Administration of the immunosuppressive drug cyclosporine A (CsA) following autologous stem cell transplantation paradoxically elicits a systemic autoimmune syndrome resembling graft-versus-host disease (GVHD). This syndrome, termed autologous GVHD, is associated with autoreactive CD8+ T cells that recognize major histocompatibility complex (MHC) class II determinants in association with a peptide from the invariant chain. To investigate the potential role of cytokines and chemokines in autologous GVHD, interleukin 2 (IL-2), IL-4, IL-10, interferon γ (IFN-γ), and macrophage inflammatory protein-1α (MIP-1α) gene expression in peripheral blood mononuclear cells (PBMCs) was determined in 36 patients treated with CsA following transplantation and correlated with the induction of cytolytic activity against autologous phytohemagglutinin-stimulated lymphocytes (PHA-blasts) and the breast cancer cell line (T47D). The determination of gene expression by real-time polymerase chain reaction (PCR) revealed that IL-10 mRNA levels by PBMCs in patients with autologous GVHD were 29-fold higher than in healthy individuals. IFN-γ (4-fold), IL-2 (3-fold), and MIP-1α (44-fold) mRNA levels were also increased in GVHD-induced patients compared with healthy individuals. The ability of PBMCs to lyse autologous PHA-blasts and T47D tumor cells exhibited an identical temporal relationship with expression of IL-10 and IFN-γ during autologous GVHD. Moreover, the susceptibility to autologous GVHD as assessed in 75 patients was significantly associated with the IL-10−1082 G/G polymorphic alleles, allelic variants in the promoter region that govern IL-10 production. These findings indicate that IL-10 may play an unexpected but critical role in autologous GVHD and could be utilized to enhance a graft-versus-tumor effect after transplantation. Interestingly, polymorphisms in the IL-10 promoter region may also explain differences in the susceptibility of patients to autologous GVHD induction.
Introduction
High-dose chemoradiotherapy combined with autologous stem cell transplantation (SCT) can be used successfully in the treatment of patients with malignant lymphoma. A number of clinical studies demonstrate that among the therapeutic options available for relapsing lymphoma, autologous SCT is the most effective approach to achieve long-term survival.1-3 However, clinical trials have failed to demonstrate any significant advantage of autologous SCT over conventional chemotherapy in the treatment of patients with either chemotherapy-resistant non-Hodgkin lymphoma (NHL), with NHL in remission with poor prognostic factors,4,5 or in patients with breast cancer with extensive lymph node involvement.6,7 Raising the dose intensity of chemotherapy facilitated by the use of autologous SCT does not necessarily improve the outcome. For patients who do not achieve sustained remission after conventional chemotherapy, novel approaches including immunotherapy are needed. Based on the findings that the relapse rate after allogeneic SCT is remarkably lower in patients with graft-versus-host disease (GVHD) compared with patients who do not develop this syndrome,8-10 one potential approach is the induction of autologous GVHD after autologous SCT.
Autologous GVHD can be induced in recipients of autologous bone marrow by administration of cyclosporine A (CsA) for a short period following transplantation.11-16 This autoaggression syndrome shares similar dermal pathology with acute GVHD after allogeneic SCT. Several initial clinical trials in patients with acute myeloid leukemia and in patients with NHL suggest that the induction of autologous GVHD can reduce the rate of relapse.17-20 Analysis of the effector mechanisms involved in autologous GVHD reveal promiscuous recognition of major histocompatibility complex (MHC) class II determinants by CD8+ T cells.21-24 Clonal expansion of autoreactive T cells are observed in both humans and in the rat model.24,25 Interestingly, the CD8+autoreactive T cells lyse myeloma, lymphoma, and breast cancer cell lines.21,22 Moreover, the antitumor effect can be enhanced by administration of interferon γ (IFN-γ) that is principally thought to be due to the up-regulation of the MHC class II target antigen (Ag) on the tumor cells.26 27
Autologous and allogeneic GVHD are multistep processes. During the “induction phase,” T cells react to Ag disparities and clonally expand (“expansion phase”). The activated T cells also release cytokines and chemokines, resulting in the recruitment of other cells such as macrophages and natural killer (NK) cells in the “recruitment phase.” Finally, the concert of T lymphocytes and other cell types mediate the pathology associated with GVHD (the “effector phase”). Cytokines drive the immune response and play a pivotal role in all phases of GVHD. However, cytokines play a complex and dual role in GVHD, and can have either protective or deleterious effects. In particular, cytokines such as interleukin 10 (IL-10) and IFN-γ may have both immunostimulatory and immunoregulatory effects leading to either exacerbation of GVHD or down-regulation of this posttransplantation complication.28,29 For instance, IL-10 can promote the growth of activated CD8+ T cells30,31 leading to the exacerbation of GVHD and the maintenance antitumor function in vivo.32,33 In contrast, IL-10 has a direct inhibitory effect on CD4+ T cells in vitro suppressing clonal expansion.34-38 Moreover, IL-10 inhibits antigen-presenting cell (APC)–dependent T-cell activation by preventing expression of MHC class II, costimulatory molecules, and chemokine ligands on APCs. 39,40
To investigate the role of cytokines and chemokines in autologous GVHD, the current study examined IL-2, IL-4, IL-10, IFN-γ, MIP-1α, RANTES, and interferon γ–inducible protein 10 (IP-10) gene expression by real-time polymerase chain reaction (PCR) in peripheral blood mononuclear cells (PBMCs) of 36 patients treated with CsA followed temporally after time points following transplantation. IL-10 mRNA levels in autologous GVHD-induced patients were 29-fold higher than in healthy individuals and 5-fold higher than in patients without clinical manifestations of autologous GVHD. IFN-γ, IL-2, MIP-1α, and IP-10 mRNA levels were also elevated, whereas expression of IL-4 and RANTES mRNA transcripts were not. IL-10, IFN-γ, MIP-1α, and IP-10 mRNA transcripts were also detected in the autologous GVHD skin lesions. Increased expression of IL-10 and IFN-γ correlated with the development of autocytolytic activity. Interestingly, the susceptibility to autologous GVHD was also significantly associated with the IL-10−1082 G/G polymorphic alleles. T-cell subset analysis revealed that IL-10 transcripts were expressed primarily in CD4+ cells and IFN-γ and IL-2 transcripts were detected principally in CD8+ cells. The cytokine and chemokine cascade in autologous GVHD is complex and involves interactions between distinct subsets of effector T cells and APCs. Understanding this cascade and the factors determining susceptibility to autologous GVHD may facilitate the development of more effective immunotherapeutic strategies to enhance the antitumor efficacy of autologous GVHD.
Patients, materials, and methods
Patients
Women between 18 and 60 years of age with metastatic breast cancer in complete or partial response to chemotherapy were included in this study, as previously described.22 41 The conduct of this trial was approved by the joint committee on clinical investigation of the Johns Hopkins Hospital. All patients provided informed written consent. Normal renal, cardiac, pulmonary, and hematopoietic reserves, in addition to an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1, were required for all patients.
Preparative regimens and induction of autologous GVHD
The patients were prepared for autologous bone marrow transplantation (BMT) by treatment with cyclosphosphamide (1.5 g/m2) and thiotepa (200 mg/m2; 4 × daily) before autologous bone marrow rescue, as previously described.22,41 Autologous GVHD was induced by the intravenous administration of CsA (2.5 mg/kg per day for 28 days; Novartis, Hanover, NJ) beginning on the day of transplantation. Recombinant IFN-γ (0.025 mg/m2; CTEP; National Cancer Institute, Bethesda, MD) was administered subcutaneously every other day from days 7 through 28 after transplantation to induce up-regulation of MHC class II determinants and enhance tumor cell recognition.26 27 One group of control patients (n = 6) underwent autologous stem cell transplantation (SCT) using the same preparative regimens but without administration of CsA and IFN-γ. There were 4 patients who underwent allogeneic SCT with development of grade IV acute GVHD and who were also compared with those patients developing autologous GVHD. Patients were evaluated daily for evidence of autologous GVHD (erythematous rash) and confirmation as grade II by skin biopsy, as previously described.22 41
Cell-mediated lympholysis assay
Lytic activity was assessed sequentially after autologous SCT using PBMCs isolated by Ficoll-Hypaque density centrifugation. Target cells used in these studies included autologous PHA-blasts cryopreserved before transplantation, the MHC class II–positive breast cancer cell line T47D,42,43 and the NK target cell line K562. The pretransplantation lymphocytes were thawed and stimulated with PHA for 72 hours (RPMI 1640; 20% normal human serum) before use as targets. The K562 cell line was grown in suspension culture in RPMI 1640 tissue culture medium supplemented with 10% fetal calf serum. The cells were washed 3 times before the cell-mediated lympholysis (CML) assay. T47D is an adherent breast cancer line grown in Dulbecco modified Eagle medium (DMEM) tissue culture medium supplemented with 5% fetal calf serum, glutamine, and sodium pyruvate.42,43Before assay, the cells were mildly trypsinzed and grown in Nalgene Teflon flasks (Thomas Scientific, Swedesboro, NJ) for 24 hours to allow for the recovery of cell surface antigens. The target cells were labeled with 250 μCi (9.25 MBq) of 51Cr for 1 hour at 37°C. The effector lymphocytes were cocultured with 2.5 × 103 labeled target cells in triplicate in round-bottom microtiter wells. After 4 hours incubation,51Cr release was assessed and the percent specific lysis was determined from triplicate cultures as previously described.22 25
RNA and genomic DNA extraction
Heparinized peripheral blood was collected from the patients after informed consent was obtained, and PBMCs were separated using density-gradient centrifugation. Monocytes were isolated by plastic adherence and gentle scraping (> 85% CD14+ by flow cytometry). Lymphocytes were also separated into distinct T-cell subsets by immunomagnetic bead separation chromatography using monoclonal antibodies (MoAbs) to the CD4+ and CD8+ cell surface determinants (Dynal Biotech, Oslo, Norway), as previously described.22,44 The purity of CD4+ or CD8+ cells was typically more than 97%. Skin biopsies (4-mm punch biopsies) were obtained with informed patient consent before transplantation and upon initial development of erythematous rash. After initial fracturing of the tissue in the presence of liquid nitrogen with pestle,44 45 total RNA was purified with Trizol reagent (Life Technologies, Gaithersburg, MD). Cell lysate was prepared from 5 × 106 PBMCs in 1 mL Trizol reagent with adequate mixing. After adding 200 μL chloroform, the solution was well mixed and centrifuged. The supernatant was collected and extracted once with chloroform. RNA was precipitated with 2-propanol and rinsed with 70% ethanol. Purified RNA was dissolved in 30 μL diethyl-pyrocarbonate–treated distilled water. Genomic DNA was prepared from PBMCs with DNAzol reagent (Life Technologies), according to the manufacturer's protocol.
Quantification of cytokine and chemokine mRNA levels by real-time PCR
Reverse transcription (RT) was conducted as follows: 32 μL water containing 1 μg total RNA was added to 0.4 μg random primers (Life Technologies) and incubated at 65°C for 10 minutes. Samples were chilled on ice and cDNA was prepared with Ready-To-Go You-Prime First-Strand kit (Amersham Pharmacia Biotech, Piscataway, NJ), according to the protocol provided by the manufacturer.
Real-time polymerase chain reactions (PCR) were performed using TaqMan assay (Applied Biosystems of PerkinElmer [ABI-PE], Foster City, CA) and PCR amplifications in ABI-PE prism 7700 sequence detection system.46,47 Briefly, a solution of TaqMan Universal PCR Master Mix (25 μL; ABI-PE) containing sense, antisense primer (300 nM each) and dual-labeled fluorogenic probes (100 nM) was prepared and aliquoted into individual MicroAmp Optical Plate (ABI-PE) and 5 μL cDNA was added to give a final volume of 50 μL. Conditions for PCR included 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds (denaturation) and 60°C for 1 minute (annealing/extension). Data were analyzed with Sequencer Detector version 1.6 software (ABI-PE). Threshold cycle (CT) during the exponential phase of amplification was determined by real-time monitoring of fluorescent emission after cleavage of sequence-specific probes by nuclease activity of Taq polymerase. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control gene. Primers and fluorogenic probes for GAPDH, IL-2, IL-4, IL-10, IFN-γ, MIP-1α, RANTES, and IP-10 were from TaqMan kits (ABI-PE). Cytokine and chemokine mRNA levels were expressed as the absolute number of copies normalized against GAPDH mRNA. Difference in amplification was determined as followed: 1/(CTcytokine – CTGAPDH).2
Relationship between cytokine production and mRNA expression
CD4+, CD8+, and monocyte subsets (2.5 × 106 cells) were diluted with 1 mL RPMI-1640 culture medium supplemented with 10% autologous serum and incubated for 3, 12, or 24 hours in 24-well cell culture plates at 37°C in 5% CO2. Concanavalin A (ConA; Sigma, St Louis, MO) was added at a final concentration of 0.4 μg/mL. The supernatants were collected by centrifugation (1500g, 10 minutes) and stored at −70°C until assay. The concentration of IL-10 or IFN-γ (mean ± SE) was determined by enzyme-linked immunosorbent assay (ELISA) using a polyclonal assay (PharMingen, San Diego, CA), as previously described.48
IL-10 polymorphisms by allele-specific polymerase chain reaction
An allele-specific polymerase chain reaction (ASPCR) was used to detect the G → A transition polymorphism at position −1082 of IL-10 gene as previously described.49,50 There were 3 primers used for ASPCR: the 3′ primer (5′-AGCAACACTCCTCGTCGCAAC-3′) was combined with either the 5′ primer (1082G: 5′-CCTATCCCTACTTCCCCC-3′), complementary to the IL-10−1082 G allele, or the 5′ primer (1082A: 5′-CCTATCCCTACTTCCCCT-3′), which is complementary to the IL-10−1082 A allele. Primers 1082G and 1082A differ only in their 3′ terminal nucleotide. Similarly, ASPCR was used to detect the C→A transition polymorphism at position −592 of IL-10 gene.51 The 3′ primer (5′-TGAGAAATAATTGGGTCCCC-3′) was combined with either the 5′ primer (592C: 5′-ATCCTGTGACCCCGCCTGTC-3′), complementary to the IL-10−592 C allele, or the 5′ primer (592A: 5′-ATCCTGTGACCCCGCCTGTA-3′), which is complementary to the IL-10−592 A allele. For microsatellite typing at position −1064, the following primers were used: the sense primer was labeled with 6-FAM fluorescent dye (IDT): sense, 5′-(6FAM)-GTCCTTCCCCAGGTAGAGCAACACTCC-3′ and antisense, 5′-CTCCCAAAGCCTTAGTAGTGTTG-3′. DNA (1 μg) was PCR-amplified through 30 cycles (95°C for 30 seconds, 60°C for 1 minute, 72°C for 1 minute) with sense primers and antisense primers. PCR products (1 μL) plus modified Genescan molecular weight makers (ABI-PE) were sized using an ABI 377 automatic sequencer equipped with the computer program Genotyper 2.0 software (ABI-PE).
Statistical analysis
Data were analyzed by the Welch t test, Fisher exact test, or ANOVA using Statview software (SAS, Cary, NC), withP values less than .05 considered statistically significant.
Results
Cytotoxic activities and autologous GVHD
Table 1 summarizes the data from patients with biopsy-confirmed autologous GVHD, patients without clinical manifestations of autologous GVHD, and control autologous SCT patients (non-CsA treated) evaluating maximal lytic activity during the course of treatment (day 12 through day 33). Cytolytic activity against autologous PHA-blasts was significantly higher in the patients who developed GVHD compared with control patients and to patients who did not develop autologous GVHD (P < .01). These data are consistent with previous data.22 41 The cytolytic activity against the T47D cell line by posttransplantation lymphocytes of patients with cutaneous GVHD was also significantly enhanced when compared with patients without GVHD (P = .047). Significant lysis of PHA-blasts, T47D cells, and K562 cells was observed in the autologous GVHD-induced patients, evaluated at the onset of GVHD versus patients without GVHD (P < .01,P < .01, P = .034, respectively).
Lymphocytotoxicity analysis
| Target cells . | Patients at onset of GVHD (N = 15) . | Patients without GVHD (N = 21) . | Controls N = 6 . |
|---|---|---|---|
| PHA-blasts | 12.9 ± 4.7* | 4.3 ± 0.9 | 1.5 ± 0.8 |
| T47D | 24.0 ± 5.0* | 12.6 ± 1.7 | 4.8 ± 1.2 |
| K562 | 53.0 ± 4.41-160 | 42.4 ± 2.4 | 50.1 ± 6.7 |
| Target cells . | Patients at onset of GVHD (N = 15) . | Patients without GVHD (N = 21) . | Controls N = 6 . |
|---|---|---|---|
| PHA-blasts | 12.9 ± 4.7* | 4.3 ± 0.9 | 1.5 ± 0.8 |
| T47D | 24.0 ± 5.0* | 12.6 ± 1.7 | 4.8 ± 1.2 |
| K562 | 53.0 ± 4.41-160 | 42.4 ± 2.4 | 50.1 ± 6.7 |
Maximum autocytolytic, anti-T47D, and natural killer cell activity observed during the interval (day 12 through day 33) for patients who developed autologous graft-versus-host disease (GVHD) confirmed by skin biopsy. Data for onset of GVHD represent the lytic activity when the patients developed clinical evidence of autologous GVHD. The results from patients without clinical manifestations of autologous GVHD and from 6 control autologous stem cell transplantation (SCT) patients (non-CsA treated) are presented for comparison. Lysis of the target cells was measured using a standard 51Cr release assay at a 100:1 effector-to-target ratio. Data expressed as mean ± SE.
P < .01,
P< .05 patients at onset of GVHD versus patients without GVHD group. PHA blasts indicates phytohemagglutinin-stimulated autologous lymphoblasts.
Gene expression of cytokines in PBMCs
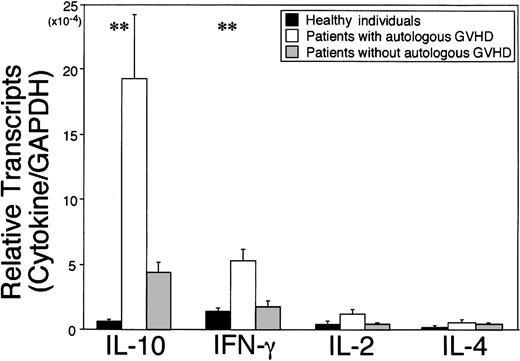
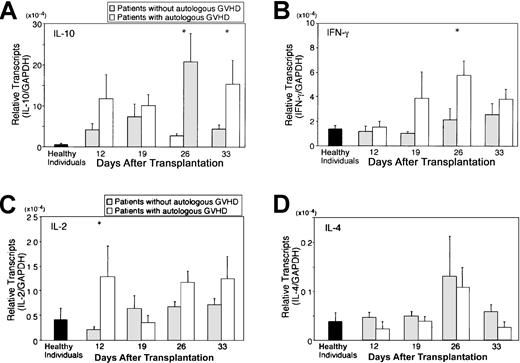
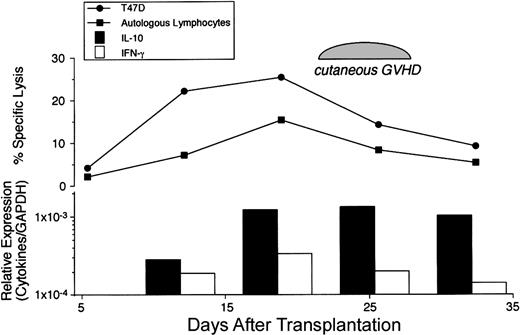
Autologous GVHD can be inducible in recipients of autologous stem cell transplantation by administration of CsA during the first 4 weeks after transplantation. Onset of disease can vary but can be correlated with the development of autocytolytic T cells detected in the peripheral blood, findings in accordance with a clonal expansion up to day 36. Therefore, PBMCs from the patients on the autologous GVHD induction protocol were serially monitored for cytokine/chemokine gene expression by real-time PCR during the course of treatment (day 12 through day 33) at 4 different time points (days 12, 19, 25, and 33 after transplantation) and compared with control autologous SCT patients (non-CsA treated), patients before transplantation, healthy individuals, and allogeneic SCT patients. Figure1 summarizes the data from patients evaluated at the onset of GVHD, patients without clinical manifestations of autologous GVHD (highest levels detected), and healthy individuals evaluating cytokine mRNA levels normalized against the housekeeping gene, GAPDH. IL-10 mRNA levels in patients at the onset of autologous GVHD were 29.1-fold higher than in healthy individuals (P < .01) and 4.5-fold higher than in those who did not develop clinical manifestations of autologous GVHD. IFN-γ or IL-2 mRNA levels were 3.8-fold and 2.8-fold higher compared with healthy individuals (P < .01 or P = .089, respectively). In contrast, IL-4 mRNA levels for all groups were comparable. No significant differences were observed in cytokine expression comparing 8 healthy individuals, 6 control autologous SCT patients (non-CsA treated), and 4 patients who were evaluated before transplantation (data not shown). Figure2A-D summarizes the temporal analysis of IL-10, IFN-γ, IL-2, and IL-4 mRNA levels for patients who developed autologous GVHD and patients who did not develop this experimental autoaggression syndrome. IL-10 and IFN-γ mRNA levels in patients with autologous GVHD were significantly higher than the levels in patients without GVHD at day 26 (P = .011 orP = .039). The difference was particularly pronounced, reflecting the development of autologous GVHD and cytolytic activity against autologous lymphocytes. Comparatively, mRNA levels for all cytokines were remarkably elevated in 4 patients following allogeneic SCT. The IL-2, IL-4, IL-10, and IFN-γ mRNA transcripts were detected in levels 160-fold, 91-fold, 730-fold, or 260-fold higher compared with healthy individuals, respectively (data not presented). The temporal changes in cytolytic activity and cytokine mRNA expression were directly assessed in PBMCs from patients with autologous GVHD following transplantation. A representative experiment evaluating lymphocytotoxicity and cytokine expression is shown in Figure3. The results reveal that the ability of PBMCs to lyse autologous PHA-blasts and the T47D tumor cells parallels the temporal relationship with IL-10 and/or IFN-γ gene expression.
Expression of cytokine mRNA in PBMCs.
Autologous GVHD can be inducible in recipients of autologous stem cell transplantation by administration of cyclosporine A during 1 to 4 weeks after transplantation. Autocytotoxic activity of PBMCs and increased cytokine message are also detectable in accordance with clonal expansion of T cells until day 36. RNA was harvested from PBMCs of healthy individuals (black bars, n = 8), patients evaluated at onset of autologous GVHD (white bars, n = 17 different time points for 15 patients) and patients without autologous GVHD (gray bars, n = 43 points for 21 patients). cDNA was analyzed for cytokine transcripts including IL-10, IFN-γ, IL-2, and IL-4 by real-time PCR. Cytokine mRNA levels were normalized against GAPDH and expressed as mean ± SE. **P < .01; patients evaluated at onset of autologous GVHD versus healthy individuals.
Expression of cytokine mRNA in PBMCs.
Autologous GVHD can be inducible in recipients of autologous stem cell transplantation by administration of cyclosporine A during 1 to 4 weeks after transplantation. Autocytotoxic activity of PBMCs and increased cytokine message are also detectable in accordance with clonal expansion of T cells until day 36. RNA was harvested from PBMCs of healthy individuals (black bars, n = 8), patients evaluated at onset of autologous GVHD (white bars, n = 17 different time points for 15 patients) and patients without autologous GVHD (gray bars, n = 43 points for 21 patients). cDNA was analyzed for cytokine transcripts including IL-10, IFN-γ, IL-2, and IL-4 by real-time PCR. Cytokine mRNA levels were normalized against GAPDH and expressed as mean ± SE. **P < .01; patients evaluated at onset of autologous GVHD versus healthy individuals.
Temporal analysis of cytokine transcription in PBMCs.
PBMCs from the patients on the autologous GVHD induction protocol were serially monitored for IL-10 (A), IFN-γ (B), IL-2 (C), and IL-4 (D) gene expression by real-time PCR during the course of treatment (day 12 through day 33). Gene expression was determined in PBMCs of 36 patients at 4 different time points (days 12, 19, 25, and 33 after transplantation) and compared with patients evaluated at onset of autologous GVHD (white bars) and patients who did not develop autologous GVHD (gray bars). *P < .05; patients evaluated at the onset of autologous GVHD versus patients who did not develop autologous GVHD.
Temporal analysis of cytokine transcription in PBMCs.
PBMCs from the patients on the autologous GVHD induction protocol were serially monitored for IL-10 (A), IFN-γ (B), IL-2 (C), and IL-4 (D) gene expression by real-time PCR during the course of treatment (day 12 through day 33). Gene expression was determined in PBMCs of 36 patients at 4 different time points (days 12, 19, 25, and 33 after transplantation) and compared with patients evaluated at onset of autologous GVHD (white bars) and patients who did not develop autologous GVHD (gray bars). *P < .05; patients evaluated at the onset of autologous GVHD versus patients who did not develop autologous GVHD.
Temporal relationship between cytolytic activity and cytokine expression.
The changes in the cytotolytic activity and cytokine mRNA expression over time were assessed in PBMCs from a patient who developed autologous GVHD following transplantation. Cytotoxicity against autologous PHA-blasts and the T47D breast cancer cell line was measured using a standard 51Cr release assay at a 100:1 effector-to-target ratio. Relative expression of IL-10 and IFN-γ mRNA levels normalized against GAPDH were determined by real-time PCR.
Temporal relationship between cytolytic activity and cytokine expression.
The changes in the cytotolytic activity and cytokine mRNA expression over time were assessed in PBMCs from a patient who developed autologous GVHD following transplantation. Cytotoxicity against autologous PHA-blasts and the T47D breast cancer cell line was measured using a standard 51Cr release assay at a 100:1 effector-to-target ratio. Relative expression of IL-10 and IFN-γ mRNA levels normalized against GAPDH were determined by real-time PCR.
Gene expression of chemokines in PBMCs
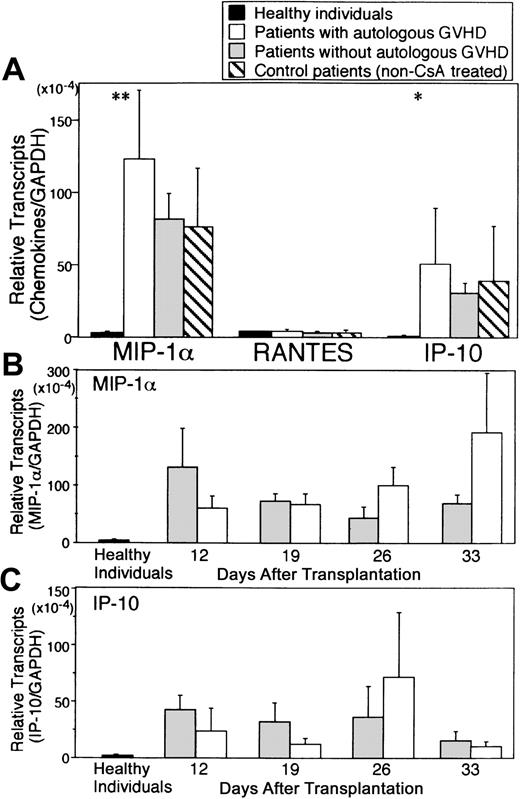
Chemokines have been implicated in the recruitment of inflammatory cells and modulate the function of both T cells and NK cells. Analysis of chemokine mRNA transcripts (MIP-1α and RANTES, both CCR5 ligands and IP-10, a CXCR3 ligand) in PBMCs is summarized in Figure 4. Compared with healthy controls, PBMCs obtained from patients at the onset of autologous GVHD exhibited significantly higher transcript levels for MIP-1α (44-fold), and IP-10 (53-fold) mRNA transcript levels for these chemokines persisted during CsA treatment (P < .01 and P = .024, respectively). Interestingly, the increase in MIP-1α and IP-10 levels was not confined to the patients developing autologous GVHD but was also observed in patients who were treated with CsA and IFN-γ but who did not develop clinical evidence of this autoaggression syndrome and in autologous SCT control recipients (not treated with CsA and IFN-γ). RANTES mRNA levels were comparable for all groups. Comparatively, mRNA levels for MIP-1α, RANTES and IP-10 in patients with grade IV allogeneic GVHD were markedly elevated (> 100-fold; data not presented).
Expression of chemokine mRNA in PBMCs.
RNA was harvested from PBMCs of healthy individuals (black bars, n = 8), patients evaluated at the onset of autologous GVHD (white bars, n = 17 different time points for 15 patients), and patients who did not develop autologous GVHD (gray bars, n = 43 points for 21 patients) (A). cDNA was analyzed for chemokine transcripts including MIP-1α, regulated upon activation in normal T cells, expressed and secreted (RANTES), interferon-gamma, and IP-10 by real-time PCR. Temporal analysis of MIP-1α and IP-10 mRNA levels for patients who developed autologous GVHD (white bars) and patients who did not (gray bars) is presented in B and C. Chemokine mRNA levels were normalized against GAPDH.
Expression of chemokine mRNA in PBMCs.
RNA was harvested from PBMCs of healthy individuals (black bars, n = 8), patients evaluated at the onset of autologous GVHD (white bars, n = 17 different time points for 15 patients), and patients who did not develop autologous GVHD (gray bars, n = 43 points for 21 patients) (A). cDNA was analyzed for chemokine transcripts including MIP-1α, regulated upon activation in normal T cells, expressed and secreted (RANTES), interferon-gamma, and IP-10 by real-time PCR. Temporal analysis of MIP-1α and IP-10 mRNA levels for patients who developed autologous GVHD (white bars) and patients who did not (gray bars) is presented in B and C. Chemokine mRNA levels were normalized against GAPDH.
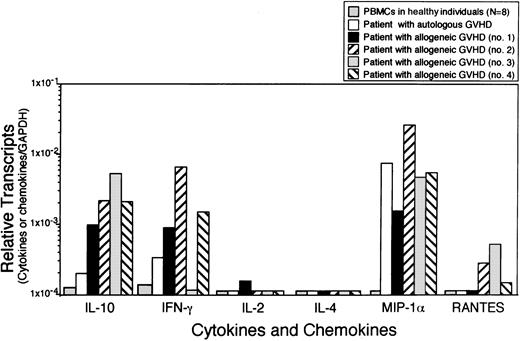
Cytokine and chemokine gene expression in skin lesions of GVHD
In order to confirm the pathogenic involvement of cytokines and chemokines in GVHD, gene expression was evaluated in the skin lesions of a patient with autologous GVHD. Comparison was also made to skin biopsies from patients with allogeneic GVHD by real-time PCR. Figure5 summarizes the data evaluating cytokine and chemokine mRNA levels normalized against GAPDH. IL-10, IFN-γ, MIP-1α, and IP-10 mRNA transcripts were detected in the skin lesions of both patients with autologous GVHD and allogeneic GVHD. Levels of IL-10 and IFN-γ mRNA in the skin from a patient with autologous GVHD were found approximately 4-fold higher compared with the levels found in PBMCs from healthy individuals. On the other hand, levels of MIP-1α mRNA transcripts were pronouncedly elevated in the autologous GVHD skin lesion with levels comparable to the levels detected in skin lesions from patients with allogeneic GVHD.
Expression of cytokine and chemokine mRNA in skin lesions.
RNA was harvested from skin biopsies of a patient with autologous GVHD and 4 patients with allogeneic GVHD. cDNA were analyzed for IL-10, IFN-γ, IL-2, IL-4, MIP-1α, and RANTES by real-time PCR. Data were normalized against GAPDH. Levels of cytokine and chemokine mRNA expression in PBMCs from healthy individuals were shown as the baseline control.
Expression of cytokine and chemokine mRNA in skin lesions.
RNA was harvested from skin biopsies of a patient with autologous GVHD and 4 patients with allogeneic GVHD. cDNA were analyzed for IL-10, IFN-γ, IL-2, IL-4, MIP-1α, and RANTES by real-time PCR. Data were normalized against GAPDH. Levels of cytokine and chemokine mRNA expression in PBMCs from healthy individuals were shown as the baseline control.
Cellular origin of cytokine transcription
To identify the cellular origin of the IL-10, IFN-γ, IL-2, and MIP-1α mRNA transcripts, CD4+ and CD8+ T cells and monocytes were separated from PBMCs of 7 patients who received CsA following autologous SCT. Gene expression was determined in each population. The results in Table2 reveal that IL-10 mRNA levels in the CD4+ subset were 2.3-fold higher compared with the levels detected in the CD8+ subset. Higher IL-10 mRNA levels in the CD4+ subset were observed in patients who developed autologous GVHD (patient no. 6 and no. 7). On the other hand, IFN-γ mRNA levels were 42-fold higher in the CD8+ subset compared with the levels detected in the CD4+ subset. IL-2 and MIP-1α mRNA levels were also found to be increased in the CD8+ subset. Higher levels of MIP-1α mRNA transcripts were detected in both CD8+ and monocytes subsets, whereas RANTES mRNA levels were detected principally in the CD8+ subset.
Expression of cytokine and chemokine mRNA in PBMC subpopulations
| Patient no. (Days after transplantation) . | Subset . | Relative transcripts (cytokines or chemokines/GAPDH) . | |||||
|---|---|---|---|---|---|---|---|
| IL-10 . | IFN-γ . | IL-2 . | MIP-1α . | RANTES . | IP-10 . | ||
| 1 | CD4 | 2.28 | 3.01 | 0.05 | 6.44 | 29.59 | 357.14 |
| Day 33 | CD8 | 1.31 | 29.59 | 0.42 | 236.97 | 1086.96 | 126.58 |
| Monocyte | 4.25 | 6.01 | — | 236.97 | 558.66 | 256.41 | |
| 2 | CD4 | 0.55 | 0.11 | 0.01 | 20.96 | NT | 14.8 |
| Day 13 | CD8 | 0.25 | 2.44 | 0.02 | 78.13 | NT | — |
| Monocyte | 0.65 | 0.01 | — | 52.91 | NT | 52.43 | |
| 3 | CD4 | 1.14 | 0.05 | — | 40.81 | NT | 36.5 |
| Day 13 | CD8 | 0.68 | 0.71 | — | 20.65 | NT | — |
| Monocyte | 1.71 | 0.22 | — | 96.15 | NT | 90.1 | |
| 4 | CD4 | 0.15 | 0.25 | — | 7.05 | NT | 166.67 |
| Day 26 | CD8 | — | 29.85 | — | 46.73 | NT | — |
| Monocyte | — | 18.18 | — | 270.27 | NT | 833.33 | |
| 5 | CD4 | 0.07 | 0.05 | 0.01 | 4.46 | 1.62 | 41.8 |
| Day 13 | CD8 | 0.07 | 1.79 | 0.04 | 4.78 | 17.99 | 20.9 |
| Monocyte | 0.09 | 0.01 | — | 12.19 | 1.19 | 47.4 | |
| 6 | CD4 | 2.62 | 0.31 | 0.06 | 126.6 | 63.3 | 20.9 |
| Day 19 | CD8 | 0.53 | 10.47 | — | 22.5 | 1538.46 | 14.8 |
| Monocyte | 2.44 | 0.07 | 0.04 | 68.1 | 97.1 | 40.3 | |
| 7 | CD4 | 4.88 | 1.85 | 0.01 | NT | NT | NT |
| Day 26 | CD8 | — | 41.84 | 0.05 | NT | NT | NT |
| MonocyteT | Too few cells to evaluate | ||||||
| Patient no. (Days after transplantation) . | Subset . | Relative transcripts (cytokines or chemokines/GAPDH) . | |||||
|---|---|---|---|---|---|---|---|
| IL-10 . | IFN-γ . | IL-2 . | MIP-1α . | RANTES . | IP-10 . | ||
| 1 | CD4 | 2.28 | 3.01 | 0.05 | 6.44 | 29.59 | 357.14 |
| Day 33 | CD8 | 1.31 | 29.59 | 0.42 | 236.97 | 1086.96 | 126.58 |
| Monocyte | 4.25 | 6.01 | — | 236.97 | 558.66 | 256.41 | |
| 2 | CD4 | 0.55 | 0.11 | 0.01 | 20.96 | NT | 14.8 |
| Day 13 | CD8 | 0.25 | 2.44 | 0.02 | 78.13 | NT | — |
| Monocyte | 0.65 | 0.01 | — | 52.91 | NT | 52.43 | |
| 3 | CD4 | 1.14 | 0.05 | — | 40.81 | NT | 36.5 |
| Day 13 | CD8 | 0.68 | 0.71 | — | 20.65 | NT | — |
| Monocyte | 1.71 | 0.22 | — | 96.15 | NT | 90.1 | |
| 4 | CD4 | 0.15 | 0.25 | — | 7.05 | NT | 166.67 |
| Day 26 | CD8 | — | 29.85 | — | 46.73 | NT | — |
| Monocyte | — | 18.18 | — | 270.27 | NT | 833.33 | |
| 5 | CD4 | 0.07 | 0.05 | 0.01 | 4.46 | 1.62 | 41.8 |
| Day 13 | CD8 | 0.07 | 1.79 | 0.04 | 4.78 | 17.99 | 20.9 |
| Monocyte | 0.09 | 0.01 | — | 12.19 | 1.19 | 47.4 | |
| 6 | CD4 | 2.62 | 0.31 | 0.06 | 126.6 | 63.3 | 20.9 |
| Day 19 | CD8 | 0.53 | 10.47 | — | 22.5 | 1538.46 | 14.8 |
| Monocyte | 2.44 | 0.07 | 0.04 | 68.1 | 97.1 | 40.3 | |
| 7 | CD4 | 4.88 | 1.85 | 0.01 | NT | NT | NT |
| Day 26 | CD8 | — | 41.84 | 0.05 | NT | NT | NT |
| MonocyteT | Too few cells to evaluate | ||||||
CD4+ cells, CD8+ cells, and monocytes were separated from PBMCs of 7 patients who received CsA following transplantation. Patients no. 6 and 7 developed clinical GVHD. Monocytes were isolated by plastic adherence and gentle scraping. Lymphocytes were also separated into distinct T-cell subsets with immunomagnetic beads. — indicates that CT (threshold cycle) could not be detected; NT, not tested. Relative transcripts were determined as follows: 1/(CTCYTOKINE − CTGAPDH) 2 × 104.
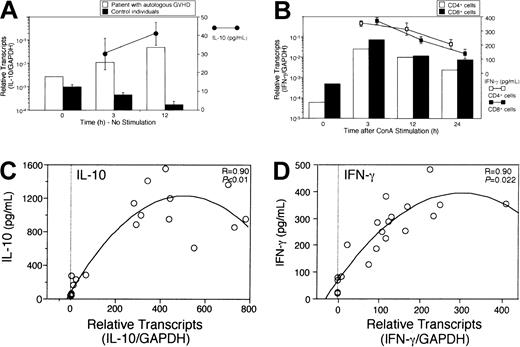
Relationship between cytokine production and mRNA expression
To quantify and correlate cytokine mRNA expression with cytokine protein production, PBMCs were isolated from a patient with autologous GVHD and from 3 control individuals. Isolated CD4+ T cells, CD8+ T cells, and monocytes were cultured separately for 3, 12, or 24 hours in the absence (nonstimulated) or presence (stimulated) of ConA. Cytokine concentrations in the culture supernatant and mRNA expression in the culture cells were measured using ELISAs or real-time PCR assays, respectively. Interestingly, IL-10 mRNA expression by PBMCs from the patient with autologous GVHD increased in the absence of any stimulus and correlated with IL-10 production assessed by ELISA. Significant IL-10 production was not detected in 3 control individuals, findings correlated with decreased IL-10 mRNA levels (Figure6A). On the other hand, IL-10 production by CD4+ cells and monocytes from control individuals in response to ConA stimulation correlated with mRNA expression of IL-10. Similarly, IFN-γ production was also detected in both CD4+ and CD8+ cells, and significantly correlated with the levels of mRNA expression for this cytokine (Figure6B). Analysis of cytokine protein production directly related to levels of mRNA expression (Figure 6C-D).
Relationship between cytokine production and mRNA expression.
2.5 × 106 cells were isolated and cultured in the absence or presence of 0.4 μg/mL concanavalin A (ConA). Cytokine concentrations (mean ± SE of triplicate cultures) in the culture supernatant and mRNA expression in the culture cells were measured using ELISA or real-time PCR assays, respectively. IL-10 mRNA expression after 3 or 12 hours of cultures in the absence of stimulus was assessed in PBMCs isolated from a patient with autologous GVHD and from 3 control individuals (A). Although IL-10 mRNA expression by PBMCs from the patient with autologous GVHD increased and correlated with IL-10 production (●–●), significant IL-10 production was not detected in 3 control individuals. IFN-γ concentrations and mRNA expression after 3, 12, or 24 hours of cultures in the presence of ConA were measured in CD4+ and CD8+ cells from a control individual (B). IL-10 protein production and mRNA expression levels were assessed in PBMCs from control individuals and from patients with autologous GVHD in the presence of ConA (C, n = 19). IFN-γ protein production and mRNA expression levels were assessed in PBMCs from control individuals or from patients with autologous GVHD in the presence of ConA (D, n = 20).
Relationship between cytokine production and mRNA expression.
2.5 × 106 cells were isolated and cultured in the absence or presence of 0.4 μg/mL concanavalin A (ConA). Cytokine concentrations (mean ± SE of triplicate cultures) in the culture supernatant and mRNA expression in the culture cells were measured using ELISA or real-time PCR assays, respectively. IL-10 mRNA expression after 3 or 12 hours of cultures in the absence of stimulus was assessed in PBMCs isolated from a patient with autologous GVHD and from 3 control individuals (A). Although IL-10 mRNA expression by PBMCs from the patient with autologous GVHD increased and correlated with IL-10 production (●–●), significant IL-10 production was not detected in 3 control individuals. IFN-γ concentrations and mRNA expression after 3, 12, or 24 hours of cultures in the presence of ConA were measured in CD4+ and CD8+ cells from a control individual (B). IL-10 protein production and mRNA expression levels were assessed in PBMCs from control individuals and from patients with autologous GVHD in the presence of ConA (C, n = 19). IFN-γ protein production and mRNA expression levels were assessed in PBMCs from control individuals or from patients with autologous GVHD in the presence of ConA (D, n = 20).
Development of autologous GVHD and IL-10 polymorphisms
Because of significantly elevated levels of IL-10 mRNA transcripts, polymorphisms in the IL-10 promoter were examined at positions 592, 1064, and 1082, which correlate with high IL-10 production. The association of allelic composition with the susceptibility to the induction of autologous GVHD is summarized in Table 3. Interestingly, the presence of an A nucleotide at either position 592 or 1082 correlates with low IL-10 production and a reduced incidence of autologous GVHD. Conversely, the presence of G/G at position 1082 correlates with high IL-10 production and an increased frequency of patients who develop autologous GVHD. In accordance are the results comparing IL-10 mRNA levels and the development of autologous GVHD with promoter polymorphisms (Figure 7). As revealed by analysis of variance, IL-10−1082 G/G homozygotes had significantly higher levels of IL-10 mRNA transcripts in patients who develop autologous GVHD (P < .01). Patients who expressed the IL-10−592 A/C and A/A genotypes had reduced levels of IL-10 mRNA transcripts and a reduced incidence of developing autologous GVHD. On the other hand, IL-10−1064 polymorphism did not correlate with the development of autologous GVHD. Attempts to correlate single nucleotide polymorphisms in other cytokine promoter regions (tumor necrosis factor alpha [TNF-α], IL-6, and IFN-γ) with the induction of autologous GVHD did not reveal any significant associations (data not shown).
Autologous GVHD correlation with IL-10 polymorphisms
| Genotype . | GVHD present . | GVHD absent . | P . |
|---|---|---|---|
| IL-10−592 | |||
| C/C | 18 | 19 | .056 |
| A/C or A/A | 11 | 29 | |
| IL-10−1064 | |||
| (7-11)i only | 12 | 12 | .170 |
| (12-15)i present | 17 | 34 | |
| IL-10−1082 | |||
| G/G | 13 | 8 | .010 |
| A/G or A/A | 16 | 35 |
| Genotype . | GVHD present . | GVHD absent . | P . |
|---|---|---|---|
| IL-10−592 | |||
| C/C | 18 | 19 | .056 |
| A/C or A/A | 11 | 29 | |
| IL-10−1064 | |||
| (7-11)i only | 12 | 12 | .170 |
| (12-15)i present | 17 | 34 | |
| IL-10−1082 | |||
| G/G | 13 | 8 | .010 |
| A/G or A/A | 16 | 35 |
Correlation of IL-10 mRNA levels and the development of autologous GVHD with IL-10−1082 polymorphisms.
Maximum IL-10 mRNA levels normalized against GAPDH by real-time PCR in PBMCs of patients are shown. **P < .01, *P < .05.
Correlation of IL-10 mRNA levels and the development of autologous GVHD with IL-10−1082 polymorphisms.
Maximum IL-10 mRNA levels normalized against GAPDH by real-time PCR in PBMCs of patients are shown. **P < .01, *P < .05.
Discussion
The present analysis of cytokine and chemokine profiles in both PBMCs and skin lesions reveal several new findings regarding the posttransplantation immune response in the pathophysiology of human autologous GVHD. The most pronounced and perhaps unexpected finding is that IL-10 mRNA levels in PBMCs from patients with autologous GVHD were 29-fold higher compared with healthy individuals. IFN-γ, IL-2, MIP-1α, and IP-10 mRNA levels were also found to be moderately increased. Comparatively, IL-4 and RANTES mRNA transcripts were not different in patients with autologous GVHD or in healthy individuals. These findings parallel the cytokine and chemokine profiles of mRNA transcripts detected in the skin lesions of both patients with autologous and allogeneic GVHD. Certainly, the elevated MIP-1α mRNA levels are consistent with the infiltration of cytokine-producing cells. Recent studies, in fact, provide evidence that activated alloreactive CD8+ T cells migrate into the skin in response to MIP-1α.52-54 These findings are in accord with the results of the present studies demonstrating elevated levels of MIP-1α in skin and PBMCs during autologous and allogeneic GVHD. Recent studies suggest that differences in cytokine secretion including IL-10 are directly related to corresponding differences in mRNA expression.55 56
Given the fact that IL-10 has powerful immunoregulatory potential, the most surprising finding of the current studies is that mRNA transcripts for this cytokine were found to be markedly elevated during the onset of autologous GVHD. One simple explanation for the findings of the current studies is that there is a regulatory response that ensues with the onset of autoaggression. The increased IL-10 mRNA levels may reflect a compensatory response correlating with the induction of autologous GVHD. However, the onset of autocytolytic activity exhibited an identical temporal relationship with elevated expression of IL-10 (and IFN-γ) mRNA transcripts. Although the present studies did not quantify IL-10 protein secretion in each patient, there still was a highly significant correlation with IL-10 mRNA transcript levels and the induction of autologous GVHD. Nevertheless, the present studies reveal that levels of cytokine in mRNA correlate with protein production. In addition, the correlation between induction of autologous GVHD and the expression of promoter polymorphisms responsible for high IL-10 production further strengthens the association of autologous GVHD with this regulatory cytokine (see below). An additional surprising finding was that IL-2 mRNA transcripts were not dramatically increased. However, the fact that increased serum levels of the soluble IL-2 receptor were detected in all patients who developed autologous GVHD (data not shown) suggests that this cytokine also plays an important role in the autoaggression syndrome.
Westendrop et al48 demonstrated that genetic factors could account for 74% of differences in IL-10 production. High IL-10 production may be a genetic risk factor for disease susceptibility.57,58 Single nucleotide polymorphisms at −1082, −592 upstream of the transcription start site have been identified with IL-10−1082 polymorphism of the G allele associated with higher in vitro IL-10 production.48IL-10−592 polymorphism of the A allele appears to be correlated with lower expression of IL-10 mRNA.59IL-10−1082 G/G homozygotes had significantly increased expression of IL-10 mRNA transcripts detected by real-time PCR in PBMCs of patients with autologous GVHD (Figure 6). Furthermore, the development of autologous GVHD was significantly associated with the IL-10−1082 G/G alleles which correlate with higher IL-10 production. These findings suggest that IL-10 may play a paradoxical role in GVHD and could even enhance the antitumor effect of this posttransplantation complication. The development of autologous GVHD correlated with inheritance of the IL-10−1082 G/G alleles. The IL-10−592 polymorphism approached significance (P = .054). The single nucleotide polymorphism (SNP) locus of IL-10−1082 revealed an effect on autologous GVHD. Interestingly, the SNP loci of IL-10−1082 and IL-10−592 are in strong linkage disequilibrium with each other.49,59 The IL-10−1082 polymorphism also associates with the specific IL-10−1064allele.50 Many transcription factor binding sites exist throughout the IL-10 promoter region. For example, the IL-10−1082 SNP appears within a putativeETS-factor binding site, while IL-10−592 may be a STAT3 binding site.60 61 Differences in these promoter regions may lead to increased IL-10 production.
IL-10 is thought to be a powerful regulatory T-helper type 2 (TH-2) cell cytokine produced by lymphocytes and monocytes that limits alloreactivity including GVHD in vivo, ostensibly inhibiting macrophage/monocyte and T-cell replication and secretion of inflammatory cytokines.62,63 The results from the present studies suggest that IL-10 may have immunostimulatory properties, particularly important in the stem cell transplantation setting. Several recent studies suggest that CsA inhibits thymic-dependent clonal deletion leading to the release of autoreactive T cells.64-66 Furthermore, CsA reduces MHC class II antigen expression in the thymus, resulting in a failure of mature T cells to recognize this Ag as self.11,67 In this setting, IL-10 may act synergistically, inducing MHC class II Ag down-regulation on thymic epithelial cells facilitating a failure to clonally delete autoreactive T cells. In support of this hypothesis is the recent finding indicating that IL-10 enhances the proliferative response of thymocytes.68 Thus, IL-10 may enhance the proliferative capacity of autoreactive T cells prematurely released from the thymus.
Alternatively, IL-10 can augment the proliferative response of IL-2– and/or IL-4–activated T cells.31 In accord are the results indicating that IL-10 increases the IL-2–stimulated cytolytic activity of CD8+ T cells.69 Interestingly, this immunoregulatory cytokine can prevent priming of autoreactive T cells, but IL-10 cannot suppress ongoing immune responses.30,70 This cytokine can even promote IL-2–independent growth of activated CD8+ T cells in the absence of costimulatory signals mediated by APCs.30 Both CD4+ and CD8+ T cells are required for the development of autologous GVHD.15 A vigorous cellular immune reaction, dominated by activated proliferating CD8+cytotoxic T lymphocytes, is mounted during both autologous GVHD15,22 and allogeneic GVHD.71,72 It is not yet clear from functional studies whether most of these activated T cells are Ag-specific expanded during GVHD or result from additional cytokine-induced activation. If activation of CD8+ effector cells by IL-10 is pivotal in the transplantation setting, such activation is most likely to account for the failure to suppress allogeneic GVHD by blocking costimulation during development of GVHD.73
Blazar and colleagues32 provide substantial evidence that IL-10 can exacerbate GVHD presumably mediated by enhanced IFN-γ production. Consistent with these studies, IL-10 and IFN-γ mRNA levels in PBMCs from patients with autologous GVHD or allogeneic GVHD were markedly elevated compared with healthy individuals. IL-10 and IFN-γ transcripts were also detected in skin lesions of these patients, suggesting that these cytokines are interactive or interdependent.
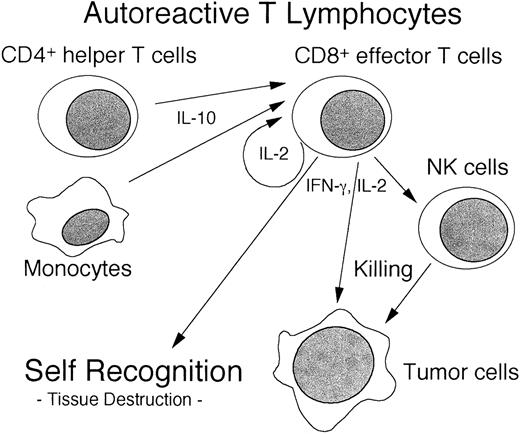
Elucidating the interactive cytokine cascade in autologous GVHD should provide novel insights into the immunobiology of this unique autoaggression syndrome. Based on the results of the current studies it appears that secretion of IL-10 by CD4+ cells and monocytes leads to the activation and proliferation of IL-2–driven CD8+ autoreactive T cells into IFN-γ–secreting cytolytic T-lymphocyte (CTL) effectors, culminating in autologous GVHD (Figure8). At present, the increase in IL-10 production as a compensatory immunoregulatory mechanism cannot be excluded. Evaluation of this hypothesis in an animal model is underway. IFN-γ and IL-2 can augment NK activity and may also lead to the subsequent amplification of lymphocytes capable of destroying tumor cells.74
Expression of chemokines such as MIP-1α and IP-10 would induce the additional recruitment of effector cells such as CTLs and NK cells, resulting in amplification of autologous GVHD. It is important to note that the up-regulation of MIP-1α and IP-10 mRNA was not restricted to patients developing autologous GVHD. Rather, mRNA levels for these chemokines were elevated in all autologous SCT patients independent of treatment with CsA and IFN-γ, suggesting an effect of SCT including the preparative regimen. Alternatively, the elevated levels of these cytokines may provide an environment that allows for the development of autologous GVHD.
Clearly, the regulation and expression of IL-10 appears to play an unexpected and critical role in the susceptibility to and intensity of autologous GVHD. Further understanding of these cytokine/chemokine pathways should promote more effective immunotherapeutic strategies for enhancing the antitumor efficacy of autologous GVHD. Moreover, polymorphic analysis may allow prediction of individual patient susceptibility to the induction of autologous GVHD and provide a possible solution (administration of IL-10) for these patients with resistant alleles.
Prepublished online as Blood First Edition Paper, June 7, 2002; DOI 10.1182/blood-2002-01-0176.
Supported by grants CA 15396, CA 82583, and AI 24319 from the National Institutes of Health, grant USAMRMC DAMD 17-99-1-9238 from the Department of Defense, and a grant from the Avon Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Allan D. Hess, Johns Hopkins University School of Medicine, Oncology Center, Bunting-Blaustein Cancer Research Building, 1650 Orleans St, Room 484, Baltimore, MD 21231; e-mail:adhess@jhmi.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal