Abstract
Recent studies have identified a role for thyroid-stimulating hormone (TSH; ie, thyrotropin) as an inductive signal for tumor necrosis factor-α (TNF-α) secretion by bone marrow (BM) cells, although the features of that activation pathway have not been defined. Using intracellular TSH staining and enzyme-linked immunoassay for detection of secreted TSH, we demonstrate that TSH synthesis in BM cells occurs within CD45+ (leukocyte common antigen) hematopoietic cells and that the majority of that activity resides in a component of CD11b+ BM cells that are not mature T cells, B cells, or Thy-1+ cells in the BM. Conversely, TSH-responsive BM cells defined by expression of TSH receptor (TSHR) using flow cytometry were selectively associated with a nonerythroid CD11b− lymphocyte precursor population. In vitro culture of magnetic-activated cell sorted CD11b− and CD11b+ cells with titrated amounts of purified TSH resulted in significantly higher levels of TNF-α secretion from CD11b− BM cells compared to non-TSH–treated cells, with no appreciable change in TNF-α production from CD11b+cells. These findings are the first to demonstrate TSH production by BM hematopoietic cells, and they demonstrate that TSH may be involved in the regulation of TNF-α by CD11b− BM cells. They also indicate that TSH-mediated regulation of TNF-α secretion within the BM most likely operates through an intrinsic network of TSH production and use between different types of BM cells, and they suggest that local TSH may be an important homeostatic regulator of hematopoiesis mediated by TNF-α.
Introduction
Although the participation of the neuroendocrine system in the regulation of immunity has been known for many years, most studies into that process have examined the involvement of glucocorticoid, steroid hormones, and reproductive hormones. Considerably less is known about how hormones of the hypothalamus-pituitary-thyroid axis affect the immune response. Evidence, however, suggests that thyroid-stimulating hormone (TSH) can be produced by cells of the immune system and that it is used by hematopoietic cells, as seen from TSH receptor (TSHR) expression on hematopoietic cells, and by its capacity to influence various immunobiologic activities in a TSH-dependent manner. Studies by Smith and coworkers demonstrated TSH secretion by human peripheral blood leukocytes stimulated with Staphylococcus enterotoxin A.1 Subsequent work revealed TSH gene expression in activated mouse spleen cells,2 and secretion of TSH by mouse splenic mononuclear cells, in particular dendritic cells (DCs) and to a lesser extent T cells and B cells.3 Human peripheral blood leukocytes have been shown to bind TSH in radiolabeled binding studies4,5 and by flow cytometry using biotin-labeled TSH.6 Similar findings have been reported for murine splenic mononuclear cells,7 collectively indicating that TSHR is selectively expressed on cells of the peripheral immune system.
In mice, TSH also been shown to influence developmental/immune regulatory functions of intestinal intraepithelial lymphocytes (IELs) involved in the recruitment and maturation of specific IEL subsets, most notably the CD8αβ T-cell receptor αβ (TCRαβ) IELs.8,9 This appears to occur through a local TSH-mediated network between TSH-producing intestinal epithelial cells and TSHR+ IELs.10 Within the bone marrow (BM), the expression of surface TSHR has been shown by immunoprecipitation,3 although the types of cells of the BM that express the TSHR was not determined, nor was the capacity of BM cells to produce TSH examined. Functionally, TSH has been shown to significantly influence BM cytokine synthesis, including tumor necrosis factor-α (TNF-α).3
Given the heterogeneity of BM cells and the basic role of the BM as a source of hematopoietic cells destined for secondary lymphoid tissue, it will be important to understand the cells involved in the production and use of TSH locally and to characterize the biologic significance of this system as a regulator of hematopoietic homeostasis. In the present study, we have defined the subsets of BM cells, which produce and use TSH, and we provide new data linking TSH-induced synthesis of TNF-α, an important cytokine with hematopoietic-regulating activities, to specific population(s) of BM cells.
Materials and methods
Mice
Female BALB/c mice, 6 to 10 weeks of age, were purchased from the National Cancer Institute (Frederick, MD) and were housed at the University of Texas Health Science Center Dental Branch.
Antibodies and reagents
Antibodies used in this study were: fluorescein isothiocyanate (FITC)–anti-CD45 (leukocyte common antigen; 30-F11); FITC–anti-CD11b (M1/70); FITC–anti-B220 (RA3-6B2); FITC–anti-TCRβ (H57-597); biotin-labeled mouse IgM (anti-TNP; G155-228); purified rat IgM (ER4-22); anti-CD16/32 Fc receptor block; streptavidin-phycoerythrin (PE) and streptavidin-cychrome (all from BD Pharmingen; San Diego, CA); rabbit antimouse TSH (Accurate Chemicals, Westbury, NY); PE–anti-Ter-119 (Caltag, South San Francisco, CA); PE–anti-Thy-1 5a-8; biotin-antirabbit antibody (Vector Laboratories; Burlingham, CA); biotin-labeled monoclonal antimouse TSHβ 1B1111; anti-Fc receptor CD16 2G2.4 tissue culture supernatant (American Type Culture Collection, Rockville, MD); and biotin-labeled human recombinant TSHβ (Sigma Chemicals, St Louis, MO); human pituitary TSHαβ (98% pure iodination grade; Calbiochem; San Diego, CA);Escherichia coli lipopolysaccharide (LPS; Sigma). Biotinylation of monoclonal antibody (mAb) 1B11 and human recombinant TSHβ was done using published protocols from our laboratory11; purification of mAb 1B11 was done as previously described.11
Intracellular TSH staining and TSHR staining
Staining for the presence of intracellular TSHβ was done according to techniques developed for intracellular cytokine staining.12 Briefly, 1.5 × 106 cells were reacted for 10 minutes at 4°C with CD16/32 Fc receptor blocking reagent (BD Pharmingen). PE- or FITC-labeled anti-CD11b, anti-CD45, anti-TCRβ, anti-B220, or anti–Thy-1 mAb was added for 20 minutes at 4°C. Cells were collected by centrifugation and suspended in 200 μL cytofix/cytoperm (BD Pharmingen) for 20 minutes at 4°C, washed twice with perm-wash (BD Pharmingen), reacted for 20 minutes with 3 μg rat IgM-blocking antibody, and washed with perm-wash. Cells were reacted for 20 minutes at 4°C with 50 μL perm-wash containing 0.3 μg biotinylated anti-TSHβ (mAb 1B11) or biotinylated mouse IgM for control staining. Cells were washed with perm-wash and reacted with streptavidin-PE or streptavidin-cychrome (BD Pharmingen) for 20 minutes at 4°C, washed, and fixed in 2% formalin. Staining for expression of surface TSHR was done by reacting 1 × 106 freshly isolated BM cells with 8 μg biotinylated recombinant TSHβ for 30 minutes at room temperature. Cells were washed and reacted with FITC–anti-CD11b or PE–anti-TER-119 plus streptavidin-PE or streptavidin-cychrome for 20 minutes at 4°C. Cells were washed and fixed in 2% formalin.
Cell purification by MACS
Purification of CD11b+ and CD11b− BM cells was done by positive and negative autoMACS magnetic-activated cell sorting (MACS) cell separation (Miltenyi Biotec, Auburn, CA). Briefly, 15 × 106 freshly isolated BM cells were reacted with 1 mL anti-CD16 tissue culture supernatant for 10 minutes at 4°C. Cells were centrifuged and washed with labeling buffer (phosphate-buffered saline [PBS], pH 7.2, supplemented with 2 mM EDTA [ethylenediaminetetraacetic acid]) and 35 μL biotin-labeled anti-CD11b was added for 20 minutes at 4°C. Cells were washed with labeling buffer and 20 μL streptavidin microbeads (Miltenyi Biotec) was added in 180 μL labeling buffer for 15 minutes at 4°C. Cells were washed, suspended in 1 mL separation buffer (PBS, pH 7.2, supplemented with 2 mM EDTA plus 0.5% bovine serum albumin [BSA]), and applied to autoMACS. Positive and negative cell populations were separated by autoMACS using the manufacturer's protocols.
In vitro cell culture, TSH, and TNF-α immunoassays
For detection of secreted TSH, MACS-purified CD11b+and CD11b− BM cells were cultured for 18 hours at a density of 1 × 106 cells/mL in RPMI 1640 containing 10% (vol/vol) fetal bovine serum (FBS), 2 mM l-glutamine, 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma). TSH induction of TNF-α was done by culturing 1 × 106 MACS-sorted CD11B+ and CD11b− BM cells for 18 hours in supplemented RPMI 1640 with graded doses of human TSH.
Cell-free BM supernatants were collected and coated overnight onto high-binding type I enzyme immunoassay/radioimmunoassay (EIA/RIA) strip plates (Costar, Corning, NY). Wells were washed with PBS containing 0.05% Tween 20 (wash buffer), blocked for 1 hour at room temperature with blocking buffer (eBioscience, San Diego, CA), and washed 3 times with wash buffer. Rabbit antimouse TSH antibody (1:200) was added for 1 hour at room temperature. Wells were washed and biotinylated antirabbit antibody (2 μg/mL) was added for 1 hour at room temperature. Wells were washed 3 times with wash buffer and 1:250 streptavidin-horseradish peroxidase (eBioscience) was added for 30 minutes at room temperature. Wells were washed andO-phenylenediamine was added for 30 minutes at room temperature; colorimetric changes were measured at 490 nM using an automated enzyme-linked immunosorbent assay (ELISA) reader (Molecular Devices, Sunnyvale, CA). Estimates of cell culture–derived TSH were determined from a standard curve of reactivity of anti-TSH antisera to serially diluted (50-1.3 ng/mL) amounts of recombinant human TSHβ. TNF-α secretion by BM cells was measured using a commercial assay (eBioscience) using the manufacturer's protocols and reagents. Colorimetric changes were measured at 450 nM. Statistical analyses of data were determined by Student t test for unpaired observations.
Reverse transcription–polymerase chain reaction analyses
Procedures for RNA extraction and cDNA preparation have been previously reported.3 10 Primers used were: TSHR forward 5′-GACTCATCTGAAGACCATACCCAGTCTTGCA-3′ and TSHR reverse 5′-CATGTAAGGGTTGTCTGTGATTTC-3′; actin forward 5′-ATGGATGACGATATCGCTG-3′ and actin reverse 5′-ATGAGGTAGTCTGTCAGGT-3′.
Amplification conditions consisted of 50 cycles with 1 minute at 95°C, 1 minute at 50°C, and 1 minute at 72°C for TSHR, and 30 cycles with 45 seconds at 95°C, 45 seconds at 50°C, and 30 seconds at 72°C for β-actin.
Results
TSH production by BM hematopoietic cells is linked to a subset of CD11b+ cells
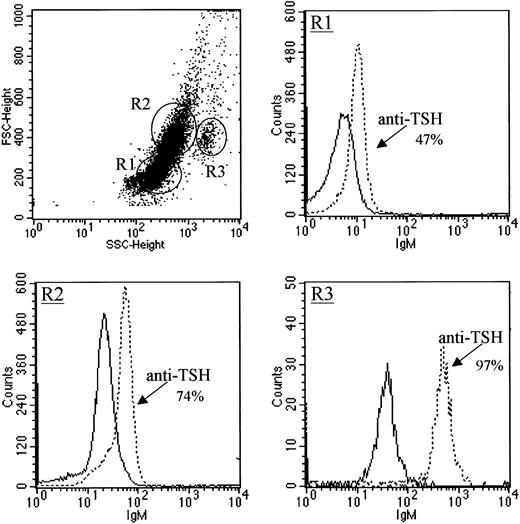
Based on our previous studies demonstrating a role for TSH in the production of BM TNF-α,3 experiments were done to define the mechanisms through which TSH is responsible for regulating TNF-α secretion. Thus, to identify population(s) of cells that might serve as a source of BM-derived TSH, BM cells were stained for intracellular TSH using procedures adapted from techniques used for intracellular cytokine staining using a mAb to mouse TSHβ. As shown in Figure1, BM cells, depending on the cell population, expressed varying amounts of intracellular TSH in that approximately half of the lymphocyte precursor population of BM cells (Figure 1, R1) expressed low levels of intracellular TSH, three quarters of the monocyte-macrophage precursor population (Figure 1, R2) expressed modest levels of intracellular TSH, and nearly all of the granulocyte precursor population (Figure 1, R3) expressed intracellular TSH at high levels.
Intracellular TSH staining of BM cells.
Cells were stained with antimouse TSHβ mAb 1B11. BM populations are shown in the scattergram as lymphocyte precursors (R1), monocyte-macrophage precursors (R2), and granulocyte precursors (R3). Staining is compared to the reactivity of biotin-labeled mouse IgM antibody for each group. Solid lines are reactivities of control antibodies. Data are representative of 3 experiments.
Intracellular TSH staining of BM cells.
Cells were stained with antimouse TSHβ mAb 1B11. BM populations are shown in the scattergram as lymphocyte precursors (R1), monocyte-macrophage precursors (R2), and granulocyte precursors (R3). Staining is compared to the reactivity of biotin-labeled mouse IgM antibody for each group. Solid lines are reactivities of control antibodies. Data are representative of 3 experiments.
To confirm that TSH-synthesizing cells in BM were hematopoietic cells and not stromal cells, freshly isolated cells were stained for intracellular expression of TSH in conjunction with antileukocyte common antigen (CD45) mAb, and with anti-CD11b staining given the high reactivity of cells in the monocyte-macrophage and granulocyte lineage populations described above. As shown in Figure2, nearly all BM intracellular TSH+ cells were CD45+, indicating that TSH production in the BM occurs from hematopoietic cells. Moreover, the majority of intracellular TSH+ cells in each group belonged to a population of CD11b+ cells, though a minor proportion (6%) of the total (82%) intracellular TSH+ cells in the monocytic precursor group were intracellular TSH+ cells (Figure 2, R2).
Characterization of TSH-producing BM cells by intracellular TSH staining with anti-CD45 (leukocyte common antigen) and anti-CD11b.
Nearly all BM cells in regions R1, R2, and R3 (described in the legend to Figure 1) that express intracellular TSH are hematopoietic cells (CD45+) and not stromal cells (CD45−) and are affiliated with a subset of CD11b+ cells. Data are representative of 2 experiments.
Characterization of TSH-producing BM cells by intracellular TSH staining with anti-CD45 (leukocyte common antigen) and anti-CD11b.
Nearly all BM cells in regions R1, R2, and R3 (described in the legend to Figure 1) that express intracellular TSH are hematopoietic cells (CD45+) and not stromal cells (CD45−) and are affiliated with a subset of CD11b+ cells. Data are representative of 2 experiments.
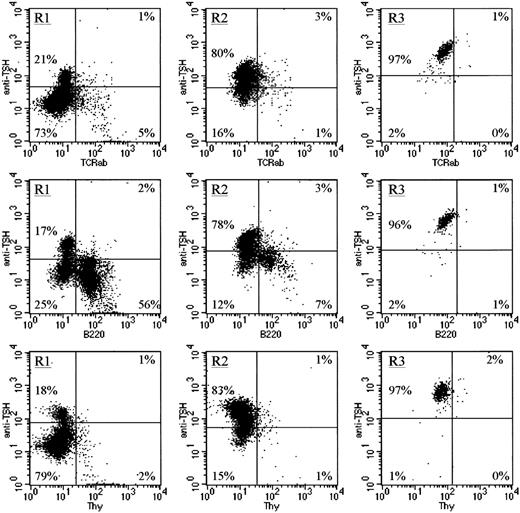
Three additional markers were used in conjunction with intracellular TSH staining. Anti-TCRβ was used to identify mature T cells,13 anti-B220 was used to identify developing B cells,14 and anti–Thy-1 was used as a marker of a subset of very early stem cells in the BM.15 Note that although a few TCRβ+ cells, B220+ cells, and Thy-1+ cells were intracellular TSH+, by far the largest number of TSH-producing cells in the BM resided within the TCRβ−, B220−, Thy-1−populations (Figure 3). These data coupled with those described in Figure 2 indicate that TSH synthesis within the BM is primarily affiliated with a population of CD11b+, TCRβ−, B220−, Thy-1− BM cells.
TSH-producing BM cells are not associated with mature T cells, developing B cells, or Thy-1+ BM cells.
To further characterize the cellular source of TSH within the BM, cells were stained with anti-TCRβ chain mAb as an indicator of mature αβ T cells, with anti-B220 mAb as an indicator of developing B cells, or with anti–Thy-1 mAb because low levels of Thy-1 are expressed on a population of early BM stem cells. Note that in all cell populations (R1, R2, and R3), only a very small percentage of the total intracellular TSH+ cells were mature T cells, developing B cells, or stem cells, further confirming that the primary source of TSH in the BM is a population of CD11b+ cells.
TSH-producing BM cells are not associated with mature T cells, developing B cells, or Thy-1+ BM cells.
To further characterize the cellular source of TSH within the BM, cells were stained with anti-TCRβ chain mAb as an indicator of mature αβ T cells, with anti-B220 mAb as an indicator of developing B cells, or with anti–Thy-1 mAb because low levels of Thy-1 are expressed on a population of early BM stem cells. Note that in all cell populations (R1, R2, and R3), only a very small percentage of the total intracellular TSH+ cells were mature T cells, developing B cells, or stem cells, further confirming that the primary source of TSH in the BM is a population of CD11b+ cells.
To better define the relationship between BM cells and TSH secretion, and to confirm that intracellular TSH+ cells actively produce TSH, freshly isolated BM cells were sorted by MACS into CD11b+ and CD11b− groups and cells from each group were cultured overnight as described in “Materials and methods.” Cell-free supernatants were recovered and screened for TSH activity by ELISA. As shown in Figure 4, although both CD11b+ and CD11b− BM cells secreted TSH, there were statistically significant (P < .01) higher levels of TSH produced by CD11b+ cells (6.12 ± 0.38 ng/mL, n = 3) compared to CD11b− cells (3.40 ± 0.18 ng/mL, n = 5). Previous studies from our laboratory reported murine TSH blood serum levels in the range of 20 to 40 ng/mL.12 Although it is difficult to equate TSH produced in vitro with that of TSH produced under normal physiologic conditions in vivo, it is interesting that the concentration of TSH produced by CD11b+ BM cells in our assay was only slightly lower than that present in the circulation of normal mice. Collectively, these findings indicate that the CD11b+ cell population is the primary source of TSH in the murine BM.
Secretion of TSH by MACS-sorted CD11b+ and CD11b− BM cells.
Cells were cultured as described in “Materials and methods.” Consistent with the findings for intracellular TSH staining (Figure 2), there was a significant increase in TSH produced by CD11b+BM cells than CD11b− cells (P < .01) indicating that CD11b+cells are a major source of TSH in the BM. Data are mean values ± SEMs of 3 or 5 samples per group.
Secretion of TSH by MACS-sorted CD11b+ and CD11b− BM cells.
Cells were cultured as described in “Materials and methods.” Consistent with the findings for intracellular TSH staining (Figure 2), there was a significant increase in TSH produced by CD11b+BM cells than CD11b− cells (P < .01) indicating that CD11b+cells are a major source of TSH in the BM. Data are mean values ± SEMs of 3 or 5 samples per group.
TSHR is primarily expressed on CD11b− BM cells
Expression of the TSHR gene was demonstrated by reverse transcription-polymerase chain reaction (RT-PCR) analyses using whole unfractionated BM cells (Figure 5A). To define the population of BM cells that express TSHR, flow cytometric analysis was done using biotinylated recombinant TSHβ in 2-color staining protocols with anti-CD11b staining. These experiments revealed a selective distribution of TSH-responsive cells in that the greatest numbers of TSHR+ cells in the BM were present in the lymphocyte precursor (R1) population of CD11b− cells (Figure 5B). Although some CD11b+ cells also were TSHR+, within regions that contained the greatest numbers of CD11b+ cells, that is, the monocyte precursor (R2) and granulocyte precursor (R3) groups, most of those cells were TSHR− (Figure 5B). These patterns were confirmed in an analysis of several BM preparations as shown in Table1, which indicates that overall there were statistically more TSHR+ cells among CD11b− cells than CD11b+ cells.
Evidence for TSHR expression in murine BM.
(A) RT-PCR analyses of TSHR in whole BM cells indicates active expression of the TSHR gene. (B) Two-color flow cytometric analyses of BM cells in regions R1, R2, and R3 (Figure 1) stained with biotinylated recombinant TSHβ and for identification of TSHR+ cells and anti-CD11b indicate a preponderance of TSHR+ cells among the lymphocyte precursor population; the overall lack of TSHR staining for Ter-119 cells indicates that cells in the region R1 are not erythrocyte precursors. Data are representative of 2 to 4 experiments.
Evidence for TSHR expression in murine BM.
(A) RT-PCR analyses of TSHR in whole BM cells indicates active expression of the TSHR gene. (B) Two-color flow cytometric analyses of BM cells in regions R1, R2, and R3 (Figure 1) stained with biotinylated recombinant TSHβ and for identification of TSHR+ cells and anti-CD11b indicate a preponderance of TSHR+ cells among the lymphocyte precursor population; the overall lack of TSHR staining for Ter-119 cells indicates that cells in the region R1 are not erythrocyte precursors. Data are representative of 2 to 4 experiments.
Expression of TSHR on BM cells
Data are mean values ± SEM of 4 experiments. Statistical analyses were done using Student t test for unpaired observations.
P < .01 when compared to CD11b+ monocyte precursors and CD11b− granulocyte precursors.
P < .01 when compared to CD11b+lymphocyte precursors and CD11b+ granulocyte precursors.
P < .05 when compared to CD11b−monocyte precursors.
P < .01 when compared to CD11b+ lymphocyte precursors.
P < .025 when compared to CD11b− and CD11b+ granulocyte precursors.
P > .05 when compared to CD11b+ monocyte precursors.
To determine whether TSHR+ cells consist of erythroid precursor population, BM cells were stained for expression of TSHR in conjunction with mAb Ter-119, a marker of murine erythroid precursors.16 Shown in Figure 5B, within all 3 cell populations (R1, R2, and R3), the majority of the TSHR+cells were located in the Ter-119− cell population, thus confirming that erythroid precursors are not a primary TSH-responsive cell population of murine BM.
TSH induces high levels of TNF-α secretion from CD11b− BM cells
The effect of TSH stimulation of BM cells was evaluated using CD11b+ and CD11b− MACS-purified cells across a range of hormone concentrations. As seen in Figure6, TSH had no significant effect on TNF-α production by CD11b+ cells when tested across a 1000-fold range of TSH concentrations. LPS at a concentration of 1000 ng/mL (determined empirically to be the optimal stimulatory concentration [data not shown]) induced high levels of TNF-α, demonstrating that although those cells did not produce TNF-α when stimulated with TSH, they were capable of TNF-α production. In contrast, to CD11b+ BM cells, CD11b− cells, the cell population with the greatest proportion of TSHR+cells, displayed a dose-dependent increase in levels of TNF-α production following TSH stimulation, which was approximately half of that produced by LPS-stimulated cells (Figure 6). Although these findings are consistent with the observation that TSH stimulates the release of TNF-α from TSHR+ CD11b− BM cells, additional work will be needed to precisely define the network of hormone production and use among BM cells.
TSH-induced secretion of TNF-α by CD11b+and CD11b− BM cells.
MACS-sorted cells were cultured with graded doses of human TSH as described in “Materials and methods.” Note the significant increase in TNF-α secretion in TSH-stimulated versus unstimulated CD11b− cells and the predominant TSHR+ cells population (P < .05) (Figure 5). Among CD11b+ cells there was no significant difference in TNF-α secretion of TSH-stimulated cells compared to unstimulated cells. TNF-α production by LPS-stimulated cultures is shown for comparison. Data are mean values ± SEMs of 2 to 5 samples per group.
TSH-induced secretion of TNF-α by CD11b+and CD11b− BM cells.
MACS-sorted cells were cultured with graded doses of human TSH as described in “Materials and methods.” Note the significant increase in TNF-α secretion in TSH-stimulated versus unstimulated CD11b− cells and the predominant TSHR+ cells population (P < .05) (Figure 5). Among CD11b+ cells there was no significant difference in TNF-α secretion of TSH-stimulated cells compared to unstimulated cells. TNF-α production by LPS-stimulated cultures is shown for comparison. Data are mean values ± SEMs of 2 to 5 samples per group.
Discussion
TNF-α has been shown to have pleiotropic effects on hematopoiesis depending on the target cells involved or the stage of development of those cells or both. Moreover, excessive levels of BM TNF-α are believed to be linked to hematopoietic failure of allogeneic BM transplantation,17 and to be involved in the pathogenesis of aplastic anemia, particularly when combined with granulocyte-macrophage colony-stimulating factor (GM-CSF).18 Developmentally, TNF-α has been reported to have stimulatory effects in interleukin 3 (IL-3)– and GM-CSF–supplemented cultures of human CD34+ hematopoietic progenitor cells (HPCs) during short-term culture,19 and to have inhibitory effects on long-term HPC cultures.20Other studies indicate that the inhibitory activity of TNF-α is mediated through the p55 TNFR and that this occurs primarily for the more mature HPC populations, whereas TNF-α–mediated inhibitory effects are associated with the p75 TNFR on early HPCs.21 22 Although these studies provide evidence for a role for TNF-α in the regulation of hematopoiesis, they do not determine how TNF-α activity is controlled locally within the BM. The finding reported here that TSH stimulation of CD11b− BM cells results in increased levels of TNF-α production (Figure 6) suggests a role for TSH in that process. It is important to note, however, that the effect of TSH on TNF-α synthesis need not be direct even though CD11b− cells express TSHR. In fact, a proportion of both CD11b+ and CD11b− cells are TSHR+ (Figure 5 and Table 1), and the potentiating effect of TSH on TNF-α activity could occur through the elaboration of TNF-α–regulating intermediates produced by TSHR+cells.
In conjunction with GM-CSF, TNF-α has been shown to play an important role in the generation of DCs from precursors in the BM. This has been demonstrated with regard to BM-derived Langerhans cells,23and for the development of DCs from early progenitor-stage cells.24 Moreover, that effect may be the consequence of TNF-α–mediated up-regulation of GM-CSF receptors on DC precursors.25 Other studies have demonstrated that TNF-α plus IL-3 in the absence of GM-CSF can mediate DC development from human cord blood,26 implying that TNF-α can act synergistically with several growth-promoting factors, or possibly that it induces the secretion of other growth-promoting factors, but nonetheless further defining an important role for TNF-α in the process of DC development within primary hematopoietic tissues. Additionally, a recent study of mouse BM-derived DC gene expression using DNA microarray assays demonstrated up-regulation of TNF-α genes in mature DCs relative to immature DCs.27 In that study, BM-derived DCs were generated by in vitro culture for 7 days with GM-CSF and IL-4; DC maturation was achieved by LPS stimulation for an additional 24 hours.23 As yet, we have not examined TSH production of BM cells after in vitro maturation, though we recently demonstrated that TSH is produced by purified splenic DCs.12 However, the relationship of those cells to the BM cells described here has not been determined, though these studies are currently under way.
Interestingly, significantly more CD11b+ BM cells, depending on the population, expressed intracellular TSH than CD11b− cells, yet there was only a 2-fold difference in the amount of secreted TSH from the former versus the latter cells. Although the basis for that difference in intracellular TSH production by those 2 populations is not yet evident, the presence of intracellular TSH in CD11b+ BM cells would imply that TSH is more rapidly available from those cells compared to CD11b− cells. It should be pointed out that our studies to date have examined TSH secretion without exogenous stimulation. Experiments are currently under way to determine kinetic TSH release from CD11b+ and CD11b− cells after culture with stimuli with mitogenic, cell-differentiating, or growth-promoting potential.
In summary, despite progress in understanding how hormones, neuroendocrines, and neuropeptides collaborate in the regulation of immunity, many basic aspects of those interactions have yet to be delineated. The findings reported here suggest that locally synthesized TSH may be involved in that process. Because hormones such as TSH are present in the blood and thus have the potential to reach a vast number of different organs and tissues, TSH-mediated effects operating across classical endocrine pathways would be difficult to regulate from an immunologic perspective, whereas the local manufacture and release of TSH disseminated across short distances in a manner analogous to the elaboration and use of cytokines and chemokines might logistically resolve this problem.
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2002-02-0544.
Supported in part by National Institutes of Health grant DK35566.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John R. Klein, University of Texas Health Science Center Houston, Department of Basic Sciences, Dental Branch, Rm 4.133, 6516 MD Anderson Blvd, Houston, TX 77030; e-mail:john.r.klein@uth.tmc.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal