Tumors of the splenic marginal zone can present in spleen or blood. The maturational status of the neoplastic B cells from each site appears heterogeneous, with either unmutated or mutated variable-region heavy chain (VH) genes. To determine an influence of tissue location, we assessed matched blood and splenic tumor cells from 4 patients and found them identical. However, one patient with unmutated VH genes in blood and spleen developed a clonally related diffuse large B-cell lymphoma in the chest wall. Strikingly, this subclone had undergone significant somatic mutation, with clear intraclonal heterogeneity. To our knowledge, this is the first case of a B-cell tumor showing initiation of somatic mutation in vivo. The finding emphasizes that the tissue microenvironment can influence tumor cell behavior and possibly affect disease progression. Importantly, because several replacement mutations were located within or close to the complementarity-determining regions (CDRs), it raises the question of a role for antigen in driving tumor growth.
Introduction
Splenic marginal zone lymphoma (SMZL) is a chronic B-cell disorder in which the splenic white pulp is infiltrated by an inner zone of small lymphocytes, frequently surrounding a residual germinal center, and an outer zone comprising larger cells with abundant pale cytoplasm and occasional blasts. Cases with blood involvement often include a proportion of cells with a characteristic villous appearance, and are diagnosed as splenic lymphoma with villous lymphocytes (SLVL).
The tumor cells are positive for surface immunoglobulin M (IgM) and IgD and express CD20, CD22, and CD79a, but not CD5, CD10, CD23, CD43, or cyclin D1. SMZL usually pursues an indolent clinical course, but transformation to a large-cell lymphoma may occur.1 The distinctive lymphocyte morphology, immunophenotype, and cytogenetic abnormalities distinguish SMZL from extranodal MZL and from the majority of cases of nodal MZL.
Analysis of mutated variable-region heavy chain (VH) genes from a small number of cases with SMZL or SLVL has shown that most have undergone somatic mutation with or without intraclonal heterogeneity.2-4 Because tissue location may influence mutational activity, we analyzed matched splenic and blood tumor cells in 4 patients. One patient who subsequently developed a clonally related transformed tumor site in the chest wall allowed the comparison of tumor cells in 3 sites. The findings indicate no difference in mutational patterns between spleen and blood, but reveal the potential for initiating mutation within the tumor clone in the appropriate environment in vivo.
Study design
Patient and clinical data
In all 4 cases, SMZL was diagnosed on splenic tissue with the use of World Health Organization criteria.5 Outer zone cells exhibited clear cytoplasm, and small satellite nodules of similar cells were present in the red pulp. Immunohistochemistry showed tumor cells to be positive for CD20 and CD79a, and negative for CD5, CD10, and CD23. Peripheral blood morphology was consistent with SLVLs in all cases.
Patient 4 presented in 1988 with splenomegaly, a lymphocyte count of 5.2 × 109/L, and lymphocyte morphology consistent with SLVL. She underwent splenectomy in 1991, and histology indicated SMZL. The white blood cell (WBC) count rose to 68 × 109/L in 1994, and blood cells from this period were available for analysis. Following 3 cycles of fludarabine, circulating tumor cells were cleared. Further administration of fludarabine in 1996 for recurrent lymphocytosis resulted in prolonged pancytopenia. In 1997, a firm mobile lump was removed from the left anterior chest wall, and histology revealed a diffuse large B-cell lymphoma (DLBCL). Chromosomal analysis of blood and splenic mononuclear cells showed an identical complex karyotype, including deletion of chromosome 7q, the most common cytogenetic abnormality in SMZL. The DLBCL revealed karyotypic evolution with additional genetic material on chromosomes 1p, 6q, and 16q, and deletion of Xq and 3q.
Analysis of VHgenes
Tumor-related VH genes were amplified from cDNA, and predominant sequences with clonally related complementarity-determining region 3 (CDR3) were identified.6 Sequence alignment was to the Entrez database (National Center for Biotechnology Information [NCBI], Bethesda, MD) and to the V Base (MRC Centre for Protein Engineering, Cambridge, United Kingdom).7
Results and discussion
Tumor VH sequences were obtained from all 4 cases, with clear clonal identity between tumor cells from different tissues of each individual (Table 1). In 3 of 4 patients, homology to the germ line sequence exceeded 98% and they were classified as unmutated.8 In these patients,VH sequences from blood and splenic tumor cells were identical. Patient 3 showed a low but significant level of somatic mutation, with intraclonal heterogeneity, in tumor cells from both blood and spleen. Mutational patterns were similar in both tissues. Data from these patients confirm the mixed origins of tumor cells in SMZL.4 They are consistent with derivation from a heterogeneous population of normal SMZ B cells, including naive B cells poised for T-cell–independent type-2 responses against bacterial polysaccharide antigens,9 and memory B cells.10 Patient 3 represents those cases of SMZL with intraclonal heterogeneity, similar to that commonly observed in follicular lymphoma (FL).11 It illustrates that cells of SMZL may be responsive to factors known to be involved in inducing somatic mutation.12 These include stromal cells, CD4+ T cells, cytokines, and ligation of the B-cell receptor (BCR). A role for antigen may exist, although analysis of mutation distributions in V-genes is no longer considered adequate to reveal this role.13 The identity of mutation pattern between blood and splenic tumor cells is consistent with the movement of tumor cells between the 2 sites.
Patient data and summary of tumorVH gene sequences
| Patient . | Sex . | Age . | WBC × 10−9/L . | sIg . | VH GL donor . | Homology (%) . | Intraclonal variation . |
|---|---|---|---|---|---|---|---|
| 1 | F | 73 | 8.4 | MDκ | V3-21 | 99.6 (B/S) | No |
| 2 | F | 65 | 14.5 | λ | V1-1 | 99.0 (B/S) | No |
| 3 | F | 54 | 7.4 | MDκ | V1-2 | 97.3* (B/S) | Yes |
| 4 | F | 68 | 12.4 | MDκ | V3-33 | 100 (B/S) | No |
| 97.6* (CW) | Yes |
| Patient . | Sex . | Age . | WBC × 10−9/L . | sIg . | VH GL donor . | Homology (%) . | Intraclonal variation . |
|---|---|---|---|---|---|---|---|
| 1 | F | 73 | 8.4 | MDκ | V3-21 | 99.6 (B/S) | No |
| 2 | F | 65 | 14.5 | λ | V1-1 | 99.0 (B/S) | No |
| 3 | F | 54 | 7.4 | MDκ | V1-2 | 97.3* (B/S) | Yes |
| 4 | F | 68 | 12.4 | MDκ | V3-33 | 100 (B/S) | No |
| 97.6* (CW) | Yes |
The VH sequences have been deposited in GenBank (Accession Nos. AF466106-42).
sIg indicates surface Ig; GL, germ line; B/S, sequences from blood and spleen; CW, sequences from chest wall.
Predominant clones.
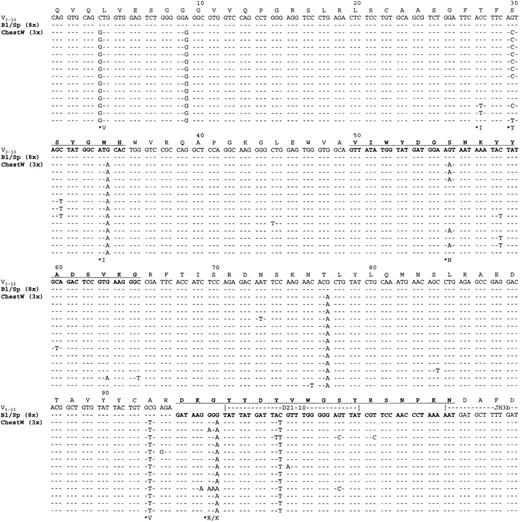
For patient 4, tumor samples were obtained from spleen, blood, and chest wall. The only phenotypic difference between the sites was the acquisition of bcl-6 expression in the chest wall biopsy. Immunohistochemical staining of this site showed no expression of CD10, but antibodies against CD21 and CD23 did reveal residual follicular structures among the diffuse infiltrate. The VHgene amplified from the spleen, and from the blood 3 years later, contained no somatic mutations. The VH sequences obtained from the DLBCL in the chest wall biopsy, obtained after a further 3 years, were clearly derived from the same tumor clone with an almost identical CDR3 sequence (Figure1). However, there had been an accumulation of somatic mutations, with continuing mutational activity indicated by intraclonal heterogeneity (Figure 1). Some mutations were common to all sequences, but others existed only in subclones. The fact that repeated mutations were present in different clones, and that no mutations were detected in multiple clones from blood or splenic tissue, confirms that these changes were derived from mutation initiated within the tumor clone. This is likely to be associated with transformation of the tumor and location in the chest wall site. Interestingly, the replacement mutations found in all clones were present in, or near to, the CDRs (Figure 1). Replacement mutations accumulating in intraclonal variants, and verified by their presence in more than 1 clone, were also in these sites. The positions involved were as follows: 30 (AGT [Ser] to ACT [Thr]); 56 (AGT [Ser] to AAT [Asn]); 97 (GGA [Gly] to AGA/AAA [Arg/Lys]); 105 (AGT [Ser] to ACT [Thr]), with the last 2 in CDR3.
Tumor-derived VH gene and deducted amino acid sequences from case 1.
VH sequences from blood (Bl), spleen (Sp), and chest wall (ChestW) are aligned against the germ line gene counterpart (V3-33). Dashes represent identity with the germline sequence. Replacement amino acid changes observed in more than one clone are starred. CDRs are in bold type and underlined. Amino acid numbering is according to Kabat et al.22
Tumor-derived VH gene and deducted amino acid sequences from case 1.
VH sequences from blood (Bl), spleen (Sp), and chest wall (ChestW) are aligned against the germ line gene counterpart (V3-33). Dashes represent identity with the germline sequence. Replacement amino acid changes observed in more than one clone are starred. CDRs are in bold type and underlined. Amino acid numbering is according to Kabat et al.22
Induction of somatic mutation in vitro in normal B cells,14 and in most cases of neoplastic B cells,15,16 requires engagement of the BCR. This presumably reflects events occurring in the normal immune responses in vivo where antigen provides this stimulus. For gastric mucosa-associated lymphoid tissue (MALT) lymphomas, there is a clear role for stimulation of tumor growth, and possibly somatic mutation, by the presence of Helicobacter pyloris.17 In the case of FL, stimulation of the BCR could occur by direct interaction between glycosylated V-regions and stromal elements.18 This may free these tumors from a requirement for conventional antigen to maintain growth. It is unlikely that somatic mutation continues indefinitely in FL.19-21SMZL is not considered to be a germinal center tumor, and glycosylation sites are not evident in published4 sequences or our own. The question of a role for interaction with antigen for stimulation of somatic mutation or for growth of tumor cells therefore remains open. The finding that a long-term in vivo tumor with unmutatedVH genes can apparently initiate somatic mutation and accumulate intraclonal heterogeneity with replacement mutations clustered in the CDRs is dramatic. It suggests that a factor in the environment, possibly able to bind to the BCR, has stimulated the cell. Alternatively, an event has occurred in the clone to activate somatic mutation independent of exogenous stimuli. Either change could be linked to the transformation to a rapidly dividing diffuse lymphoma, with karyotypic evolution. Transformation is an uncommon event in SMZL and we need to investigate whether this route is the exception or the rule.
Prepublished online as Blood First Edition Paper, May 31, 2002; DOI 10.1182/blood-2002-01-0169.
Supported by the Cancer Research Campaign UK and by Tenovus UK.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Freda K. Stevenson, Molecular Immunology Group, Tenovus Laboratory, Southampton University Hospitals, Southampton SO16 6YD, United Kingdom; e-mail: fs@soton.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal