Abstract
During human development, hematopoiesis is thought to be compartmentalized to the fetal circulation, liver, and bone marrow. Here, we show that combinations of cytokines together with bone morphogenetic protein-4 and erythropoietin could induce multiple blood lineages from human skeletal muscle or neural tissue. Under defined serum-free conditions, the growth factors requirements, proliferation, and differentiation capacity of muscle and neural hematopoiesis were distinct to that derived from committed hematopoietic sites and were uniquely restricted to CD45−CD34− cells expressing the prominin AC133. Our study defines epigenetic factors required for the emergence of hematopoiesis from unexpected tissue origins and illustrates that embyronically specified microenvironments do not limit cell fate in humans.
Introduction
Mammalian development involves a series of complex cellular events that originates from a single totipotent cell.1,2 As these cells proliferate and differentiate, groups of daughter cells are organized by morphogenetic movements to pattern and specify tissues necessary for the formation and function of the developing animal.3 During this process, lineage potential becomes increasingly limited and is restricted to the majority of cell types found at a given anatomical site.2However, the precise epigenetic signals required for lineage specification or whether commitment encompasses all cells comprising a given tissue remains unknown. This limited understanding of cell fate commitment during mammalian development is exemplified by observations in the mouse that suggest that the differentiation potential of cells is not restricted to the tissue source from which they reside, eg, the ability to generate neural cell types from cells harvested from bone marrow (BM).4-7 Although there are several interpretations of the cellular basis of these observations, it is clear that anatomical tissue/organ sites that have been specified during development are not exclusively restricted and are capable of unexpected cell fates. Therefore, similar to specific lineage induction during embryonic development, the molecular signals governing development of unique lineages from unrelated tissue sites has yet to be characterized.
Because most of these fundamental observations are predicated from experimental evidence in the mouse, it remains unknown if human tissue displays similar properties. Distinctions between mouse and human progenitor behavior is epitomized by the successful expansion and gene transfer into hematopoietic repopulating cells in the mouse, which has enjoyed limited success in the human scenario.8,9Human hematopoiesis represents one of the most rigorously studied and characterized processes of proliferation, differentiation, and lineage commitment. In the mouse, hematopoietic cells are first detected in the yolk sac, followed by definitive hematopoiesis originating in the aorta-gonad-mesonephros (AGM) region10,11 and is sustained in committed hematopoietic tissue sites such as fetal liver and BM. These established sites of hematopoiesis contain primitive blood stem/progenitors capable of sustained development into multiple blood lineages.10,12,13 In humans, characterizing the initiation of hematopoiesis has been limited; however, studies indicate that hematopoietic progenitors can be detected in similar regions to the mouse AGM and later on in the fetal circulation, liver, and BM.14-16
Observations of unexpected cell fate from a given tissue have been demonstrated in complex in vivo environments,5,17 thereby preventing further elucidation of the factors governing this cellular process.5,7,18 In vitro, most observations of unexpected cell fate have been documented by using uncharacterized culture conditions containing either conditioned media or serum extracts.17,19 20 These previous approaches have provided pioneering observations but limit our ability to understand cell fate progression because of the lack of defined systems required to elucidate the specific factors and cell types responsible for unexpected cell fate emergence. By using serum-free culture systems, we show that hematopoietic lineage development can be induced from human muscle and neural tissue sites under the control of hematopoietic-associated cytokines and combinations of bone morphogenetic protein-4 (BMP-4) and erythropoietin (EPO). Both qualitative and quantitative analyses indicate that emergence from either muscle or neural tissue is distinct to that demonstrated previously in accepted sites of hematopoiesis. Our study indicates that epigenetic signals are capable of inducing muscle and neural hematopoiesis and that tissue microenvironments do not limit cell fate potential during human development.
Materials and methods
Human tissues
Samples of 16- to 22-week gestational age human brain and muscle tissues were obtained in conjunction with local ethical and biohazard authorities of the University of Western Ontario and London Health Sciences Centre. Skeletal muscle was removed from the femur bone and cut into fine pieces in Hanks buffered salt solution (HBSS; Gibco, Burlington, Ontario, Canada). Collagen was added to a final concentration of 0.2%, and tissue was incubated for 20 minutes at 37°C followed by 10 minutes in 0.25% Trypsin (Gibco). Neural tissue was triturated in HBSS, and both tissues were forced through needles of increasing gauge.18,21 22 Cell suspensions were filtered through sterile 70-μm cell strainers and plated in tissue culture plates. After 1 hour, nonadherent cells were removed, counted, and replated in fibronectin-coated plastic. Fetal blood was washed twice with HBSS and filtered through a 70-μm cell strainer, counted, and plated.
In vitro culture of human fetal cells
Counted cells were cultured in 5 different conditions. A medium contained epidermal growth factor (EGF), fibroblast growth factor (FGF), and nerve growth factor (NGF) with added 10% chick embryo extract and retinoic acid. Base serum-free essential media contained bovine serum albumin (BSA), insulin, and transferrin in Iscoves modified Dulbecco medium (IMDM) (Stem Cell Technologies). Hematopoietic growth factors (HGFs) added to BIT (BSA, insulin, transferrin) media included 300 ng/mL stem cell factor (SCF), FLT-3 ligand, 50 ng/mL granulocyte-colony-stimulating factor (G-CSF), and 10 ng/mL interleukin-3 (IL-3), IL-6. To assess contribution of candidate extrinsic factors, 25 ng/mL BMP-4 (R & D Systems) and/or 3 U/mL EPO were added to essential media containing growth factors. Cells were incubated at 37°C and 5% CO2, and media and growth factors were replenished every 2 to 3 days.
Flow cytometric analysis of human muscle and neural cells
Fetal tissues were prepared as mentioned above and resuspended at 10 × 106 cells/mL in phosphate-buffered saline (PBS). The following fluorochrome antibodies were used for the detection of hematopoietic-associated surface proteins: human CD45-fluorescein isothiocyanate (FITC; Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA), human AC133-phycoerythrin (PE; Miltenyi Biotech), and CD34-allophycocyanin (APC; BDIS), vascular endothelial (VE)–Cadherin-FITC (Alexis Biochemicals, CA), and mouse immunoglobulin G1(IgG1) tails conjugated to each fluorochrome for isotype controls (BDIS). Cells were then stained with AC133-PE–conjugated antibody, and 5 μg/mL propidium iodide (Sigma) for detection of viable overlapping populations. Human neural cells expressing surface AC133 were isolated and used for colony-forming cell assays.
Immunohistochemistry of human muscle and neural cells
Cultured cells were washed several times with PBS and fixed with 70% EtOH and 0.15 M NaCl for 10 minutes at room temperature and washed 3 times with PBS. Fixed cells were blocked with 10% goat serum for 30 minutes at room temperature and washed 3 times with PBS. Neural cells were incubated with a rabbit polyclonal antibody specific for nestin and monoclonal microtubule-associated protein-2 (MAP-2) antibody, whereas muscle cells were stained with monoclonal antiserum directed against mysoin heavy chain and a monoclonal specific for myogenin. Antibody staining was done in PBS with 10% BSA for 1 hour at room temperature. Cells were rinsed with PBS 3 times and incubated for 1 hour at room temperature with FITC-conjugated goat antibody to rabbit IgG (nestin), Cy5-conjugated goat antibody to mouse IgG (MAP-2, myogenin), or FITC-conjugated goat antibody to mouse IgG (myosin) (1:100; Jackson Immunochemicals). After 3 final washes in PBS, coverslips were mounted with mounting medium and viewed and photographed with a Zeiss photomicroscope. Omission of primary antibody or antiserum resulted in no detectable staining.
Colony assays
Human clonogenic progenitor assays were performed by plating 500 000 to 1 000 000 de novo–isolated muscle and neural cells into serum-free Methocult H4236 (Stem Cell Technologies) containing 50 ng/mL rhu-SCF, 10 ng/mL rhu-granulocyte macrophage colony-stimulating factor (GM-SCF) and rhu–IL-3, and 3 U/mL rhu-EPO, or with or without BMP-4 (25 ng/mL). Cultured cells were harvested, washed with PBS, and counted; 5000 cells were plated in serum containing Methocult. Differential colony counts were assessed by morphology following incubations for 10 to 14 days at 37°C and 5% CO2 in a humidified atmosphere.
Transplantation of human muscle and neural cells and analysis of NOD/SCID mice
Cells were intravenously injected into mice that were sublethally irradiated at 335 cGy by using a 137 Cs γ-irradiator at cell doses indicated. Mice were killed 4 to 8 weeks after transplantation, and BM cells were recovered from each mouse. Other tissues were collected at time of death and kept frozen for DNA extraction and subsequent analysis. Analysis of human cell engraftment was evaluated by analyzing genomic DNA extracted from all murine tissues collected. Extracted DNA was used for polymerase chain reaction (PCR) amplification by using human Cart-1–specific primers (Gibco). PCR products were resolved on 1.4% agarose and transferred onto nylon membrane (Amersham). Membranes were used for southern hybridization with a 32dCTP–labeled Cart-1 DNA fragment.
Flow cytometric analysis of murine BM
BM cells were harvested by flushing removed femur, tibia, and iliac crests of mice that underwent transplantation. Cells were stained with monoclonal antibodies at 4°C for 30 minutes and washed several times prior to analysis by flow cytometry using a FACSCalibur (BDIS) and Cell Quest software (BDIS). Antibody specific to human CD45 was conjugated to FITC for detection of human hematopoietic cells.
Results
Phenotypic characterization of human muscle and neural tissues using hematopoietic-associated cell surface markers
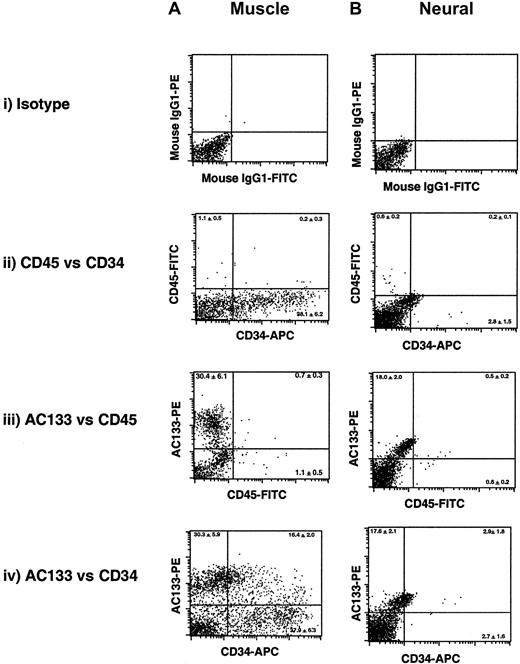
To phenotypically characterize the nature of human muscle or neural cells in the context of hematopoietic potential, we examined surface markers associated with the hematopoietic lineage. Little to no de novo–isolated human muscle (Figure1Aii) or neural (Figure 1Bii) tissue demonstrated expression of CD45, the pan-leukocyte marker expressed on all committed hematopoietic tissue.21 Because of the absence of appreciable CD45-expressing cells in either muscle or neural tissue, substantial numbers of AC133+ or CD34+ muscle (Figure 1Aii) or neural (Figure 1Bii) cells coexpressing CD45 could not be detected in any samples (n = 12). However, both muscle and neural human tissues were shown to exhibit expression for cell surface markers AC133 and CD34, previously associated with primitive human hematopoietic cells23-25(Figure 1Aiv and Figure 1Biv). Interestingly, the percentage and extent of expression of AC133 and CD34 was substantially higher in human muscle tissue, although both tissue types contained double-positive (AC133+CD34+) cells (Figure 1A-B, iv). These analyses indicate that human markers considered to be restricted to hematopoietic tissue are capable of more ubiquitous tissue expression and are shared among both muscle and neural tissues in humans, suggesting that phenotype alone is inefficient at making predictions of cell fate commitment and/or restriction without comparative functional analysis.
Flow cytometric analysis of cell surface phenotype of cells derived from human muscle and neural tissues.
Flow cytometry was used to evaluate the cell surface expression of the human-specific proteins associated with hematopoietic tissue: AC133, and cell differentiation markers CD34 and CD45. A representative FACS analysis of de novo–isolated muscle (A) and neural (B) cells is shown, and the percentage of subpopulations expressing single or coexpressing human hematopoietic markers is shown as the average mean ± SEM (n = 4) in each quadrant. Specificity of hematopoietic antibody staining and background signal was determined by comparing muscle and neural cells stained with mouse IgG1 (Ai and Bi) to establish positive quadrant levels to omit fluorescence because of nonspecific binding and auto fluorescence properties of the cells. Results are based on data from 4 independent samples of muscle and neural tissues analyzed in duplicate.
Flow cytometric analysis of cell surface phenotype of cells derived from human muscle and neural tissues.
Flow cytometry was used to evaluate the cell surface expression of the human-specific proteins associated with hematopoietic tissue: AC133, and cell differentiation markers CD34 and CD45. A representative FACS analysis of de novo–isolated muscle (A) and neural (B) cells is shown, and the percentage of subpopulations expressing single or coexpressing human hematopoietic markers is shown as the average mean ± SEM (n = 4) in each quadrant. Specificity of hematopoietic antibody staining and background signal was determined by comparing muscle and neural cells stained with mouse IgG1 (Ai and Bi) to establish positive quadrant levels to omit fluorescence because of nonspecific binding and auto fluorescence properties of the cells. Results are based on data from 4 independent samples of muscle and neural tissues analyzed in duplicate.
Human muscle and neural tissues are devoid of hematopoietic-reconstituting function
To functionally examine if human muscle or neural tissue was capable of pluripotent hematopoietic reconstituting capacity, immune-deficient nonobese diabetic (NOD)/severe combined immunodeficiency disease (SCID) mice received transplants intravenously of de novo–isolated fetal cells in single cell suspensions derived from neural and muscle tissues and were compared with cells obtained from previously accepted sites of human hematopoiesis such as liver, blood, and BM. Because more mature human hematopoietic progenitors cannot be detected in this model,22,26 this biologic assay discriminates between human hematopoietic stem cells from hematopoietic progenitors devoid of repopulating function. This human-mouse xeno-transplantation approach has previously allowed for the development of an assay for candidate human hematopoietic stem cells,27 including fetal hematopoietic stem cells from liver, blood, and BM.16
Human chimerism was evaluated in the BM of mice that underwent transplantation between 4 to 8 weeks after transplantation (n = 46). A representative analysis of the BM of NOD/SCID mice that underwent transplantation stained for the human hematopoietic specific marker CD45 is shown in Figure 2. Compared with isotype control (Figure 2A), fetal liver (2B), blood (2C), and BM (2D) demonstrated human hematopoietic engraftment in vivo, whereas both skeletal muscle (2E) and neural (2F) tissues were unable to repopulate recipient BM to similar levels, using the same range of cell doses (2 × 106 to 5 × 106 cells). From 2.5 × 106 to 6.5 × 106 cells derived from muscle or neural tissue were cultured for 5 days and then transplanted into NOD/SCID without any human hematopoietic chimerism detected in recipient BM. Therefore, in contrast to fetal liver, blood, and BM tissue at the same stage of human development, cells derived from human muscle or neural tissue were devoid of repopulating function by using this in vivo transplantation model.
Human chimerism in the tissue of immune-deficient mice that received intravenous transplants of human fetal tissues.
Comparison of human hematopoietic chimerism from hematopoietic and nonhematopoietic sources. Scatterplots show FACS analysis of mouse BM stained with the pan-leukocyte marker CD45 for the detection of human hematopoietic cells (gated box) after transplantation with isotype control (A), fetal blood (B), fetal liver (C), fetal BM (D), fetal muscle (E), and fetal neural cells (F). Cell doses ranged between 2 × 106 and 5 × 106 to up to 15 × 106 for muscle and neural transplants (n = 43).
Human chimerism in the tissue of immune-deficient mice that received intravenous transplants of human fetal tissues.
Comparison of human hematopoietic chimerism from hematopoietic and nonhematopoietic sources. Scatterplots show FACS analysis of mouse BM stained with the pan-leukocyte marker CD45 for the detection of human hematopoietic cells (gated box) after transplantation with isotype control (A), fetal blood (B), fetal liver (C), fetal BM (D), fetal muscle (E), and fetal neural cells (F). Cell doses ranged between 2 × 106 and 5 × 106 to up to 15 × 106 for muscle and neural transplants (n = 43).
At lower limits of detection (0.1%-0.01%), microchimerism of human cells was detected in several organs after 4 to 8 weeks after transplantation of fetal muscle and neural cells in NOD/SCID mice. This range of detection is indicative of approximately 1 human cell in 10 to 50 000 mouse cells at a given site and is similar to levels of neural engraftment from mouse BM.28 29 Although it is possible that human cells engrafting these murine tissue sites have undergone cell fate alteration, the microchimerism of human cells detectable (1 in 10 to 50 000 cells) suggests that the cells present are insufficient to be physiologically relevant to the recipient animal. Similar levels of microchimerism detected by this PCR method did not detect human chimerism in multiple organs of NOD/SCID mice that received transplants of purified CD34−Lin−cells derived from fetal blood (data not shown), suggesting that this level of organ chimerism is unique to transplantation of muscle and neural cells. Furthermore, the presence of cells derived from human muscle and neural tissues expressing VE-cadherin suggests the endothelial cells present in the transplanted products derived from muscle or neural tissue could account for the low level of human chimerism detected by PCR. On the basis of our results and previous murine studies, alternative methods other than complex in vivo systems are required to study the potential of hematopoiesis arising from unexpected origins of human muscle or neural tissue.
Induction of muscle and neural hematopoiesis
Primary samples of cells derived from muscle and neural tissues were harvested from human fetal skeletal muscle and brain. Single cell suspensions of human muscle and neural cells were cultured under a variety of conditions. As shown in Figure3A-B, cells derived from muscle and neural tissues were cultured in serum-free media containing essential amino acids, albumin, insulin, and transferrin, together with specific hematopoietic-associated cytokines SCF, FLT-3L, IL-3, IL-6, and G-CSF (HGF, where HGF refers to the combination of these hematopoietic growth factors), and addition of EPO and BMP-4. Because factors such as BMP-4 or EPO have been shown to be involved in the induction of a hematopoietic cell fate during embryogenesis of invertebrates and mammals30-32 and initiation of primitive hematopoiesis,32,33 we hypothesized that these proteins may serve as candidate regulators of hematopoietic specification from nonhematopoietic tissue. After 4 days of culture, cells were examined by light microscopy and in situ immunohistochemical analysis for specific lineage markers. Human muscle tissue (Figure 3Ai) expressed both structural myosin heavy chain protein34 (Figure 3Aii) and myogenin DNA binding protein (Figure 3Aiii) that are specific to the muscle lineage,35,36 whereas human neural tissue (Figure 3Bi) showed expression of both the neural progenitor marker, nestin37 (Figure 3Bii), and MAP-238(Figure 3Aiii).
Immunohistochemical and morphologic analysis of human fetal muscle and neural cells using tissue-specific markers and emergence of functional hematopoietic progenitors.
Light microscopy was used to visualize cells comprising human muscle (Ai) and neural (Bi) tissues, showing morphologic features associated with these tissue types. Human muscle tissue specificity was analyzed by immunohistochemistry for the expression of muscle-specific markers recognizing heavy chain of myosin (Aii) and the nuclear DNA binding factor, myogenin (Aiii), whereas human neural tissue demonstrated expression of neural progenitor-specific marker, nestin (Bii) and MAP-2 (Biii), respectively. A representative panel of multilineage hematopoietic colonies derived from muscle (Aiv) and neural (Biv) tissues cultured in the presence of HGF together with BMP-4 and EPO is shown. Human hematopoietic colony types generated from muscle and neural tissues include erythroid burst-forming unit (BFU-E), CFU-granulocyte (G), -macrophage (M), and tetrapotent mixed colonies (granulocyte, erythroid, macrophage, megakaryocyte [GEMM]). Similar results were obtained from 9 independent samples of muscle and neural tissue samples. Magnification × 200.
Immunohistochemical and morphologic analysis of human fetal muscle and neural cells using tissue-specific markers and emergence of functional hematopoietic progenitors.
Light microscopy was used to visualize cells comprising human muscle (Ai) and neural (Bi) tissues, showing morphologic features associated with these tissue types. Human muscle tissue specificity was analyzed by immunohistochemistry for the expression of muscle-specific markers recognizing heavy chain of myosin (Aii) and the nuclear DNA binding factor, myogenin (Aiii), whereas human neural tissue demonstrated expression of neural progenitor-specific marker, nestin (Bii) and MAP-2 (Biii), respectively. A representative panel of multilineage hematopoietic colonies derived from muscle (Aiv) and neural (Biv) tissues cultured in the presence of HGF together with BMP-4 and EPO is shown. Human hematopoietic colony types generated from muscle and neural tissues include erythroid burst-forming unit (BFU-E), CFU-granulocyte (G), -macrophage (M), and tetrapotent mixed colonies (granulocyte, erythroid, macrophage, megakaryocyte [GEMM]). Similar results were obtained from 9 independent samples of muscle and neural tissue samples. Magnification × 200.
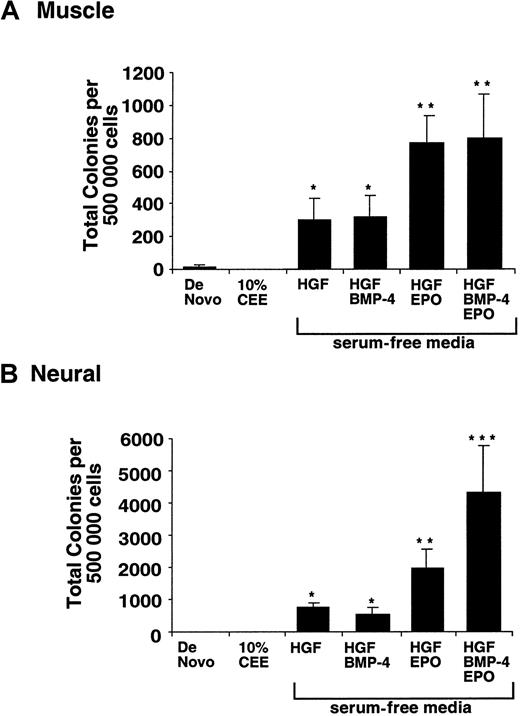
Although cultured muscle and neural cells showed morphologic and phenotypic commitment, we examined whether these cells possessed hematopoietic potential. Cultured cells from muscle and neural tissues were evaluated for functional hematopoietic progenitor capacity by using hematopoietic colony-forming unit (CFU) assays.23 39-41 Cells from respective liquid cultures were harvested and seeded into methylcellulose containing hematopoietic growth factors under serum-free conditions and examined for clonal hematopoietic potential after 14 days. Both muscle (Figure 3Aiv) and neural (Figure 3Biv) cells cultured in serum-free media with specific growth factors were able to give rise to multiple hematopoietic progenitors of erythroid (BFU-E), granulocytic (CFU-G), macrophage (CFU-M), and hematopoietic clones with tetrapotent lineage capacity (CFU-GEMM [granulocyte, erythroid, macrophage, megakaryocyte]). On the basis of unexpected hematopoietic potential arising from human muscle and neural tissues, we further examined this observation by using quantitative analysis to better understand the basis of hematopoietic lineage emergence.
Single-cell suspensions derived from muscle and neural tissues were further examined for de novo hematopoietic potential and after in vitro culture with and without specific extrinsic factors as shown (Figure 4). To determine if cells within human muscle or neural tissue possessed hematopoietic potential on de novo isolation, individual samples were seeded into clonal hematopoietic progenitor assays. Several initial experiments indicated that de novo–isolated muscle or neural tissue were completely devoid of clonal hematopoietic potential (data not shown), suggesting that cells with hematopoietic progenitor function are absent in human muscle or neural tissue. To ensure that seeding of muscle and neural cells into methylcellulose hematopoietic clonal assays was in the linear range of response, dilutions of input cells were carried out, ranging from 10 000 to 1 000 000 cells. When human muscle or neural cell input was increased to more than 500 000 cells/well, a small number of hematopoietic progenitors could be detected from de novo–isolated muscle (Figure 4A) and neural tissues (Figure 4B). The total number of hematopoietic progenitor detectable among 500 000 cells derived from muscle or neural tissue was 8 ± 2 and 1 ± 1, respectively (Table1). To confirm that muscle or neural cells did not inhibit CFU formation, we seeded bona fide hematopoietic cells enriched for a known frequency of blood progenitors with or without muscle and neural cells. There was no difference in the number of CFU progenitors in hematopoietic progenitor assays with or without the addition of muscle or neural cells, suggesting the large number of input neural or muscle cells does not effect the detection of hematopoietic progenitor in this assay system (data not shown). Our results suggest that hematopoietic potential of muscle and neural tissues during human development is nearly absent and optimally only represents 0.0016% and 0.0002%, respectively.
Evaluation of human hematopoietic cell fate potential of de novo and cultured muscle and neural cells.
Primary human muscle (A) and neural (B) tissues were examined for hematopoietic progenitor capacity (CFU) at isolation (day 0) or after 5 days of in vitro culture in essential media containing 10% CEE, or human hematopoietic growth factors (HGF) alone or with BMP-4 or EPO, as single additions or in combination as indicated. In all culture conditions, media and cytokines were replenished every other day. Single, double, and triple asterisks indicate substantial differences within measured groups of P < .01. Data shown is based on 6 to 13 independent samples analyzed in culture conditions indicated.
Evaluation of human hematopoietic cell fate potential of de novo and cultured muscle and neural cells.
Primary human muscle (A) and neural (B) tissues were examined for hematopoietic progenitor capacity (CFU) at isolation (day 0) or after 5 days of in vitro culture in essential media containing 10% CEE, or human hematopoietic growth factors (HGF) alone or with BMP-4 or EPO, as single additions or in combination as indicated. In all culture conditions, media and cytokines were replenished every other day. Single, double, and triple asterisks indicate substantial differences within measured groups of P < .01. Data shown is based on 6 to 13 independent samples analyzed in culture conditions indicated.
Comparison of total hematopoietic progenitors emerging from muscle and neural tissue to changes in total number of specific phenotypically characterized subsets
| . | De novo . | 10% CEE . | HGF . | HGF + BMP-4 + EPO . |
|---|---|---|---|---|
| Muscle tissue | ||||
| Total cells | 500 000 | 920 000 | 330 000 | 320 000 |
| AC133+ | 190 000 | 0 | 11 612 | 28 139 |
| CD34+ | 23 500 | 87 400 | 46 054 | 41 195 |
| CD45+ | 1 025 | 0 | 0 | 5 165 |
| CD34+CD45+ | 600 | 0 | 0 | 59 |
| Total hematopoietic progenitors | 8 | 4 | 182 | 630 |
| Neural tissue | ||||
| Total cells | 500 000 | 120 000 | 110 000 | 82 000 |
| AC133+ | 97 450 | 291 | 1 116 | 658 |
| CD34+ | 21 650 | 1 087 | 283 | 789 |
| CD45+ | 4 850 | 2 388 | 9 843 | 9 574 |
| CD34+CD45+ | 95 | 669 | 541 | 132 |
| Total hematopoietic progenitors | 1 | 5 | 507 | 2 475 |
| . | De novo . | 10% CEE . | HGF . | HGF + BMP-4 + EPO . |
|---|---|---|---|---|
| Muscle tissue | ||||
| Total cells | 500 000 | 920 000 | 330 000 | 320 000 |
| AC133+ | 190 000 | 0 | 11 612 | 28 139 |
| CD34+ | 23 500 | 87 400 | 46 054 | 41 195 |
| CD45+ | 1 025 | 0 | 0 | 5 165 |
| CD34+CD45+ | 600 | 0 | 0 | 59 |
| Total hematopoietic progenitors | 8 | 4 | 182 | 630 |
| Neural tissue | ||||
| Total cells | 500 000 | 120 000 | 110 000 | 82 000 |
| AC133+ | 97 450 | 291 | 1 116 | 658 |
| CD34+ | 21 650 | 1 087 | 283 | 789 |
| CD45+ | 4 850 | 2 388 | 9 843 | 9 574 |
| CD34+CD45+ | 95 | 669 | 541 | 132 |
| Total hematopoietic progenitors | 1 | 5 | 507 | 2 475 |
Flow cytometry was used to assess the expression of human-specific cell surface proteins AC133, CD34, CD45, and double-positive CD34/CD45 before and after in vitro culture in serum-free media containing either 10% chicken embryonic extract (CEE) or human hematopoietic growth factors (HGFs) alone or with bone morphogenetic protein-4 (BMP-4) and erythropoietin (EPO) for a total of 500 000 human muscle– and human neural–derived cells. Values show average absolute number of cells of each phenotype. Absolute number of detected hematopoietic progenitors after culture in each medium is given. Treated human muscle and neural cells maintained normal expression of tissue-specific markers (myosin for muscle and nestin for neural tissue) irrespective of changes in hematopoietic cell surface phenotype as shown in Figure 3.
To provide an extrinsic environment conducive of the embryonic development and promotion of intrinsic cell fate of cells derived from muscle and neural tissues, 10% chick embryonic extract (10% CEE) was added to defined serum-free media cultures.18,42 The addition of 10% CEE media, shown previously in murine studies to promote hematopoietic cell fate induction,18 had no affect on hematopoietic potential of either muscle or neural cells in humans (Figure 4A-B). However, exposure of cells derived from muscle and neural tissues cultured in serum-free media with combinations of HGFs was capable of inducing hematopoietic progenitors (Figure 4A-B). The addition of BMP-4 and EPO induced hematopoietic progenitors from cells derived from muscle and neural tissues by an additional 4- to 5-fold, with development into multiple hematopoietic lineages (Figure 3Aiv and Biv). Our results demonstrate that neither muscle nor neural tissue are capable of hematopoiesis de novo, but development into multiple hematopoietic lineages can be induced under the control of specific epigenetic signaling factors.
Known phenotypic characteristics of hematopoietic progenitors do not correlate with muscle and neural hematopoietic potential
On the basis of the ability of HGFs and BMP-4 + EPO treatment in serum-free media to induce hematopoietic progenitors, we examined changes in cell number and absolute number of phenotypic subsets previously associated with hematopoietic progenitor function. By using a starting population of 500 000 cells derived from muscle and neural tissues (de novo), an average number of AC133, CD34, CD45, and double-positive CD34/CD45 cells were calculated from 13 independent human fetal samples and compared with totals after in vitro culture in essential serum-free media with 10% CEE, HGF,43 44 or HGF treatment in combination with BMP-4 and EPO (Table 1). Human muscle or neural cells cultured in 10% CEE lose AC133 expression, whereas in the presence of HGF combinations a larger number of AC133+cells were detectable in both cultures of muscle and neural cells. AC133 expression was further augmented in the presence of HGF together with BMP-4 and EPO by more than 2-fold compared with HGF-treated muscle cultures but had little effect on neural-derived cells (Table 1). Changes in total AC133 cells within treated cultures do not correlate with increases in hematopoietic progenitors for either muscle or neural tissue (Table 1). Cell surface expression of CD34 or CD45 on either muscle- or neural-derived cells did not correlate with hematopoietic progenitor function. Despite the presence of greater numbers of total CD34 cells in muscle and neural cultures treated with 10% CEE, hematopoietic progenitors were nearly undetectable from these cultures. CD45, a marker of hematopoietic lineage commitment, or double-positive CD34+CD45+ cells considered to be enriched for hematopoietic progenitors were completely absent in muscle cultures treated with HGFs, whereas hematopoietic progenitor potential of an average of 182 progenitors was detectable. The inability to correlate presence of specific subsets of CD34 or CD34+CD45+ cells within the neural-treated cultures was consistent. The detection of an average of 630 and 2475 hematopoietic progenitors in cultures of muscle and neural cells containing HGFs and BMP-4 + EPO corresponded to as few as 59 and 132 CD34+CD45+ cells. On the basis of comparative quantitative analysis among de novo–isolated and treated cultures, our data indicate that surrogate markers for hematopoietic progenitors do not apply to hematopoietic potential induced from muscle and neural tissues and suggest that emergence of hematopoiesis from these unexpected sources is unique to other forms of hematopoietic lineage progression that have been characterized to date.
Epigenetic signals required for emergence of muscle and neural hematopoiesis are distinct from circulating fetal hematopoietic progenitors
During human fetal development, hematopoietic progenitors can be isolated from the circulation between 16 and 22 weeks of gestation.16 On the basis of our observations that hematopoietic progenitors can arise from cells derived from muscle and neural tissues in response to HGFs together with BMP-4 and EPO (Figure4 and Table 1), we performed side-by-side comparisons of the proliferative and differentiation potential of hematopoietic progenitors isolated from fetal blood (FB), fetal muscle, and fetal neural tissues obtained from the same human donors and cultured under identical defined culture conditions (Figure5).
Comparative analysis of human hematopoietic-, muscle-, and neural-derived hematopoietic progenitors.
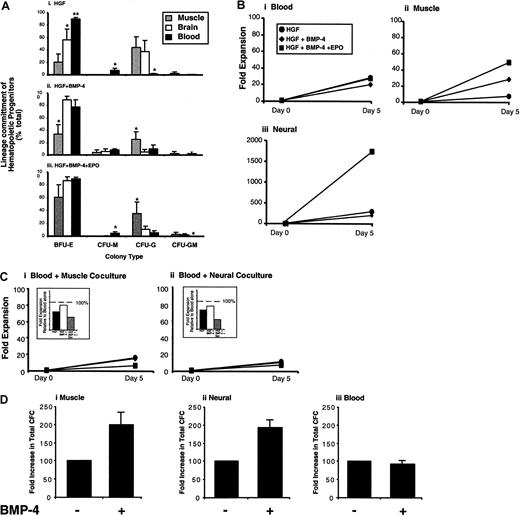
Human tissues indicated were harvested under identical conditions, and functional hematopoietic progenitor capacity was evaluated at day 0 (de novo–isolated tissues) and again at day 5 of culture in HGF conditions with or without BMP-4 or BMP-4 together with EPO. (A) Developmental potential of hematopoietic progenitors was compared among various human tissues by enumerating colony types as a measure of hematopoietic lineage commitment that include erythroid (BFU-E), monocytic (CFU-M), granulocytic (CFU-G), and myelocytic (CFU-GM). Composition of CFU types generated for each tissue is expressed as mean percentage ± SEM (n = 5) of the total colonies for muscle (gray), neural (white), and blood (black) cells cultured in essential media containing HGF (i), BMP-4 (ii), or BMP-4 and EPO in combination (iii). Single and double asterisks indicate substantial differences within measured groups ofP < .01, (n = 5). (B) Fold changes in total hematopoietic progenitor expansion was compared in response to culture conditions indicated and compared with day 0 of cultured fetal blood (i), muscle (ii), and neural (iii) cells. Each graph shows the increase in progenitors after culture in essential media containing HGF (●), BMP-4 (♦), or BMP-4 and EPO (▪). (C) Fold expansion of fetal blood cultured for 5 days in the absence or presence of cells derived from fetal muscle (i) or fetal neural (ii) tissue using cytokine combinations indicated. Cells were cocultured using 5.0 × 105 fetal blood cells to equal numbers of fetal muscle or neural cells to maintain the same cell density used in previous experiments shown in panel B. Insets show the relative fold expansion to fetal blood cells cultured alone compared with the various coculture treatments. (D) Hematopoietic progenitors assayed from single cells suspensions of muscle (i), neural (ii), and blood (iii) in the absence or presence of BMP-4 directly added to methylcellulose used in this clonal assay. Graphs show number of clonogenic progenitor of de novo–isolated tissues in the absence of BMP-4 (control) shown standardized as 100% and in the presence of BMP-4 as a percentage relative to control ± SEM. Results are based on a total of 5 independent samples.
Comparative analysis of human hematopoietic-, muscle-, and neural-derived hematopoietic progenitors.
Human tissues indicated were harvested under identical conditions, and functional hematopoietic progenitor capacity was evaluated at day 0 (de novo–isolated tissues) and again at day 5 of culture in HGF conditions with or without BMP-4 or BMP-4 together with EPO. (A) Developmental potential of hematopoietic progenitors was compared among various human tissues by enumerating colony types as a measure of hematopoietic lineage commitment that include erythroid (BFU-E), monocytic (CFU-M), granulocytic (CFU-G), and myelocytic (CFU-GM). Composition of CFU types generated for each tissue is expressed as mean percentage ± SEM (n = 5) of the total colonies for muscle (gray), neural (white), and blood (black) cells cultured in essential media containing HGF (i), BMP-4 (ii), or BMP-4 and EPO in combination (iii). Single and double asterisks indicate substantial differences within measured groups ofP < .01, (n = 5). (B) Fold changes in total hematopoietic progenitor expansion was compared in response to culture conditions indicated and compared with day 0 of cultured fetal blood (i), muscle (ii), and neural (iii) cells. Each graph shows the increase in progenitors after culture in essential media containing HGF (●), BMP-4 (♦), or BMP-4 and EPO (▪). (C) Fold expansion of fetal blood cultured for 5 days in the absence or presence of cells derived from fetal muscle (i) or fetal neural (ii) tissue using cytokine combinations indicated. Cells were cocultured using 5.0 × 105 fetal blood cells to equal numbers of fetal muscle or neural cells to maintain the same cell density used in previous experiments shown in panel B. Insets show the relative fold expansion to fetal blood cells cultured alone compared with the various coculture treatments. (D) Hematopoietic progenitors assayed from single cells suspensions of muscle (i), neural (ii), and blood (iii) in the absence or presence of BMP-4 directly added to methylcellulose used in this clonal assay. Graphs show number of clonogenic progenitor of de novo–isolated tissues in the absence of BMP-4 (control) shown standardized as 100% and in the presence of BMP-4 as a percentage relative to control ± SEM. Results are based on a total of 5 independent samples.
Biologic differences between FB, muscle, and neural hematopoietic lineage potential were examined by comparing the relative frequencies of erythroid, granulocytic, monocytic, and myelocytic hematopoietic progenitors detected. Relative frequencies of hematopoietic progenitors from each human tissue displayed their own distinct profiles (Figure 5Ai-iii). Most strikingly, cells derived from muscle and neural tissues possessed less erythroid hematopoietic potential (BFU-E) but demonstrated enhanced granulocytic (CFU-G) development than FB. To compare proliferative expansion of hematopoietic progenitors derived from FB versus muscle and neural tissues, single-cell suspensions of each tissue were harvested and seeded at identical concentrations into essential media containing HGFs alone and compared HGF with BMP-4 or BMP-4 and EPO (Figure 5B). Hematopoietic progenitor expansion from FB averaged 18-fold when cultured in the presence of HGF (Figure 5Bi), whereas HGF induced an 8-fold increase in muscle-derived hematopoietic progenitors (Figure 5Bii) and a 200-fold expansion of neural-derived hematopoietic progenitors (Figure 5Biii). Addition of BMP-4 or BMP-4 and EPO in combination had no effect on changes in the expansion of hematopoietic progenitors derived from FB (Figure 5Bi). In contrast, addition of BMP-4 and EPO resulted in a 6-fold and 8-fold augmentation of hematopoietic progenitor expansion induced from muscle (Figure 5Bii) and neural (Figure 5Biii) tissues, respectively. These results demonstrate that muscle- and neural-derived hematopoietic progenitors are responsive to BMP-4 and EPO, whereas BMP-4 and EPO are unable to affect hematopoietic progenitor expansion from circulating FB. To further examine whether differential responsiveness of muscle and neural hematopoietic progenitor expansion was not due to progenitors from fetal circulation uniquely responding via an indirect effect of muscle and neural cells in the culture, we performed direct coculture experiments shown in Figure 5C. Equal numbers of neural and muscle cells were cocultured with FB cells and treated with or without BMP-4 or BMP-4 and EPO. In the presence of either muscle (Figure 5Ci) or neural (Figure 5Cii) coculture, blood cells derived from the fetal circulation remained unresponsive to either BMP-4, or BMP-4 and EPO and did not demonstrate enhanced progenitor expansion as compared with treatment with HGFs alone. These results suggest that muscle and neural cells are unable to indirectly affect fold expansion of hematopoietic progenitors that originate from fetal blood sources, decreasing the likelihood of differential cytokine response shown among muscle or neural hematopoietic progenitors to be arising from contaminating fetal blood cells.
To address whether BMP-4 was able to affect the hematopoietic lineage induction from individual muscle or neural clones, muscle and neural cells were plated into defined, serum-free methylcellulose assays for hematopoietic progenitor detection, in the absence and presence of BMP-4, and were compared with progenitors derived from circulating fetal blood. Direct addition of BMP-4 to clonal assay systems was able to increase the total number of hematopoietic progenitors detectable from both muscle and neural tissues by 2-fold, whereas the frequency of fetal blood progenitors was unaffected (Figure 5D). These results indicate that hematopoietic progenitors can be induced from de novo–isolated muscle and neural cells at the single cell level.
Taken together, our findings illustrate biologic differences in human hematopoiesis arising from nonhematopoietic tissue compared with committed blood sources in (1) developmental program of hematopoietic lineages, (2) hematopoietic progenitor expansion, and (3) responsiveness to instructive factors such as BMP-4 and EPO. Quantitative and qualitative differences between fetal blood and hematopoietic emergence from human muscle and neural tissues indicate that the origin and biologic nature of hematopoiesis from muscle and neural tissues is distinct to embryonically specified hematopoietic sites.
Purification of cells with human hematopoietic potential
The nature of candidate cells responsible for progenitor function from various tissues has been prospectively isolated by using the presence or absence of cell surface markers.45-47 Human blood cells with the ability to reconstitute the BM of NOD/SCID mice were shown to be restricted to a subfraction expressing the prominin AC133, similar to sustained chimerism of the brain of neonatal NOD/SCID mice implanted with human neural cells.45 These combined studies suggest that AC133 expression serves as a marker for the identification of human hematopoietic and neural stem/progenitor cells.48
With the use of viability stains such as 7AAD, live cells devoid of CD34 cell surface expression were isolated, further gated for CD45− cells, and divided into AC133+ and AC133− subfractions. A representative gating strategy is shown in Figure 6A. AC133+CD34−CD45− and AC133−CD34−CD45− cells were cultured in essential media containing HGFs, combined with BMP-4 and EPO for 5 days. The proliferation of AC133+ cells was greater than the AC133− subset over the culture period. In addition, phase contrast light microscopy indicated that AC133+ cells developed into large spheric clusters and established projections, whereas poor growth of AC133−cells was accompanied with lack of notable projections or cluster formation (Figure 6Ai-ii). After 5 days, cells resulting from AC133+CD34−CD45− and AC133−CD34−CD45− cultures were harvested and placed into serum-free hematopoietic progenitor assays. Functional hematopoietic potential of producing progenitors of multiple blood lineages, similar to that shown from unpurified samples (Figure3), was restricted to the AC133+CD45−CD34− subset (Figure6C). No hematopoietic colonies could be detected among AC133−CD45−CD34− cells in any experiments using 4 independent neural samples (Figure 6C). The frequency of AC133+CD45−CD34−cells originating from neural tissue with primitive hematopoietic progenitor potential was 1 in 787 in contrast to the AC133−CD45−CD34− subset in which no hematopoietic progenitors were detectable using as many as 500 000 purified cells. Our results indicate that the population of human AC133-expressing cells possess greater lineage potential than their AC133− counterpart. In addition to AC133 expression providing a marker for human hematopoietic and neural stem cells,23 45 our study indicates this prominin family member enriches for a population cells derived from human neural tissue that is capable of hematopoietic progenitor function.
Isolation of AC133+ and AC133−subsets from human embryonic tissues.
Human fetal neural cells were stained with human-specific antibodies raised to the prominin AC133, CD34, and CD45. AC133+ and AC133− cells were isolated according to sorting gates shown in Figure 1, from the population of cells devoid of CD34 and the hematopoietic marker CD45. (A) Purified subpopulations of AC133+CD34−CD45− (Bi) and AC133−CD45−CD34− (Bii) cells from human neural tissue were cultured under serum-free conditions shown to induce neural hematopoiesis. Magnification × 200 (Bi) and × 400 (Bii). Hematopoietic colonies of multiple lineages were detected from AC133+CD34−CD45− cells. The composition of colonies was similar to that shown in Figure 4. (C) Quantitative analysis of hematopoietic colonies arising from either AC133+ or AC133− subsets from the CD34−CD45− population. Cells were cultured in HGF with BMP-4 and EPO and were then collected and plated into colony-forming assays. Colonies were scored after 12 to 14 days and shown as the average number of colonies per 50 000 cell input ± SEM. Averages shown are based on 4 independent samples.
Isolation of AC133+ and AC133−subsets from human embryonic tissues.
Human fetal neural cells were stained with human-specific antibodies raised to the prominin AC133, CD34, and CD45. AC133+ and AC133− cells were isolated according to sorting gates shown in Figure 1, from the population of cells devoid of CD34 and the hematopoietic marker CD45. (A) Purified subpopulations of AC133+CD34−CD45− (Bi) and AC133−CD45−CD34− (Bii) cells from human neural tissue were cultured under serum-free conditions shown to induce neural hematopoiesis. Magnification × 200 (Bi) and × 400 (Bii). Hematopoietic colonies of multiple lineages were detected from AC133+CD34−CD45− cells. The composition of colonies was similar to that shown in Figure 4. (C) Quantitative analysis of hematopoietic colonies arising from either AC133+ or AC133− subsets from the CD34−CD45− population. Cells were cultured in HGF with BMP-4 and EPO and were then collected and plated into colony-forming assays. Colonies were scored after 12 to 14 days and shown as the average number of colonies per 50 000 cell input ± SEM. Averages shown are based on 4 independent samples.
Discussion
Here we report that de novo–isolated cells from human muscle and neural tissues show nearly undetectable hematopoietic properties, suggesting that these localized tissue environments are not permissive to hematopoiesis. However, cells derived from muscle and neural tissues were capable of inducible hematopoietic progenitor function into multiple lineages once treated with specific extrinsic factors under serum-free conditions. These epigenetic signals and the phenotypic nature of cells capable of hematopoiesis were distinct from hematopoiesis arising from expected, bona fide hematopoietic tissue such as circulating fetal blood. Observations from historical experiments in which whole explants of tissue were removed from one site of a developing embryo and reimplanted into an alternative site have established concepts of lineage commitment.49 The inability of these removed tissues to be respecified to adopt the fate of the new environment while retaining its original cell fate potential describes the paradigm of tissue specification during embryonic development.50 In contrast to classical embryonic explant experiments using intact sections, our approach liberates the individual cells from the extrinsic influences of neighboring cells or soluble factors in the original parent tissue. On the basis of our results, we suggest that parent microenvironment imposes cell fate restriction and/or prevents cells from responding to atypical hematopoietic factors; therefore, a 2-step process of both removal and treatment of single-cell suspensions in serum-free media allows emergence of hematopoietic progenitors from muscle and neural tissues.
Human cells derived from muscle and neural tissues displayed distinct responsiveness to the combinations of EPO and BMP-4. Hematopoietic differentiation from muscle-derived cells was greatest in the presence of EPO, whereas the addition of BMP-4 was less effective. In contrast, human neural tissue showed the greatest response in the presence of both BMP-4 and EPO. Treatment of committed fetal blood cells with hematopoietic-inducing factors such as BMP-4 and EPO had no effect, unlike primitive hematopoiesis arising from muscle- and neural-derived cells. These functional differences in cellular response suggest that emergence of hematopoietic potential from muscle and neural tissues are physiologic distinct and represent unique origins of hematopoiesis. This is further supported by the distinct nature of hematopoietic progenitors arising from muscle and neural tissues that are devoid of CD45 or CD34 cell surface expression. Previous studies from our laboratory indicate that primitive CD34− cells from cord blood (CB) are capable of hematopoietic cell fate.23 In contrast to CD34− cells derived from muscle or neural human tissue, de novo–isolated CD34− CB-derived cells that coexpress AC133 are (1) highly enriched for hematopoietic progenitor function and do not require further ex vivo culture, (2) capable of engrafting the BM site of NOD/SCID mice that underwent transplantation, and (3) express cell surface CD45.23 Our laboratory is currently optimizing methods to enrich similar subpopulation from nonhematopoietic fetal tissues so that adequate numbers of these cells can be assayed for SCID-repopulating cells (SRC) function in vivo. Our current study in humans supports the notion that preconceived relationships between tissue restriction and anatomical position are more complex than previously appreciated. Our results indicate that regulatory mechanisms responsible for emergence of hematopoietic progenitors from unexpected tissue origins can be controlled by extrinsic influences that are sufficient to induce neogenic blood formation.
We suggest that the cellular origin of hematopoiesis from muscle or neural tissue arises from novel precursors within muscle or neural tissue capable of hematopoietic progenitor function. Subpopulations of cells within specific tissues may remain unrestricted and possess pluripotent potential that is reminiscent of embryonic stem cells.4,36,51 The inability to generate reconstituting hematopoietic stem cells from murine embryonic stem (ES) cell parents in vitro, in contrast to the ease in inducing hematopoietic progenitors, provides biologic precedence for this property in pluripotent cells.52,53 Therefore, unlike bona fide hematopoietic tissue that has been committed during development of the embryo, hematopoiesis induced from nonhematopoietic tissue does not give rise to detectable repopulating stem cells. This notion is further supported by previous mouse studies in which hematopoiesis from muscle or neural sites could be demonstrated in vivo but failed to meet several criteria used to define bona fide murine hematopoietic stem cells such as long-term reconstitution, self-renewal properties, and protection after lethal irradiation.54 Similar to muscle- and neural-derived hematopoietic progenitors shown in our current study, recent studies by Kaufman et al55 demonstrate that hematopoietic progenitors arising from human ES cell lines are devoid of cell surface CD45 expression, further supporting a common functionality among human ES cells and blood progenitors emerging from human muscle and neural tissues. Multifunctional signals supplied by parent tissue sites limits differentiation potential of these cells; therefore, removal of these restrictions, combined with the introduction of unique growth factor combinations found in alternative tissue microenvironments, is sufficient in instructing unique developmental programs. Defined serum-free in vitro systems used in this study has allowed us to identify critical factors that include BMP-4 and EPO, that are central to the emergence of hematopoietic lineages from muscle and neural tissues, and that do not affect committed blood cells. The utility of newly identified inducing factors capable of instructing hematopoiesis from unexpected tissue origins provides a system to further characterize cell fate restriction of primary human cells.
We thank Amgen (Thousand Oaks, CA) for cytokines and the staff of the labor and delivery departments of St Joseph's Hospital and London Health Sciences, London, ON, Canada, and especially the assistance of Dr Fraser Fellows. In addition, we would like to thank Dr M. Underhill for critically reviewing this manuscript and Drs J. M. Verdi, G. F. Pickering, R. Hammond, M. Bani-Yahoub, and I. Skerjanc for assistance in developing immunohistochemical protocols and providing muscle- and neural-specific antibodies.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-02-0502.
Supported by a grant (MT-15063) from the Canadian Institutes of Health Research, Ontario, Canada, and a scholarship award (MSH-35681) from the Canadian Institutes of Health Research, Ontario, Canada, (M.B.) and studentship from the Stem Cell Network, Canadian National Centre of Excellence Program (K.J.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mickie Bhatia, The John P. Robarts Research Institute, Stem Cell Biology and Regenerative Medicine, 100 Perth Dr, London, Ontario, N6A 5K8, Canada; e-mail: mbhatia@rri.ca.


![Fig. 3. Immunohistochemical and morphologic analysis of human fetal muscle and neural cells using tissue-specific markers and emergence of functional hematopoietic progenitors. / Light microscopy was used to visualize cells comprising human muscle (Ai) and neural (Bi) tissues, showing morphologic features associated with these tissue types. Human muscle tissue specificity was analyzed by immunohistochemistry for the expression of muscle-specific markers recognizing heavy chain of myosin (Aii) and the nuclear DNA binding factor, myogenin (Aiii), whereas human neural tissue demonstrated expression of neural progenitor-specific marker, nestin (Bii) and MAP-2 (Biii), respectively. A representative panel of multilineage hematopoietic colonies derived from muscle (Aiv) and neural (Biv) tissues cultured in the presence of HGF together with BMP-4 and EPO is shown. Human hematopoietic colony types generated from muscle and neural tissues include erythroid burst-forming unit (BFU-E), CFU-granulocyte (G), -macrophage (M), and tetrapotent mixed colonies (granulocyte, erythroid, macrophage, megakaryocyte [GEMM]). Similar results were obtained from 9 independent samples of muscle and neural tissue samples. Magnification × 200.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/9/10.1182_blood-2002-02-0502/4/m_h82123310003.jpeg?Expires=1765917751&Signature=MIV9b-mxiW1DsmL7n627mo5novq3LUDwPLJArDmG4dRMnXamQdPFB9gbhpOnsSXeW4V450PqwF91JBB9VfwiU~IRLaW9FvyUouFW1wacwX3X1~yaJmKkGcqrn4l5cpI7U35jHM25kfKmDibOMV97wlwOcmIscfrgUJB8BD8RNJZ-wXNRb~WErpQam6v5ZoQTIV3TzGBgc1-CZCCVN9K7RDzQUIDPjGcsCHJYgI8~4CUlWlpQsAAyfuUHZl2cf2K0paIybf6yKfnCTZ6HGOt3iyC7rmyhOitKGXjFSEGj-eTy-ZeX0wOGV~5Jl~FNurVOOTF7~R30TP9B37asOjOUkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal