Following primary infection, human cytomegalovirus (CMV) establishes a lifelong latent infection in bone marrow–derived myeloid lineage cells. Although downmodulation of major histocompatibility complex (MHC) class I and class II protein levels occurs during active viral replication, little is known about the modulation of these proteins during latent infection. When analyzed by flow cytometry, latently infected adherent cells collected from granulocyte macrophage progenitor (GM-P) cultures exhibited a striking reduction in MHC class II antigen present on the cell surface starting very early after exposure to virus that continued for more than 2 weeks. In comparison, cell surface levels of the monocyte cell surface marker CD14 remained unaltered in these cells. A recombinant virus (RV798) lacking the virus genes US2-US11 retained the ability to downmodulate MHC class II levels during latent infection. Immunoblot and immunofluorescent antibody staining analyses showed that the reduction in MHC class II surface levels during latency was associated with a block in protein trafficking. HLA-DR was retained within cytoplasmic vesicles that also contained HLA-DM. Thus, downmodulation remained independent of all previously characterized MHC class I and class II immunomodulatory viral gene products and involved a mechanism not previously ascribed to any viral function. These data show that latent infection is accompanied by reduced cell surface expression of MHC class II proteins, a strategy that would afford the virus escape from immunosurveillance and increase the chances for lifelong latent infection.
Introduction
Human cytomegalovirus (CMV), a species-specific herpesvirus that infects a majority of individuals worldwide, is an important cause of congenital infection leading to neurological damage such as hearing impairment. The increasing impact of CMV disease in immunocompromised transplant recipients as well as the AIDS-affected population has spawned interest in viral latency and reactivation.1 Following resolution of primary infection by the host immune response, the virus establishes lifelong latency in monocytes and their progenitors.2-11 Subclinical reactivation of productive replication may occur spontaneously in immunocompetent individuals and leads to asymptomatic viral shedding in body secretions. Reactivation and amplification following reactivation are major contributors to the incidence of disease in immunocompromised individuals.1 Over the last decade, specific cellular immune clearance mechanisms as well as viral immune evasion mechanisms that impact the outcome of productive replication have been characterized. Although not yet studied in any detail, latency and reactivation are likely to be influenced by host immune clearance mechanisms. Myeloid lineage cells remain CMV DNA-positive prominent sites of latent infection throughout life.2-11 The CMV genome is apparently maintained as an extrachromosomal plasmid8 at a low copy number of 1 to 10 copies/cell7 and viral gene expression is highly restricted.9,10 CMV latency-associated transcripts (CLTs) encoded from the major immediate early (ie1/ie2) region of the viral genome are expressed in both experimental and natural latent infection of granulocyte macrophage progenitor (GM-P) cells.5,7,9-11 Expression of latency-associated genes is restricted to a subset of viral DNA-positive cells7 and their functions have yet to be defined.12 Serum antibody responses to open reading frames (ORFs) found on latent transcripts have been detected in a majority of healthy seropositive blood donors, which suggests that latency-associated proteins are expressed during natural infection.10 13
During active CMV infection, host innate and adaptive immune responses recognize and clear productively infected cells. Individuals with deficits in cell-mediated immune responses are at a higher risk for CMV persistence and disease than individuals with defects in the antiviral humoral response.1 MHC class I– and class II– restricted CMV-specific CTL responses are generated and are believed to be important for clearance.14,15 MHC class I–restricted CTLs, in particular, are capable of protecting allograft recipients from CMV disease when transferred along with virus-specific MHC class II–restricted T cells.15,16 To balance the impact of the host immune response, CMV encodes at least 4 immunomodulatory functions (US2, US3, US6, and US11) whose roles are to limit antigen presentation by cell surface MHC class I and II proteins during productive replication17-27 (David Johnson, oral personal communication, July 2002). US2, US3, US6, and US11 have each been shown to down-regulate cell surface MHC class I expression by interfering with specific steps in the assembly and transport pathway.17-23 CMV inhibits interferon-gamma (IFN-γ)–mediated induction of MHC class II expression on productively infected endothelial cells, human fibroblasts (HFs), and U373-MG astrocytoma cells,24,25 and down-regulates MHC class II levels on cells that have been engineered to express this protein.26 The viral US2 and US3 functions have been implicated in the down-regulation of MHC class II levels due to a capacity to degrade or mislocalize components of this assembly pathway26 (David Johnson, oral personal communication, July 2002). A recent study extended this work to productively infected macrophages, confirming the importance of US1-US11 region gene products but also suggesting that other, as-yet-unidentified viral gene(s), may also function in down-regulating MHC class II levels in these cells.27 Thus, multiple mechanisms enable CMV to avoid immune recognition in cells that are permissive and support active infection. Immunomodulation clearly plays an important role in both persistence and pathogenesis of this virus.
During latency, CMV is sequestered in myeloid lineage cells that exhibit low levels of cell surface MHC class I but high levels of MHC class II proteins.28 These cells are important partners for antigen presentation to T and B lymphocytes during the induction of the antiviral immune response and may be targeted by immune clearance mechanisms. In work that focused on identification of latently infected cell types, CD34, CD33, CD14, CD15, CD10, CD1a, CD11a, CD11b, CD29, CD31, CD44, CD49d, CD54 (ICAM-1), ICAM-2, E-cadherin, and s-LE-x were found to be expressed at similar levels on mock- and CMV-infected GM-P cells.11 The impact of latent CMV infection on MHC class II expression had not been assessed. In this study we sought to establish whether MHC class II expression is altered on experimentally infected cells in GM-P cultures and have applied a combination of flow cytometry, immunoblot detection, and immunofluorescence analysis to assess expression levels and patterns. We show that cell surface MHC class II levels are reduced on latently infected adherent cells in GM-P cultures but that viral genes associated with immunomodulation during productive infection are not responsible for this effect.
Materials and methods
Cells and virus culture
Human fetal liver hematopoietic cells were cultured in GM-P medium.5 Briefly, fetal liver cells (from 16- to 20-week abortuses) were separated on Lymphoprep (GIBCO/BRL, Rockville, MD) and cultured in Iscove modified Dulbecco medium, 5% conditioned medium from the 5637 bladder carcinoma cell (ATCC HTB9), penicillin G (100 units/mL), and streptomycin (100 μg/mL). On day 4 of culture, nonadherent cells were collected and split into 2 flasks. One flask of cells was not exposed to CMV (mock infected) and the other was exposed to CMV at a multiplicity of infection (MOI) of 3 for 3 hours, washed 3 times in Hanks balanced salt solution (HBSS), and placed into a 37°C incubator in a humidified atmosphere containing 5% CO2. Nonadherent GM-Ps were collected and transferred to fresh 25-cm2 flasks every 3 days. Newly adherent cells (ie, those that had become adherent over the preceding 2 days) were collected for analysis by gentle scraping. The high passage human CMV strains TownevarRIT329,30 and a low passage of strain Toledo31 were used in these experiments. CMV RV798 is a US2-US11 deletion mutant derived from strain AD169varATCC.17 Primary human foreskin fibroblasts (HFs) were used for virus propagation and plaque assay.
Quantitative competitive–polymerase chain reaction (QC-PCR)
Cells (1.3 × 104) were suspended in 10 μL of a previously described lysis buffer,5 incubated for 16 hours at 65°C to degrade proteins followed by 10 minutes at 98°C to inactivate proteinase K and denature nucleic acids. Cell lysates were supplemented with between 3 × 103 and 3 × 105 copies of a denatured CMVie1/ie2 cDNA competitor pON23475 and polymerase chain rection (PCR) amplification was performed for 30 cycles (94°C for 1 minute, 62°C for 1 minute, and 72°C for 2 minutes) with primers IEP3C and IEP4BII.5 7 After amplification, 20% of each reaction was subjected to electrophoresis in a 3% agarose gel. The relative quantities of PCR products derived from genomic and competitor templates were estimated after staining with ethidium bromide by density integration using a Stratagene Eagle Eye II/Eagle Sight system.
Antibodies
Monoclonal antibodies (CalTag, Burlingame, CA) specific for human MHC class II (HLA-DR; clone TU36), tagged with phycoerythrin (PE), and human CD14 (clone TUK4), tagged with fluoresceine isothiocyanate (FITC), were used for flow cytometry. Murine monoclonal antibody to human MHC class II (HLA-DR, DP, DQ; clone IQU9) (Novacastra, Newcastle upon Tyne, United Kingdom), rabbit anti–HLA-DM (SU-36), rabbit anti–human CD14 (M-305), FITC-conjugated goat anti–rabbit IgG (CalTag), Texas Red (TR)–conjugated goat anti–mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) and mouse anti–cytomegalovirus late antigen (clone SL20; Accurate Chemical and Scientific, Westbury, NY) were used in immunofluorescent staining and confocal microscopy. Immunoblots were performed with an HLA-DRα chain–specific rabbit antibody DA6.147.32
Immunoblot analysis
Cell lysates were prepared and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) in 12.5% gels.33 Electrotransfer was onto Immobilon-P polyvinylidene diflouride membranes (Millipore, Bedford, MA), which were incubated in blocking solution (5% nonfat milk in phosphate-buffered saline [PBS]) for 1 hour followed by incubation with anti–HLA-DRα antibody diluted at 1:300 in blocking solution. Secondary goat anti–mouse immunoglobulin G-horseradish peroxidase conjugate (Amersham, Buckinghamshire, England) was used at 1:2000 dilution and detection was by enhanced chemiluminscence (ECL; Amersham, Buckinghamshire, England).
Flow cytometry
Cells (1 × 105) were first washed and then suspended in 100 μL of FACS staining buffer (PBS with 1% fetal bovine serum, 0.2% sodium azide). Anti–HLA-DR-PE, anti–CD14-FITC, or isotype control antibody was added to a final 1:20 dilution and incubated for 30 minutes at 4°C. In all experiments, mock- and CMV-infected cells were incubated with isotype control antibodies to control for nonspecific antibody binding. Cells were washed with FACS staining buffer and suspended in orthofixative (PBS with 1% EM-grade formaldehyde) for evaluation with a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ). In each flow cytometry run, 10 000 events were collected. All calculations were performed using Cell Quest software (Becton Dickinson).
Immunofluorescence and confocal microscopy
Cells (1-5 × 104) were spotted onto glass slides, air-dried, fixed and permeabilized with acetone at 4°C for 15 minutes, washed in PBS, incubated with blocking buffer (10% normal goat serum in PBS) at 37°C for 30 minutes, washed 3 times with PBS, and incubated with primary anti–human MHC class II, rabbit anti–HLA-DM, rabbit anti–CD14 or isotype control antibodies (diluted 1:50 in blocking buffer) for 30 minutes at 37°C. Slides were washed 3 times in PBS and the secondary antibodies were added for 30 minutes in the dark at 37°C. Secondary antibodies included TR-conjugated goat anti–mouse IgG (1:100) and FITC-conjugated goat anti–rabbit IgG (1:100) diluted in blocking solution. After 3 washes in PBS, slides were mounted with Syva mounting fluid (Behring Diagnostics, San Jose, CA) and examined using an Optiscan (Nottinghill, Australia) laser scanning confocal microscope. In all immunofluorescence staining experiments controls included mock-infected cells incubated with the specific antibody of interest and CMV- and mock-infected cells incubated with appropriate isotype control antibodies.
Results
Impact of latent CMV infection on cell surface MHC class II levels
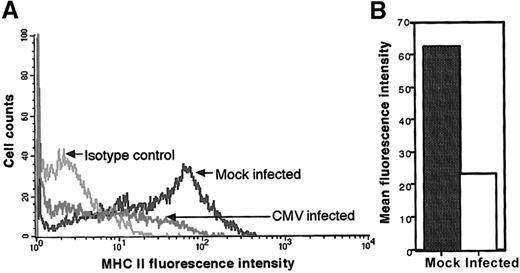
Flow cytometry was used to evaluate changes in MHC class II levels on the surface of latently infected cells. Human fetal liver–derived GM-Ps, grown in suspension culture5 and divided into 2 equal portions, were either mock- or CMV-infected. Previous studies11 showed that CMV-infected GM-Ps continued to express many cell surface markers characteristic of the GM-P lineage. Levels of cell surface CD34, CD33, CD14, CD15, CD10, CD1a, CD11a, CD11b, CD29, CD31, CD44, CD49d, CD54 (ICAM-1), ICAM-2, E-cadherin, and s-LE-x on CMV-infected GM-Ps were found to be similar to mock-infected cells. The impact of CMV infection on MHC class II levels was not evaluated previously. On day 10 after infection with CMV strain Toledo (MOI of 3), cells that had become adherent over the preceding 2 days were harvested, stained for MHC class II (HLA-DR) expression using mouse monoclonal antibody TU36, and subjected to flow cytometric analysis. A markedly reduced level of cell surface MHC class II was observed on CMV-infected cells compared with mock-infected GM-Ps (Figure 1A). Staining patterns with isotype-matched control antibodies showed that the change was specific. As shown in Figure 1B, the mean fluorescence intensity (MFI) of MHC class II–specific staining in the mock-infected culture (MFI of 64) was dramatically reduced when compared with the parallel infected culture (MFI of 23). A similar 2- to 3-fold reduction in the MFI was consistently observed in a further 3 experiments comparing mock- versus CMV-infected cells. Thus, CMV-infected GM-Ps sustained reduced levels of MHC class II on their surface.
Flow cytometry analysis of MHC class II protein levels on CMV latently infected adherent cells from GM-P cultures.
GM-Ps were infected with CMV strain Toledo (MOI of 3) or mock infected. Newly adherent cells were harvested and stained with anti–MHC class II (HLA-DR) antibody TU36 at day 10 after infection (A). The MFI of cell surface MHC class II levels is shown (B).
Flow cytometry analysis of MHC class II protein levels on CMV latently infected adherent cells from GM-P cultures.
GM-Ps were infected with CMV strain Toledo (MOI of 3) or mock infected. Newly adherent cells were harvested and stained with anti–MHC class II (HLA-DR) antibody TU36 at day 10 after infection (A). The MFI of cell surface MHC class II levels is shown (B).
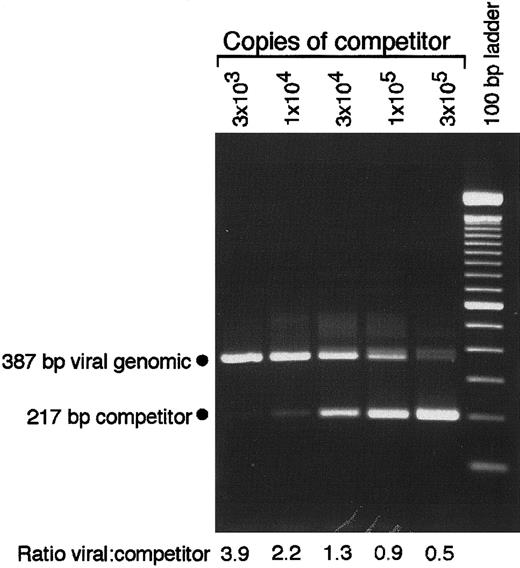
Down-regulation of MHC class II has been associated with productive CMV replication.26,27 Although viral gene expression has been shown to be highly restricted and spontaneous reactivation has never been observed in GM-Ps,5,7,10,11 we confirmed that attached cells in GM-P cultures remained free of infectious CMV. At 2 weeks after infection, aliquots of CMV-infected GM-P culture supernatants, cell lysates, and intact cells were seeded onto permissive HFs and monitored by culture for 14 days without the appearance of cytopathic effect (data not shown). When subjected to immunofluorescence analysis with late viral antigen-specific monoclonal antibody SL20, 104 attached cells from GM-P cultures were found to be negative (data not shown). This series of experiments was consistent with previous reports that failed to detect productive viral replication or gene products in CMV-infected GM-P cultures.5,7,10,11 To assess viral genome copy number, cell lysates prepared from 1.3 × 104 adherent GM-Ps were subjected to previously described5 QC-PCR amplification using primers IEP3C and IEP4BII along with a competitor template (Figure 2). This analysis revealed an average of between 2 and 8 viral genomes per cell in the culture, which was similar to previous, more extensive analyses that characterized the levels and distribution of viral DNA in latently infected GM-Ps.7
CMV DNA copy number determination by QC-PCR.
Lysates of 1.3 × 104 cells from a Toledo-infected GM-P culture (MOI of 3, day 10 after infection) were analyzed in the presence of the indicated copies of pON2347 competitor. The positions of the 387-bp product from CMV genomic DNA and the 217-bp product from the competitor template are indicated. The ratio of viral genomic to competitor template was determined by density integration using a Stratagene Eagle Eye II/ Eagle Sight system.
CMV DNA copy number determination by QC-PCR.
Lysates of 1.3 × 104 cells from a Toledo-infected GM-P culture (MOI of 3, day 10 after infection) were analyzed in the presence of the indicated copies of pON2347 competitor. The positions of the 387-bp product from CMV genomic DNA and the 217-bp product from the competitor template are indicated. The ratio of viral genomic to competitor template was determined by density integration using a Stratagene Eagle Eye II/ Eagle Sight system.
Viral strain and time course of MHC class II down-regulation by CMV
The Toledo strain is a low-passage virus that retains virulence characteristics.31 In order to determine whether a laboratory-passaged viral strain would alter MHC class II expression, GM-Ps were infected with the attenuated strain CMV TownevarRIT3 at a MOI of 3. Cells were cultured as previously described5 and examined over a time course of 15 days. Recently, adherent cells were harvested 2, 5, 8, 12, and 15 days after infection for flow cytometric analysis with monoclonal antibodies to MHC class II (HLA-DR) as well as the monocyte/macrophage marker CD14. At all time points, cells from CMV-infected GM-P cultures were found to express markedly less cell surface MHC class II than mock-infected cells from parallel cultures (Figure3). These data demonstrate that CMV infection leads to a consistent MHC class II down-regulation that persists over an extended time period and that strains Toledo and TownevarRIT3 are equivalent in their ability to modulate MHC class II expression in adherent cells from GM-P cultures.
Time course of MHC class II levels on mock- and CMV-infected adherent cells in GM-P cultures.
GM-Ps were infected with CMV strain TownevarRIT3 (MOI of 3) or mock infected, and newly adherent cells were harvested for staining with anti–MHC class II (HLA-DR) antibody TU36 at days 2, 5, 8, 12, and 15 after infection (AI). Isotype control antibody was also included at all time points.
Time course of MHC class II levels on mock- and CMV-infected adherent cells in GM-P cultures.
GM-Ps were infected with CMV strain TownevarRIT3 (MOI of 3) or mock infected, and newly adherent cells were harvested for staining with anti–MHC class II (HLA-DR) antibody TU36 at days 2, 5, 8, 12, and 15 after infection (AI). Isotype control antibody was also included at all time points.
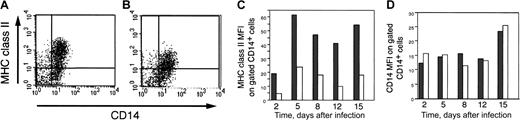
To determine whether down-regulation of MHC class II levels occurred on monocyte lineage cells, we assessed the monocyte marker CD14 in parallel with MHC class II levels (Figure4). In this experiment, cells were first gated for the expression of CD14 and these CD14+ cells were then subjected to further analysis. Although a similar percentage of mock- and CMV-infected gated CD14+ cells expressed MHC class II on day 15 (63% and 55%, respectively), the amount of MHC class II expressed on gated CD14+ cells in latently infected GM-P cultures (expressed as MFI) was dramatically reduced (Figure 4A-B). Although there were variable levels of MHC class II cell surface expression on gated CD14+ cells from both mock and infected cultures, at all time points tested (days 2, 5, 8, 12, and 15 after infection), CMV infection resulted in consistent, 2-5 fold lower levels of cell surface MHC class II on gated CD14+ GM-Ps (Figure 4C). In contrast, the MFI of the CD14 marker showed little or no alteration in response to CMV infection11 (Figure 4D). In 3 independent experiments, CMV-infected GM-Ps exhibited a reduction in the MFI of cell surface MHC class II without any significant change in CD14 MFI levels. These results show that CMV infection specifically reduced the levels of cell surface MHC class II on CD14+cells during experimental latent infection.
MHC class II and CD14 levels on adherent cells from GM-P cultures.
(A) Flow cytometry of MHC class II and CD14 on mock-infected cells at day 15 after infection. (B) Flow cytometry of MHC class II and CD14 on CMV-infected cells at day 15 after infection. (C) Flow cytometry MFI analysis of MHC class II levels on mock-infected and CMV-infected cells at days 2, 5, 8, 12, and 15 after infection on gated CD14+cells. (D) Flow cytometry MFI analysis of CD14 protein levels on mock-infected and CMV-infected cells at days 2, 5, 8, 12, and 15 after infection on gated CD14+ cells. GM-P cultures were infected with CMV strain TownevarRIT3 (MOI of 3) and adherent cells were stained with anti–MHC class II (HLA-DR) antibody TU36 and anti–CD14 antibody TUK4. Grey bars, mock-infected samples; white bars, CMV-infected samples.
MHC class II and CD14 levels on adherent cells from GM-P cultures.
(A) Flow cytometry of MHC class II and CD14 on mock-infected cells at day 15 after infection. (B) Flow cytometry of MHC class II and CD14 on CMV-infected cells at day 15 after infection. (C) Flow cytometry MFI analysis of MHC class II levels on mock-infected and CMV-infected cells at days 2, 5, 8, 12, and 15 after infection on gated CD14+cells. (D) Flow cytometry MFI analysis of CD14 protein levels on mock-infected and CMV-infected cells at days 2, 5, 8, 12, and 15 after infection on gated CD14+ cells. GM-P cultures were infected with CMV strain TownevarRIT3 (MOI of 3) and adherent cells were stained with anti–MHC class II (HLA-DR) antibody TU36 and anti–CD14 antibody TUK4. Grey bars, mock-infected samples; white bars, CMV-infected samples.
Effect of deletion of US2-US11 region on MHC class II expression
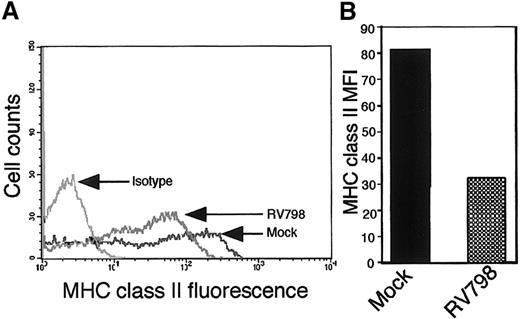
The US2-US11 region of the viral genome encodes functions that modulate cell surface expression of MHC class II proteins during productive infection26 27 (David Johnson, oral personal communication, July 2002). We sought to address the role of US2-US11 region genes in altering MHC class II expression on cells from GM-P cultures. Mutant RV798-infected GM-Ps (MOI of 3) were evaluated for MHC class II (HLA-DR) levels at day 11 after infection. Lower levels of cell surface MHC class II were detected in RV798 latently infected GM-Ps compared with mock-infected cells (Figure5A-B). These data show that the propensity of CMV to reduce MHC class II levels on latently infected cells was independent of known viral immunomodulatory functions. The result with the US2-US11 region mutant RV798 was not unexpected as there is no evidence that this region is expressed during the latent phase of infection.
MHC class II levels in adherent cells from GM-P cultures infected with US2-US11 mutant RV798.
Parallel cultures of GM-Ps were either mock infected or infected with CMV US2-US11 deletion mutant RV798. On day 11 after infection, cells were stained for MHC class II (HLA-DR) with TU36. (A) Flow cytometry plot. (B) MFI of cell surface MHC class II levels.
MHC class II levels in adherent cells from GM-P cultures infected with US2-US11 mutant RV798.
Parallel cultures of GM-Ps were either mock infected or infected with CMV US2-US11 deletion mutant RV798. On day 11 after infection, cells were stained for MHC class II (HLA-DR) with TU36. (A) Flow cytometry plot. (B) MFI of cell surface MHC class II levels.
Accumulation of MHC class II protein in CMV latently infected GM-Ps
Assembled MHC class II protein is a heterodimer of one α and one β chain that binds to an antigenic peptide generated by proteolytic degradation of antigens in cytoplasmic vesicles. After MHC class II peptide binding takes place in cytoplasmic vesicles, the assembled complex trafficks to the cell surface.34 On day 12 and day 15 after infection, lysates of 5 × 104 GM-Ps were prepared from mock- and CMV-infected GM-P cultures and proteins were separated by SDS-PAGE on 12.5% gels. Immunoblot analysis was carried out with the anti–MHC class II (HLA-DRα) antibody DA6.147 in conjunction with the ECL detection system. A 33-kd fragment corresponding to the HLA-DRα chain was readily detected in mock- and CMV-infected samples at both time points (Figure6A-B). Densitometric scanning of this immunoblot revealed that the relative amounts of total MHC class II α chain did not significantly change in response to CMV infection on either day 12 or day 15 after infection (data not shown). Thus, the reduction of cell surface MHC class II expression on latently infected cells did not apparently result from altered MHC class II α chain synthesis or degradation.
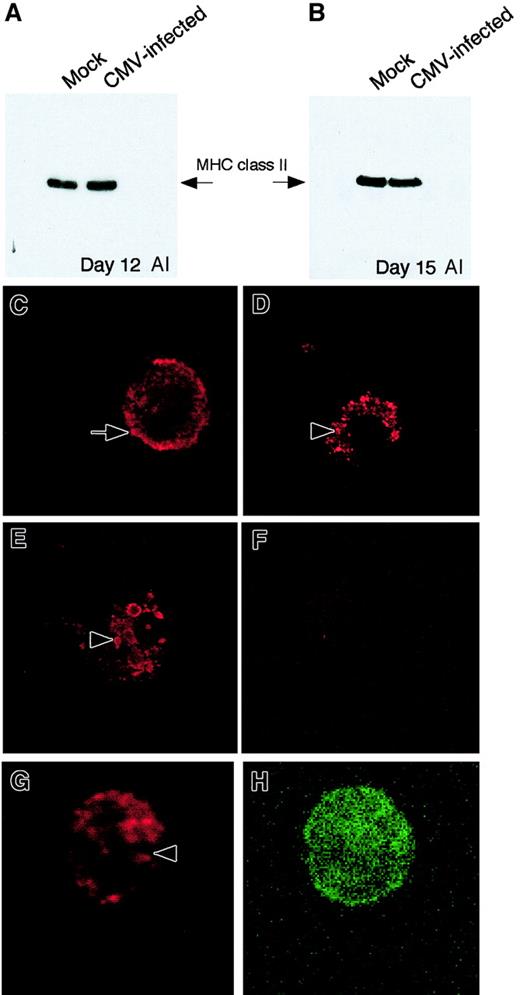
Expression of MHC class II protein in adherent cells from GM-P cultures by immunoblot and immunofluorescence analyses.
(Top panels) Immunoblot analysis. (A) MHC class II (HLA-DRα) immunoblot performed on whole cell lysates from mock-infected cells and CMV-infected cells at day 12 after infection. (B) MHC class II (HLA-DRα) immunoblot performed on whole cell lysates from mock-infected cells and CMV-infected cells at day 15 after infection. (Bottom panels) Immunofluorescence analysis of MHC class II protein expression in CMV latently infected cells on day 15 after infection. (C) Mock-infected cells stained with MHC class II antibody IQU9. (D) CMV strain Toledo–infected cells stained with MHC class II antibody IQU9. (E) CMV mutant RV798-infected cells stained with MHC class II antibody IQU9. (F) CMV strain Toledo–infected cells stained with isotype control antibody. (G, H) CMV strain Toledo–infected cell dual stained for MHC class II (G) and CD14 (H). Anti–MHC class II (red staining) and CD14 (green staining) antibodies were detected with TR- and FITC-conjugated secondary antibodies, respectively. Arrow points to the cell surface of a mock-infected cells and arrowheads point to accumulation of MHC class II in latently infected cells. Original magnification, × 600.
Expression of MHC class II protein in adherent cells from GM-P cultures by immunoblot and immunofluorescence analyses.
(Top panels) Immunoblot analysis. (A) MHC class II (HLA-DRα) immunoblot performed on whole cell lysates from mock-infected cells and CMV-infected cells at day 12 after infection. (B) MHC class II (HLA-DRα) immunoblot performed on whole cell lysates from mock-infected cells and CMV-infected cells at day 15 after infection. (Bottom panels) Immunofluorescence analysis of MHC class II protein expression in CMV latently infected cells on day 15 after infection. (C) Mock-infected cells stained with MHC class II antibody IQU9. (D) CMV strain Toledo–infected cells stained with MHC class II antibody IQU9. (E) CMV mutant RV798-infected cells stained with MHC class II antibody IQU9. (F) CMV strain Toledo–infected cells stained with isotype control antibody. (G, H) CMV strain Toledo–infected cell dual stained for MHC class II (G) and CD14 (H). Anti–MHC class II (red staining) and CD14 (green staining) antibodies were detected with TR- and FITC-conjugated secondary antibodies, respectively. Arrow points to the cell surface of a mock-infected cells and arrowheads point to accumulation of MHC class II in latently infected cells. Original magnification, × 600.
To determine whether CMV infection altered protein localization within cells, MHC class II antigen distribution was assessed by immunofluorescence analysis in mock- and CMV-infected GM-P cultures. GM-Ps were infected with CMV strain Toledo or mutant RV798 at a MOI of 3 and newly adherent cells were analyzed for MHC class II (HLA-DR, DP, DQ) expression at day 15 after infection using monoclonal antibody IQU9 and a TR-conjugated goat anti–mouse IgG as the secondary antibody. MHC class II antigen showed a cell surface and cytoplasmic distribution pattern in mock-infected cells (Figure 6C). In contrast, the pattern of MHC class II staining was altered in cells latently infected with either strain of virus (Figure 6D-E). A punctate vesicular pattern was observed in the cytoplasm and this pattern correlated with the dramatic loss of detectable cell surface staining we observed by flow cytometry. Isotype control antibodies did not stain either CMV- or mock-infected cells (Figure 6F and data not shown). To confirm that the MHC class II retention phenotype was in myeloid lineage cells, we dual-stained cells from latently infected cultures for MHC class II and CD14. As expected, cells staining for CD14 also exhibited an accumulation of MHC class II (Figure 6G-H).These results suggest that MHC class II protein is retained within cytoplasmic vesicles in latently infected cells.
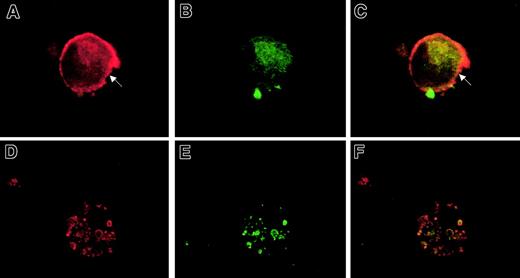
HLA-DM is an accessory protein that plays a critical role in the MHC class II pathway by catalyzing the removal of MHC class II–associated Ii (invariant chain) during loading of antigenic peptides.35 36 Given the importance of HLA-DM in the MHC class II biosynthesis pathway, we sought to determine whether latent CMV infection altered HLA-DM localization. Parallel GM-P cultures that had been mock- or CMV strain Toledo–infected were harvested at day 15 after infection. Newly adherent cells were subjected to immunofluorescence analysis for MHC class II with mouse monoclonal antibody IQU9 followed by a goat anti–mouse TR-conjugated secondary antibody as well as for HLA-DM with a rabbit polyclonal primary antibody followed by a goat anti–rabbit FITC-conjugated secondary antibody. Mock-infected cells exhibited MHC class II and HLA-DM distribution in a pattern that was consistent with a majority of the HLA-DR-DP-DQ on the cell surface and cytoplasm and the majority of the HLA-DM largely restricted to the cytoplasm (Figure7A-C). In contrast, adherent cells from latently infected GM-P cultures had a pattern where MHC class II and HLA-DM colocalized to punctate, cytoplasmic vesicles (Figure 7D-F). Staining was not detected with isotype control antibodies in CMV- or mock-infected cells (data not shown). These results show both MHC class II and HLA-DM antigens are retained in cytoplasmic vesicles, preventing appearance of MHC class II on the cell surface, although the nature of the vesicles remains to be determined.
Analysis of MHC class II (DR, DP, DQ) and HLA-DM protein localization in CMV latently infected cells.
Immunofluorescence staining of mock-infected (A-C) and CMV-infected cells (D-F) at day 15 after infection. Cells were dual stained for MHC class II (A and D; red staining) and HLA-DM (B and E; green staining). Fluorescent images were digitally overlaid to assess colocalization (C, F). Arrow indicates cell surface periphery MHC class II staining on a mock-infected cell. Original magnification, × 600.
Analysis of MHC class II (DR, DP, DQ) and HLA-DM protein localization in CMV latently infected cells.
Immunofluorescence staining of mock-infected (A-C) and CMV-infected cells (D-F) at day 15 after infection. Cells were dual stained for MHC class II (A and D; red staining) and HLA-DM (B and E; green staining). Fluorescent images were digitally overlaid to assess colocalization (C, F). Arrow indicates cell surface periphery MHC class II staining on a mock-infected cell. Original magnification, × 600.
Discussion
This study provides the first demonstration of immune evasion during the latent phase of CMV infection. Cell surface levels of MHC class II are modulated in latently infected myeloid progenitor cells. The mechanism is different from any that have been previously implicated in modulating MHC class I and class II levels during productive infection and the gene(s) controlling this process appear to be novel. The US2-US11 region plays no role in modulating MHC class II levels during latency although US2 and US3 have been ascribed roles in modulating MHC class II levels in productively infected cells26 (David Johnson, oral personal communication, July 2002). Down-regulation during latency involves retention of MHC class II in cytoplasmic vesicles and not an alteration in protein synthesis or degradation.
Like all herpesviruses, the success of CMV as a pathogen is largely dependent on its ability to maintain lifelong latency, reactivate sporadically, and transmit efficiently within the population. During primary or recurrent infection, replicating CMV encodes a range of functions to impede host immune clearance. Many reports have focused on disruption of MHC class I and class II presentation pathways during replication.17-27,37 Immune evasion strategies may also be important during the latent phase of infection. Like gammaherpesviruses such as Epstein-Barr virus (EBV) that target B lymphocytes, but unlike alphaherpesviruses that target immune-privileged sensory neurons, CMV confronts the challenge of infecting cells that are themselves components of the immune system. Although the response to CMV latency-associated proteins has not been studied, EBV latency in B lymphocytes has been shown to be associated with a CD4 response to the EBV nuclear antigen 1 (EBNA-1), via MHC class II presentation.38 Although a CD4+ T-cell response is documented, little is known about the impact of EBV gene products on MHC class II expression. Our demonstration that CMV down-regulates cell surface MHC class II levels during latency implicates immune surveillance by CD4+ T cells during latency in myeloid progenitors. This may extend through their differentiation to monocytes, macrophages, and dendritic cells. Thus, our observations are relevant to latent CMV infection as well as to the process of host cell differentiation leading to reactivation. Given the likelihood that peptides generated from either productive or latent gene products would be presented in the context of MHC class II, the virus may have evolved separate mechanisms to influence immune recognition in both of these settings within a single cell lineage.
Interference between T-cell receptor–bearing cells and target cells with viral peptides presented on MHC class II proteins may certainly confer a survival advantage on the virus and ensure that a sufficient reservoir of CMV becomes established in the host. The US2-US11 functions appear to dominate modulation of MHC class I and MHC class II during primary infection. Tomazin and coworkers26 reported that the CMV US2 gene product functions to down-regulate MHC class II during productive infection of a permissive glioblastoma cell line (U373-MG) which had been engineered to express MHC class II. Odeberg and Soderberg-Naucler27 extended this work to show that US1-US11 region genes of CMV were responsible for down-regulation of MHC class II in mixed populations of productively and abortively infected monocyte-derived macrophages. A US1-US11–resistant population was also implicated when infected cultures were evaluated a few days after exposure to the virus. The mechanism we have described in uniformly infected GM-P cultures may be related to some aspects of the investigations in monocyte-derived macrophages27; however, it will be important to establish whether activated monocytes that down-regulate MHC class II over a prolonged period are productively, abortively, or latently infected.
The identity of the CMV gene or genes involved in the down-regulation of MHC class II during latent infection remains to be determined. We have previously shown that only 2% to 3% of latently infected cells in the GM-P model of latency express sense latency associated transcripts (CLTs)7, yet more than 50% of the cells in the current study show a reduction in cell surface MHC class II. It is therefore tempting to speculate that an additional, as-yet-unidentified viral gene or genes may mediate this phenotype. Indeed, the vast majority of the CMV genome is yet to be assessed for expression during the latent phase and the functions of those ORFs that have been identified as expressed during latency remain to be defined.12 A CMV homologue (encoded by UL111.5A) of the IL-10 (cmvIL-10) has recently been described39,40 and shown to be expressed during productive infection of human embryonic fibroblast cells. The EBV homologue of IL-10 first characterized over a decade ago41,42 is expressed during productive replication where it is believed to modulate the host adaptive immune response in a manner very much like host IL-1043 which includes down-regulation of MHC class II on antigen presenting cells. Although there is currently no evidence that cmvIL-10 is expressed during latent CMV infection, it is possible that this immunomodulatory function may play some role in our observations. Additional studies will define the contribution (if any) of UL111.5A to immune evasion during latency and the assessment of the whole viral genome for gene expression and functions during the latent phase of infection are important goals of future work.
Immunofluorescence and confocal microscopy demonstrated that MHC class II antigens accumulate within the cytoplasm of cells from latently infected cultures, and this accumulation is frequently detected as discrete punctate foci. Taken together with the immunoblot data showing no difference in the total amount of MHC class II (HLA-DRα) antigen comparing mock- and CMV-infected cells, our results suggest that MHC class II proteins are retained within latently infected cells. HLA-DM localizes to cytoplasmic areas where newly synthesized MHC class II proteins pass as they are processed and transported to the cell surface.44 The continued accumulation of HLA-DM during latent infection and the high degree of colocalization with MHC class II suggests that latent virus encodes a function which blocks a stage where MHC class II and accessory proteins remain associated in latently infected cells.
In summary, this study provides the first evidence that CMV encodes an immune evasion strategy during the latent infection and suggests additional mechanisms for viral maintenance during latency. The mechanism of MHC class II down-regulation during latent infection differs from those characterized to occur during productive infection. Continued studies to further define viral and cellular molecular processes during latency may ultimately lead to the development of therapies aimed at lessening the impact of CMV disease in allogeneic hematopoietic cell transplant settings.
The authors would like to thank Dr Tom Jones for the US2-US11 deletion virus (RV798), and Allen Cheung, Chrissie Jenkins, Gavin Morrow, and Dr Josh Stern for their assistance.
Supported in part by NH&MRC project grant no. 153873 (B.S.) and US Public Health Service grants NIH RO1 AI33852 and NIH PO1 CA49605 (E.S.M). A.A. was supported by the Katherine McCormick Fellowship, the Stanford University School of Medicine Dean's Postdoctoral Award, and a grant from the University of Sydney Medical Foundation. B.S. was supported in part by a fellowship from the American Heart Association, Western States Affiliate, and the University of Sydney Rolf Edgar Lake Fellowship.
B.S. and A.A. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Barry Slobedman, Centre for Virus Research, Westmead Millennium Institute, PO Box 412, Westmead NSW, 2145, Australia; e-mail: barry_slobedman@wmi.usyd.edu.au.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal