Idiopathic myelofibrosis (IMF) is a chronic myeloproliferative disorder characterized by megakaryocyte hyperplasia and bone marrow fibrosis. Biologically, an autonomous megakaryocyte growth and differentiation is noticed, which contributes to the megakaryocyte accumulation. To better understand the molecular mechanisms involved in this spontaneous growth, we searched for genes differentially expressed between normal megakaryocytes requiring cytokines to grow and IMF spontaneously proliferating megakaryocytes. Using a differential display technique, we found that the immunophilin FKBP51 was 2 to 8 times overexpressed in megakaryocytes derived from patients' CD34+ cells in comparison to normal megakaryocytes. Overexpression was moderate and confirmed in 8 of 10 patients, both at the mRNA and protein levels. Overexpression of FKBP51 in a UT-7/Mpl cell line and in normal CD34+ cells induced a resistance to apoptosis mediated by cytokine deprivation with no effect on proliferation. FKBP51 interacts with both calcineurin and heat shock protein (HSP)70/HSP90. However, a mutant FKBP51 deleted in the HSP70/HSP90 binding site kept the antiapoptotic effect, suggesting that the calcineurin pathway was responsible for the FKBP51 effect. Overexpression of FKBP51 in UT-7/Mpl cells induced a marked inhibition of calcineurin activity. Pharmacologic inhibition of calcineurin by cyclosporin A mimicked the effect of FKBP51. The data support the conclusion that FKBP51 inhibits apoptosis through a calcineurin-dependent pathway. In conclusion, FKBP51 is overexpressed in IMF megakaryocytes and this overexpression could be, in part, responsible for the megakaryocytic accumulation observed in this disorder by regulating their apoptotic program.
Introduction
Idiopathic myelofibrosis (IMF), also called myelofibrosis with myeloid metaplasia or agnogenic myeloid metaplasia, is a chronic myeloproliferative disease. It is a clonal stem cell disorder1,2 that leads to a megakaryocyte (MK) hyperplasia and to the release in the bone marrow environment of fibrotic cytokines.3,4 The development of a collagen fibrosis in place of normal hematopoietic tissue is responsible for most symptoms of this disease, that is, an extramedullary hematopoiesis, which predominates in the spleen and an ineffective hematopoiesis, which progresses to pancytopenia. Thus, the hallmark of this disease is an MK hyperplasia.5-7 This is further sustained by the fact that induction of an MK proliferation by a deregulated synthesis of thrombopoietin (TPO) leads in the mouse to a disease very similar to IMF.8-10 However, in the human disease, MK hyperplasia occurs in the absence of a recognizable physiologic stimulus. It has been shown that MK progenitors in IMF are hypersensitive to cytokines,11,12 a part of them differentiating in the absence of TPO.13,14 Therefore, it can be postulated that this defective regulation of MK proliferation is the first event in the occurrence of the disease. However, monocytes/macrophages may also play an important role in this disease. A spontaneous activation of the nuclear factor-κB (NF-κB) pathway has been described in patient monocytes15; second, although TPO induced a myelofibrosis in severe combined immunodeficient (SCID) mice, no myelofibrosis could be observed in nonobese diabetic-SCID (NOD-SCID) mice.8 Because NOD-SCID mice have also a monocyte defect, this result suggests that monocytes in combination with MKs are required to induce a myelofibrosis.
With the exception of chronic myeloid leukemia (CML), the molecular mechanisms responsible for the clonal proliferation in myeloproliferative diseases are unknown.16 Little is known about the mechanism of spontaneous growth and differentiation observed in these disorders. Cytogenetic studies in IMF have not found any specific recurrent chromosomal abnormalities17 18; thus, there is no straightforward approach for the identification of candidate genes involved in the pathogenesis of the disease. To go further in the molecular mechanisms, we performed a differential gene expression approach between spontaneously grown MKs from IMF and their normal counterparts obtained after cytokine stimulation. We focused our study on spontaneously growing MKs for 2 reasons: First, normal clones may persist in this disease and spontaneously growing MKs likely derive from the malignant clone. Second, our goal was to identify genes primarily involved in the cytokine hypersensitivity and spontaneously growing MKs delineate a homogenous cell population with this phenotype. By this approach, we found that the FKBP51 transcript and protein were overexpressed in MKs from IMF and that its overexpression in normal cells promotes cell survival after cytokine deprivation.
Patients, materials, and methods
Patients
The diagnosis of IMF was made on bone marrow biopsy for all patients. Moreover, all patients presented characteristic teardrops on blood smears and the presence of circulating myeloid precursor cells. Table 1 illustrates the clinical features of these patients at the time of diagnosis (Lille score19) and blood cell counts at time of investigation.
Clinical description of patients at diagnosis
| Patient . | Lille score at diagnosis . | Time since diagnosis (y) . | Transfusion requirements . | Therapy prior to blood sampling . | Leukocyte count (103/mL) . | Percentage of immature cells in the blood . | Hemoglobin level (g/dL) . | Platelet count (106/mL) . | Analysis in DDRT-PCR . | Analysis in semiquantitative PCR . | Analysis in quantitative PCR . | Analysis in Western-blot . | Analysis in flow cytometry . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | Int | 5 | No | No | 35 000 | 25 | 11.0 | 550 | + | + | + | + | |
| P2 | High | 8.5 | Yes | HU + PU | 74 100 | 39 | 9.9 | 82 | + | + | + | + | |
| P3 | Int | 4 | Yes | HU | 21 000 | 15 | 7.7 | 92 | + | + | + | ||
| P4 | Low | 0 | No | No | 12 700 | 9 | 11.8 | 253 | + | + | + | + | |
| P5 | Low | 0 | No | HU | 9 300 | 5 | 11.4 | 174 | + | + | + | ||
| P6 | High | 2 | No | HU + PU | 17 400 | 43 | 11.4 | 203 | + | + | + | + | |
| P7 | Low | 2 | No | HU | 9 500 | 6 | 12.7 | 256 | + | ||||
| P8 | Int | 1 | Yes | HU | 4 600 | 15 | 11.4 | 69 | + | ||||
| P9 | Int | 1 | Yes | HU | 13 500 | 12 | 10.2 | 107 | + | ||||
| P10 | Low | 6 | No | HU | 18 000 | 5 | 12.8 | 420 | + |
| Patient . | Lille score at diagnosis . | Time since diagnosis (y) . | Transfusion requirements . | Therapy prior to blood sampling . | Leukocyte count (103/mL) . | Percentage of immature cells in the blood . | Hemoglobin level (g/dL) . | Platelet count (106/mL) . | Analysis in DDRT-PCR . | Analysis in semiquantitative PCR . | Analysis in quantitative PCR . | Analysis in Western-blot . | Analysis in flow cytometry . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | Int | 5 | No | No | 35 000 | 25 | 11.0 | 550 | + | + | + | + | |
| P2 | High | 8.5 | Yes | HU + PU | 74 100 | 39 | 9.9 | 82 | + | + | + | + | |
| P3 | Int | 4 | Yes | HU | 21 000 | 15 | 7.7 | 92 | + | + | + | ||
| P4 | Low | 0 | No | No | 12 700 | 9 | 11.8 | 253 | + | + | + | + | |
| P5 | Low | 0 | No | HU | 9 300 | 5 | 11.4 | 174 | + | + | + | ||
| P6 | High | 2 | No | HU + PU | 17 400 | 43 | 11.4 | 203 | + | + | + | + | |
| P7 | Low | 2 | No | HU | 9 500 | 6 | 12.7 | 256 | + | ||||
| P8 | Int | 1 | Yes | HU | 4 600 | 15 | 11.4 | 69 | + | ||||
| P9 | Int | 1 | Yes | HU | 13 500 | 12 | 10.2 | 107 | + | ||||
| P10 | Low | 6 | No | HU | 18 000 | 5 | 12.8 | 420 | + |
Presented are the clinical description of patients at diagnosis (Lille score19), time since diagnosis, transfusion requirement at the time of blood collection, therapy prior to blood collection, leukocyte count at the time of blood collection, and experiments performed with each sample.
HU indicates hydroxyurea; PU, 6-mercaptopurine.
Cell cultures and preparation of total RNA
Heparinized blood samples (20-50 mL) were obtained from 10 patients (P1-P10) with IMF after informed consent was obtained. Aliquots of cytapheresis products were obtained from patients with nonhematologic disease after mobilization by chemotherapy and granulocyte colony-stimulating factor (G-CSF) after informed consent was obtained. Mononuclear cells were separated on a Ficoll gradient (Lymphoprep; Nycomed Pharma, Oslo, Norway). Low-density cells (<1.077g/cm3) were recovered and washed, and CD34+ cells were separated using a magnetic cell sorting system (miniMACS; Miltenyi Biotec, Bergisch Gladbach, Germany) in accordance with the manufacturer's recommendations. The purity of recovered cells was determined by flow cytometry using PE–anti-CD34 mAb (PE-HPCA2; Becton Dickinson, Le Pont du Claix, France) and was over 80%. The immunophenotype of CD34+ cells isolated from patients and leukapheresis were quite similar when studied with antibodies against CD38, CD33, and CD19.
Cells (1 × 105 cells/mL) were cultured in Iscove modified Dulbecco medium (IMDM) supplemented with penicillin, streptomycin, glutamine, and 11.5 μM α-thioglycerol (all from Sigma-Aldrich, St Quentin Fallavier, France), 1.5% bovine serum albumin (BSA; Cohn fraction V; Sigma-Aldrich), sonicated lipids, and iron-saturated human transferrin. For patient samples, cultures were performed in the absence of any cytokine. For normal CD34+ cells, cultures were performed in the presence of 10 ng/mL pegylated recombinant human MK growth and development factor (PEG-rhuMGDF, a truncated form of TPO; a generous gift from Kirin Laboratories, Tokyo, Japan) and 25 ng/mL recombinant human stem cell factor (SCF; a generous gift from Amgen, Thousand Oaks, CA).
Megakaryocytes were purified between day 8 and 11 with a CD41 monoclonal antibody (mAb) using a magnetic cell sorting system (miniMACS; Miltenyi Biotech) in accordance with the manufacturer's recommendations.
Preparation of total RNA
Total RNA was prepared from 10-day cultured purified MKs with the RNA+ reagent (Quantum; Appligene, Illkirch, France). RNA concentrations were determined spectrophotometrically. RNA was then repurified using the Genhunter kit (Genhunter, Nashville, TN) according to the manufacturer's instructions.
Differential display and cDNA isolation
First-strand cDNA was synthesized from 0.2 μg total RNA with each of the 3 anchored oligodeoxythymylic acid primers (H-T11-N) from Genhunter. The reaction (20 μL) was carried out at 37°C for 60 minutes. For polymerase chain reaction (PCR) amplification, 1 μL cDNA served as the template in an RNA image kit (Genhunter) with the same anchored primer used in the cDNA synthesis. The 5′ arbitrary primer length (AP primers) was used for differential display reverse-transcription–PCR (DDRT-PCR), carried out according to the manufacturer's protocol on total RNA. These RNAs were prepared from spontaneously grown MKs (3 patients with primitive myelofibrosis [PMF]) or 2 pools of normal MKs grown in presence of cytokines. Samples were subjected to 40 cycles of amplification under the following conditions: denaturing at 94°C for 30 seconds, annealing at 40°C for 2 minutes, extension at 72°C for 30 seconds, and a final extension at 72°C for 5 minutes. The resulting PCR products were resolved on a denaturing polyacrylamide gel and visualized by autoradiography of the dried gel. PCR products of interest were excised from the gel, and the DNA was eluted and reamplified by PCR using the same primers. However, to obtain blunt PCR products, PCR was performed using another polymerase Taqpfu (Stratagene, La Jolla, CA) and its recommended buffer. The PCR product was ligated into the TOPO-cloning vector (Invitrogen, Groningen, The Netherlands). Clones of the PCR-generated fragments were obtained by transformation of Escherichia coli strain TOP10 (Invitrogen). DNA sequences were determined for at least 3 independent clones of each fragment.
A total of 144 primer sets (AP primers) was used. For the gene of interest (fragment 49) generated with primers AP-76 and H-T11-A, the reamplified PCR product of differential display fragment 49 was subcloned with the TOPO-cloning system (Invitrogen). Several sublones of the 49 fragment were sequenced. Comparisons to the GenEmbl database were performed by using Infobiogen Package and BLAST2 (National Center for Biotechnology Information, Bethesda, MD) software.
Semiquantitative RT-PCR
Semiquantitative RT-PCR was performed using 2 different patients and normal samples than those previously used for DDRT-PCR. Primers were designed inside the sequence (3′ end) found by DDRT-PCR and were as follows: 5′ ACTCCCTCCACACCACAGTC 3′ and 5′ CCAGTAGCTTCCCAAAACCA 3′ for the antisense. Another set of primers was designed in the 5′ end of the cDNA (5′ TTTGACTGCAGAGATGTGGC 3′ and 5′ AAGGCAGCAAGGAGAAATGA 3′) to confirm this differential expression. The internal controls were actin and the MK-specific gene GPIIb for which primers were 5′-GTACCACAGGCATTGTGATG-3′ and 5′-GCAACATAGCACAGCTTCTC-3′ and 5′-TGACTGGCACACAGCTCTATGG-3′ and 5′-CACTCTGACCCAGGAACACCA-3′, respectively. PCR was performed as follows: denaturing at 94°C for 30 seconds, annealing at 40°C for 2 minutes, extension at 72°C for 30 seconds for 27, 29, 32, or 35 cycles for the FKBP51 and GPIIb mRNA and 25, 27, 29, and 32 cycles for actin mRNA.
Real-time RT-PCR
Primers and internal probes for amplification of GPIIb and FKBP51 mRNA were determined using the Primer Express Software (Perkin-Elmer Applied Biosystems, Foster City, CA). Primers sequences were 5′-GGGTCACTAATGAAAAAGGAACAGA-3′ and 5′-CTCTTCCCTCCTTGGCGTG-3′ for FKBP51 and 5′-TGACTGGCACACAGCTCTATGG-3′ and 5′-CACTCTGACCCAGGAACACCA-3′ for GPIIb. The sequences of internal probes for FKBP51 and GPIIb were 5′-CAATGGAAGAAGAGAAACCTGAGGGCCAC-3′ and 5′-ACCCCTGGGCGACCTCGACC-3′, respectively. The 18S primers were provided by Perkin-Elmer Applied Biosystems. All probes were labeled with FAM fluorescent. PCRs were carried out using the ABI Prism GeneAmp 5700 Sequence Detection System (Perkin-Elmer Applied Biosystems). All samples were treated with DNAse using the Genhunter kit to prevent amplification of any possible contaminating gDNA. Briefly PCR amplification was performed using the TaqMan Universal PCR Master Mix (Perkin-Elmer Applied Biosystems) containing primers (1.2 μM) and probes (0.1 μM). Conditions for PCR reactions included 2 minutes at 50°C, 10 minutes at 95°C, and 40 cycles of denaturation at 95°C for 15 seconds, and annealing/extension at 60°C for 1 minute. The SDS software was used to analyze fluorescent signals and calculate the cycle threshold. Quantitative normalization of cDNA in each sample was performed using expression of GPIIb and 18S genes (ribosomal control kit; Perkin-Elmer Applied Biosystems).
Immunofluorescence
Normal and IMF grown MKs were double-labeled. After cytocentrifugation, cells were fixed with 2% paraformaldehyde, permeabilized with 0.1% Triton, and labeled with an anti-FKBP51 mAb (FFI; gift from D. F. Smith, Mayo Clinic, Scottsdale, AZ) and a polyclonal anti–von Willebrand factor (VWF) antibody (Dako, Glostrup, Denmark). Donkey tetrarhodamine isothiocyanate (TRITC)–labeled antimouse, and fluorescein isothiocyanate (FITC)–labeled antirabbit goat F(ab′)2 fragments (Jackson Immunoresearch, West Grove, PA) were used as secondary antibodies.
Localization of FKBP51 in the different MK samples was analyzed using a Nikon fluorescence microscope (Nikon Eclipse E 600; Tokyo, Japan) equipped with filter sets for FITC and TRITC.
Samples were also analyzed by flow cytometry. Briefly, cells were collected, washed in phosphate-buffered saline (PBS) and resuspended in fixation medium (Harlan, Sera-Lab, Loughborough, England) for 15 minutes, then washed, incubated for 30 minutes at room temperature with mouse antihuman anti-FKBP51 mAb (HI57; gift from D. F. Smith) or control IgG1 (Becton Dickinson) and rabbit antihuman anti-VWF antibody in the permeabilization buffer. Cells were washed in PBS and resuspended in PBS with the FITC goat antimouse antibody and the phycoerythrin (PE) goat antirabbit antibody (Southern Biotechnology, Birmingham, AL and Silenius, Melbourne, Australia, respectively) and analyzed with a FACSort machine (Becton Dickinson).
Western blots
Soluble proteins obtained from approximately 5 × 104 to 5 × 106 cells lysed in Laemmli buffer (0.125 mM Tris [tris(hydroxymethyl)aminomethane], pH 6.8, 4% sodium dodecyl sulfate [SDS], 20% glycerol, 10% mercaptoethanol, 1 μg/mL aprotinin, and 0.02% bromophenol blue) were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE; 12%) and then transferred electrophoretically (130 mA, 90 minutes) onto a nitrocellulose membrane (Biorad, Hercules, CA) in a buffer containing 25 mM Tris, 192 mM glycine, 0.01% SDS, and 20% methanol. Nonspecific binding was inhibited by a preincubation with dried milk overnight at 4°C. The membranes were incubated for 90 minutes at room temperature with the different primary antibodies, including a rabbit polyclonal directed against GPIIb-IIIa (generous gift from D. Pidard, INSERM U485, Institut Pasteur, France), a rabbit antiactin antibody (Sigma-Aldrich), a rabbit polyclonal anticaspase-3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and a mouse mAb antihuman FKBP51 (gift from D. F. Smith). After 2 washes with TBS-Tween (10 mM Tris, pH 7.5,150 mM NaCl,0.1% Tween, 0.02% NaN3), the membranes were incubated for 30 minutes at room temperature with either peroxidase-conjugated goat antirabbit or sheep antimouse antibodies diluted at 1:5000 and 1:10 000, respectively (Amersham, Saclay, France). The bands were developed with an enhanced chemiluminescence system (ECL kit; Amersham).
Generation of the retrovirus and retroviral transduction
The following 3 constructs were used: Migr (long terminal repeat-internal ribosome entry site-enhanced green fluorescent protein [LTR-IRES-EGFP]; a generous gift from J. Miller, Philadelphia, PA) and Migr-FKBP51 (LTR-FKBP51-IRES-EGFP). The human FKBP51 and mutant (FKBP51/414) cDNA were both obtained from D. F. Smith and then inserted into the Migr retroviral vector. The FKBP51/414 mutant corresponds to normal FKBP51 cDNA deleted in the heat shock protein (HSP) binding site and previously demonstrated as inefficient to bind to the HSP70/HSP90 complex.
The retrovirus-producing cell line 293 EBNA (Invitrogen, Carlsbad, CA) was maintained in Dulbecco medium containing 10% fetal calf serum (FCS; Gibco BRL, Cergy-Ponbise, France). Vesicular stomatitis virus-G (VSV-G) pseudotyped retroviruses were produced by transient transfection of 293 EBNA cells by means of the Exgen reagent (Euromedex, Mundolsheim, France) and 3 plasmids: pCMV-G (VSV-G coding sequence); pCMV-gag-pol (both kindly supplied by Dr J. Morgenstern, Millenium, Boston, MA); and the Migr vector. Supernatant containing infectious retroviral particles was recovered and concentrated 20-fold by means of an Amicon (Millipore, Bedford, MA). Viral titers were determined by limiting dilution assay on NIH 3T3 cells according to the EGFP fluorescence, and ranged from 106 to 107viral particles per milliliter. UT-7/Mpl cells or normal cytapheresis CD34+ cells were cultured in IMDM supplemented with 10% FCS with penicillin-streptomycin and 50% retroviral supernatants. Granulocyte-macrophage colony-stimulating factor (GM-CSF; a gift from Novartis, Basel, Switzerland; 10 ng/mL) or the combination of interleukin 3 (IL-3; 100 UI/mL), Flt-3 ligand (50 ng/mL; gifts from Immunex, Seattle, WA), SCF (50 ng/mL; gift from Amgen), and PEG-rhuMGDF (10 ng/mL; gift from Kirin Laboratories) were added, respectively. Three days after infection, cells were washed and EGFP+cells were purified using a FACSVantage cytometer (Becton Dickinson).
Cytokine deprivation test
The UT-7/Mpl cells overexpressing FKBP51 and their controls (UT-7/Mpl expressing GFP) were amplified in IMDM with GM-CSF. Cells were washed 3 times in PBS and then plated at 105 cells/mL in IMDM supplemented with 10% FCS in the absence of cytokine. Each day for 1 to 7 days, cells were counted using the trypan blue exclusion test to measure proliferation or an methyl-thiazol-diphenyl-tetrazolium (MTT) assay for survival. CD34+ cells were cultured in serum-free medium without cytokine at 104 cells/mL. Each day for 7 days, cells were counted using the trypan blue exclusion test.
Annexin V labeling
The UT-7/Mpl cells overexpressing FKBP51 and their controls were deprived of cytokines for 24 hours and labeled by annexin-V–PE for 15 minutes at room temperature in the dark and counterstained with 7-amino actinomycin D (7-AAD), according to the manufacturer's instructions (Becton Dickinson). Cells were analyzed by flow cytometry.
Chemotherapy-induced apoptosis
The UT-7/Mpl cells overexpressing FKBP51 and their controls were amplified in IMDM with GM-CSF. Cells were washed 3 times in PBS and plated at 105cells/mL in IMDM supplemented with 10% FCS and GM-CSF (10 ng/mL) and with or without doxorubicin (Dakota Pharm, Sanofi Winthrops, France; 5 μM); 24 hours later viable cells were counted using the trypan blue exclusion test.
Calcineurin activity measurement
The UT-7/Mpl cells overexpressing FKBP51 and their controls were amplified in Dulbecco modified Eagle medium (DMEM) with GM-CSF. Cells were washed 3 times in PBS and plated at 105 cells/mL in IMDM supplemented with 10% FCS without cytokine. Twenty-four hours after cytokine deprivation, cells were counted and aliquots of 1.5 × 105 cells were frozen until used.
To determine the calcineurin (PP2B) activity in cells, we used a nonradioactive enzyme assay based on a peptide dephosphorylation kinetic. The activity is defined as the initial velocity determined on the first 6 minutes of its Hill kinetic.20
Briefly, 1.5 × 105 frozen cells were resuspended in 20 μL lysis buffer consisting of KCl 1 M, Tris-HCl 1 M, pH 8.3, Nonidet P40, and Tween 20. Lysates were mixed with 140 μL assay buffer containing 50 mM Tris-HCl, pH 7.0, 0.1 mM EGTA (ethyleneglycoltetraacetic acid), 0.5 mM dithiothreitol, 0.3 mg/mL BSA, 0.1 μM calmodulin, 1 mM MnCl2, 1 mM CaCl2, and 500 nM okadaic acid. Addition of okadaic acid inhibits PP1 and PP2A activity and omission of magnesium inhibits PP2C activity. Thus, selective inhibition of these 3 phosphatases gives a specificity to the calcineurin activity determination.
The substrate used was a synthetic 19–amino acid peptide corresponding to a portion of the regulatory subunit of type II (RII) cyclic adenosine monophosphate (cAMP)–dependent protein kinase (Bachem, Bubendorf).
The spectrophotometric detection of peptide dephosphorylation by calcineurin was performed using high-performance liquid chromatography (HPLC) on a RP 18 column (Lichrocart 125-4 Lichrospher 100 RP-18, 5-μm; Merck, Darmstadt, Germany). The mobile phase composed of a KH2PO4 buffer, 10 mM, pH 5.9, and acetonitrile (ACN) was run under gradient conditions (initial: 87/13 KH2PO4/ACN, 83/17 KH2PO4/ACN for 6-11 minutes and return at initial conditions for 11-14 minutes).
Results
Identification of FKBP51 cDNA as an MK gene differentially expressed between controls and patients with IMF
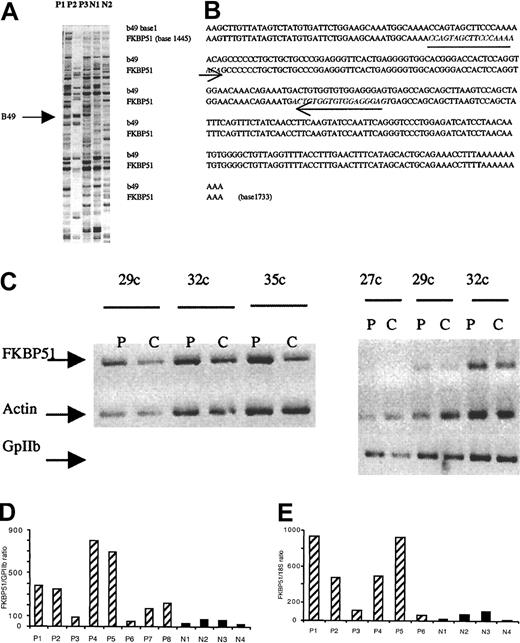
A DDRT-PCR technique was used to identify genes that could be involved in the abnormal cytokine response of IMF MKs. A major advantage of DDRT-PCR is its capability to amplify minute amounts of transcripts and to rapidly determine their nucleotide sequence. The following protocol was used. Purified CD34+ cells were cultured in serum-free, cytokine-free medium for the pathologic samples or in the presence of SCF and TPO for samples from individuals without hematologic disease. At day 10 of culture, CD41+ MKs were purified immunomagnetically and total RNA was harvested. The purity in MKs was over 90% in all samples studied after reanalysis by flow cytometry. To prevent artifacts, samples from 3 patients (P1, P2, and P3) were compared to those of 2 pools from healthy subjects. One band, designated b49, was found to be overexpressed in the 3 patients and was cloned and sequenced (Figure 1A). Sequence analysis using BLAST-2 analysis demonstrated that the cloned PCR product corresponded to the 3′ end of human FKBP51 (sequence homology, Figure 1B).
Characterization of FKBP51 as a gene overexpressed in PMF megakaryocytes.
(A) PCR differential screening. mRNA from normal or PMF spontaneously grown megakaryocytes were reverse transcribed and amplified by PCR using AP76 and H-T11-A primers (Genhunter). Amplified cDNAs were run side by side on a sequencing gel. The arrow indicates b49 band only amplified in myelofibrosis samples. (B) Alignment of b49 (288 bp) and FKBP51. Alignment was performed using LFASTAn-LALIGNn program from Infobiogen (Villejuif, France). Sequence homology was 99.6%; b49 alignment was located at the 3′end of the cDNA (base 1445-1733). The 2 18mer initially used to confirm the differential expression in semiquantitative RT-PCR are underlined. (C) Semiquantitative RT-PCR from normal (C) and pathologic (P) MKs. Internal differential display primers were designed from b49 and 27, 29, 32, and 35 cycles were performed to amplify actin and GPIIb. Similar results were obtained with 2 other pathologic samples. (D,E) Real-time quantitative RT-PCR. Real-time quantitative RT-PCR was performed with RNA extracted from spontaneously growing MKs from 8 patients (P1-P8) and MKs grown in presence of SCF and TPO from 4 controls (N1-N4). MKs were immunomagnetically purified; purity of recovered cells was determined by flow cytometry and was over 90%. Ratio of FKBP51 expression on GPIIb mRNA expression showed a 2- to 10-fold increase in FKBP51 expression except in one patient (P6). P6 presented an acute transformation of the disease. The same results were obtained with a ratio between FKBP51 expression and 18S in 6 patients (P1-P6; panel E).
Characterization of FKBP51 as a gene overexpressed in PMF megakaryocytes.
(A) PCR differential screening. mRNA from normal or PMF spontaneously grown megakaryocytes were reverse transcribed and amplified by PCR using AP76 and H-T11-A primers (Genhunter). Amplified cDNAs were run side by side on a sequencing gel. The arrow indicates b49 band only amplified in myelofibrosis samples. (B) Alignment of b49 (288 bp) and FKBP51. Alignment was performed using LFASTAn-LALIGNn program from Infobiogen (Villejuif, France). Sequence homology was 99.6%; b49 alignment was located at the 3′end of the cDNA (base 1445-1733). The 2 18mer initially used to confirm the differential expression in semiquantitative RT-PCR are underlined. (C) Semiquantitative RT-PCR from normal (C) and pathologic (P) MKs. Internal differential display primers were designed from b49 and 27, 29, 32, and 35 cycles were performed to amplify actin and GPIIb. Similar results were obtained with 2 other pathologic samples. (D,E) Real-time quantitative RT-PCR. Real-time quantitative RT-PCR was performed with RNA extracted from spontaneously growing MKs from 8 patients (P1-P8) and MKs grown in presence of SCF and TPO from 4 controls (N1-N4). MKs were immunomagnetically purified; purity of recovered cells was determined by flow cytometry and was over 90%. Ratio of FKBP51 expression on GPIIb mRNA expression showed a 2- to 10-fold increase in FKBP51 expression except in one patient (P6). P6 presented an acute transformation of the disease. The same results were obtained with a ratio between FKBP51 expression and 18S in 6 patients (P1-P6; panel E).
We focused our study on FKBP51 because FKBP51 is a member of a highly homologous multigene family containing at least 5 other proteins that can bind FK506 (FK506 binding protein). FKBP51 can inhibit calcineurin when complexed with FK506.21 In addition, FKBP51 binds to HSP90/HSP70 and associates to steroid receptors.22 The precise function of FKBP51 in hematopoietic cells is presently unknown; however, as a consequence of its protein interactions, FKBP51 may play an important role in cell survival, proliferation, and differentiation.
To verify the initial differential display results, oligonucleotides specific for human FKBP51 gene were designed both in the 3′ and 5′ ends of the FKBP51 cDNA sequence. A semiquantitative RT-PCR technique was applied on samples from 3 other patients (P4, P5, and P6) as well as from 2 previously studied patients (P1 and P2). Using the 2 sets of primers, identical results were obtained confirming a moderate overexpression (1.5- to 8-fold) of FKPB51 in all but one (P6) PMF- derived MK (Figure 1C). In fact, this last patient was in an accelerated phase of the disease with an acute transformation 3 weeks later. Because the differences were overall moderate, we developed a real-time quantitative RT-PCR using TaqMan probes to confirm this differential level of FKPB51 transcripts. Regression curve was linear allowing quantification. The GPIIb (an MK-specific gene) and 18S (an ubiquitous reporter gene) transcripts were used for normalization in 6 and 8 patients, respectively. The results illustrated in Figure 1, panels D and E, showed a 2- to 10-fold increase in the FKBP51 transcripts present in IMF MKs from all patients except P6. The sample from patient P3 was the one with the lowest increase in FKBP51 transcript. However, the FKBP51/18S ratio was 2-fold higher than normal control (87.7 versus 46.1 for the patient sample when compared to the median of normal samples). Moreover, a similar result was obtained when results were normalized to GPIIb (FKBP51/GPIIb ratio 117.4 for P3 and 52.6 in median for normal controls). Thus, results of the semiquantitative and real-time RT-PCR analyses were concordant, affording evidence that these differences in FKBP51 level were significant. However, because MKs were obtained in 2 different conditions (in the presence of cytokine for normal MKs and without cytokines for IMF MKs), it was possible that FKBP51 transcripts could be down-regulated by cytokines. To eliminate this hypothesis, normal MKs were grown in the presence of SCF and TPO. At day 9 of culture, half of the cultured cells were deprived of cytokines overnight and FKBP51 transcripts were quantified by real-time quantitative RT-PCR in the 2 samples. No difference was observed (data not shown) suggesting that overexpression of FKBP51 transcript could be related to the molecular mechanism of the disease.
The FKBP51 protein is increased in IMF MKs
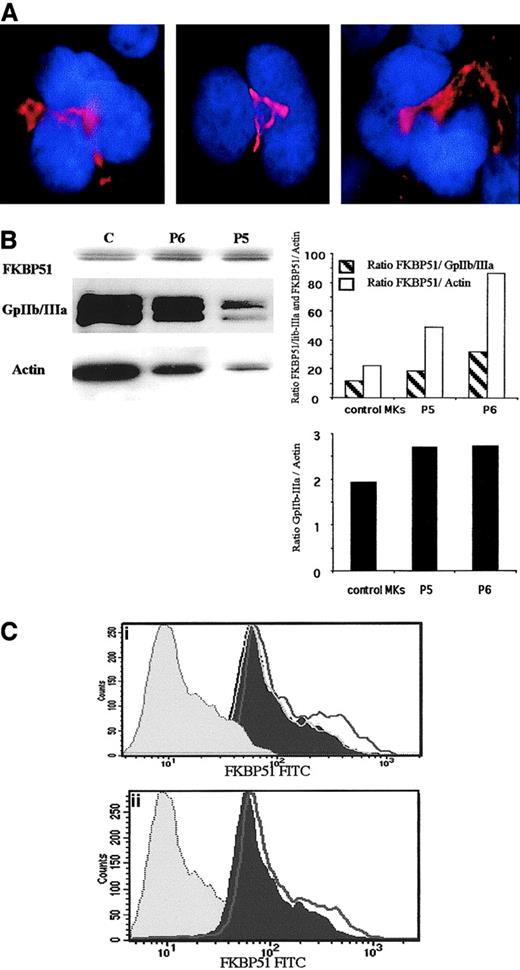
To confirm that FKBP51 mRNA overexpression in IMF MKs leads to an increase in the protein level, immunofluorescence and Western blot analysis were performed. In normal and IMF MKs, FKBP51 was present in the cytoplasm in an area located near the nucleus (Figure2A). Western blots were subsequently performed and FKBP51 protein appeared as a doublet at around 50 kDa. To compare the level of FKBP51 in normal and IMF MKs, blots were sequentially rehybridized with an anti–GPIIb-IIIa antibody and an antiactin antibody. The FKBP51 band was scanned and normalized to the level of the GPIIb band and actin band. In the 2 patient samples, a 2- to 3-fold increase in the FKBP51/GPIIb and the FKBP51/actin ratios was observed in comparison to normal MKs. Moreover, the GpIIb/actin ratio was calculated to eliminate a possible contamination of MKs by other cells and was not markedly different among samples (Figure 2B). To further confirm that the observed increase was not due to the presence of rare contaminant cells (FKBP51 being broadly expressed), flow cytometry was performed using a double-staining technique with an anti-VWF polyclonal antibody to identify MKs and an anti-FKBP51 mAb. An increase in the FKBP51 protein expression in IMF MKs was noticed in 4 of 6 patient samples when compared to normal MKs. Results from 2 typical samples are illustrated in Figure 2C. These moderate differences were significant because FKBP51 expression was found exactly identical in MKs derived from 3 normal samples (Figure2C).
Expression of FKBP51 in normal and IMF MKs.
(A) Immunofluorescence localization of FKBP51 in normal MKs. Normal MKs were cultured from CD34+ cells in the presence of TPO and SCF. After cytocentrifugation, cells were fixed, permeabilized, and labeled with an anti-FKBP51 mAb. TRITC-labeled donkey antimouse F(ab′)2 was used as secondary antibody. Cells were counterstained with DAPI (4,6 diamidino-2-phenylindole). Localization of FKBP51 in the different MK samples (in red) was analyzed using a fluorescence microscope. A cytoplasmic labeling was observed with a peculiar pattern, which could correspond to the Golgi apparatus. Original magnification, × 1000. (B) FKBP51 expression in normal and pathologic MKs studied by Western blot. Protein extracts from spontaneously growing MKs from 2 patients (P5 and P4) and one normal leukapheresis product, CD34+cell-derived MKs (cultured in presence of TPO), were separated by SDS-PAGE. Immunoblotting was performed with the anti-FKBP51 antibody, anti–GpIIb-IIIa antibody, and an antiactin antibody in the same membrane. Ratios of FKBP51/GpIIb and FKBP51/actin are illustrated as well as GpIIb/actin ratio. An increase in the FKBP51/GpIIb and FKBP51/actin ratios was observed in the 2 samples derived from PMF, whereas the GpIIb/actin was almost constant. (C) Differential FKBP51 protein expression in normal and IMF MKs by flow cytometry. Spontaneously growing MKs from IMF patients and normal MKs were doubly labeled with an anti-VWF polyclonal antibody and an anti-FKBP51 mAb by indirect immunofluorescence. Analysis of FKBP51 expression was performed in the VWF+e gate. In panels Ci and Cii, the histogram filled in gray illustrates the control isotype(IgG-FITC). The histogram filled in black illustrates the graph of a healthy control. The unfilled curve in black line shows the graph of 2 different patients. In panel Ci, 2 other controls are shown (gray lines). The 3 control sample curves are superimposed. The 2 illustrated patient MKs express a low but significant increase in FKBP51 expression.
Expression of FKBP51 in normal and IMF MKs.
(A) Immunofluorescence localization of FKBP51 in normal MKs. Normal MKs were cultured from CD34+ cells in the presence of TPO and SCF. After cytocentrifugation, cells were fixed, permeabilized, and labeled with an anti-FKBP51 mAb. TRITC-labeled donkey antimouse F(ab′)2 was used as secondary antibody. Cells were counterstained with DAPI (4,6 diamidino-2-phenylindole). Localization of FKBP51 in the different MK samples (in red) was analyzed using a fluorescence microscope. A cytoplasmic labeling was observed with a peculiar pattern, which could correspond to the Golgi apparatus. Original magnification, × 1000. (B) FKBP51 expression in normal and pathologic MKs studied by Western blot. Protein extracts from spontaneously growing MKs from 2 patients (P5 and P4) and one normal leukapheresis product, CD34+cell-derived MKs (cultured in presence of TPO), were separated by SDS-PAGE. Immunoblotting was performed with the anti-FKBP51 antibody, anti–GpIIb-IIIa antibody, and an antiactin antibody in the same membrane. Ratios of FKBP51/GpIIb and FKBP51/actin are illustrated as well as GpIIb/actin ratio. An increase in the FKBP51/GpIIb and FKBP51/actin ratios was observed in the 2 samples derived from PMF, whereas the GpIIb/actin was almost constant. (C) Differential FKBP51 protein expression in normal and IMF MKs by flow cytometry. Spontaneously growing MKs from IMF patients and normal MKs were doubly labeled with an anti-VWF polyclonal antibody and an anti-FKBP51 mAb by indirect immunofluorescence. Analysis of FKBP51 expression was performed in the VWF+e gate. In panels Ci and Cii, the histogram filled in gray illustrates the control isotype(IgG-FITC). The histogram filled in black illustrates the graph of a healthy control. The unfilled curve in black line shows the graph of 2 different patients. In panel Ci, 2 other controls are shown (gray lines). The 3 control sample curves are superimposed. The 2 illustrated patient MKs express a low but significant increase in FKBP51 expression.
Overexpression of FKBP51 protein induces prolonged cell survival after cytokine deprivation
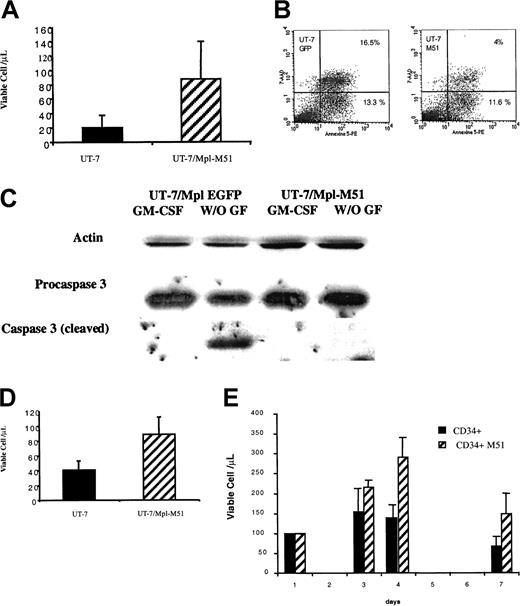
To examine the consequences of FKBP51 overexpression on the response to cytokines, we constructed a bicistronic retrovirus (Migr) containing the entire coding region of FKBP51 and the GFP. Both cDNAs are under the control of the viral LTR and are separated by an EMCV1 IRES. Retrovirus encoding a VSV-G envelope was produced and used to overexpress FKBP51 in UT-7/Mpl cells, a factor-dependent human cell line with an MK phenotype.23 24 Infected cells were selected on the EGFP expression and were tested for their proliferation, differentiation, and survival capacities. No differences in thymidine uptake were observed between parental or transduced cells when cells were stimulated by cytokines (GM-CSF or TPO), even at low cytokine concentration (data not shown). No change in the expression of differentiation antigens such as CD41 and glycophorin A was observed in the cytokine-deprived UT-7/Mpl cells overexpressing FKBP51 (data not shown). In contrast, FKBP51 overexpressing UT-7/Mpl cells were markedly resistant to cytokine deprivation in comparison to the UT-7/Mpl cells infected by the control vector (Migr-EGFP; Figure3A). Indeed, cells remained alive for more than 5 days after cytokine deprivation, whereas 50% of the control cells died in 2 days. After 36 hours of cytokine deprivation, the percentage of annexin-V+ cells was markedly decreased in the UT-7/Mpl cell line overexpressing FKBP-51 cells in comparison to control cells (Figure 3B). This resistance to apoptosis was further confirmed when we studied the cleavage of procaspase-3. Cleavage was detected in control cells but not in UT-7/Mpl overexpressing FKBP51 after 24 hours of cytokine deprivation (Figure 3C). In the absence of cytokine, almost no increase in cell number was observed suggesting that FKBP51 overexpression essentially counteracts cytokine deprivation-induced apoptosis but has no main effect on proliferation. In favor of this hypothesis, cells overexpressing FKBP51 were also less sensitive to other apoptotic agents such as doxorubicin (Figure 3D). Similar results were observed after 3 independent retroviral infections of UT7/Mpl cells.
Increased survival in FKBP51 overexpressing cells.
(A) UT-7/Mpl cell survival in cytokine-free medium. UT-7/Mpl cells were infected with a retrovirus encoding both FKBP51 and EGFP or a control vector containing EGFP alone. EGFP+ cells were sorted and permanent cell lines were obtained in the presence of GM-CSF. Cells were deprived in GM-CSF and living cells were counted at day 2 by trypan blue exclusion test or MTT test. Results from the trypan blue exclusion test are presented. EGFP+ cells and the wild-type UT-7/Mpl cells have similar survival, whereas FKBP51 overexpressing UT-7 stay alive for up to 5 days after cytokine deprivation (P = .0035). (B) Increased survival of UT-7/Mpl overexpressing FKBP51 is related to a resistance to apoptosis. UT-7/Mpl expressing EGFP and UT-7/Mpl overexpressing FKBP51 cells (UT-7/Mpl-M51) were grown in the presence of GM-CSF. Cytokine deprivation was performed for 36 hours, and binding of annexin V was assessed by flow cytometry. Cells dead by apoptosis (positive for both 7-AAD and annexin V) were markedly reduced in UT-7/Mpl-M51 cells (4%) in comparison to UT-7/Mpl-EGFP cells (16.5%). (C) Inhibition of procaspase-3 cleavage by overexpression of FKBP51. Western blots were performed in UT-7/Mpl expressing EGFP and UT-7/Mpl cells overexpressing FKBP51 cultured with GM-CSF or without growth factor for 24 hours. Cleavage of procaspase-3 was noticed in normal cells after cytokine withdrawal (lane 2) but not in UT-7/Mpl cells overexpressing FKBP51 (lane 4). (D) UT-7/Mpl cell survival in GM-CSF after daunorubicin treatment. UT-7/Mpl expressing EGFP and UT-7/Mpl overexpressing FKBP51 cells (UT-7/Mpl-M51) were grown in the presence of GM-CSF and treated by 5 μM daunorubicin. Cell survival was evaluated 24 hours later (P = .023). (E) CD34+ cell survival in serum-free cytokine-free medium. Normal CD34+ cells were transduced by retroviral vector encoding both FKBP51 and EGFP or EGFP alone after 48 hours of stimulation by cytokines. Cells were then cultured in serum-free medium at 50 000 cells/mL. Cell survival was evaluated by counting cells each day using the trypan blue exclusion test. Results are the average of 3 experiments (day 3,P = .083; day 4, P = .027; day 7,P = .023).
Increased survival in FKBP51 overexpressing cells.
(A) UT-7/Mpl cell survival in cytokine-free medium. UT-7/Mpl cells were infected with a retrovirus encoding both FKBP51 and EGFP or a control vector containing EGFP alone. EGFP+ cells were sorted and permanent cell lines were obtained in the presence of GM-CSF. Cells were deprived in GM-CSF and living cells were counted at day 2 by trypan blue exclusion test or MTT test. Results from the trypan blue exclusion test are presented. EGFP+ cells and the wild-type UT-7/Mpl cells have similar survival, whereas FKBP51 overexpressing UT-7 stay alive for up to 5 days after cytokine deprivation (P = .0035). (B) Increased survival of UT-7/Mpl overexpressing FKBP51 is related to a resistance to apoptosis. UT-7/Mpl expressing EGFP and UT-7/Mpl overexpressing FKBP51 cells (UT-7/Mpl-M51) were grown in the presence of GM-CSF. Cytokine deprivation was performed for 36 hours, and binding of annexin V was assessed by flow cytometry. Cells dead by apoptosis (positive for both 7-AAD and annexin V) were markedly reduced in UT-7/Mpl-M51 cells (4%) in comparison to UT-7/Mpl-EGFP cells (16.5%). (C) Inhibition of procaspase-3 cleavage by overexpression of FKBP51. Western blots were performed in UT-7/Mpl expressing EGFP and UT-7/Mpl cells overexpressing FKBP51 cultured with GM-CSF or without growth factor for 24 hours. Cleavage of procaspase-3 was noticed in normal cells after cytokine withdrawal (lane 2) but not in UT-7/Mpl cells overexpressing FKBP51 (lane 4). (D) UT-7/Mpl cell survival in GM-CSF after daunorubicin treatment. UT-7/Mpl expressing EGFP and UT-7/Mpl overexpressing FKBP51 cells (UT-7/Mpl-M51) were grown in the presence of GM-CSF and treated by 5 μM daunorubicin. Cell survival was evaluated 24 hours later (P = .023). (E) CD34+ cell survival in serum-free cytokine-free medium. Normal CD34+ cells were transduced by retroviral vector encoding both FKBP51 and EGFP or EGFP alone after 48 hours of stimulation by cytokines. Cells were then cultured in serum-free medium at 50 000 cells/mL. Cell survival was evaluated by counting cells each day using the trypan blue exclusion test. Results are the average of 3 experiments (day 3,P = .083; day 4, P = .027; day 7,P = .023).
To confirm these results on normal cells, the same approach was performed on CD34+ cells. Overexpression of FKBP51 induced a significant increase in survival of CD34+ cells after cytokine deprivation and cells could be kept in culture for more than 7 days (Figure 3 E).
We next investigated if the effects of FKBP51 were dependent on its expression level. For this purpose, we cloned UT-7/Mpl on the expression of EGFP by cell sorting. Only the clones, which express intermediate or high levels of EGFP, were resistant to cytokine deprivation (data not shown).
This result demonstrates that FKBP51 overexpression impairs apoptosis. Thus, we investigated which of the 2 known protein interactions (HSP70/HSP90 complex and calcineurin) could be involved in this antiapoptotic effect.
Resistance to cytokine deprivation after FKBP51 overexpression is not related to an activation of the HSP70/HSP90 pathway
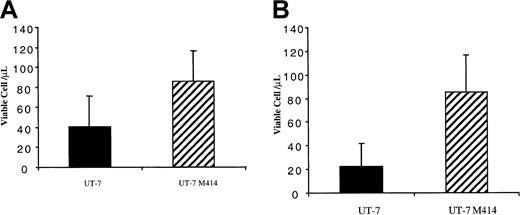
To determine if the HSP70/HSP90 interaction was involved in the antiapoptotic activity mediated by FKBP51, a FKBP51 mutant (FKBP51-414) that does not bind to HSP70/HSP90 was overexpressed in the UT-7/Mpl cell line using the Migr retroviral vector. FKBP51-414 overexpressing cell lines were obtained by cell sorting in 3 independent retroviral infections. These 3 cell lines were much more resistant to cytokine deprivation or doxorubicin treatment than the control (Figure4A,B). Thus, a FKBP51 deleted in the TPR domain and then having lost its HSP70/HSP90 binding capability kept the antiapoptotic activity. This suggests that resistance to cytokine deprivation mediated by FKBP51 is not due to HSP binding.
FKBP51 lacking the TPR sequence continues to promote cell survival.
UT-7/Mpl cells were infected with a retrovirus encoding both the FKBP51 mutant (FKBP51-414) and EGFP or a control retrovirus encoding EGFP alone. EGFP+ cells were sorted and permanent cell lines were obtained in the presence of GM-CSF. (A) At 24 hours, UT-7 cell survival in GM-CSF after daunorubicin treatment. Cells were deprived in GM-CSF and living cells were counted at day 2 by the trypan blue exclusion test or MTT test. Results from the trypan blue exclusion test are presented. EGFP+ cells and the wild-type UT-7 cells have similar survival, whereas UT-7/Mpl 414 cells stay alive for up to 5 days after cytokine deprivation (P = .00037). (B) UT-7/Mpl cell survival in cytokine-free medium. Cells were deprived in GM-CSF and living cells were counted at day 2 by trypan blue exclusion test or MTT test. Results from the trypan blue exclusion test are presented. EGFP+ cells and the wild-type UT-7 cells have similar survival whereas FKBP51 overexpressing UT-7 stay alive for up to 5 days after cytokine deprivation.
FKBP51 lacking the TPR sequence continues to promote cell survival.
UT-7/Mpl cells were infected with a retrovirus encoding both the FKBP51 mutant (FKBP51-414) and EGFP or a control retrovirus encoding EGFP alone. EGFP+ cells were sorted and permanent cell lines were obtained in the presence of GM-CSF. (A) At 24 hours, UT-7 cell survival in GM-CSF after daunorubicin treatment. Cells were deprived in GM-CSF and living cells were counted at day 2 by the trypan blue exclusion test or MTT test. Results from the trypan blue exclusion test are presented. EGFP+ cells and the wild-type UT-7 cells have similar survival, whereas UT-7/Mpl 414 cells stay alive for up to 5 days after cytokine deprivation (P = .00037). (B) UT-7/Mpl cell survival in cytokine-free medium. Cells were deprived in GM-CSF and living cells were counted at day 2 by trypan blue exclusion test or MTT test. Results from the trypan blue exclusion test are presented. EGFP+ cells and the wild-type UT-7 cells have similar survival whereas FKBP51 overexpressing UT-7 stay alive for up to 5 days after cytokine deprivation.
FKBP51 overexpression induces a decrease in calcineurin phosphatase activity
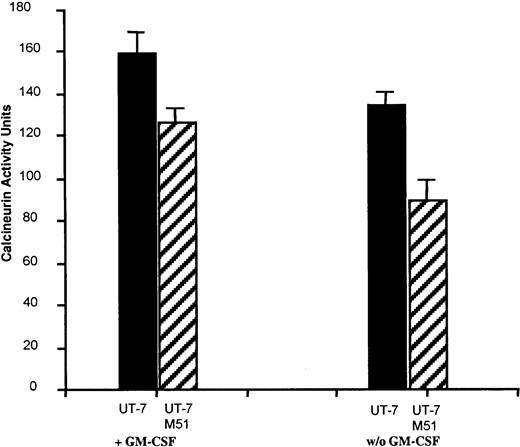
Subsequently, we examined if overexpression of FKBP51 could inhibit the calcineurin pathway in UT-7/Mpl cells. Calcineurin activity was measured in UT-7 cells overexpressing GFP and UT-7/Mpl cells overexpressing FKBP51 stimulated or not by cytokine. A high calcineurin phosphatase activity was detected in control UT-7/Mpl cells stimulated by GM-CSF. This activity was in the same order of magnitude as in activated lymphocytes.20 After cytokine deprivation, this level slightly decreased. In contrast, calcineurin activity was low in the different cell lines overexpressing FKBP51 either in the presence or the absence of cytokines (Figure 5) indicating that FKBP51 inhibits calcineurin activity.
Calcineurin activity in UT-7/Mpl cells.
Calcineurin activity was measured in 1.5 × 105 UT-7/Mpl EGFP cells or cells overexpressing FKBP51 cultured with or without GM-CSF. Three independent experiments were performed in triplicate. Results represented the mean of these experiments. These results are similar to those obtained with normal lymphocytes for the EGFP UT-7 cells and with FK506 or CsA-treated patients lymphocytes for the FKBP51 overexpressing cells.20
Calcineurin activity in UT-7/Mpl cells.
Calcineurin activity was measured in 1.5 × 105 UT-7/Mpl EGFP cells or cells overexpressing FKBP51 cultured with or without GM-CSF. Three independent experiments were performed in triplicate. Results represented the mean of these experiments. These results are similar to those obtained with normal lymphocytes for the EGFP UT-7 cells and with FK506 or CsA-treated patients lymphocytes for the FKBP51 overexpressing cells.20
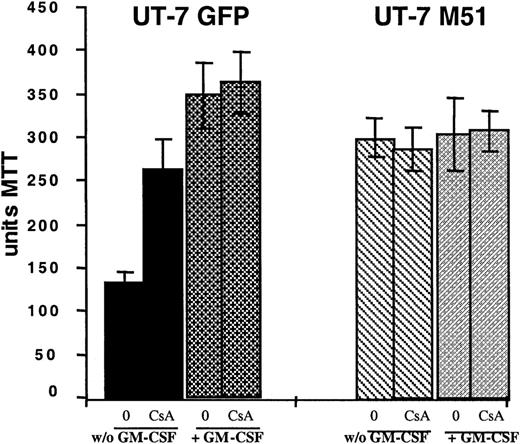
To determine whether the antiapoptotic effect of FKBP51 was related to an inhibition of calcineurin, we treated parental UT-7/Mpl with the immunosuppressive drug cyclosporin A (CsA), known to inhibit calcineurin activity.25 As illustrated in Figure6, CsA treatment at a dosage of 1 μg/mL was not toxic for the cells; although treatment did not increase the survival of UT-7/Mpl M51 cells, it demonstrates a significant increment in the survival of the parental UT-7/Mpl cells deprived of cytokine. The extent of survival of the CsA-treated parental cell line was similar to what seen in the transfected cells.
Effect of CsA on UT-7/Mpl cell survival.
UT-7 cells overexpressing EGFP or FKBP51 were cultured with or without GM-CSF and with or without CsA (1 μg/mL) for 48 hours. The MTT test was performed. Cell survival was tested by an MTT test. CsA partially reverted cell death induced by cytokine withdrawal in normal cells but had no significant effect on the survival of UT-7/Mpl cells overexpressing FKBP51.
Effect of CsA on UT-7/Mpl cell survival.
UT-7 cells overexpressing EGFP or FKBP51 were cultured with or without GM-CSF and with or without CsA (1 μg/mL) for 48 hours. The MTT test was performed. Cell survival was tested by an MTT test. CsA partially reverted cell death induced by cytokine withdrawal in normal cells but had no significant effect on the survival of UT-7/Mpl cells overexpressing FKBP51.
Thus, these results support the conclusion that FKBP51 induces resistance to apoptosis through an inhibition of the calcineurin signal transduction pathway. The precise signaling pathways modified by this calcineurin inhibition will require further investigation.
Discussion
Idiopathic myelofibrosis is a myeloproliferative disease, characterized by a clonal MK proliferation and a secondary bone marrow fibrosis. Myelofibrosis is associated with an osteosclerosis that is responsible for most clinical symptoms of the disease including extramedullary hematopoiesis and pancytopenia. There is evidence that fibrosis is due to the release of MK-derived fibrotic cytokines such as transforming growth factor-β1,26,27platelet-derived growth factor,28,29 and platelet factor 4,30,31 which are able to stimulate fibroblast proliferation and collagen deposition.32 In addition, MKs also synthesize vascular endothelial growth factor and thrombospondin, 2 cytokines that are able to stimulate the bone marrow angiogenesis associated with the myelofibrosis.33 However, the mechanisms that lead to the release of these cytokines by MKs in the marrow environment are poorly understood. It has been suggested that this was due (1) to dysplastic MKs that die in the marrow,5 (2) to cell death induced by polymorphonuclear cells after their emperipolesis by MKs,34,35 or (3) to a spontaneous release of the MK α-granule content. However, another hypothesis was proposed that placed monocytes as a central key in the pathogenesis of fibrosis.8,15 Therefore, it is not excluded that the combination of an MK hyperplasia and a monocyte activation is required to obtain the development of a myelofibrosis. Whatever the precise mechanism, MK hyperplasia is a key phenomenon in the development of IMF. This suggests that the primary mechanisms responsible for the development of IMF could be a defective megakaryocytic regulation, which leads to an MK accumulation.36 It has been previously demonstrated that IMF MK progenitors are hypersensitive to TPO and a part of them are able to differentiate in the absence of cytokine.13,37Hypersensitivity to cytokines of hematopoietic progenitors appears to be a general biologic hallmark of the different myeloproliferative disorders. Understanding the mechanisms of cytokine hypersensitivity in IMF is important for the comprehension of the molecular mechanism(s) responsible for this disease and for the development of new therapeutic approaches. Indeed, most treatments of IMF are symptomatic. Allogeneic hematopoietic stem cell transplantation may be the only treatment capable of curing the disease, but it can be applied to a minority of patients.38
To investigate the mechanisms responsible for spontaneous MK growth, we performed a differential display approach and found FKBP51 as a gene overexpressed in spontaneously grown MKs from the 8 of 10 patients with IMF studied. The comparison was performed with normal cytokine-stimulated MKs, a population that clearly differs from the spontaneously growing MKs by the absence of selection pressure. However, we could eliminate that FKBP51 expression was regulated primarily by cytokines, thus suggesting that this deregulated expression was involved in the spontaneous growth mechanism.
Expression of FKBP51 was considered to be mainly restricted to the T-cell lineage in the mouse but it is more ubiquitously expressed in humans.21,39 Its overexpression in a cytokine-dependent MK cell line and normal CD34+ cells leads to an increased cell survival after cytokine deprivation. FKBP51 is known as an immunophillin that can regulate FK506-induced calcineurin inhibition, a calcium–calmodulin-regulated serine/threonine phosphatase21,39,40 and binds to the HSP70/HSP90 complex.41-45 In our report, we show that FKBP51 overexpression per se inhibits calcineurin activity. This result suggests that FKBP51 physiologically regulates calcineurin activity either alone or by complexing an unknown cellular protein. In addition, we present some evidence that the antiapoptotic effect of FKBP51 is mediated through inhibition of calcineurin activity. Indeed, this effect could be due to the interaction of FKBP51 with the HSP70/HSP90 complex. HSP70 plays a marked role in the apoptotic process by antagonizing apoptosis-inducing factor.46 HSP90 is also an antiapoptotic molecule by stabilizing several kinases such as Raf-147 and Src.48 Nevertheless, an FKBP51 mutant, lacking the tetratricopeptide repeat (TPR) sequence involved in binding to the HSP complex, retains the antiapoptotic effect of the wild-type molecule, thus suggesting that the HSP pathway is not involved in the antiapoptotic properties of FKBP51. However, we cannot totally exclude that such a mutant could still interfere with HSP70 and HSP90, but from our experiment it seems more likely that inhibition of calcineurin was responsible of the antiapoptotic effects of FKBP51. Calcineurin may have apoptotic or antiapoptotic effects depending on the cellular environment because many of its effects are mediated through activation by dephosphorylation of transcription factors such as nuclear factor of activated T cells (NF-AT).49-51 The role of the calcineurin pathway in the myeloid lineages is poorly understood. However, results obtained in a myeloid cell line suggest that it has a predominant apoptotic effect.52 Calcineurin may have direct apoptotic effects by inducing dephosphorylation of Bad.53 In addition, there is a cross-talk between calcineurin and several signaling pathways. Calcineurin regulates the NF-κB pathway by dephosphorylating Iκ-B and preventing its degradation by the proteasome.51 Thus, it inhibits NF-κB activation, which regulates several antiapoptotic molecules such as Bcl-xL.54,55 Recently, it has been shown that calcineurin can also control the STAT pathway. Calcineurin dephosphorylates STAT3 and an inhibitor of calcineurin could induce prolonged STAT-mediated signals.56,57 In addition, we have further preliminary evidence that the effects of FKBP51 are related to calcineurin inhibition because pharmacologic inhibition of calcineurin by CsA also induces an increase survival of UT-7/Mpl cells after cytokine deprivation. However, CsA has several biologic effects that are independent of calcineurin.58 Further studies will be required by using a target inhibition of calcineurin to confirm these results.
The present results provide new evidence that myeloproliferative diseases are associated with an increase cell survival signal in hematopoietic cells. In CML, it has been shown that the BCR-ABL protein induces cell cycle progression and also cell survival. A sustained expression of Bcl-xL after cytokine deprivation has been detected in polycythemia vera.59
The findings that overexpression of an FKBP member is associated with IMF opens new avenues in investigations of the mechanisms involved in myeloproliferative disorders. The development of murine model with an overexpression of FKBP51 in the MK lineage may be helpful to precisely understand the precise role of FKB51 in the development of myelofibrosis and the therapeutic potential of calcineurin activators.
The authors are grateful to Francoise Wendling, Anne Galy, and Jonathan Dundo (INSERM U362) for helpful discussions and improving the English manuscript. They also want to thank Catherine Boccaccio (Institut Gustave Roussy, Villejuif, France) for providing leukapheresis samples.
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2002-02-0485.
Supported by grants from the INSERM and la Ligue Nationale contre le Cancer “équipe labellisée 2000” and fellowships from the Research Ministry (H.C).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stéphane Giraudier, Central Hematology Laboratory, Hôpital Henri Mondor, Avenu du Maréchal de Lattre de Tassigny, Créteil 94000, France; e-mail:sgiraudi@igr.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal