Store-operated Ca++ entry (SOCE) is thought to comprise the major pathway for Ca++ entry in platelets. Recently, a number of transient receptor potential (TRP) proteins, which have been divided into 3 groups (TRPC, TRPM, and TRPV), have been suggested as SOCE channels. We report the expression and function of TRPC proteins in human platelets. TRPC6 is found at high levels and TRPC1 at low levels. Using purified plasma (PM) and intracellular membranes (IM), TRPC6 is found in the PM, but TRPC1 is localized to the IM. Using Fura-2–loaded platelets, we report that, in line with TRPC6 expression, 1-oleoyl-2-acetyl-sn-glycerol (OAG) stimulated the entry of Ca++ and Ba2+ independently of protein kinase C. Thrombin also induced the entry of Ca++ and Ba2+, but thapsigargin, which depletes the stores, induced the entry of only Ca++. Thus, thrombin activated TRPC6 via a SOCE-independent mechanism. In phosphorylation studies, we report that neither TRPC6 nor TRPC1 was a substrate for tyrosine kinases. TRPC6 was phosphorylated by cAMP-dependent protein kinase (cAMP-PK) and associated with other cAMP-PK substrates. TRPC1 was not phosphorylated by cAMP-PK but also associated with other substrates. Activation of cAMP-PK inhibited Ca++ but not Ba2+ entry induced by thrombin and neither Ca++ nor Ba2+entry stimulated by OAG. These results suggest that TRPC6 is a SOCE-independent, nonselective cation entry channel stimulated by thrombin and OAG. TRPC6 is a substrate for cAMP-PK, although phosphorylation appears to not affect cation permeation. TRPC1 is located in IM, suggesting a role at the level of the stores.
Introduction
Platelet activation forms an integral component of hemostasis and contributes to the events leading to thrombosis. Complete activation of platelets by all stimulatory agents leads to an increase of cytosolic Ca++ levels, which triggers many intracellular signaling processes important for the expression of functional responses.1 Conversely, the vasodilators prostacyclin (PGI2) and nitric oxide (NO) inhibit platelet function, with inhibition of Ca++elevation an identified mechanism.2 Cytosolic Ca++ elevation occurs as a consequence of release of the cation from intracellular stores and influx from the outside medium. Whilst the mechanism for Ca++ release from the stores in nonexcitable cells is well accepted, Ca++ entry mechanisms are less understood. The key elements involved in Ca++signaling include activated surface receptors that lead to the stimulation of phospholipase C (PLC), resulting in the hydrolysis of the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) to release inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds to the IP3 receptor (IP3R) on intracellular stores, releasing Ca++, and DAG is a potent activator of protein kinase C (PKC). Ca++ entry is thought to occur predominantly as a consequence of store depletion and has been referred to as store-operated Ca++ entry (SOCE) or capacitative Ca++ entry (CCE).3 However, the details of the SOCE pathway or the identity of the SOCE channel in the plasma membrane (PM) has remained elusive. Many models have been proposed, which include a conformational coupling mechanism involving the IP3R at the stores signaling to the PMchannel,4 release of a soluble Ca++ influx factor (CIF) from the stores that may trigger the opening of the cation channel in the PM,5 involvement of tyrosine kinases,6,7 and modifications of the conformational coupling mechanism that include a secretionlike mechanism involving rearrangements of the cytoskeleton8 and possible channel insertion.9 Importantly, there is as yet no consensus regarding the identity of the SOCE channel, and until such identification is established, the mechanisms for its regulation will remain contentious. There is also variability in the cation selectivity of the SOCE pathway. A Ca++ release–activated Ca++ current (ICRAC) that is highly selective for Ca++ has been described in hematopoietic cells.10 Endothelial cells have been reported to express SOCE activities11 that have moderate selectivity for Ca++, while some smooth muscle cell types express relatively nonselective SOCE pathways.12 Thus, the identities of the SOCE channels themselves may vary between cells.
At the molecular level, the transient receptor potential (TRP) proteins have been proposed as candidates for SOCE channels. TRP was first described as a Drosophila mutant that had an impaired visual transduction response (hence, transient receptor potential).13 Subsequent to cloning,14 theDrosophila (d)TRP protein was shown in vitro to be operated by a SOCE mechanism, although in vivo its gating mechanism remains to be established.15 There are currently known to be 3Drosophila TRP genes (trp, trpl, andtrpγ) and at least 20 TRP genes in mammalian systems.16,17 Using sequence identities, TRP proteins have been divided into 3 groups: the TRPC group, the TRPM group, and the TRPV group. There is considerable information on the TRPC group, which is the most closely related to dTRP, and overexpression studies have suggested most to be receptor-activated, nonselective cation channels.18 They have a widespread distribution, which suggests functional significance in mammalian systems. Their gating by a store depletion–dependent mechanism is controversial except for TRPC4, which was first cloned from bovine tissues19 and recently in the TRPC4 knockout mouse, where in endothelial cells, the absence of TRPC4 resulted in a reduced SOCE current.20Further, the reduced SOCE activity resulted in impaired agonist-dependent vasorelaxation, suggesting an importance of TRPC4 in vascular reactivity.
In platelets and megakaryocytic cells, there is currently information on the expression of TRPC proteins. Using reverse transcriptase–polymerase chain reaction (RT-PCR), we first reported that megakaryocytes (using the cell lines MEG01, DAMI, and HEL) expressed TRPC1, 2, and 3.21 Our studies have been recently confirmed and extended to include TRPC6 and TRPC4 mRNA in megakaryocytic cells and also in platelets, if very large numbers of cells are used to extract the mRNA.22 Recently, Rosado and Sage23 24 have reported the detection of TRPC1 protein in platelets and suggested its coupling to the type II IP3R upon depletion of intracellular stores. In this study, we sought to further examine the expression, role, and phosphorylation of TRPC proteins in human platelets. Using a range of highly specific antibodies, we report the high expression of TRPC6 and low expression of TRPC1. Further, we report that TRPC6 is activated by thrombin and diacylglycerol by a SOCE-independent mechanism and that it is a substrate for cAMP-dependent protein kinase (cAMP-PK), making it a target for physiologically important vasodilators. We also report that, surprisingly, TRPC1 is located in platelet intracellular membranes, suggesting a primary role at the level of the Ca++ stores.
Materials and methods
Materials
All chemical reagents were obtained from Sigma Chemical (Dorset, United Kingdom) unless otherwise stated. Electrophoresis reagents were obtained from National Diagnostics (Hull, United Kingdom), and nitrocellulose membranes were from Schleicher and Schuell (London, United Kingdom). Sp-5,6-DCl-cBiMPS (BIMPS), which activates cAMP-PK; 8pCPT-cGMP (8pCPT), which activates cGMP-dependent protein kinase (cGMP-PK); and Rp-8-pCPT-cGMPS, an inhibitor of cGMP-PK, were obtained from Biolog Life Science Institute, Bremen, Germany. A cAMP-PK inhibitor peptide 14-22 amide was obtained from CN Biosciences (Nottingham, United Kingdom). Tissue culture media were obtained from GIBCO-BRL (Paisley, United Kingdom). [32P]Pi and γ-[32P]-adenosine triphosphate (ATP) was from ICN (Basingstoke, United Kingdom).
Antibodies
Ank is a polyclonal antibody that was raised to the sequence (LNEKLFLLACDKGDYYM), which resides in the first ankyrin domain of hTRPC1.25 The peptide was coupled to thyroglobulin and, along with Freunds complete adjuvant, subcutaneously injected into rabbits for antibody production. After 2 further boosts, rabbits were bled and serum prepared. Affinity purification was carried out using the antigenic peptide linked to an ω-aminohexyl agarose column using N-γ-maleimidobutyrlloxy-succinimide ester (Pierce & Warriner, Chester, United Kingdom). Diluted antiserum (1:1 with phosphate-buffered saline [PBS]) was incubated with the column for 4 hours, washed with 1 M guanidine HCl, 50 mM Tris pH 7.4, followed by antibody elution with 4.5 M MgCl2, 100 mM Tris pH 8.0, 0.1% bovine serum albumin (BSA). The eluted antibody was dialyzed against PBS for 1 hour at room temperature and overnight with fresh PBS containing 0.005% NaN3. An antihemagglutinin (HA) epitope antibody was obtained from Roche Diagnostics (Lewes, East Sussex, United Kingdom). Anti-Xenopus TRP-1 (XTRP-1) was raised to a C-terminal peptide of XTRP-1 and has been previously characterized.26Anti-TRPC1 and anti-TRPC6 antibodies were obtained from Alomone Laboratories (Jerusalem, Israel). Anti-bTRPC4 antibody, which was raised to a C-terminal sequence from bTRPC4 and also recognizes TRPC5, has been previously characterized.27 Anti-αTRPC3 antibody was raised to the N-terminal 213 amino acids of hTRPC3 and previously described.28 The antiphosphotyrosine antibody 4G10 was obtained from TCS Biologicals (Botolph Claydon, United Kingdom). A polyclonal antibody recognizing the vasodilator-stimulated phosphoprotein (VASP)29 was a generous gift from Professor U. Walter (Wurzburg, Germany).
Cell culture and transfection of TRPC constructs
cDNA for hTRPC1, mTRPC2, and hTRPC3 were subcloned into the vector pcDNA3.30 hTRPC1 contained an HA epitope at the N-terminus and hTRPC3 at the C-terminus. cDNA for mTRPC4α, mTRPC4β, mTRPC5, and mTRPC6 were subcloned into the pIRESneo vector. Amplification of the plasmids was carried out using Escherichia coli JM109 and maxipreps prepared using a Qiagen Maxi preparation kit as described by the manufacturer (Qiagen, Crawley, West Sussex, United Kingdom). DNA purity of the constructs was checked using restriction enzyme digestion.
Transfections were carried out using QBI-293A cells (Qbiogene, Livingstone, United Kingdom), which are a subclone of HEK-293 cells selected for improved transfection properties. The QBI-293A cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 110 mg/L sodium pyruvate, 10% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were maintained in T175cm2 flasks until they were approximately 70% confluent and were transfected using a standard calcium phosphate precipitation protocol with TRPC constructs (30-60 μg plasmid DNA in 900 μL, 1 mL of 2 X BES buffer, 100 μL of 2.5 M CaCl2per T175cm2 flask). The medium was replaced 24 hours later, and the cells were allowed to grow for a further 24 hours, after which they were harvested and frozen at −80°C until required. The cell pellets were thawed on ice and resuspended in sonication medium (0.34 M sorbitol, 10 mM HEPES [N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid] pH 7.2 with a cocktail of inhibitors including aprotinin [0.2 U/mL], pepstatin A [10 μg/mL], PMSF [phenylmethylsulfonyl fluoride] [0.1 mM], and soybean trypsin inhibitor [0.2 mg/mL]). Homogenization was carried out on ice using a Vibra Cell ultrasonicator (Jencons Scientific, Leighton Buzzard, United Kingdom), with 2 × 10-second bursts at an amplitude setting of 30. The homogenates were centrifuged at 100 000g for 90 minutes to generate a microsomal pellet that was resuspended in sonication medium plus 5% glycerol at approximately 2 mg/mL. These were used for all subsequent analyses, including Western blotting and phosphorylation by exogenous kinases.
Preparation of Fura-2–labeled platelets
Blood was taken from donors, who denied taking any medication for the last 9 days, into 0.1 volume 3.2% trisodium citrate and platelet-rich plasma (PRP) was isolated after centrifugation at 200g for 15 minutes. Fura-2–labeling was carried out by incubating PRP (acidified to pH 6.5 using 0.3 M citric acid) at 37°C with 3 μM Fura-2/AM (acetoxymethyl ester) for 1 hour. The PRP was then allowed to cool to room temperature and centrifuged at 1200g for 15 minutes. The platelet pellet was resuspended at 1-1.5 × 108 cells/mL in a medium consisting of 10 mM HEPES, 140 mM NaCl, 5 mM KCl, 1 mM MgSO4, 10 mM glucose, 3 μM indomethacin, and 1 U/mL apyrase (grade III; Sigma Chemical, United Kingdom). Cation elevation studies were carried out using 2-mL suspensions at 37°C with sample mixing carried out by cuvette inversion. Fluorescence measurements were made using a rotating wheel spectrofluorimeter (Cairns Research, Faversham, Kent, United Kingdom) with excitation at 340 and 380 nm and emission at 510 nm. [Ca++]i or [Ba2+]i is reported as the 340 nm:380 nm ratio (R340/380). Additions of agents were made as stated in the “Results” section or legends to the relevant Figures. Autofluorescence was estimated by addition of 2 mM MnCl2 in the presence of 10 μM ionomycin. Where shown, calculation of [Ca++]i was carried out as described by Sage.31
Preparation of highly purified platelet plasma and intracellular membranes using high-voltage free flow electrophoresis
Platelet plasma membranes (PMs) and intracellular membranes (IMs) were prepared as described in considerable detail in previous publications.32 33 Briefly, platelets were separated from human blood and treated with neuraminidase (type X, 0.05 U/mL) for 20 minutes at 37°C. After washing, platelets were sonicated at 4°C (with 2 × 10-second bursts, amplitude setting at 50) in sonication medium and centrifuged at 42 000g for 90 minutes on a linear (1-3.5 M) sorbitol density gradient to obtain a mixed membrane (MM) fraction (free of granular contamination). MMs were concentrated by centrifugation (100 000g, 60 minutes), resuspended in 0.4 M sorbitol, 10 mM triethanolamine pH 7.2 with a conductivity of 750 μS/cm, and separated into PM and IM by free flow electrophoresis (FFE) using an Octopus FFE apparatus (Dr Weber, Gmbh, Germany) running at 750 V, 100 mA. Two peaks comprising ofPM (less electronegative) and IM (more electronegative) were obtained. Tops of peaks were pooled, centrifuged (100 000g, 60 minutes), and resuspended in 0.4 M sorbitol, 10 mM triethanolamine pH 7.2.
Labeling of intact platelets with [32P]Pi and immunoprecipitation
Freshly prepared platelets were resuspended in a wash buffer consisting of 36 mM citric acid, 103 mM NaCl, 5 mM KCl, 5 mM glucose, 1mM EDTA (ethylenediaminetetraacetic acid) pH6.5 with 60 nM prostacyclin (PGI2). They were then incubated for 90 minutes at 37°C with 0.25 mCi (9.3 MBq) carrier-free [32P]Pi/mL and washed twice with fresh wash buffer before resuspension in a HEPES tyrode medium containing 10 mM HEPES, 145 mM NaCl, 5 mM KCl, 1 mM MgSO4, 5 mM glucose pH 7.4 at 2 × 109 cells/mL. Incubations were carried out in aggregation cuvettes using a Peyton dual channel aggregometer (ISMS; Dorking, Surrey, United Kingdom) with 500 μL suspensions at 37°C with mixing. Additions of stimulatory or inhibitory agonists were for the times stated in the “Results” section and reactions stopped by addition of equal volume of Triton X-100 solubilization buffer with composition 1% Triton, 10 mM EGTA (ethyleneglycoltetraacetic acid), 150 mM NaCl, 40 mM Tris pH 7.8, 2 mM PMSF, 2 mM sodium vanadate, 0.2 mM leupeptin, 10 μg/mL pepstatin A, 0.4 U/mL aprotinin, 0.2 mg/mL soybean trypsin inhibitor, and 10 μM E64d. After 30 minutes' rotating at 4°C, the mixtures were centrifuged at 17 900g for 5 minutes at 4°C and the supernatants used for immunoprecipitation. Supernatants were first precleared with 0.1 volume 20% protein-G sepharose (PGS) for 30 minutes. After centrifugation at 420g, 5 minutes at 4°C (in an eppendorf centrifuge), the respective primary antibody was added to the supernatant and incubated overnight rotating at 4°C. The following morning, 50 μL 20% PGS was added and incubated for at least 4 hours at 4°C. The immunoadsorbant was spun down, washed 4 times with washing buffer (0.2% Triton X100, 0.1% BSA, 0.01% sodium azide in PBS), with the last wash for at least 4 hours or overnight and then once with buffer lacking BSA. Sodium dodecyl sulfate (SDS)–Laemmli sample buffer was added to the pellet and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), followed by Western blotting and autoradiography.
Western blotting
Samples were applied to SDS-PAGE gradient gels (5%-15%) following the method of Laemmli34 and subjected to electrophoresis. Separated proteins were transferred onto nitrocellulose membrane by semidry blotting using a current density of 0.8 mA/cm2 for 1.5 hours, and the nitrocellulose was then blocked for 3 hours (or overnight) in blocking medium (5% dried milk, 1% normal goat serum [NGS] in TBS-Tween [20 mM Tris pH 7.4, 500 mM NaCl, 0.2% Tween]). Filters were washed 3 times in TBS-Tween, followed by incubation with the corresponding primary antibody (Ank, anti-TRPC6, etc) in TBS-Tween + 20 mg/mL BSA for at least 1 hour or overnight. After washing, the membranes were incubated with an appropriate second antibody (eg, goat antirabbit) conjugated to horseradish peroxidase in TBS-Tween + 2 mg/mL BSA for 1 hour, followed by detection using enhanced chemiluminescence (ECL) reagents.
Phosphorylation of QBI-293A microsomes by catalytic subunit of cAMP-PK
Phosphorylation of overexpressed hTRPC1 or mTRPC6 containing microsomes was carried out using a procedure used to phosphorylate platelet membranes.35 The reaction mixture consisted of 50 mM HEPES buffer (pH 7.4), 10 mM MgCl2, 1 mM dithiothreitol (DTT), 0.2 mM EGTA, 500 μg membrane protein, 50 μM ATP containing 10 μCi (0.37 MBq)γ-[32P] ATP, and, where indicated, 350 U catalytic subunit (CAT) or 1 μM cAMP-PK inhibitor peptide. The reaction was initiated by adding ATP and CAT, mixing and incubation at 30°C for the time stated. The reaction was terminated by the addition of cold solubilization buffer (at final concentration of 0.5% Triton X-100, 20 mM Tris pH 7.8, 5 mM EDTA, 75 mM NaCl, 1 mM PMSF, 1 mM sodium vanadate, 0.1 mM leupeptin, 5 μg/mL pepstatin, 0.2 U/mL aprotinin, 0.1 mg/mL soybean trypsin inhibitor, and 5 μM E64d). After 30 minutes' rotating at 4°C, the samples were centrifuged at 17 900g for 5 minutes. The supernatant was precleared and immunoprecipitated as described above.
All Western blots and autoradiographs shown represent typical results obtained with at least 3 different platelet or membrane preparations, and analysis of statistical significance (Student t test using Microsoft Excel, Seattle, WA), where appropriate, is reported in figure legends.
Results
Antibodies and the expression of TRPC proteins in human platelets
Figure 1 presents the characterization of an affinity-purified polyclonal antibody (Ank) that was raised to a peptide sequence in the first ankyrin domain of hTRPC1. Ank recognized hTRPC1 overexpressed in QBI-293A cells with the protein migrating at approximately 80 kDa (Figure 1A). The overexpressed protein also contained an HA epitope at the N terminus, allowing its detection with an anti-HA epitope antibody (results not shown). Ank also was effective at immunoprecipitating overexpressed hTRPC1, as determined by probing the immunoprecipitates with the HA-epitope antibody in Western blots. This confirmed the size of the overexpressed protein as approximately 80 kDa (Figure 1B). Ank is specific for hTRPC1, as it did not recognize mTRPC2, hTRPC3, mTRPC4α, mTRPC5, or mTRPC6, all overexpressed in QBI-293A cells (results not shown).
Characterization of the TRPC1 antibody Ank and expression of TRPC proteins in human platelets.
(A) A 50 μg microsomal preparation of nontransfected and hTRPC1-transfected QBI-293A cells (QBI) was probed for Western blot detection with Ank antibody (5 μg/mL). (B) QBI-293A cells overexpressing hTRPC1 were lysed, immunoprecipitated (IP) with Ank, and probed with anti-HA (0.5 μg/mL). (C) Lysates of resting platelets were probed with Ank (left panel) or immunoprecipitated with Ank and probed with the Ank antibody (right panel). (D) 50 μg nontransfected and mTRPC6-transfected QBI-293A cells (left panel) and 50 μg platelet mixed membranes (right panel) were probed with anti-TRPC6 (1/400). (E) 50 μg mTRPC4α and mTRPC5-transfected QBI-293A cells and 100 μg mixed membranes from platelets and the megakaryocytic cell lines DAMI and CHRF-288 were probed with anti-bTRPC4 (1/200). (F) 50 μg nontransfected and hTRPC3-transfected QBI-293A cells and 50 μg platelet mixed membranes were probed with anti-αTRPC3 (1/2000). Molecular size markers are noted in kilodaltons (kDa) and TRPC protein sizes indicated with arrows. All Western blots are representative of at least 3 distinct determinations.
Characterization of the TRPC1 antibody Ank and expression of TRPC proteins in human platelets.
(A) A 50 μg microsomal preparation of nontransfected and hTRPC1-transfected QBI-293A cells (QBI) was probed for Western blot detection with Ank antibody (5 μg/mL). (B) QBI-293A cells overexpressing hTRPC1 were lysed, immunoprecipitated (IP) with Ank, and probed with anti-HA (0.5 μg/mL). (C) Lysates of resting platelets were probed with Ank (left panel) or immunoprecipitated with Ank and probed with the Ank antibody (right panel). (D) 50 μg nontransfected and mTRPC6-transfected QBI-293A cells (left panel) and 50 μg platelet mixed membranes (right panel) were probed with anti-TRPC6 (1/400). (E) 50 μg mTRPC4α and mTRPC5-transfected QBI-293A cells and 100 μg mixed membranes from platelets and the megakaryocytic cell lines DAMI and CHRF-288 were probed with anti-bTRPC4 (1/200). (F) 50 μg nontransfected and hTRPC3-transfected QBI-293A cells and 50 μg platelet mixed membranes were probed with anti-αTRPC3 (1/2000). Molecular size markers are noted in kilodaltons (kDa) and TRPC protein sizes indicated with arrows. All Western blots are representative of at least 3 distinct determinations.
Ank was then used to determine the expression of TRPC1 in human platelets. Figure 1C shows that in Western blots of platelet lysates, Ank recognized 3 bands between 65 and 105 kDa migration positions, namely at 100, 80, and 70 kDa. These bands may reflect different glycosylation states or alternatively spliced forms of TRPC1 in platelets. Although the migration size of overexpressed hTRPC1 is 80 kDa, recent studies in the literature have reported endogenous mammalian TRPC1 to be 100, 92, 80, and 65 kDa,36,23,26suggesting that more than one detectable form may be present (hTRPC1β differs from the full-length protein by 34 amino acids37). We emphasize that Western blotting detection of TRPC1 in human platelets is very weak, with similar analyses obtained using anti-XTRP-1 antibody38 and also by the commercially available anti-TRPC1 antibody from Alomone Laboratories (results not shown). Both anti–XTRP-1 and anti-TRPC1 detected the overexpressed hTRPC1 in QBI-293A cells (results not shown). Immunoprecipitation analysis was carried out using Ank to see if TRPC1 could be extracted for better detection. The immunoprecipitates revealed a prominent peptide band at 100 kDa with only faint staining of 65- and 80-kDa bands (Figure 1C). The 100-kDa band was further recognized by the anti–XTRP-1 antibody, confirming it to be TRPC1 (results not shown). Similar findings were seen if immunoprecipitation was carried out using lysates of the megakaryocytic cell line DAMI, except that the level of the 100-kDa peptide was lower than that observed in platelets. This is in agreement with our previous demonstration of the expression of TRPC1 mRNA in DAMI cells.21
Platelets also were tested for the presence of other TRPC proteins, using a range of polyclonal antibodies, each specifically recognizing its corresponding protein. An anti-TRPC6 antibody (raised to an N-terminal sequence of rTRPC6) recognized mTRPC6 overexpressed in QBI-293A cells as a doublet protein band migrating at 100 and 110 kDa, and also recognized well a 110-kDa band in platelet mixed membranes, suggesting a good expression of this cation channel in platelets (Figure 1D). We believe that the doublet band of overexpressed mTRPC6 is due to full and partial glycosylation of the protein, as treatment of solubilized membranes with N-glycosidase F (Boehringer Mannheim, Mannheim, Germany; to remove attached carbohydrate residues) resulted in the detection of only the 100-kDa band (results not shown). An antibody raised to the C-terminal sequence of bovine (b)TRPC4 recognized overexpressed mTRPC4α and mTRPC5 (again in QBI-293A cells) but did not recognize a corresponding band from platelet mixed membranes or from membranes from the megakaryocytic cells DAMI and CHRF-288 (Figure 1E). This suggested that platelets and these cell lines did not express TRPC4 and TRPC5 or that the levels were too low to detect with this antibody. Figure 1F shows that an antibody raised to the N-terminal 213 amino acids of hTRPC328 recognized overexpressed hTRPC3 in QBI-293A cells but did not recognize a band at 92 kDa in platelet mixed membranes. This again suggested that TRPC3 was not expressed in platelets or that the levels were too low for detection. Taken together, the data suggest that human platelets express high levels of TRPC6 and much lower levels of TRPC1, with TRPC7 not examined in this study.
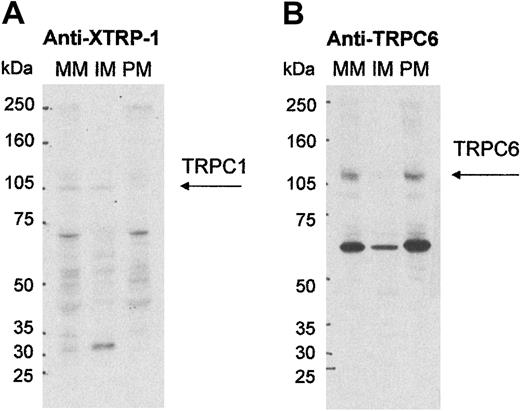
Our laboratory has, for a number of years, used the technique of FFE to separate PM and IM from human platelets. Full characterization of these membrane fractions has been described in many previous publications.32 39 Using this technique, we investigated the localization of TRPC1 and TRPC6. Figure2B shows that detection of the 110-kDa TRPC6 protein band was observed in mixed membranes (MM) prior to separation by FFE, and in PM after electrophoresis, but there was little or no detection of the protein in IM. Surprisingly, TRPC1 (100 kDa) with, again, weak detection, was located in MM and IM, suggesting that its predominant location was not in the PM and therefore may not play a direct role in cation entry in platelets.
Localization of TRPC1 and TRPC6 in highly purified platelet membranes.
Shown are Western blot analyses of platelet mixed membranes (MM) prior to high-voltage free flow electrophoresis (FFE) and purified plasma (PM) and intracellular membranes (IM) prepared using FFE. (A) 100 μg protein was probed with anti–XTRP-1 (1 μg/mL) and (B) 50 μg protein was probed with anti-TRPC6 (1/400). Molecular size markers are noted in kilodaltons (kDa) and the TRPC protein indicated with arrows.
Localization of TRPC1 and TRPC6 in highly purified platelet membranes.
Shown are Western blot analyses of platelet mixed membranes (MM) prior to high-voltage free flow electrophoresis (FFE) and purified plasma (PM) and intracellular membranes (IM) prepared using FFE. (A) 100 μg protein was probed with anti–XTRP-1 (1 μg/mL) and (B) 50 μg protein was probed with anti-TRPC6 (1/400). Molecular size markers are noted in kilodaltons (kDa) and the TRPC protein indicated with arrows.
Cation influx studies using Fura-2–loaded platelets
The above results indicated that human platelets express TRPC6 in the platelet PM. Recent studies suggest that in heterologous systems, members of the TRPC3, 6, and 7 subfamilies can be activated by the DAG analog 1-oleoyl-2-acetyl-sn-glycerol (OAG) to stimulate cation entry.18,40-43 We therefore carried out studies on Fura-2–labeled platelets to determine if OAG stimulated endogenous TRPC6 to allow calcium entry. Figure3 shows the results. In the presence of 1 mM extracellular Ca++, addition of OAG stimulated a dose-related increase of (340/380 ratio) fluorescence of Fura-2–loaded platelets, signifying an increase of cytosolic [Ca++] (Figure 3A). Minimal increase was observed if no Ca++ was added to the incubation medium, suggesting that the major component of Ca++ increase was due to entry of the cation (results not shown). Ca++ increase induced by OAG was steady and near linear over time, whereas that by thrombin was rapid, reaching a peak followed by a slow decline (Figure 3B). The mechanism of action of OAG on the TRPC3, 6, and 7 subfamilies is not known but has been suggested to act via a membrane-delimiting manner and independent of PKC activity.18,40 The latter has been reported to be activated maximally by 75 μM OAG.44 In our experiments, incubation of platelets with a PKC inhibitor bisindolylmaleimide I (Bis I, 0.5 μM) 5 minutes prior to addition of OAG did not significantly affect the rate of Ca++ entry induced by 60 μM OAG and confirmed that this cation entry was independent of PKC (increase of ratio units above basal 4 minutes after OAG addition was 0.44 ± 0.09 [n = 4] and 0.57 ± 0.04 [n = 3] in the presence and absence of Bis I respectively, P = .30). However, 60 μM OAG–induced platelet aggregation (which is known to be dependent on PKC) was totally inhibited by 0.5 μM Bis I (results not shown).
Ca++ influx in response to oleoyl acetyl glycerol (OAG) and thrombin.
Fura-2–loaded platelets were incubated at 37°C in the presence of 1 mM extracellular Ca++ (addition of Ca++marked on traces). Agonists were then added, and the elevations of [Ca++]i were monitored using the 340:380 nm ratio. A calibrated [Ca++]i (nM) scale is shown on the right. Figures given below represent increase in ratio units above basal level after 5 minutes. (A) Cells stimulated with 30, 60, and 90 μM OAG showed linear elevation of [Ca++]i of 0.78 ± 0.156, 0.98 ± 0.162, and 1.08 ± 0.175 ratio units, respectively (n = 11). (B) Cells stimulated with 1 U/mL thrombin showed rapid [Ca++]i increase with a peak value of 2.4 ± 0.787 ratio units (n = 6), followed by a slow decrease to a plateau value after 5 minutes of 1.35 ± 0.57 ratio units (n = 6).
Ca++ influx in response to oleoyl acetyl glycerol (OAG) and thrombin.
Fura-2–loaded platelets were incubated at 37°C in the presence of 1 mM extracellular Ca++ (addition of Ca++marked on traces). Agonists were then added, and the elevations of [Ca++]i were monitored using the 340:380 nm ratio. A calibrated [Ca++]i (nM) scale is shown on the right. Figures given below represent increase in ratio units above basal level after 5 minutes. (A) Cells stimulated with 30, 60, and 90 μM OAG showed linear elevation of [Ca++]i of 0.78 ± 0.156, 0.98 ± 0.162, and 1.08 ± 0.175 ratio units, respectively (n = 11). (B) Cells stimulated with 1 U/mL thrombin showed rapid [Ca++]i increase with a peak value of 2.4 ± 0.787 ratio units (n = 6), followed by a slow decrease to a plateau value after 5 minutes of 1.35 ± 0.57 ratio units (n = 6).
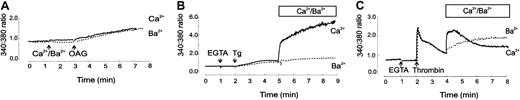
We further investigated if OAG led to the activation of entry of other cations, particularly Ba2+, as this has been used to monitor the activity of heterologously expressed TRPC3.41 43 Figure 4A shows that 60 μM OAG was effective at inducing the entry of Ba2+into platelets at a rate similar to that of Ca++, further correlating the expression of TRPC6 in the platelet PM. We then went on to determine the contribution of TRPC6 to SOCE and receptor-activated cation entry pathways. For these studies, the cells were either stimulated with thapsigargin, which inhibits SERCA pumps and depletes intracellular stores, or with thrombin, which acts on its receptor and induces G protein–dependent phospholipase C signaling. Experiments were carried out with the agonist added in the absence of extracellular Ca++ to monitor release from stores, followed by addition of the respective cation to determine entry (Figure 4B-C). Addition of thapsigargin (3 μM) caused a slow release of Ca++ from stores and a rapid entry of Ca++ upon addition of extracellular Ca++, but was ineffective at inducing sufficient entry of Ba2+ (Figure 4B). This demonstrated that in platelets, SOCE is selective for Ca++over Ba2+. In similar experiments, addition of 1 U/mL thrombin showed rapid release of Ca++ from intracellular stores and the rapid entry of either Ca++ or Ba2+ (Ca++ > Ba2+) upon either cation addition (Figure 4C). The kinetics of Ba2+entry (ie, increase to a plateau) suggested that unlike Ca++, Ba2+ was a poor substrate for platelet Ca++ pumps. These results indicated that thrombin signaling to PLC and the resultant IP3 and DAG formation activated not only SOCE channels by IP3-induced depletion of the stores but also non-SOCE channels such as TRPC6 that could be monitored by the DAG (OAG)-induced entry of Ba2+.
Cation influx in response to oleoyl acetyl glycerol (OAG), thapsigargin (Tg), and thrombin.
Fura-2–loaded platelets were incubated at 37°C with additions (marked with arrows), and the ratio (340:380 nm) fluorescence increases were measured in experiments using extracellular Ca++(continuous line) or Ba2+ (dashed line). (A) 1 mM Ca++ or Ba2+ was added to platelets followed by 60 μM OAG 2 minutes later. The increase in ratio fluorescence 4 minutes after OAG addition was 0.588 ± 0.072 (n = 13) and 0.58 ± 0.056 (n = 9) ratio units, for Ca++ and Ba2+, respectively; P = .93. (B) 100 μM EGTA was added to platelets, followed by 3 μM Tg 1 minute later. 1 mM Ca++ or 1 mM Ba2+ was added 3 minutes later, and fluorescence recordings continued for a further 4 minutes. Increase of ratio fluorescence 4 minutes after cation addition was 3.24 ± 0.354 (n = 4) and 0.46 ± 0.05 (n = 6) for Ca++ and Ba2+, respectively;P < .001 (C) 1 U/mL thrombin was added 1 minute after 100 μM EGTA. 2 minutes later either 1 mM Ca++ or Ba2+ was added. Ratio fluorescence increases with 1 mM Ca++ were at peak (0.5 minutes) 1.2 ± 0.082 and plateau (4 minutes) 0.46 ± 0.138 (n = 4); and for Ba2+ at 0.5 minutes was 0.29 ± 0.02 and at plateau 0.66 ± 0.053 (n = 6); at 0.5 minutes P < .001, at 4 minutesP = .153.
Cation influx in response to oleoyl acetyl glycerol (OAG), thapsigargin (Tg), and thrombin.
Fura-2–loaded platelets were incubated at 37°C with additions (marked with arrows), and the ratio (340:380 nm) fluorescence increases were measured in experiments using extracellular Ca++(continuous line) or Ba2+ (dashed line). (A) 1 mM Ca++ or Ba2+ was added to platelets followed by 60 μM OAG 2 minutes later. The increase in ratio fluorescence 4 minutes after OAG addition was 0.588 ± 0.072 (n = 13) and 0.58 ± 0.056 (n = 9) ratio units, for Ca++ and Ba2+, respectively; P = .93. (B) 100 μM EGTA was added to platelets, followed by 3 μM Tg 1 minute later. 1 mM Ca++ or 1 mM Ba2+ was added 3 minutes later, and fluorescence recordings continued for a further 4 minutes. Increase of ratio fluorescence 4 minutes after cation addition was 3.24 ± 0.354 (n = 4) and 0.46 ± 0.05 (n = 6) for Ca++ and Ba2+, respectively;P < .001 (C) 1 U/mL thrombin was added 1 minute after 100 μM EGTA. 2 minutes later either 1 mM Ca++ or Ba2+ was added. Ratio fluorescence increases with 1 mM Ca++ were at peak (0.5 minutes) 1.2 ± 0.082 and plateau (4 minutes) 0.46 ± 0.138 (n = 4); and for Ba2+ at 0.5 minutes was 0.29 ± 0.02 and at plateau 0.66 ± 0.053 (n = 6); at 0.5 minutes P < .001, at 4 minutesP = .153.
Phosphorylation status of TRPC proteins in human platelets
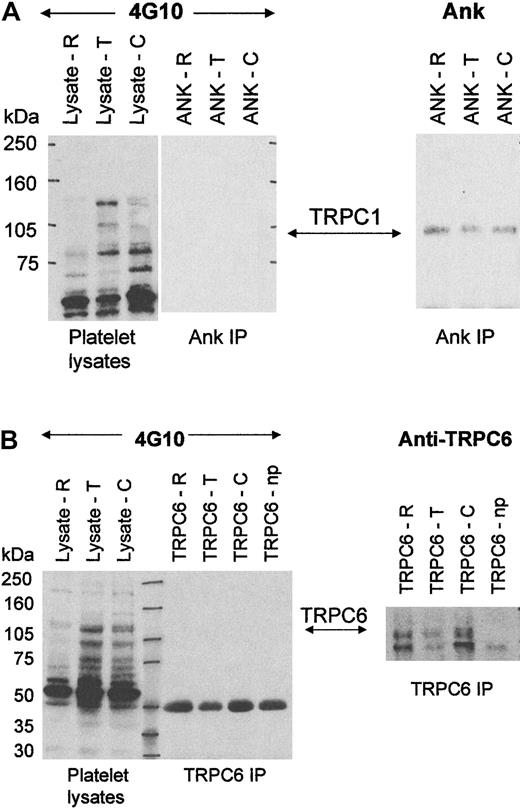
Our above findings suggested that platelet TRPC6 was activated upon thrombin addition. We next sought to examine if the phosphorylation status of TRPC proteins changed when platelets were treated with stimulatory or inhibitory agonists. Analysis of the primary sequence of hTRPC1 and hTRPC6 suggest that both proteins contain consensus sequences for cAMP-PK, cGMP-PK, PKC, casein kinase II, and tyrosine kinases (Prosite, ExPasy Molecular Biology Server, Geneva, Switzerland, http://www.expasy.ch/). We first examined tyrosine phosphorylation of TRPC proteins, as a number of studies have suggested that tyrosine kinases play a role in Ca++ entry when platelets undergo activation.6 7 Studies were carried out on freshly isolated platelets in aggregation tubes and, after stimulation with appropriate agents, lysates were prepared and immunoprecipitation carried out using the Ank and anti-TRPC6 antibodies (to extract TRPC1 and TRPC6, respectively). Western blots were probed with the antiphosphotyrosine antibody 4G10. Analysis of platelet lysates after cell stimulation with thrombin and collagen revealed that there was an increase of tyrosine-phosphorylated proteins, particularly of molecular sizes 140, 105, and 80 kDa, as observed by many investigators previously (Figure 5A). However, immunoprecipitates of TRPC1 using Ank suggested that TRPC1 (at 100 kDa) did not undergo tyrosine phosphorylation when platelets were stimulated with 2 U/mL thrombin or 30 μg/mL collagen, even though significant extraction of the 100-kDa protein was evident. TRPC6 also did not undergo tyrosine phosphorylation when platelets were stimulated with thrombin or collagen (Figure 5A-B). This suggested that direct tyrosine phosphorylation of TRPC1 or TRPC6 was not a mechanism for triggering cation entry into platelets. In experiments involving TRPC6, we have noticed a decrease in the level of the TRPC6 protein in Triton X-100 soluble lysates, its extraction by the antibody, and an increase of the protein in the insoluble pellet after stimulation of platelets with thrombin but not with collagen (results not shown). This may reflect an increased association with the cytoskeleton upon thrombin stimulation, and currently it is not known whether the TRPC6 protein associated with the Triton insoluble pellet undergoes tyrosine phosphorylation. However, this issue was not further investigated in this study.
Tyrosine phosphorylation of TRPC1 and TRPC6.
Western blot analysis of lysates from resting platelets (R), platelets activated with 2 U/mL thrombin (T), or activated with 30 μg/mL collagen (C). (A) Western blot analysis of 50 μL platelet lysates and Ank immunoprecipitations probed with the antiphosphotyrosine antibody 4G10 (1 μg/mL) and Ank (5 μg/mL). (B) Western blot analysis of 50 μL platelet lysates and anti-TRPC6 immunoprecipitations probed with the antiphosphotyrosine antibody 4G10 (1 μg/mL) and anti-TRPC6 (1/400). Np reflects control immunoprecipitations with anti-TRPC6 without platelet lysates. Molecular size markers are noted in kilodaltons (kDa) and the TRPC protein identifications indicated with arrows.
Tyrosine phosphorylation of TRPC1 and TRPC6.
Western blot analysis of lysates from resting platelets (R), platelets activated with 2 U/mL thrombin (T), or activated with 30 μg/mL collagen (C). (A) Western blot analysis of 50 μL platelet lysates and Ank immunoprecipitations probed with the antiphosphotyrosine antibody 4G10 (1 μg/mL) and Ank (5 μg/mL). (B) Western blot analysis of 50 μL platelet lysates and anti-TRPC6 immunoprecipitations probed with the antiphosphotyrosine antibody 4G10 (1 μg/mL) and anti-TRPC6 (1/400). Np reflects control immunoprecipitations with anti-TRPC6 without platelet lysates. Molecular size markers are noted in kilodaltons (kDa) and the TRPC protein identifications indicated with arrows.
Studies were then carried out to determine if the TRPC proteins underwent changes in serine or threonine phosphorylation upon platelet activation or inhibition using [32P]Pi-labeled cells. Activation was achieved using 2 U/mL thrombin or 3 μM thapsigargin; inhibition using 500 μM BIMPS, 500 μM 8PCPT, or 10 μM PGE1. As with the tyrosine phosphorylation experiments, incubations were carried out in aggregation tubes, followed by lysate preparation and extraction of TRPC proteins using the Ank and antiTRPC6 antibodies. Detection of phosphorylation was carried out by autoradiography of the dried-down gels or Western blots. Figure6A shows that under conditions where thrombin caused complete aggregation of platelets, examination of lysates by autoradiography showed the phosphorylation of pleckstrin at 45 kDa, a well-established substrate of protein kinase C (lane T, Figure 6A). Thapsigargin stimulation also caused aggregation of platelets and phosphorylation of pleckstrin (results not shown). BIMPS and 8pCPT did not cause aggregation of platelets or increase phosphorylation of pleckstrin but did stimulate the phosphorylation of the 50-kDa protein VASP, which is a well-established substrate of cAMP-PK and cGMP-PK (lanes B and 8P, Figure 6A).29 BIMPS- and 8PCPT-induced VASP phosphorylation also was detected using a polyclonal antibody that recognized both the 50-kDa (phosphorylated) and 46-kDa (nonphosphorylated) forms, confirming that cAMP-PK and cGMP-PK were activated with these 2 agents (results not shown). Autoradiographic analysis of immunoprecipitates of TRPC1 from resting platelets showed phosphoproteins of molecular size 250 and 120 kDa but not at 100 kDa (the latter being the migration size of extracted TRPC1, Figure 6B). Upon stimulation of the cells with thrombin, these bands were completely dephosphorylated. In the presence of BIMPS or 8pCPT, the TRPC1 antibody Ank extracted phosphoproteins at molecular sizes 250, 120, 85, and 70 kDa. Occasionally, a phosphoprotein of a size greater than 250 kDa was detected and other minor bands at 55 and 30 kDa (Figure 6B). Although the 85-kDa phosphoprotein migrated at the size of overexpressed hTRPC1, this identity from platelet extracts was not confirmed, as probing the Western blots with Ank (or XTRP-1 antibody) revealed only detection of the 100-kDa immunoprecipitated band. This suggested that in platelets, TRPC1 was not phosphorylated by PKC, cAMP-PK, or cGMP-PK, but associated with a number of substrates of cAMP-PK and cGMP-PK. The identity of these substrates was not further examined in this study. In separate experiments, the incubation of [32P]Pi-labeled platelets with 100 μM sodium nitroprusside (which acts as a NO donor to elevate cGMP in platelets, resulting in activation of cGMP-PK) also resulted in the extraction by Ank of phosphoproteins of molecular size 250, 120, 85, 70, and 55 kDa (results not shown). Further, the inclusion of a partially permeable specific inhibitor of cGMP-PK (Rp-8-pCPT-cGMPS) inhibited the phosphorylation of these bands. This suggested that the phosphorylation of these substrates was physiologically relevant and, because of their association, they may be involved in the regulation or modulation of TRPC1 function. Figure 6C shows that the TRPC6 antibody immunoprecipitated weakly phosphorylated bands at 250, 120, 110, and 85 kDa from resting platelets. In the presence of BIMPS or 8PCPT, these proteins underwent further phosphorylation, while if platelets were stimulated with thrombin, they were totally dephosphorylated. The 110-kDa band was immunologically identified as TRPC6 by Western blotting, suggesting that unlike TRPC1, it is a substrate for cAMP-PK and cGMP-PK.
Serine or threonine phosphorylation of TRPC1 and TRPC6.
[32P]Pi-labeled platelets were used in a resting state (R) or incubated with 2 U/mL thrombin (T), 500 μM BIMPS (B), or 500 μM 8PCPT (8P). (A) Autoradiography (of dried-down gel) using 50 μL platelet lysates per lane. (B) Autoradiography of Ank immunoprecipitation blot. (C) Autoradiography of anti-TRPC6 immunoprecipitation blot. In (B) and (C), lower panels represent immune detection of blots with Ank and anti-TRPC6, respectively. Molecular size markers are noted in kilodaltons (kDa), TRPC and coimmunoprecipitated phosphoproteins are indicated with arrows.
Serine or threonine phosphorylation of TRPC1 and TRPC6.
[32P]Pi-labeled platelets were used in a resting state (R) or incubated with 2 U/mL thrombin (T), 500 μM BIMPS (B), or 500 μM 8PCPT (8P). (A) Autoradiography (of dried-down gel) using 50 μL platelet lysates per lane. (B) Autoradiography of Ank immunoprecipitation blot. (C) Autoradiography of anti-TRPC6 immunoprecipitation blot. In (B) and (C), lower panels represent immune detection of blots with Ank and anti-TRPC6, respectively. Molecular size markers are noted in kilodaltons (kDa), TRPC and coimmunoprecipitated phosphoproteins are indicated with arrows.
Studies were then carried out to determine if overexpressed hTRPC1 or mTRPC6 could be phosphorylated by exogenous addition of the CAT of cAMP-PK. Microsomal membranes from QBI-293A cells overexpressing hTRPC1 or mTRPC6 were incubated with γ[32P]ATP ± CAT. After 10 minutes' incubation, reactions were stopped with lysis buffer, hTRPC1, or mTRPC6 extracted as before and exposed to Western blotting and autoradiography. We have previously shown that under these conditions, CAT stimulates the phosphorylation of many platelet membrane cAMP-PK substrates.35 32 Figure7 shows that addition of CAT to membranes with overexpressed mTRPC6 resulted in increased [32P] incorporation into the doublet protein band, but there was little incorporation into hTRPC1. Inclusion of a peptide inhibitor of CAT (100 μM 14-22 amide) further inhibited phosphorylation of mTRPC6 under control conditions, suggesting that a low level of cAMP-PK existed in the QBI-293A cells. These results further support our platelet findings, suggesting that TRPC6 is phosphorylated directly, but TRPC1 is not a good substrate of cAMP-PK.
Phosphorylation of overexpressed hTRPC1 and mTRPC6 using CAT of cAMP-PK.
500 μg membrane proteins from QBI-293A cells overexpressing either hTRPC1 or mTRPC6 were incubated with γ[32P] ATP in the absence of CAT (CON), presence of 350 U CAT (+PKA CAT), or presence of 1 μM cAMP-PK inhibitor peptide (+PKA INHIB). (A) Autoradiography of Ank immunoprecipitations of overexpressed hTRPC1 and subsequent probing of the Western blot with the Ank antibody (5 μg/mL). (B) Autoradiography of anti-TRPC6 immunoprecipitations of overexpressed mTRPC6 and probing with anti-TRPC6 antibody (1/400). Note both partial and fully glycosylated forms of mTRPC6 undergo phosphorylation. Molecular size markers are noted in kilodaltons (kDa) and the TRPC protein identifications indicated with arrows.
Phosphorylation of overexpressed hTRPC1 and mTRPC6 using CAT of cAMP-PK.
500 μg membrane proteins from QBI-293A cells overexpressing either hTRPC1 or mTRPC6 were incubated with γ[32P] ATP in the absence of CAT (CON), presence of 350 U CAT (+PKA CAT), or presence of 1 μM cAMP-PK inhibitor peptide (+PKA INHIB). (A) Autoradiography of Ank immunoprecipitations of overexpressed hTRPC1 and subsequent probing of the Western blot with the Ank antibody (5 μg/mL). (B) Autoradiography of anti-TRPC6 immunoprecipitations of overexpressed mTRPC6 and probing with anti-TRPC6 antibody (1/400). Note both partial and fully glycosylated forms of mTRPC6 undergo phosphorylation. Molecular size markers are noted in kilodaltons (kDa) and the TRPC protein identifications indicated with arrows.
Studies were then designed to test if phosphorylation of TRPC6 by cAMP-PK resulted in any effect on cation entry into Fura-2–loaded platelets. Many previous studies have demonstrated an action of cAMP-PK on both a reduction of agonist-stimulated IP3formation, an inhibition of Ca++ release from intracellular stores, and the promotion of resequestration and extrusion (for a review, see Authi1). As this study focused on entry channels, experiments were carried out adding 250 μM BIMPS to platelet suspensions after stores had been depleted but before the addition of extracellular Ca++. Figure8A shows that activation of cAMP-PK 2 minutes prior to addition of extracellular Ca++ resulted in a 54% reduction of thrombin-stimulated Ca++ entry (P < .001), even though depletion of stores was similar to vehicle-treated control platelets. This clearly suggested that cAMP-PK inhibited calcium entry at a step downstream of store depletion and possibly at the level of the entry channel. Similar experiments, adding extracellular Ba2+ after store depletion, showed that BIMPS had little or no effect on thrombin-stimulated Ba2+ entry (Figure 8B). This result was surprising, as TRPC6 was phosphorylated by cAMP-PK in both platelets and overexpression systems, and suggested that phosphorylation by cAMP-PK did not affect channel function. However, addition of BIMPS 2 minutes prior to thrombin did inhibit both Ca++ release from stores (via an inhibition of PLC and stimulation of resequestration) and entry of Ba2+ (Figure 8C). Further, addition of the PLC inhibitor U73122 (10 μM) 2 minutes prior to thrombin drastically inhibited Ca++ release from stores and entry of Ba2+(increase of ratio units 4 minutes after Ba2+ addition was 1.26 ± 0.02 [n = 3] and 0.69 ± 0.02 [n = 3] in the absence and presence of U73122 [P < .001]), suggesting that inhibition of thrombin-stimulated PLC reduced channel activation. BIMPS also was used to determine if cAMP-PK affected OAG-induced cation entry. No effect was observed on Ba2+ entry (Figure 8D) or Ca++ entry (results not shown), confirming that phosphorylation of TRPC6 by cAMP-PK did not affect cation permeability through the channel.
Effect of cAMP-PK activation (using BIMPS) on cation influx in platelets mediated by thrombin or OAG.
Fura-2–loaded platelets were incubated at 37°C with additions (marked with arrows or overhead bars), and the elevations of [Ca++]i or [Ba2+]iwere monitored using the 340:380 nm ratio. (A) In the absence of extracellular Ca++ (addition of 100 μM EGTA), release of Ca++ from stores was activated by the addition of 0.5 U/mL thrombin and subsequent addition of 250 μM BIMPS (red line) or dimethyl sulfoxide (DMSO) vehicle (black line). Influx was recorded with the addition of 1 mM Ca++. Peak values upon addition of extracellular Ca++ were 0.46 ± 0.015 (n = 3) and 1.06 ± 0.009 (n = 4), BIMPS and vehicle, respectively;P < .001. (B) As in (A) above, with the addition of 1 mM Ba2+, 5-minute plateau values were 1.065 ± 0.171 (n = 4) and 0.874 ± 0.167 (n = 5), BIMPS and vehicle, respectively; P = .46. (C) As in (B), except BIMPS or vehicle was added 2 minutes prior to thrombin, 4-minute plateau values of [Ba2+]i were 0.62 ± 0.01 (n = 3) and 1.13 ± 0.02 (n = 3) BIMPS and vehicle, respectively;P < .001. (D) In the presence of 1 mM extracellular Ba2+, cells were incubated with 250 μM BIMPS (red line) or DMSO vehicle (black line) before addition of 60 μM OAG. Ratio values at 5 minutes were 0.61 ± 0.057 (n = 3) and 0.54 ± 0.059 (n = 3), BIMPS and vehicle, respectively;P = .44.
Effect of cAMP-PK activation (using BIMPS) on cation influx in platelets mediated by thrombin or OAG.
Fura-2–loaded platelets were incubated at 37°C with additions (marked with arrows or overhead bars), and the elevations of [Ca++]i or [Ba2+]iwere monitored using the 340:380 nm ratio. (A) In the absence of extracellular Ca++ (addition of 100 μM EGTA), release of Ca++ from stores was activated by the addition of 0.5 U/mL thrombin and subsequent addition of 250 μM BIMPS (red line) or dimethyl sulfoxide (DMSO) vehicle (black line). Influx was recorded with the addition of 1 mM Ca++. Peak values upon addition of extracellular Ca++ were 0.46 ± 0.015 (n = 3) and 1.06 ± 0.009 (n = 4), BIMPS and vehicle, respectively;P < .001. (B) As in (A) above, with the addition of 1 mM Ba2+, 5-minute plateau values were 1.065 ± 0.171 (n = 4) and 0.874 ± 0.167 (n = 5), BIMPS and vehicle, respectively; P = .46. (C) As in (B), except BIMPS or vehicle was added 2 minutes prior to thrombin, 4-minute plateau values of [Ba2+]i were 0.62 ± 0.01 (n = 3) and 1.13 ± 0.02 (n = 3) BIMPS and vehicle, respectively;P < .001. (D) In the presence of 1 mM extracellular Ba2+, cells were incubated with 250 μM BIMPS (red line) or DMSO vehicle (black line) before addition of 60 μM OAG. Ratio values at 5 minutes were 0.61 ± 0.057 (n = 3) and 0.54 ± 0.059 (n = 3), BIMPS and vehicle, respectively;P = .44.
Discussion
In this study we have shown for the first time expression of TRPC6 in the human platelet PM, its involvement in cation entry by the surface receptor–activating agent thrombin, in addition to direct activation by OAG, and we have also shown that it is a substrate for cAMP-PK.
Compared with Ca++ release from intracellular stores, Ca++ entry is still little understood. SOCE has been suggested as the major mechanism responsible in nonexcitable cells, but neither the identity of the entry channels nor the mechanism for their gating are established. Members of the TRP family of ion channels represent the first potential candidates for SOCE channels. However, information accumulated from overexpression systems suggest that most members of the TRP family form receptor-activated channels acting independently from store regulation.16 17 Most cells are postulated to contain more than one member of the TRP family contributing either part or all of the entry channels in thePM. Analysis at the protein level, however, is incomplete and mostly to be determined. Here we show that a low level of TRPC1 is present in platelet IM and a higher expression of TRPC6 in thePM. TRPC4 and 5 were undetectable using an antibody that detected both overexpressed TRPC4 and TRPC5, and TRPC3 was also undetectable using a similarly high-affinity antibody. Currently we do not have an antibody that recognizes TRPC7.
Recently, Rosado and Sage23,24 reported the presence of TRPC1 protein in platelets and suggested that it mediated Ca++ entry via a store depletion–dependent coupling to the type II IP3R. They presented their findings as evidence for a secretionlike conformational coupling mechanism with the type II IP3R at the stores linking with TRPC1 (presumed to be at the PM) only when stores were depleted. Further, they reported that Jasplakinolide, which induced cortical actin assembly and inhibited Ca++ entry, also disrupted the coupling of TRPC1 with the type II IP3R.24 TRPC1 has been suggested to play a role in SOCE in a number of other cell types, including salivary gland cells,45 A549 and pulmonary artery cells,46 and vascular smooth muscle cells,47 although the mechanism of gating in these cells has not been elucidated. Our studies present new evidence that supports an indirect role for TRPC1 in cation entry in platelets. We propose a function for TRPC1 in calcium signaling at the level of the stores that we and others have shown to contain the type I and type II IP3R.32,48 The possibility that it may become inserted into the PM upon cell activation, as has been described in Xenopus oocytes,9 cannot be ruled out. Further, a recent study has described that in a HEK-293 cell line expressing hTRPC3, Jasplakinolide (and calyculin-A) inhibited Ca++ entry by the internalization of TRPC3 constitutively associated with the type III IP3R and other Ca++ regulatory proteins without disruption of the multimolecular complex.49 Thus, membrane fusion to and internalization from the PM could possibly provide a mechanism for regulation of Ca++ entry. However, our studies suggest that SOCE in platelets is selective for Ca++ over Ba2+ entry and thus should be mediated by an ion channel bearing these properties. TRPC1, however, is known to be a nonselective channel with similar permeabilities37,50 for Ca++, Na+, Cs+, and Ba2+ and thus on its own is unlikely to account for the Ca++ selectivity of SOCE function at thePM. The reported properties of CaT1 and CaT2 (TRPV6 and TRPV5, respectively) and the suggestion that CaT1 forms a part or all of ICRAC51 52 should necessitate a study of the expression of these proteins in platelets.
TRPC6 is well expressed in platelets and located in the PM. In heterologous systems, TRPC6 has been shown to be a nonselective cation channel (though favoring Ca++ over Na+by 5 to 1) that is activated by receptors linked to phospholipase C signaling18 but independent of store depletion. TRPC6 is closely related to TRPC3 and TRPC7, with which it shares approximately 75% sequence identity and, along with these members, it can be directly activated by DAG analogs. Indeed, we have shown that in line with the expression of TRPC6, OAG activates both Ca++ and Ba2+ entry into platelets. The ability of TRPC6, 3, and 7 to allow Ba2+ passage has proved useful in examining the gating properties of these channels, and our finding that thrombin induces Ba2+ entry is highly indicative of the activation of TRPC6 by surface receptor–activating stimuli. This therefore represents (as far as we are aware) the first demonstration of the activation of an identified endogenous cation channel in thePM of platelets. TRPC6 has been shown to be important for the α1-adrenoreceptor–activated Ca++ permeable cation channel in rabbit portal vein smooth muscle cells,53 thus it may represent an important cation channel in a number of vascular cells. The mechanism of activation of the TRPC6, 3, and 7 subgroup by surface receptor activation downstream of phospholipase C is not known. The most obvious route is via the formation of DAG, which directly activates this subgroup through a membrane-delimiting manner independent of PKC.18 This suggests that DAG would have a specific binding site on either the channel itself or on a regulatory protein closely associated with it. Recently, Zhang and Saffen54 described 3 forms of the rTRPC6 cDNA (A, B, and C), where rTRPC6A coded for the full-length protein, rTRPC6B coded for a variant that lacked the N-terminal amino acids 3-56, and rTRPC6C lacked amino acids 3-56 and 735-802 at the C terminus of the transcribed protein. OAG was found to be effective in activating rTRPC6A only while surface receptor activation gated rTRPC6A and B, suggesting that amino acids 3-56 conferred OAG sensitivity and that the C-terminal region was important in coupling reactions necessary for activation by surface receptors.54 However, the sequence present in amino acids 3-56 of TRPC6 is absent in both TRPC3 and TRPC7, which also are sensitive to OAG, suggesting that this is unlikely to be the only region involved in OAG recognition. From the apparent molecular size, we predict platelets to express the full-length TRPC6 that shows sensitivity to DAG. Currently, the GenBank database cites 2 splice variants of hTRPC6 (hTRPC6D1, accession number AJ271067; and hTRPC6D2, accession number AJ271068) in addition to the full-length version, with hTRPC6D1 lacking amino acids 316-431 and hTRPC6D2 lacking amino acids 371-431. As both splice variants lack the predicted first transmembrane domain, it is likely that they are nonfunctional. There exists serious controversy regarding the role (if any) of the IP3Rs in the activation of TRPC3, and because of the high sequence identity, possibly of TRPC6 and 7, although there is currently no study describing positive regulation of the latter 2 channels by IP3. Gill's group have suggested that gating of TRPC3 in heterologous systems does not require IP3Rs, as TRPC3 overexpressed in DT40 cells lacking all IP3Rs can be activated to allow Ba2+ entry independent of store depletion, either by agonists initiating PLC signaling or by OAG directly.41 However, Putney's group reports in the same system that TRPC3 can act as a SOCE channel (thapsigargin-initiated Ba2+ entry in TRPC3 expressing DT40 wild-type cells) with a partial requirement for the IP3R and that surface receptor–mediated activation of TRPC3 had a total requirement for the IP3R.43 Whilst only further experimentation will resolve this contentious issue in heterologous systems, our studies suggest that in an endogenous system that expresses TRPC6 (ie, the platelet), thapsigargin does not initiate sufficient Ba2+ entry. Thus, thrombin-induced Ba2+ entry is independent of store depletion and most probably by the PLC-mediated formation of DAG, as U73122 was effective at inhibiting Ba2+ entry. Therefore, thrombin activates a minimum of 2 channels, a SOCE channel allowing predominantly Ca++ entry and a non-SOCE channel (TRPC6) permeable to both Ca++ and Ba2+. The slower kinetics of Ba2+ entry may suggest a role for TRPC6 in the middle and later stages of platelet activation. Recently, Rosado and Sage55 reported that high concentrations of PMA (1 μM) stimulated Ca++ and Sr2+ entry via a noncapacitative but PKC-dependent pathway. This study is apparently in contrast to many previous observations that PKC provides a negative-feedback role on agonist-mediated signaling that includes Ca++ elevation.56 In our studies, preincubation of platelets with the PKC inhibitor Bis I did not affect OAG-induced cation entry and, as expected, enhanced thrombin-stimulated Ba2+ entry (results not shown).
The finding that TRPC6 and (to a lesser extent) TRPC1 were present in human platelets prompted us to examine the phosphorylation status of these proteins during activation and inhibition of platelet function. Both TRPC6 and TRPC1 contain consensus sequences for a number of protein kinases including tyrosine kinases, PKC, casein kinase II, cAMP-PKs, and cGMP-PKs. Although controversial and little understood, tyrosine kinases have been suggested to play a role in SOCE-mediated Ca++ entry,7,57 with a recent study that suggests an action by tyrosine kinases on the cytoskeleton and further, that tyrosine kinase inhibitors prevented actin polymerization.58 Our experiments revealed little evidence of tyrosine phosphorylation of either TRPC6 or TRPC1 under conditions where thrombin and collagen had induced the widespread tyrosine phosphorylation of platelet proteins, suggesting that the channels themselves were not direct targets of tyrosine kinases. In [32P]Pi-labeled platelets, TRPC6 was found to be a substrate of cAMP-PK and cGMP-PK and associated with 250-, 120-, and 85-kDa phosphoproteins. TRPC1 was not a good substrate for this kinase but was similarly associated with a number of cAMP-PK and cGMP-PK substrates. These findings are significant, as the vasodilators NO and PGI2 inhibit platelet function predominantly via cGMP-PK and cAMP-PK, respectively. TRPC6 contains 2 sites for cyclic nucleotide kinase action at RRQT70 and KKLS,322 and currently it is not known whether both sites are phosphorylated or what their relationship is to the regulation of TRPC6 function. Our studies using Fura-2–labeled cells show that under conditions where store depletion had occurred, activation of cAMP-PK resulted in inhibition of Ca++ entry induced by thrombin but not of Ba2+entry. As we have shown that Ba2+ entry occurred by a SOCE-independent mechanism, most probably mediated by DAG, this suggested that cAMP-PK acted on the SOCE pathway, at a site downstream of store depletion, either on the gating mechanism or on the SOCE channel itself. Targets for cAMP-PK previously identified include PLC activation,59 the IP3R itself,32the Ca++ pumps,60 and many more (for a review, see Koesling et al61). Inhibition of Ba2+entry was seen if BIMPS was added 2 minutes prior to thrombin addition, reflecting phospholipase C inhibition. Rosado et al62 have recently reported an action of cAMP on tyrosine phosphatases that may be involved with the SOCE mechanism and suggested that it was independent of cAMP-PK. However, there is currently controversy regarding the action and efficacy of the KT series of cyclic nucleotide kinase inhibitors in intact platelets,63 and therefore further studies are required to clarify these suggestions. Interestingly, direct activation of TRPC6 with OAG also was not affected by cAMP-PK activation, further confirming our suggestion that thrombin acts on TRPC6 via the formation of DAG. Our results raise the question, “Why is TRPC6 a substrate for cAMP-PK if after phosphorylation there is no effect on channel activity?” Currently, the answer to this question is not known, but cAMP-PK may affect other functions of TRPC6 not tested in this study, such as its association to other proteins and particularly to components of the cytoskeleton. Alternatively, the possibility that Ba2+ entry resulted from the presence of another Ca++/Ba2+ channel cannot be ruled out. In this study, we have not addressed the identities of the phosphoproteins that associate with either TRPC6 or TRPC1. Clearly, suitable candidates for the 250-kDa protein are the IP3Rs and the actin-binding protein filamin. The possibility that the 120-kDa phosphoprotein could be the newly described IP3R-associated G-kinase substrate (IRAG)64 and known to be involved with Ca++regulation is equally fascinating. Our future studies will investigate the relationship of components of this multimolecular complex with the cAMP-PKs and cGMP-PKs.
In conclusion, our studies report the presence of high levels of TRPC6 and low levels of TRPC1 in platelet membranes. The presence of TRPC6 in the plasma membrane, its activation by thrombin, and its demonstration as a substrate of cAMP-PK represent the first identification of a non-SOCE–regulated cation channel in human platelets. A similar search for other members of the TRP family may reveal the molecular identity of the SOCE channel and a better understanding of how information from the stores leads to their gating. Our studies also suggest a role for TRPC1 at the level of the intracellular Ca++ stores. During the review of this manuscript, Mori et al65 reported that TRP1 knock-out in the DT40 cell line resulted in a reduction of agonist-mediated release of Ca++ from the stores and a reduction of IP3-mediated Ca++ release from membranes. This further supports a role for TRPC1 at the level of the intracellular stores.
We thank Professor G. Barritt (Adelaide, Australia) for the generous gift of anti–XTRP-1 antibody, and Professor C. Montell (Baltimore, MD) for the generous gift of anti-αTRPC3.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-03-0723.
Supported by grants from the British Heart Foundation and from the National Institutes of Health (GM54235, M.X.Z.).
Correspondence:Kalwant S. Authi, Centre for Cardiovascular Biology and Medicine, King's College London, New Hunt's House, Guy's Campus, London SE1 1UL; e-mail: kalwant.authi@kcl.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 3. Ca++ influx in response to oleoyl acetyl glycerol (OAG) and thrombin. / Fura-2–loaded platelets were incubated at 37°C in the presence of 1 mM extracellular Ca++ (addition of Ca++marked on traces). Agonists were then added, and the elevations of [Ca++]i were monitored using the 340:380 nm ratio. A calibrated [Ca++]i (nM) scale is shown on the right. Figures given below represent increase in ratio units above basal level after 5 minutes. (A) Cells stimulated with 30, 60, and 90 μM OAG showed linear elevation of [Ca++]i of 0.78 ± 0.156, 0.98 ± 0.162, and 1.08 ± 0.175 ratio units, respectively (n = 11). (B) Cells stimulated with 1 U/mL thrombin showed rapid [Ca++]i increase with a peak value of 2.4 ± 0.787 ratio units (n = 6), followed by a slow decrease to a plateau value after 5 minutes of 1.35 ± 0.57 ratio units (n = 6).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/8/10.1182_blood-2002-03-0723/4/m_h82023238003.jpeg?Expires=1769640937&Signature=TNXXseXgv1cP2fp4QAi~SkxhxF2D-LbQ~P2wVr0KLDDOdKLzGmPI6hjeMdiDPo4eBPYIJg3R0y1odHSGzt43fruo1qsYhC4QFpHRchmoeEJTgZ0EILBmIIpjxOjjGKDUns3JgLwzEETkoTFny2B03XMHgs89z7EVCIkiVhxOxHOzKDSLJSt2X-i~Ow-SVi1g~nnpcb2FUJAEnqclJ1iDxL8wAzoYfUNOlDRcwxWVf2TSk4Q-yQKRDhuGplcaNl23paJxdkWf-4-wjZ5hz4Uw366xltg3Vuv-WWAJ1zpM4TLeUj8srZE4yYdzQRGKIef8o7K9wMVzOUbeongMb0d0Hg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Serine or threonine phosphorylation of TRPC1 and TRPC6. / [32P]Pi-labeled platelets were used in a resting state (R) or incubated with 2 U/mL thrombin (T), 500 μM BIMPS (B), or 500 μM 8PCPT (8P). (A) Autoradiography (of dried-down gel) using 50 μL platelet lysates per lane. (B) Autoradiography of Ank immunoprecipitation blot. (C) Autoradiography of anti-TRPC6 immunoprecipitation blot. In (B) and (C), lower panels represent immune detection of blots with Ank and anti-TRPC6, respectively. Molecular size markers are noted in kilodaltons (kDa), TRPC and coimmunoprecipitated phosphoproteins are indicated with arrows.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/8/10.1182_blood-2002-03-0723/4/m_h82023238006.jpeg?Expires=1769640937&Signature=tiwldYBwn7bnzQNEpnWHqYzmf~CNugmygAIN44AOwV7RHUbbu8aaE~qUt~CV1Ipnc~gpxgP1r4P9x2YP4tIjiVQDcNGige87t1AV~Pu-MLY6zlddnnU2C~umIubLNmqfXPOzY1Tfdqz~HaznTHlROuEL3gNSsvnDHFQdUZIOeCuEYZ-uBAJod3QyGz8ObH8036i8iTjjn2ypFc7pnH89e7vDBgLmBlEry~ggQFLLHU3TVHxGmUq-Rvnt-fISN5NDE8Ws46HP-GaROGj79bN2HxfFTmjJqvM6V969qmGekG02v13O7L0GioQckxi7FdlNi~T2midOIVovRIdPBJn2Zw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Phosphorylation of overexpressed hTRPC1 and mTRPC6 using CAT of cAMP-PK. / 500 μg membrane proteins from QBI-293A cells overexpressing either hTRPC1 or mTRPC6 were incubated with γ[32P] ATP in the absence of CAT (CON), presence of 350 U CAT (+PKA CAT), or presence of 1 μM cAMP-PK inhibitor peptide (+PKA INHIB). (A) Autoradiography of Ank immunoprecipitations of overexpressed hTRPC1 and subsequent probing of the Western blot with the Ank antibody (5 μg/mL). (B) Autoradiography of anti-TRPC6 immunoprecipitations of overexpressed mTRPC6 and probing with anti-TRPC6 antibody (1/400). Note both partial and fully glycosylated forms of mTRPC6 undergo phosphorylation. Molecular size markers are noted in kilodaltons (kDa) and the TRPC protein identifications indicated with arrows.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/8/10.1182_blood-2002-03-0723/4/m_h82023238007.jpeg?Expires=1769640937&Signature=nTmJ9WzDsgtJu9EKJiVfVjk2CQSKyDsw1-23z8UHOsTm4KiOsL5xs8RggfqGyeHPyHXQ5I2Gf60-NwPD66lMJJnwRtOqPhtyvZ3G3XSEVJAJO89Zr7bltajipoLWl7lkQmfeRjp2IWh0sd4w-~oDP55Akhf5aksUWTY2d0kWH1bMTp2jM-c6z8~1KEoQV~s5VbT1S-tM7S0z8UDDcH3OetdXDSyFT6l~QCpGmsfHW~LIV2t~8lIETMTzC42OjYQooAeY5ZIqQGBRNK-Rd1svUfMxxzkY70GfZcK4UcBLeoKQy0hNm64mNYT7aaRY3PCig8EQdG4k3GUX6492xXecRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Effect of cAMP-PK activation (using BIMPS) on cation influx in platelets mediated by thrombin or OAG. / Fura-2–loaded platelets were incubated at 37°C with additions (marked with arrows or overhead bars), and the elevations of [Ca++]i or [Ba2+]iwere monitored using the 340:380 nm ratio. (A) In the absence of extracellular Ca++ (addition of 100 μM EGTA), release of Ca++ from stores was activated by the addition of 0.5 U/mL thrombin and subsequent addition of 250 μM BIMPS (red line) or dimethyl sulfoxide (DMSO) vehicle (black line). Influx was recorded with the addition of 1 mM Ca++. Peak values upon addition of extracellular Ca++ were 0.46 ± 0.015 (n = 3) and 1.06 ± 0.009 (n = 4), BIMPS and vehicle, respectively;P < .001. (B) As in (A) above, with the addition of 1 mM Ba2+, 5-minute plateau values were 1.065 ± 0.171 (n = 4) and 0.874 ± 0.167 (n = 5), BIMPS and vehicle, respectively; P = .46. (C) As in (B), except BIMPS or vehicle was added 2 minutes prior to thrombin, 4-minute plateau values of [Ba2+]i were 0.62 ± 0.01 (n = 3) and 1.13 ± 0.02 (n = 3) BIMPS and vehicle, respectively;P < .001. (D) In the presence of 1 mM extracellular Ba2+, cells were incubated with 250 μM BIMPS (red line) or DMSO vehicle (black line) before addition of 60 μM OAG. Ratio values at 5 minutes were 0.61 ± 0.057 (n = 3) and 0.54 ± 0.059 (n = 3), BIMPS and vehicle, respectively;P = .44.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/8/10.1182_blood-2002-03-0723/4/m_h82023238008.jpeg?Expires=1769640937&Signature=IOYb26yF95~JkoZ7SOFf3l~NQr7iS79bDMiJ0iRqkFPaYMUuOG-uh~N~vvYX~yf2S4H5qQ3-k6TFjQUREQ01QCycOZcVl3Ev97vwgLZKA7By~dMeTMFA1aVF2PfetM77QPIh5y88ftGtnAol3IJ7QcB4H8Nav1E9f3CHk8~xtaAGke2dQuw90wffw0g2xL9EhBxWsqjfzqPXVg1PCoC8773MI~bnL0v0KvXfqvtI46v34tETgAXGsHMxXyLroZIHadNyiC4aYIBGc1QLDcoZ63G~KoRcMchykRUS9Z86msBbVtdybCr4lO85MoBDvcooSgPLWSYKJr06uvmesBiCZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal