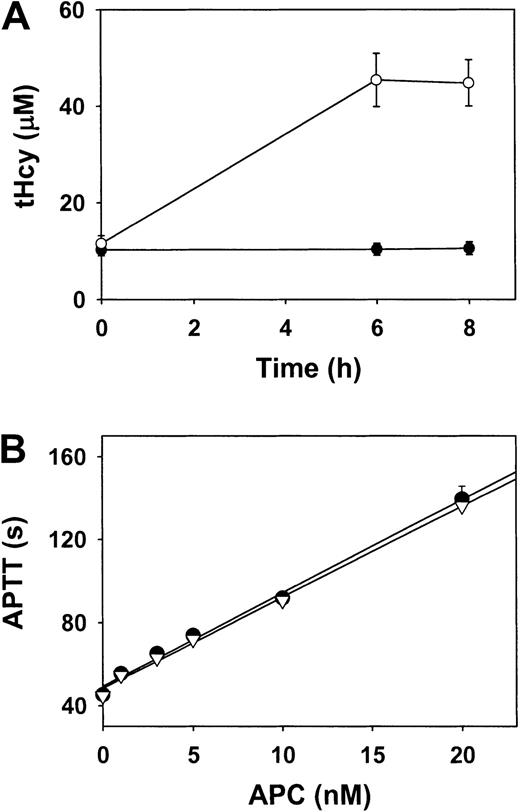
To determine whether acute hyperhomocysteinemia impairs the sensitivity of human FV to APC, 10 healthy human volunteers received an oral l-methionine (100 mg/kg) load in a placebo-controlled, randomized, double-blind crossover study. Plasma tHcy levels increased from 11.5 ± 1.6 μM to 45.4 ± 5.5 μM (P < .001) 6 hours after administration of L-methionine (Figure 4A). Plasma tHcy levels did not change significantly (10.2 ± 1.2 μM to 10.5 ± 1.3 μM;P > .05) after administration of placebo. The sensitivity of plasma FV to APC was essentially identical in samples collected 6 hours after administration of L-methionine and in samples collected from the same volunteers 6 hours after administration of placebo (Figure 4B). These observations show that marked elevation of plasma tHcy in humans does not acutely alter the susceptibility of FV to APC.
Plasma tHcy and sensitivity of FV to APC in human volunteers.
(A) Plasma tHcy levels after oral administration of L-methionine (open circles) or placebo (solid circles). (B) Plasma was collected 6 hours after oral administration of L-methionine (open triangles) or placebo (solid circles). Samples were diluted 1:10 in human FV–deficient plasma, and APTT was measured in the presence of the indicated concentrations of human APC. Values are mean ± SE.
Plasma tHcy and sensitivity of FV to APC in human volunteers.
(A) Plasma tHcy levels after oral administration of L-methionine (open circles) or placebo (solid circles). (B) Plasma was collected 6 hours after oral administration of L-methionine (open triangles) or placebo (solid circles). Samples were diluted 1:10 in human FV–deficient plasma, and APTT was measured in the presence of the indicated concentrations of human APC. Values are mean ± SE.
Discussion
A great deal of epidemiologic evidence indicates that hyperhomocysteinemia is a risk factor for thrombotic vascular disease,1-3 but the mechanisms responsible for vascular pathology in hyperhomocysteinemia are not completely understood. Homocysteine may create a predisposition to thrombosis and other adverse cardiovascular events through several different mechanisms, including impairment of the protein C anticoagulant system.5,6 13 Because much of the evidence for homocysteine-induced impairment of the protein C system has been obtained from experiments performed in vitro, we tested the hypotheses that activation of protein C and susceptibility of FV to APC are altered during hyperhomocysteinemia in vivo. Our major findings were that thrombin-induced activation of endogenous protein C is not significantly impaired in hyperhomocysteinemic monkeys and that plasma FV does not become resistant to APC during hyperhomocysteinemia in monkeys, mice, or humans.
The hyperhomocysteinemic diet that we fed to monkeys produced about a 2-fold increase in plasma tHcy. Although moderate, this degree of hyperhomocysteinemia is likely to be pathophysiologically relevant because it is very similar to that obtained in a previous study in which we observed significant impairment of endothelium-dependent vasomotor function in hyperhomocysteinemic monkeys.10Abnormal endothelial function has also been observed in CBS-deficient mice with mild elevations of plasma tHcy (about 10 μM),27 and relatively small increases in plasma tHcy are associated with adverse cardiovascular events in humans.1Our finding that normal APC responses to thrombin were preserved during hyperhomocysteinemia in monkeys suggests that impairment of protein C activation is unlikely to be a major mechanism of vascular pathologic conditions in mild hyperhomocysteinemia. The thrombin-infusion protocol is a reliable method for detecting abnormalities of endogenous protein C activation in monkeys: we previously used this protocol to demonstrate impairment of the protein C system in hypercholesterolemic monkeys.15 16
Although there was no difference in activation of protein C when monkeys were fed the control diet compared with when they were fed the hyperhomocysteinemic diet, we did observe a trend toward less prolongation of APTT in response to thrombin during the hyperhomocysteinemic diet (Figure 1B). This effect was probably not caused by resistance of FV to APC because in vitro assays of the sensitivity of plasma FV to human APC demonstrated equivalent dose-response relations in control and hyperhomocysteinemic monkeys (see below). It is also unlikely that this effect was caused by resistance of FVIII or other plasma factors to APC because prolongation of APTT in response to infusion of APC is not impaired in monkeys with combined hyperhomocysteinemia and hypercholesterolemia.15It remains possible that hyperhomocysteinemia may alter the change in APTT in response to thrombin through APC-independent mechanisms. Exploration of such mechanisms will require additional studies with larger numbers of animals.
The activated form of coagulation FV (FVa) is a major substrate for APC, and impaired inactivation of FVa by APC in patients with FV Leiden is associated with an increased risk of venous thrombosis.14 Homocysteine can react rapidly with free cysteine residues of FV in vitro, resulting in “homocysteinylation” of FV.13 Homocysteinylation of FV does not alter its rate of activation by thrombin, but homocysteinylated FVa is resistant to inactivation by APC.13 Using a direct assay for APC resistance in which human APC was added to undiluted monkey plasma, we did not detect resistance to APC in a previous study of monkeys with combined hyperhomocysteinemia and hypercholesterolemia.15In the current study, we used a modified assay for APC resistance in which monkey plasma was diluted 1:10 into human FV–deficient plasma to measure the APC sensitivity of FV specifically.20 We found that the sensitivity of monkey FV to APC when monkeys were fed a control diet was identical to that when monkeys were fed a hyperhomocysteinemic diet (Figure 2). This finding shows that FVa from hyperhomocysteinemic monkeys is not resistant to inactivation by APC.
To determine the effect of higher levels of plasma tHcy on the sensitivity of FVa to APC, we performed APC-sensitivity assays with plasma from CBS+/+ or CBS+/− mice that were fed hyperhomocysteinemic diets. We observed no significant differences in the APC sensitivity of FV between CBS+/+ and CBS+/− mice or between mice fed control and hyperhomocysteinemic diets, despite plasma tHcy levels of up to 93 ± 16 μM in CBS+/− mice fed a high-methionine/low-folate diet. The absence of APC resistance in hyperhomocysteinemic mice probably was not caused by a species-specific difference in the susceptibility of FV to homocysteinylation because all the cysteine residues near the APC cleavage site in the A2 domain are conserved in murine and human FV.28 It is possible, however, that species-specific differences in the plasma composition of homocysteine mixed disulfides in humans and rodents29 could contribute to differences in reactivity with FV.
To test the hypothesis that acute hyperhomocysteinemia produces resistance to APC in humans, we conducted APC-sensitivity assays of plasma obtained 6 hours after administration of an oral methionine load. A limitation of this experimental approach is that the hemostatic effects of acute hyperhomocysteinemia induced by oral methionine loading in healthy volunteers may differ from those in patients with chronic hyperhomocysteinemia. Nevertheless, oral methionine loading does produce impairment in endothelium-dependent vasomotor responses in the brachial artery of healthy humans.18 Moreover, because homocysteinylation of purified FV occurs within minutes in vitro,13 we anticipated that resistance of plasma FV to APC should be apparent within minutes to hours after oral methionine loading. However, despite a large increase in plasma tHcy (45 ± 6 μM versus 11 ± 1 μM), the sensitivity of plasma FV to APC was essentially identical in samples collected 6 hours after oral administration of methionine and in those obtained 6 hours after oral administration of placebo.
Our observations that hyperhomocysteinemia in vivo in monkeys, mice, and humans does not induce resistance to APC in circulating FV is in contradiction to the hypothesis generated from the in vitro studies of Undas et al.13 This discrepancy may be explained by variations in the reactivity of FV with homocysteine in an in vitro purified system compared with an in vivo environment. Undas et al13 found that purified FV was homocysteinylated in vitro by the reduced thiol DL-homocysteine (30 μM-1.0 mM), presumably through a copper-dependent oxidation reaction. Homocysteinylation of FV was also detected when human plasma was exposed to a high concentration (10 mM) of DL-homocysteine that far exceeded clinical pathologic levels. Disulfide forms of homocysteine account for more than 95% of plasma tHcy, even in patients with moderate hyperhomocysteinemia.21 Therefore, the plasma concentration of the reduced thiol form of homocysteine that is available to react with FV through oxidative reactions in vivo is at least 2 to 3 orders of magnitude lower than that used in the in vitro plasma experiments. Furthermore, homocysteinylation of FV in vivo may not depend on direct oxidation of homocysteine because conversion of homocysteine to its disulfide forms in plasma is mediated mainly by thiol-disulfide exchange reactions rather than by copper-dependent oxidation.30 Thiol-disulfide exchange appears to be a major mechanism for homocysteinylation of albumin,30 but it is not known whether FV is susceptible to homocysteinylation through this mechanism.
In summary, our findings in monkeys, mice, and human volunteers constitute evidence against a major effect of hyperhomocysteinemia on activation of protein C or on inactivation of FVa by APC. Therefore, these mechanisms are unlikely to be clinically important in most patients with thrombosis or other adverse cardiovascular events associated with mild to moderate hyperhomocysteinemia. Our data do not, however, exclude the possibility that impairment of the protein C anticoagulant pathway may occur in some patients with more severe hyperhomocysteinemia. This possibility could perhaps be addressed in studies of patients with end-stage renal disease or hereditary homocystinuria due to homozygous CBS deficiency.21
We thank Lorie Leo for technical assistance. Genotyping was performed by Lucinda Robbins, Norma Sinclair, and Patricia Lovell in the University of Iowa Transgenic Facility under the direction of Curt D. Sigmund.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2002-03-0727.
Supported by the Office of Research and Development, Department of Veterans Affairs, and National Institutes of Health grants HL63943, DK25295, HL58972, HL52246, and HL62984.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Steven R. Lentz, Department of Internal Medicine, C303 GH, University of Iowa, Iowa City, IA 52242; e-mail:steven-lentz@uiowa.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal