The adapter protein SLP-76 is a critical mediator of signal transduction via the platelet collagen receptor glycoprotein VI (GPVI) and its coreceptor FcRγ. We tested the hypothesis that SLP-76 is required for collagen-induced procoagulant responses in murine platelets. Platelets from SLP-76 null (SLP-76−/−) or heterozygous (SLP-76+/−) mice were activated with the GPVI agonist convulxin, and surface expression of P-selectin (a marker of granule release) and annexin V binding (a marker of procoagulant phospholipid) were determined by flow cytometry. Convulxin induced surface expression of P-selectin in SLP-76+/− platelets, but not SLP-76−/− platelets (P < .01), and failed to stimulate annexin V binding to either SLP-76+/−or SLP-76−/− platelets. Platelet procoagulant activity was measured in a prothrombinase assay. Convulxin did not stimulate procoagulant activity in either SLP-76+/− or SLP-76−/− platelets, but fibrillar collagen produced a 1.9-fold increase in procoagulant activity in both SLP-76+/− and SLP-76−/− platelets (P < .001 versus unstimulated platelets). Similar results were obtained with platelets from FcRγ null mice, for which collagen, but not convulxin, induced procoagulant activity (P < .01). Costimulation with thrombin and collagen produced a further (2.3-fold) increase in procoagulant activity in SLP-76+/− platelets (P < .05), but not in SLP-76−/− platelets. SLP-76−/− platelets also exhibited less annexin V binding than SLP-76+/−platelets after costimulation with thrombin and convulxin (P < .05). These findings demonstrate that an intact GPVI/FcRγ/SLP-76 signal transduction pathway is not essential for platelet procoagulant activity induced by collagen but is necessary for maximal procoagulant response to costimulation with thrombin plus collagen. Thus, both GPVI-dependent and GPVI-independent pathways contribute to collagen-induced platelet procoagulant activity.
Introduction
Collagen is a major physiologic agonist for platelet activation, mediating shape change, granule release, aggregation, and expression of procoagulant activity. Several putative collagen receptors have been identified on platelets,1 but the major signal transducing collagen receptor is a member of the immunoglobulin superfamily, glycoprotein VI (GPVI).2,3Signal transduction through GPVI is dependent on coexpression of the Fc receptor γ chain (FcRγ),4,5 which contains immunoreceptor tyrosine-based activation motifs (ITAMs) that become phosphorylated when platelets are stimulated with fibrillar collagen.6
The hematopoietic cell adaptor protein SLP-76 is a critical component of the GPVI/FcRγ signal transduction pathway in platelets.7 SLP-76 becomes tyrosine phosphorylated in response to stimulation with collagen or GPVI agonists in both human and murine platelets, and platelets from SLP-76 null mice fail to aggregate or undergo granule release in response to collagen.8-10 SLP-76 is also necessary for normal signal transduction from integrin αIIbβ3 to the platelet cytoskeleton.11 SLP-76 null mice exhibit a bleeding diathesis and decreased perinatal survival.8,12,13 The bleeding diathesis can be reversed by reconstitution of SLP-76 into bone marrow cells of SLP-76–deficient mice, which confirms that expression of SLP-76 in hematopoietic cells is necessary for normal hemostasis.14
Although collagen is known to be a potent agonist for the platelet procoagulant response, the signal transduction pathways leading to expression of platelet procoagulant activity are poorly characterized.15 The procoagulant activity of activated platelets is mediated by expression of anionic membrane phospholipids that support the assembly and activity of procoagulant enzyme complexes,16 and can be detected by functional assays17 or annexin V binding.18 It is not known whether SLP-76 is necessary for collagen-induced expression of platelet procoagulant activity.19
In this study, we tested the hypothesis that SLP-76 is required for collagen-induced expression of platelet procoagulant activity in murine platelets. Platelets were isolated from SLP-76–deficient mice, and the procoagulant response to collagen was measured by annexin V binding and a prothrombinase assay. Our results demonstrate that collagen induces procoagulant responses in murine platelets despite the absence of SLP-76 and that the GPVI agonist convulxin20 fails to produce detectable platelet procoagulant activity in murine platelets. SLP-76 is required, however, for maximal procoagulant activity when platelets are stimulated simultaneously with thrombin and collagen. These findings demonstrate that the GPVI/FcRγ/SLP-76 signal transduction pathway is not required for platelet procoagulant activity induced by collagen as a single agonist but is necessary for maximal procoagulant responses when platelets are stimulated by multiple agonists.
Materials and methods
Reagents and mice
Bovine prothrombin, factor Va, factor Xa, and α-thrombin were obtained from Haematological Technologies (Essex Junction, VT). Chromozym TH was obtained from Roche Diagnostics (Indianapolis, IN). Ionomycin was obtained from Sigma Chemicals (St Louis, MO). Collagen (type l, equine fibrillar) was obtained from Chrono-log (Havertown, PA). Convulxin, purified from Crotalus durissus terrificusvenom,20 was purchased from Pentapharm (Basel, Switzerland). Fluorescein isothiocyanate (FITC)–conjugated rat antimouse CD62P (P-selectin), FITC-conjugated annexin V, FITC-conjugated rat IgG1, and phycoerythrin (PE)–conjugated rat antimouse CD49b (α2 integrin) were obtained from BD Pharmingen (San Diego, CA). SLP-76–deficient mice12 were bred to produce SLP-76 null (SLP-76−/−) and heterozygous (SLP-76+/−) littermates. Genotyping for the targeted SLP-76 allele was performed by polymerase chain reaction.12 SLP-76+/− and SLP-76−/− mice were studied at 3 to 12 months of age and were matched for age and sex. FcRγ null (FcRγ−/−) mice21 were purchased from the Jackson Laboratory (Bar Harbor, ME). The protocol was approved by the University of Iowa Animal Care and Use Committee.
Platelet isolation and aggregation
Washed platelets were isolated from acid-citrate-dextrose anticoagulated whole blood as described,14 and suspended in modified Tyrode buffer (134 mM NaCl, 2.9 mM KCl, 0.34 mM Na2HPO4, 12 mM NaHCO3, 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 1.0 mM MgCl2, 5.0 mM glucose, pH 7.35). Aggregation of prewarmed platelets (2 × 108/mL) was measured by optical aggregometry at 37°C using a Chrono-log 560VS aggregometer. Platelets were activated with convulxin (250 ng/mL) or bovine α-thrombin (0.5 U/mL).
Flow cytometry
Washed platelets (1 × 106/reaction) were suspended in modified Tyrode buffer containing 2.0 mM CaCl2, and either left unstimulated (“control”) or stimulated with convulxin (250 ng/mL), thrombin (0.5 U/mL), thrombin plus convulxin, or ionomycin (3 μM) for 15 minutes at 37°C without stirring. Platelets were then incubated for 10 minutes at 23°C with PE-conjugated rat antimouse CD49b and either FITC-conjugated rat antimouse CD62P, FITC-conjugated annexin V, or FITC-conjugated rat IgG1 (isotype control). Platelets were analyzed on a Becton Dickinson (San Diego, CA) FACScan flow cytometer as described previously.14 Platelets were gated by light scatter and expression of CD49b (α2 integrin).
Platelet procoagulant activity
Platelet procoagulant activity was measured in a prothrombinase assay that was modified from a method originally developed for use with human platelets.22 Washed murine platelets (6 × 106/reaction) were either left unstimulated (“control”) or stimulated with convulxin (250 ng/mL), type I fibrillar collagen (40 μg/mL), thrombin (0.5 U/mL), thrombin plus collagen, or ionomycin (3 μM) at 37°C, without agitation but with slow mixing (100 rpm), in modified Tyrode buffer containing 2.9 mM CaCl2 and 0.05% (wt/vol) fatty acid-free bovine serum albumin (BSA). After 10 minutes, factor Va (6 nM) and factor Xa (3 nM) were added, followed 1 minute later by prothrombin (4 μM). The concentration of CaCl2 in the final reaction mixture was 2.0 mM. The rate of thrombin generation was measured by subsampling the reaction mixture into 0.05 M Tris (tris(hydroxymethyl)aminomethane)–HCl, 120 mM NaCl, 2.0 mM EDTA (ethylenediaminetetraacetic acid), pH 7.5, and measuring thrombin activity using the chromogenic substrate Chromozym TH (0.32 mM) in a Spectramax 190 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA).
To verify that the rate of thrombin generation was dependent on the concentration of procoagulant phospholipid, control experiments were performed with murine platelets that had been subjected to 3 cycles of freezing and thawing to expose anionic membrane phospholipids. The initial rate of thrombin generation demonstrated a logarithmic relationship to the amount of added platelet phospholipid.
Confocal microscopy
Glass coverslips (12 mm) were coated with 1.0 mg/mL human fibrinogen (Sigma Chemicals) as described previously.14After 2 rinses with phosphate-buffered saline (PBS), the coverslips were blocked with Tyrode buffer containing 2% BSA for 30 minutes at room temperature. Washed platelets (1 × 105/well) in modified Tyrode buffer containing 2.0 mM CaCl2 were added to the fibrinogen-coated coverslips, and then stimulated with thrombin (0.5 U/mL), collagen (50 μg/mL), or thrombin plus collagen for 30 minutes at 37°C in the presence of FITC anti-CD49b and PE-conjugated annexin V. Platelets were then fixed with 1% paraformaldehyde, washed with PBS, and analyzed by confocal microscopy (Bio-Rad 1024 confocal microscope; Hercules, CA).
Statistical analysis
The unpaired, 2-tailed Student t test was used to compare values in SLP-76+/− and SLP-76−/−mice. The paired, 2-tailed Student t test was used to compare values obtained with different platelet agonists. A value ofP < .05 was used to define statistical significance. Values are reported as mean ± SE.
Results
Platelet aggregation and granule release
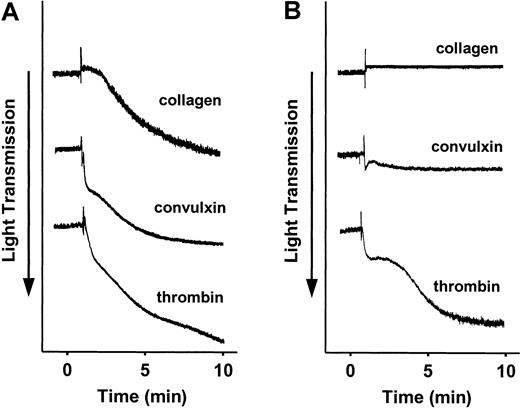
Platelets were isolated from SLP-76+/− or SLP-76−/− mice, and aggregation was measured in response to equine fibrillar collagen, the GPVI agonist convulxin, or bovine α-thrombin. SLP-76+/− platelets aggregated normally in response to all 3 agonists (Figure 1A). SLP-76−/− platelets aggregated normally in response to thrombin, but failed to aggregate in response to collagen or convulxin (Figure 1B).
Effect of deficiency of SLP-76 on platelet aggregation.
Aggregation of SLP-76+/− (A) or SLP-76−/−(B) platelets was measured in response to stimulation with 40 μg/mL collagen, 250 ng/mL convulxin, or 0.5 U/mL thrombin.
Effect of deficiency of SLP-76 on platelet aggregation.
Aggregation of SLP-76+/− (A) or SLP-76−/−(B) platelets was measured in response to stimulation with 40 μg/mL collagen, 250 ng/mL convulxin, or 0.5 U/mL thrombin.
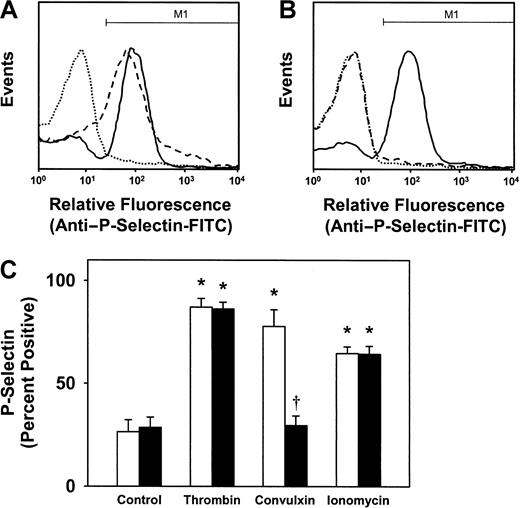
To monitor granule release, SLP-76+/− and SLP-76−/− platelets were stimulated with thrombin, convulxin, or the calcium ionophore ionomycin, and surface expression of the α-granule protein P-selectin was measured by flow cytometry. Thrombin induced an approximate 5-fold increase in surface expression of P-selectin in both in SLP-76+/− and SLP-76−/− platelets (Figure2A-B). After stimulation with thrombin, surface staining for P-selectin was detected on 87% ± 4% of SLP-76+/− platelets and 86% ± 3% of SLP-76−/− platelets, compared with less than 30% of unstimulated control platelets (P < .01; Figure 2C). Convulxin induced a 4.5-fold increase in surface expression of P-selectin in SLP-76+/− platelets (Figure 2A), with surface P-selectin detected on 78% ± 8% of SLP-76+/−platelets after stimulation with convulxin (Figure 2C). In contrast, convulxin did not induce surface expression of P-selectin in SLP-76−/− platelets (Figure 2B-C). Ionomycin induced a significant increase in P-selectin expression in both SLP-76+/− and SLP-76−/− platelets (Figure2C).
Effect of deficiency of SLP-76 on granule release.
Granule release in SLP-76+/− (A) or SLP-76−/− (B) platelets was monitored by flow cytometric measurement of surface expression of P-selectin in unstimulated platelets (dotted line) or after stimulation with 0.5 U/mL thrombin (solid line) or 250 ng/mL convulxin (dashed line). (C) The percentage of platelets staining positively for P-selectin, defined as region M1 as shown in panels A and B, was determined for SLP-76+/−(open bars; n = 7) or SLP-76−/− (filled bars; n = 7) platelets that were either left unstimulated (control) or stimulated with thrombin (0.5 U/mL), convulxin (250 ng/mL), or ionomycin (3 μM). *P < .05 versus control; †P < .05 versus SLP-76+/− platelets.
Effect of deficiency of SLP-76 on granule release.
Granule release in SLP-76+/− (A) or SLP-76−/− (B) platelets was monitored by flow cytometric measurement of surface expression of P-selectin in unstimulated platelets (dotted line) or after stimulation with 0.5 U/mL thrombin (solid line) or 250 ng/mL convulxin (dashed line). (C) The percentage of platelets staining positively for P-selectin, defined as region M1 as shown in panels A and B, was determined for SLP-76+/−(open bars; n = 7) or SLP-76−/− (filled bars; n = 7) platelets that were either left unstimulated (control) or stimulated with thrombin (0.5 U/mL), convulxin (250 ng/mL), or ionomycin (3 μM). *P < .05 versus control; †P < .05 versus SLP-76+/− platelets.
Annexin V binding
To measure surface exposure of anionic phospholipids, platelets from SLP-76+/− and SLP-76−/− mice were stimulated with thrombin, convulxin, or ionomycin, and annexin V binding was measured by flow cytometry. Stimulation with thrombin enhanced annexin V binding to both SLP-76+/− and SLP-76−/− platelets (Figure3A-B). After stimulation with thrombin, 32% ± 4% of SLP-76+/− platelets and 39% ± 5% of SLP-76−/− platelets stained positively for annexin V binding, compared with less than 25% of unstimulated control platelets (P < .05; Figure 3C). In contrast, convulxin failed to induce annexin V binding to either SLP-76+/− or SLP-76−/− platelets. Ionomycin induced a large increase in annexin V binding to both SLP-76+/− and SLP-76−/− platelets, with more than 85% of platelets staining positively for annexin V after stimulation with ionomycin (Figure 3C).
Effect of deficiency of SLP-76 on annexin V binding.
Annexin V binding to SLP-76+/− (A) or SLP-76−/− (B) platelets was measured by flow cytometry in unstimulated platelets (dotted line) or after stimulation with thrombin (solid line), or convulxin (dashed line). (C) The percentage of platelets staining positively for annexin V binding, defined as region M1 as shown in panels A and B, was determined for SLP-76+/− (open bars; n = 7) or SLP-76−/−(filled bars; n = 7) platelets that were either left unstimulated (control) or stimulated with thrombin (0.5 U/mL), convulxin (250 ng/mL), or ionomycin (3 μM). *P < .05 versus control.
Effect of deficiency of SLP-76 on annexin V binding.
Annexin V binding to SLP-76+/− (A) or SLP-76−/− (B) platelets was measured by flow cytometry in unstimulated platelets (dotted line) or after stimulation with thrombin (solid line), or convulxin (dashed line). (C) The percentage of platelets staining positively for annexin V binding, defined as region M1 as shown in panels A and B, was determined for SLP-76+/− (open bars; n = 7) or SLP-76−/−(filled bars; n = 7) platelets that were either left unstimulated (control) or stimulated with thrombin (0.5 U/mL), convulxin (250 ng/mL), or ionomycin (3 μM). *P < .05 versus control.
Platelet procoagulant activity
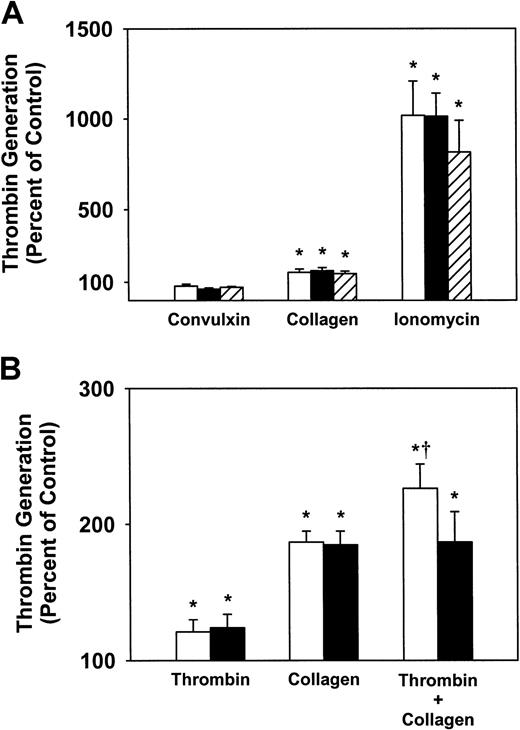
Platelet procoagulant activity was measured in a modified prothrombinase assay. After stimulation with various agonists, the ability of platelets to support the generation of thrombin from prothrombin was measured in the presence of exogenous factor Va and factor Xa. Convulxin failed to stimulate a detectable increase in prothrombinase activity with platelets from SLP-76+/− or SLP-76−/− mice, whereas collagen produced a significant increase in prothrombinase activity with platelets from either SLP-76+/− or SLP-76−/− mice (P < .05 versus unstimulated platelets; Figure4A). These results suggested that the GPVI/FcRγ/SLP-76 pathway is not absolutely necessary for collagen-induced prothrombinase activity. Further support for this finding was obtained with platelets from FcRγ−/− mice, in which prothrombinase activity was induced by collagen but not convulxin (Figure 4A). Ionomycin produced a large (> 10-fold) increase in prothrombinase activity with SLP-76+/−, SLP-76−/− , or FcRγ−/− platelets (P < .01).
Effect of deficiency of SLP-76 or FcRγ on platelet procoagulant activity.
(A) Prothrombinase activity was measured with SLP-76+/−(open bars; n = 5), SLP-76−/− (filled bars; n = 4), or FcRγ−/− (hatched bars; n = 4) platelets that were stimulated with collagen (40 μg/mL), convulxin (250 ng/mL), or ionomycin (3 μM). (B) Prothrombinase activity was measured with SLP-76+/− (open bars; n = 10) or SLP-76−/−(filled bars; n = 10) platelets that were stimulated with thrombin (0.5 U/mL), collagen (40 μg/mL), or thrombin plus collagen. Values are expressed as percent of unstimulated control platelets. *P < .05 versus unstimulated platelets. †P < .05 versus SLP-76+/− platelets stimulated with collagen.
Effect of deficiency of SLP-76 or FcRγ on platelet procoagulant activity.
(A) Prothrombinase activity was measured with SLP-76+/−(open bars; n = 5), SLP-76−/− (filled bars; n = 4), or FcRγ−/− (hatched bars; n = 4) platelets that were stimulated with collagen (40 μg/mL), convulxin (250 ng/mL), or ionomycin (3 μM). (B) Prothrombinase activity was measured with SLP-76+/− (open bars; n = 10) or SLP-76−/−(filled bars; n = 10) platelets that were stimulated with thrombin (0.5 U/mL), collagen (40 μg/mL), or thrombin plus collagen. Values are expressed as percent of unstimulated control platelets. *P < .05 versus unstimulated platelets. †P < .05 versus SLP-76+/− platelets stimulated with collagen.
To further define the role of SLP-76 in platelet procoagulant activity, platelets from SLP-76+/− and SLP-76−/−littermates (n = 10) were stimulated with thrombin or collagen, or costimulated with thrombin plus collagen (Figure 4B). Thrombin produced a 1.3-fold increase in prothrombinase activity with both SLP-76+/− and SLP-76−/− platelets (P < .05 versus unstimulated platelets). Collagen produced a 1.9-fold increase in prothrombinase activity with both SLP-76+/− and SLP-76−/− platelets (P < .01 versus unstimulated platelets). A larger, 2.3-fold, increase in prothrombinase activity was observed when SLP-76+/− platelets were costimulated with thrombin and collagen (P < .05 versus SLP-76+/− platelets stimulated with collagen alone). In contrast, costimulation of SLP-76−/− platelets with thrombin and collagen did not increase prothrombinase activity over that produced when SLP-76−/− platelets were stimulated with collagen alone (Figure 4B). In additional control experiments, the prothrombinase activity observed when SLP-76+/+ platelets stimulated with thrombin plus collagen was identical to that of SLP-76+/−platelets (data not shown). These findings indicated that SLP-76 is not essential for platelet procoagulant activity induced by thrombin or collagen alone, but is necessary for maximal procoagulant activity when platelets are stimulated with multiple agonists.
Annexin V binding after costimulation with thrombin and convulxin
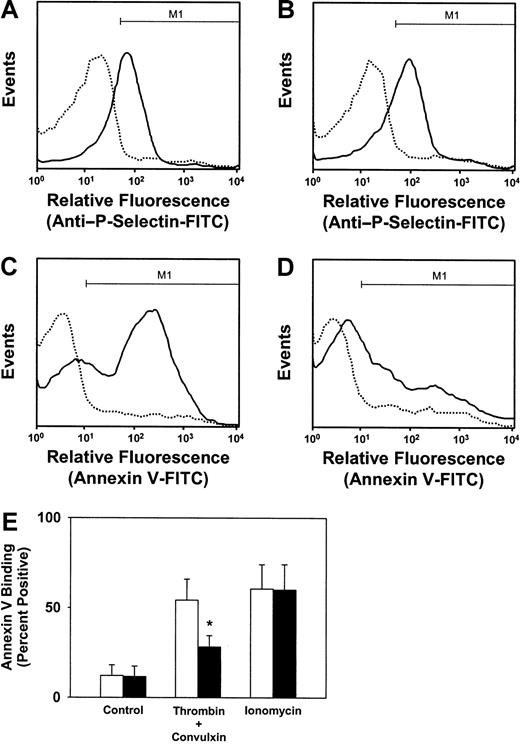
We next measured annexin V binding with platelets that were costimulated with thrombin and convulxin. After simultaneous activation with thrombin plus convulxin, SLP-76+/− and SLP-76−/− platelets exhibited equivalent surface expression of P-selectin (Figure 5A-B), but SLP-76−/− platelets had markedly less annexin V binding than SLP-76+/− platelets (Figure 5C,D). The flow cytometric pattern of annexin V binding observed with SLP-76−/− platelets after costimulation with thrombin and convulxin (Figure 5D) was very similar to that observed when SLP-76−/− platelets were stimulated with thrombin alone (Figure 3B). This result was confirmed with platelets from several additional pairs of SLP-76+/− and SLP-76−/−mice (Figure 5E). These findings demonstrate that the GPVI/SLP-76 signal transduction pathway does contribute to anionic phospholipid exposure when platelets are stimulated simultaneously with thrombin and convulxin.
Effect of deficiency of SLP-76 on granule release and annexin V binding after costimulation with thrombin and convulxin.
Granule release in SLP-76+/− (A) or SLP-76−/− (B) platelets was monitored by flow cytometric measurement of surface expression of P-selectin in unstimulated platelets (dotted line) or after costimulation with 0.5 U/mL thrombin and 250 ng/mL convulxin (solid line). Annexin V binding to SLP-76+/− (C) or SLP-76−/− (D) platelets was measured by flow cytometry in unstimulated platelets (dotted line) or after costimulation with thrombin and convulxin (solid line). (E) The percentage of platelets staining positively for annexin V binding, defined as region M1 as shown in panels C and D, was determined for SLP-76+/− (open bars; n = 4) or SLP-76−/−(filled bars; n = 4) platelets that were either left unstimulated (control) or stimulated with thrombin (0.5 U/mL) plus convulxin (250 ng/mL), or ionomycin (3 μM). *P < .05 versus SLP-76+/− platelets.
Effect of deficiency of SLP-76 on granule release and annexin V binding after costimulation with thrombin and convulxin.
Granule release in SLP-76+/− (A) or SLP-76−/− (B) platelets was monitored by flow cytometric measurement of surface expression of P-selectin in unstimulated platelets (dotted line) or after costimulation with 0.5 U/mL thrombin and 250 ng/mL convulxin (solid line). Annexin V binding to SLP-76+/− (C) or SLP-76−/− (D) platelets was measured by flow cytometry in unstimulated platelets (dotted line) or after costimulation with thrombin and convulxin (solid line). (E) The percentage of platelets staining positively for annexin V binding, defined as region M1 as shown in panels C and D, was determined for SLP-76+/− (open bars; n = 4) or SLP-76−/−(filled bars; n = 4) platelets that were either left unstimulated (control) or stimulated with thrombin (0.5 U/mL) plus convulxin (250 ng/mL), or ionomycin (3 μM). *P < .05 versus SLP-76+/− platelets.
Annexin V binding after costimulation with thrombin and collagen
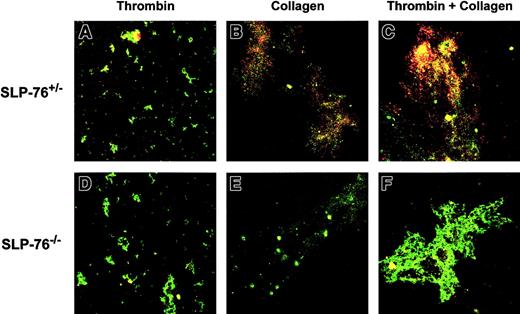
Annexin V binding was examined with fibrinogen-adherent platelets by confocal microscopy (Figure 6). Following adherence to fibrinogen-coated coverslips, platelets were stimulated with thrombin, collagen, or thrombin plus collagen. Adherent platelets and platelet aggregates were identified by staining with FITC anti-CD49b (green fluorescence) and annexin V binding was detected by staining with PE-conjugated annexin V (red fluorescence). A low level of annexin V staining was detected with both SLP-76+/− and SLP-76−/− platelets after stimulation with thrombin (Figure 6A,D). Moderate staining for annexin V was observed with SLP-76+/− platelets after stimulation with collagen (Figure 6B) and strong staining for annexin V was seen with SLP-76+/− platelets after costimulation with thrombin and collagen (Figure 6C). With SLP-76−/− platelets, however, stimulation with collagen (Figure 6E) or costimulation with thrombin and collagen (Figure 6F) did not produce an evident increase in annexin V staining over that observed after stimulation with thrombin alone (Figure 6D). These findings indicate that SLP-76 is necessary for maximal anionic phospholipid exposure when fibrinogen-adherent platelets are stimulated with collagen or costimulated with thrombin and collagen.
Confocal photomicrographs of annexin V binding with fibrinogen-adherent platelets.
Following adherence to fibrinogen-coated coverslips, SLP-76+/− (A-C) or SLP-76−/− (D-F) platelets were stimulated with thrombin (0.5 U/mL; A,D), collagen (50 μg/mL; B,E), or thrombin plus collagen (C,F). Adherent platelets and platelet aggregates were identified by staining with FITC anti-CD49b (green fluorescence) and annexin V binding was detected by staining with PE-conjugated annexin V (red fluorescence). Yellow fluorescence indicates costaining for both CD49b and annexin V binding. Original magnification × 200.
Confocal photomicrographs of annexin V binding with fibrinogen-adherent platelets.
Following adherence to fibrinogen-coated coverslips, SLP-76+/− (A-C) or SLP-76−/− (D-F) platelets were stimulated with thrombin (0.5 U/mL; A,D), collagen (50 μg/mL; B,E), or thrombin plus collagen (C,F). Adherent platelets and platelet aggregates were identified by staining with FITC anti-CD49b (green fluorescence) and annexin V binding was detected by staining with PE-conjugated annexin V (red fluorescence). Yellow fluorescence indicates costaining for both CD49b and annexin V binding. Original magnification × 200.
Discussion
The adapter protein SLP-76 is essential for platelet activation responses that are downstream of the major signal transducing collagen receptor GPVI.7 Platelets that lack SLP-76 exhibit marked impairment of several activation responses to collagen, including shape change, aggregation, and granule release.8 9 We hypothesized, therefore, that deficiency of SLP-76 also may impair platelet procoagulant responses to collagen. Contrary to our expectations, we found that SLP-76 was not necessary for platelet procoagulant activity when platelets in suspension were stimulated by collagen as a single agonist. Consistent with this finding, we also found that deficiency of FcRγ, which is essential for GPVI signaling, did not impair the platelet procoagulant response to collagen and that the GPVI agonist convulxin failed to induce annexin V binding or increase prothrombinase activity despite producing full aggregation and granule release. Deficiency of SLP-76 did, however, cause impairment of maximal procoagulant responses when platelets were costimulated with thrombin plus collagen or thrombin plus convulxin. These results demonstrate that both GPVI-dependent and GPVI-independent pathways contribute to collagen-induced platelet procoagulant activity.
Our finding that stimulation of murine platelets with convulxin as a single agonist did not induce detectable annexin V binding or expression of procoagulant activity is discordant with the observations of Furihata et al, who found an association between GPVI content and convulxin-induced procoagulant activity in human platelets.23 This discordancy may be related to differences between murine and human platelets or to differences in the sensitivities of the procoagulant activity assays to platelet-derived factor V. Because the prothrombinase assay used by Furihata and coworkers relied on activated platelets as a source of factor V, it is very likely that a component of the platelet procoagulant activity detected in their study was due to convulxin-induced release of factor V from α granules, with subsequent conversion of factor V to factor Va by exogenous factor Xa. The platelet procoagulant activity assay that we used in the current study is more specific for procoagulant phospholipid, because both factor Va and factor Xa are provided exogenously. We suspect, therefore, that stimulation of platelet GPVI with convulxin may augment prothrombinase activity through release of platelet factor V, but have little effect on exposure of procoagulant phospholipid. This interpretation is consistent with recent observations by Siljander et al, who found that GPVI agonists stimulated full aggregation but only minimal exposure of anionic phospholipids in human platelets in suspension.24
There is considerable evidence that GPVI is a major signal transducing collagen receptor on platelets, but it is likely that other surface receptors also contribute to platelet-collagen interactions.6 The initial tethering of platelets to collagen surfaces under conditions of high shear stress is dependent on an interaction between collagen-bound von Willebrand factor (VWF) and the glycoprotein Ib/V/IX complex.25 VWF-mediated tethering facilitates subsequent interactions between collagen and GPVI and other platelet surface receptors such as α2β1integrin, which promotes adhesion.6 The α2β1 integrin appears to be essential for firm adhesion of murine platelets to collagen.26Stimulation of GPVI may up-regulate the activity of α2β1, leading to reinforcement of adhesive interactions with collagen.27 Several additional collagen receptors, including CD3628 and platelet glycoprotein V,29 have been identified on platelets, and current models propose that some of these receptors may function as accessory molecules with GPVI to produce full activation responses to fibrillar collagen.1 30 Our results extend these models by providing evidence for a GPVI-independent pathway for collagen-induced platelet procoagulant activity.
Although our results clearly demonstrate that collagen can induce platelet procoagulant activity in the absence of SLP-76, it is evident that the GPVI/FcRγ/SLP-76 signal transduction pathway is necessary to generate maximal procoagulant responses on costimulation of murine platelets with thrombin and collagen. This combination of agonists is likely to be physiologically important, because exposure of platelets to both collagen and thrombin occurs during primary hemostasis in vivo. We speculate, therefore, that impaired platelet procoagulant responses to costimulation with thrombin and collagen may contribute to the bleeding phenotype observed in SLP-76 null mice.8,12,13Interestingly, the combination of thrombin and collagen also has been found to be a potent inducer of procoagulant activity in human platelets. Human platelets that are costimulated with collagen plus thrombin, or convulxin plus thrombin, express higher levels of surface factor V and procoagulant phospholipid than platelets stimulated with either thrombin or convulxin alone.31 SLP-76 also appears to be required for maximal exposure of anionic phospholipid when fibrinogen-adherent platelets are stimulated with collagen (Figure 6). This observation suggests that, like costimulation with thrombin and collagen, costimulation with fibrinogen and collagen may be a potent stimulus for platelet procoagulant activity. This finding also is consistent with a critical role for SLP-76 in fibrinogen-induced signal transduction through the αIIbβ3integrin.14 32
In agreement with previous results with human platelets,24 31 we found that the calcium ionophore ionomycin is a potent inducer of annexin V binding and prothrombinase activity. The annexin V binding response to ionomycin was similar to that observed after costimulation with thrombin and collagen (Figure5), which suggests that a very potent calcium mobilization signal is sufficient to generate maximum platelet procoagulant activity. Alternatively, ionomycin may produce membrane effects that result in exposure of anionic phospholipids independently of its effects on calcium influx.
In summary, we have found that stimulation of murine platelets with the GPVI agonist convulxin produces aggregation and granule release, but little procoagulant activity. In contrast, stimulation with fibrillar collagen produces substantial platelet procoagulant activity in addition to aggregation and granule release. SLP-76 is necessary for aggregation and granule release, which is consistent with a major role for GPVI in these activation responses to collagen. SLP-76 is dispensable for procoagulant activity when platelets in suspension are stimulated by collagen as a single agonist, but required for maximal procoagulant activity induced by costimulation with thrombin and collagen. We conclude that both GPVI-independent (activated by collagen alone) and GPVI-dependent (activated by collagen plus thrombin) signaling events contribute to platelet procoagulant activity.
We thank Todd Goldman for technical assistance and Justin Fishbaugh, Technical Director of the University of Iowa Holden Comprehensive Cancer Center Flow Cytometry Facility, for expert advice. Confocal microscopy was performed in the University of Iowa Central Microscopy Research Facility.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-04-1234.
Supported by National Institutes of Health grants HL063017, DK025295, HL004460, HD027748, and HL063943, and the Office of Research and Development, Department of Veterans Affairs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Steven R. Lentz, Department of Internal Medicine, C303 GH, University of Iowa, Iowa City, IA 52242; e-mail:steven-lentz@uiowa.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal