Abstract
We describe a von Willebrand disease (VWD) variant characterized by the persistence of von Willebrand factor (VWF) propeptide as a result of a C>T transition at nucleotide 2527 in exon 17 of the VWF gene. This mutation, which was present in the proband and his father, predicts the substitution of Cys for Arg at position 760 of pre–pro-VWF, 4 residues before the propeptide cleavage site belonging to a consensus sequence for substrate recognition by the processing enzyme paired dibasic amino acid–cleaving enzyme (PACE)/furin. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) documented the presence of both processed and unprocessed VWF in the patient's plasma, with unprocessed VWF relatively less represented. The patient's hemostatic phenotype was characterized by a mild decrease in plasma factor VIII (FVIII) and VWF, a decrease in plasma VWF multimers, and a mild reduction in the FVIII binding capacity of VWF. The FVIII binding defect was more pronounced in the proband than in the father because he also inherited the type 2N Arg91Gln mutation from his mother. The persistence of VWF propeptide did not impair VWF synthesis because platelet VWF content was normal, nor did it compromise VWF storage in endothelial cells, because of the normal post–1-deamino-8-D-arginine vasopressin (DDAVP) increase in plasma VWF. Coexpression of wild-type and Arg760Cys VWF into a Furin-producing BHK cell line resulted in decreased VWF secretion and a defect in the FVIII binding capacity of VWF, together with the persistence of VWF propeptide. These findings confirm that a normal consensus sequence for VWF propeptide cleavage and efficient cleavage are required in vivo for normal FVIII binding capacity of VWF.
Introduction
Von Willebrand factor (VWF) is a large multifunctional glycoprotein that serves as a carrier of factor VIII (FVIII)1 and promotes platelet adhesion and aggregation at the site of vascular injury.2 VWF biosynthesis in megakaryocytes and endothelial cells involves a series of complex processes, including the disulfide binding of VWF dimers into multimers,3-5 and the cleavage of propeptide to form mature VWF.4-6 In the trans-Golgi compartment, the 741–amino acid VWF propeptide promotes the multimerization of VWF dimers through a CXXC sequence present in the D1 and D2 domains that resembles the functional site of thiol disulfide acidoreductases.6 Deletion of the VWF propeptide abolishes VWF multimerization, but not its dimerization.7 In endothelial cells, VWF multimerization proceeds to a high degree before cleavage of VWF propeptide, most of which is cleaved prior to VWF secretion8-10; this process, however, is not necessary for multimerization.10 Cleavage of VWF propeptide requires paired dibasic amino acid–cleaving enzyme (PACE)–furin as a processing enzyme11 and a consensus sequence within 4 amino acids before the cleavage site.12
After synthesis, VWF is released from endothelial cells through 2 pathways: a constitutive release that assures the normal plasma VWF concentration and involves about 90% of the newly synthesized VWF, and a regulated or acute pathway, stimulated by a variety of agonists (ie, thrombin, adrenalin, histamine, B-adrenergic agonists, desmopressin)13,14; for its development the latter pathway requires the presence of VWF stored in Weibel-Palade bodies.15,16 Targeting of VWF to Weibel-Palade bodies seems to require components of both the pro-VWF and the mature subunit.15
A decrease or abnormality in VWF causes von Willebrand disease (VWD), a very heterogeneous bleeding disorder.17 Type 1 and type 3 VWD are defined as a quantitative VWF defect18; type 2 is a functional VWF abnormality that includes defects in VWF binding to platelet GPIb (2A, 2B, 2M) or impaired FVIII binding function (2N).17 19-21 In this report we describe a VWD variant characterized by the persistence of pro-VWF at the heterozygous level, associated with a decrease in plasma VWF levels and a defect in the binding of FVIII to VWF.
Patients, materials, and methods
Patients and volunteers were studied following their informed consent in accordance with the declaration of Helsinki
Blood samples were anticoagulated by using sodium citrate (3.8%, 1/10 vol/vol) or sodium citrate supplemented with 50 mM EDTA (ethylenediaminetetraacetic acid), 60 mM NEM (N-ethylmaleimide), and 50 IU/mL Aprotinin.
Platelet-rich plasma (PRP) was obtained by centrifuging blood at 180g for 10 minutes. Platelet-poor plasma (PPP) was obtained by centrifugation of blood samples at 800g for 15 minutes; the plasma was then centrifuged at 12 000g for 4 minutes to eliminate all cellular fragments and stored at −80°C until use, but for no longer than 2 months. Bleeding time (BT), activated partial thromboplastin time (aPTT), and ristocetin-induced platelet aggregation (RIPA) were determined as described.22 RIPA was evaluated by using a ristocetin concentration of 1.2 mg/mL or 1.5 mg/mL.
VWF antigen (VWF:Ag) was assayed by enzyme-linked immunosorbent assay (ELISA) by using horseradish peroxidase (HRP)–conjugated anti-VWF polyclonal antibody (Dako, Amsterdam, The Netherlands).23 VWF ristocetin cofactor activity (VWF:RCo) was determined by an aggregometer method using normal washed formalin-fixed platelets.24 FVIII activity was measured by a one-stage method, as previously described.25 FVIII binding activity of VWF (VWF:FVIIIB) was evaluated by using an ELISA method as previously reported.26 Briefly, after coating anti-VWF polyclonal antibody to microtiter plates, plasma VWF was added and incubated for 1 hour at room temperature. After washings, bound endogenous FVIII was removed by CaCl2; 1 U/mL recombinant FVIII (rFVIII) (Helixate; Aventis, Malbourg, Germany) was added and the plates were incubated for 1 hour. After washing, the amount of bound rFVIII was evaluated with an anti-FVIII HRP-conjugated polyclonal antibody (Enzyme Research, South Bend, IN). The values were expressed in units per deciliter, taking the optical density in the first dilution of normal pooled plasma as 100. The collagen binding activity of VWF (VWF:CB) was evaluated by an ELISA test using type I (95%) and type III (5%) collagen (Horm, Munchen, Germany), as previously described.27 Platelet VWF:Ag was measured by an ELISA method.23
For genetic analysis, genomic DNA was extracted from peripheral blood leukocytes using the Easy DNA extraction kit (Invitrogen, Carlsbad, CA). Exons 17 and 20 of the VWF gene were amplified from 100 ng of genomic DNA by polymerase chain reaction (PCR) using AmpliTaq DNA polymerase (Perkin Elmer, Warrington, Great Britain) in a Perkin Elmer model 2400 thermal cycler. The PCR reaction mixture contained 5 μL of 10× PCR buffer, 1.25 pmol of each deoxynucleoside triphosphate (dNTP), 12.5 pmol of each primer, and 1 U of Taq polymerase in a total volume of 50 μL. Samples were denatured at 94°C for 5 minutes and amplified for 30 cycles of 30 seconds at 94°C, 30 seconds at 64°C, and 30 seconds at 72°C before a final step of 7 minutes at 72°C. The following sense and antisense primers were chosen according to the VWFgene sequence determined by Mancuso et al28: 17A sense primer, 5′-GTGGAGGAGGCAGCGAGTATAG-3′ (14/150, intron 16); 17B antisense primer, 5′-CGTGAGGAATCTGGGCAGG-3′ (14/342 intron 17); 20A3, 5′-AAGTCCACACTCCACGCTAC-3′ (17/97 intron 19); 20B4, 5′-GCAGACAGATCCACAGAACC-3′ (17/467 intron 20). Primer pairs 17A/17B and 20A3/20B4 yielded PCR products of 210 bp and 390 bp, respectively. PCR products were purified by ultracentrifugation with a Centricon 100 filter (Amicon, Bedford, MA) and eluted in 10 μL H2O. The amplified fragments were sequenced by the dideoxy method using the Big Dye Terminator sequencing kit (Perkin Elmer), precipitated with ethanol and sodium acetate to remove the excess dye terminator, and analyzed in an ABI Prism 310 Genetic Analyzer. We also amplified and sequenced exons 18 to 27 by using the method described above adapted to a panel of published primers.29
For expression studies, plasmid pSVvWFA, containing the normal human full-length cDNA of VWF (kindly provided by Dr C. Mazurier, Lille, France)30 was mutated by recombinant PCR using the following primers: Exp 1 (nucleotide [nt] 2081-2098), 5′-ACATTCGAGGCCTGCCAT-3′; Exp 2a (nt 2576-2599), 5′-CCCCTGTCTCATTGCAGCAAAAGGA-3′; Exp 3a (nt 2599-2576), 5′-TCCTTTTGCTGCAATGAGACAGGGG-3′; Exp 4 (nt 3250-3267), 5′-TGCTTCAGGACCACGGAG-3′. The cDNA nucleotides are numbered according to Ginsburg et al31; the underlined nucleotides are those that differ from the normal wild-type sequence. In the first step, 50 ng pSVvWFA was amplified by using primers Exp 1 and Exp 3a in a Perkin Elmer Cetus model 2400 thermal cycler programmed as follows: after 5′ denaturation at 94°C, 30 cycles of 94°C for 45 seconds, 58°C for 45 seconds, and 72°C for 45 seconds; in parallel, a second fragment was amplified from the plasmid by using primers Exp 2a and Exp 4 under the same conditions with the exception that the annealing temperature was 66°C. The amplified products were purified with Microcon filters (Amicon) to remove unincorporated primers and dNTPs and then mixed in equal quantities (5-10 ng) and submitted to 30 cycles of PCR by using the external primers Exp 1 and Exp 4 with an annealing temperature of 60°C and an elongation time of 1 minute, 10 seconds. The 1186-bp mutated fragment was digested with NotI and EagI (NEB, Beverly, MA) and ligated into pSVvWFA that had been digested with the same enzymes to remove the wild-type sequence, resulting in plasmid pSVR760CvWF. The fragment obtained from PCR and the site of insertion were completely sequenced to verify the presence of the desired mutation and to exclude any other mutations.
For expression studies, vectors pSVvWFA and pSVR760CvWF were transfected transiently into baby hamster kidney (BHK) cells stably transfected with furin (FUR4BHK) (kindly provided by Dr E. J. Sadler, St Louis, MO). The cells were grown in Optimem 1 medium (Life Technologies, Inchnnan, Scotland) supplemented with 2% fetal calf serum. One day before transfection, 1 × 106 cells were seeded in 75-cm2 flasks, so that the cells were 50% to 60% confluent the next day. The cells were transfected with 6 μg plasmid DNA per culture flask by using the Fugene transfection reagent (Roche, Mannheim, Germany). For cotransfection experiments, 3 μg of each plasmid was used. After 72 hours the transfection media were removed and concentrated 10-fold with a Centricon filter; conditioned medium from BHK cells transfected with 6 μg DNA vector was used as a control. In the text, the expressed recombinant VWF (rVWF) produced from plasmids pSVvWF and pSVR760CvWF in BHK cells are referred to as wild type (rWTVWF) and rR760CVWF, respectively. After medium removal, the cells were washed twice with phosphate-buffered saline (PBS; without Ca++ and Mg++), exposed to 0.25% trypsin for 8 minutes, and then centrifuged at 800g for 15 minutes. Pelleted cells were lysed in a solution containing 2% Triton-X100 and centrifuged at 12 000g for 4 minutes to remove membrane fragments; resulting supernatants were recovered for analysis of VWF.
Patients
The proband is a 32-year-old man with a mild bleeding history, in whom hemophilia A was diagnosed in the past. He suffered from epistaxis, bleeding gums, and bleeding after tooth extractions. At 11 years of age, he experienced a severe hemorrhage after tonsillectomy that required blood transfusions; at that time, hemophilia A was diagnosed. The father is 64 years old, and epistaxis is his main bleeding symptom; prior to the present study, he was never examined. The mother is 63 years old, asymptomatic, and classified as a hemophilia A carrier following the diagnosis of hemophilia A in her son.
Results
Hemostatic findings
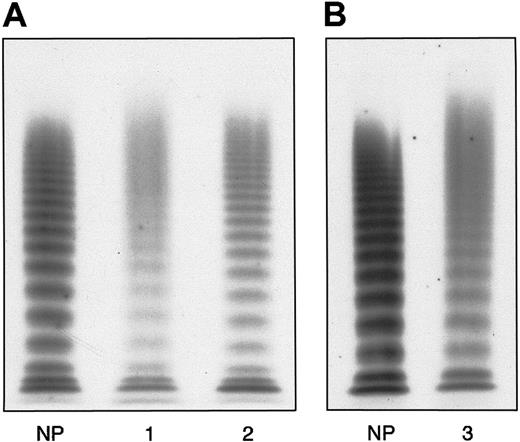
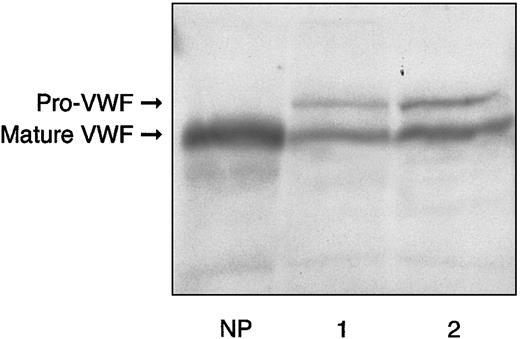
The main hemostatic findings in the family members are shown in Table 1. In the proband, BT was normal; RIPA was almost absent using 1.2 mg/mL ristocetin, and about 16% using 1.5 mg/mL. FVIII, VWF:Ag, and VWF:RCo levels were slightly decreased, with a mild reduction in the FVIII-to-VWF:Ag ratio (0.75 versus normal > 0.8). Accordingly, the binding capacity of VWF:FVIIIB was investigated and found to be decreased both as an absolute value (11.9 U/dL versus normal range, 70-140 U/dL) and a ratio (0.23 versus normal > 0.75). VWF:CB was also decreased (27.8 U/dL versus normal 70-140 U/dL) with an almost normal ratio (0.78 versus normal > 0.8). Platelet VWF:Ag content was normal (118 U/dL versus normal range, 70-140 U/dL). The plasma VWF multimer pattern showed all oligomers present, together with unusually large VWF multimers (Figure1A). Each single multimer appeared less resolved than its normal counterpart because of the presence of a slight smear between the contiguous oligomers. Moreover, starting from the sixth-seventh bands, the multimer pattern exhibited a continuous smear, in contrast to the discontinuous one observed in healthy volunteers and a type 1 VWD patient showing a similar decrease in plasma VWF as the proband (Figure 1A). Furthermore, each multimer, especially the smaller ones, appeared to migrate more slowly than the corresponding normal counterpart, suggesting an increase in molecular weight. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) demonstrated that circulating VWF was represented as 2 forms: one was similar to normal mature VWF, with an apparent size of 225 kDa, and the other, relatively less represented, corresponded to pro-VWF (Figure2). The hemostatic pattern of the father was similar to that of the son, except for the normal plasma FVIII/VWF:Ag ratio (0.99) and the less pronounced decrease in FVIII binding capacity (22.2 U/dL, with a ratio of 0.45). The multimer pattern resembled that of his son (Figure 1B), and SDS-PAGE demonstrated the persistence of pro-VWF (Figure 2). Hemostatic parameters in the mother were all normal except for VWF:FVIIIB, which was decreased both as an absolute value (45.6 U/dL) and ratio (0.43), despite normal plasma FVIII levels and a normal FVIII/VWF:Ag ratio.
Main hemostatic findings of the patients studied
| Patients . | BT, min . | PTT, s . | RIPA,*% . | FVIII, U/dL . | VWF:Ag, U/dL . | VWF:RCo, U/dL . | VWF:FVIIIB, U/dL . | VWF:CB, U/dL . | Platelet VWF:Ag, U/dL . |
|---|---|---|---|---|---|---|---|---|---|
| Proband | 2 min 23 s | 39.7 | 2.5 | 39 | 51 | 45.5 | 11.9 (0.23) | 38 (0.73) | 120 |
| Father | 1 min 59 s | 39 | 21 | 48.1 | 49.3 | 35 | 22.2 (0.45) | 33.8 (0.93) | 100.5 |
| Mother | 3 min 10 s | 31.8 | 77.6 | 98 | 106 | 77 | 45.6 (0.43) | 85 (0.43) | 107.5 |
| Normal range | <4 min | 30-40 | 60-84 | 60-160 | 60-160 | 60-130 | 70-140 (>0.75) | 70-140 (>0.75) | 70-140 |
| Patients . | BT, min . | PTT, s . | RIPA,*% . | FVIII, U/dL . | VWF:Ag, U/dL . | VWF:RCo, U/dL . | VWF:FVIIIB, U/dL . | VWF:CB, U/dL . | Platelet VWF:Ag, U/dL . |
|---|---|---|---|---|---|---|---|---|---|
| Proband | 2 min 23 s | 39.7 | 2.5 | 39 | 51 | 45.5 | 11.9 (0.23) | 38 (0.73) | 120 |
| Father | 1 min 59 s | 39 | 21 | 48.1 | 49.3 | 35 | 22.2 (0.45) | 33.8 (0.93) | 100.5 |
| Mother | 3 min 10 s | 31.8 | 77.6 | 98 | 106 | 77 | 45.6 (0.43) | 85 (0.43) | 107.5 |
| Normal range | <4 min | 30-40 | 60-84 | 60-160 | 60-160 | 60-130 | 70-140 (>0.75) | 70-140 (>0.75) | 70-140 |
The values in parentheses represent the VWF:FVIIIB/VWF:Ag or VWF:CB/VWF:Ag ratios.
RIPA 1.2 mg/mL.
Plasma VWF multimer pattern obtained by electrophoresis of denatured, nonreduced samples in a 1.2% agarose gel containing 0.1% SDS.
After running, fixing, and washing, multimers were detected by125I–anti-VWF polyclonal antibody. At the top are large multimers, at the bottom the small forms. (A) Proband's pattern (1) compared with normal plasma (NP) and a type 1 VWD (2). (B) The father's pattern (3) compared with NP. The patterns in A and B represent different gels electrophoresed under the same experimental conditions. In the proband and his father only the first 6 to 7 oligomers are detectable, even though to a lesser extent than the normal counterpart and type 1 VWD, whereas the higher forms appear as a continuous smear.
Plasma VWF multimer pattern obtained by electrophoresis of denatured, nonreduced samples in a 1.2% agarose gel containing 0.1% SDS.
After running, fixing, and washing, multimers were detected by125I–anti-VWF polyclonal antibody. At the top are large multimers, at the bottom the small forms. (A) Proband's pattern (1) compared with normal plasma (NP) and a type 1 VWD (2). (B) The father's pattern (3) compared with NP. The patterns in A and B represent different gels electrophoresed under the same experimental conditions. In the proband and his father only the first 6 to 7 oligomers are detectable, even though to a lesser extent than the normal counterpart and type 1 VWD, whereas the higher forms appear as a continuous smear.
Electrophoresis of plasma from proband and his father in 5% reducing SDS-PAGE, as compared with normal plasma.
Lane 1 sample is derived from the proband; lane 2, from the proband's father; NP indicates normal plasma. The bands were detected by125I–anti-VWF antibody.
Electrophoresis of plasma from proband and his father in 5% reducing SDS-PAGE, as compared with normal plasma.
Lane 1 sample is derived from the proband; lane 2, from the proband's father; NP indicates normal plasma. The bands were detected by125I–anti-VWF antibody.
Effect of DDAVP infusion
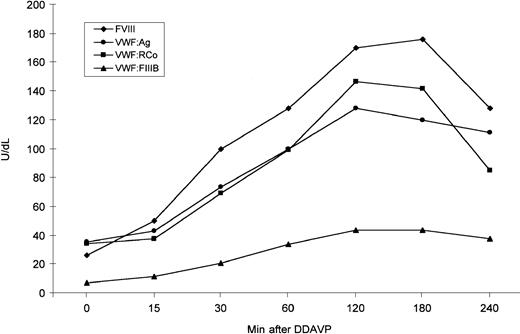
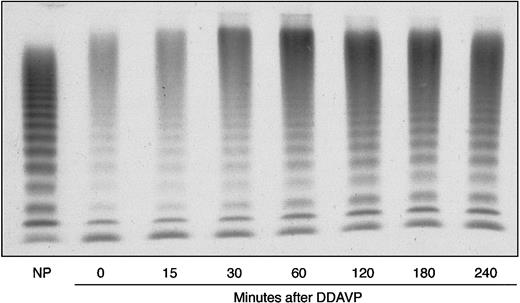
VWF release by endothelial cells was evaluated in the proband by 1-deamino-8-D-arginine vasopressin (DDAVP) administration. The post-DDAVP peaks of VWF:Ag and VWF:RCo were observed after 120 minutes and showed a 3.6- and 4.2-fold increase, respectively; at the peak, FVIII showed a 6.5-fold increase (Figure3). The VWF:FVIIIB values ranged from 6.95 U/dL before DDAVP to 43.7 U/dL at the post-DDAVP peak, with a ratio ranging from 0.19 to 0.34; thus, they still remained abnormal (Figure 3). All multimers were also more represented after DDAVP; the unusually large forms were further increased and appeared so large that they accumulated between the stacking and resolving gel (Figure4). The smear between the oligomers was also more evident.
DDAVP-induced changes in plasma FVIII, VWF:Ag, VWF:RCo, and VWF:FVIIIB as observed in the proband.
DDAVP was administered subcutaneously, at a concentration of 0.3 μg/kg body weight.
DDAVP-induced changes in plasma FVIII, VWF:Ag, VWF:RCo, and VWF:FVIIIB as observed in the proband.
DDAVP was administered subcutaneously, at a concentration of 0.3 μg/kg body weight.
Proband's DDAVP-induced modifications in plasma VWF multimer pattern, as compared with NP.
Electrophoresis was carried out in a 1.2% agarose gel under nonreducing conditions. The blood samples were collected before (0 minutes) and 15, 30, 60, 120, 180, and 240 minutes after administration of DDAVP.
Proband's DDAVP-induced modifications in plasma VWF multimer pattern, as compared with NP.
Electrophoresis was carried out in a 1.2% agarose gel under nonreducing conditions. The blood samples were collected before (0 minutes) and 15, 30, 60, 120, 180, and 240 minutes after administration of DDAVP.
Genetic analysis
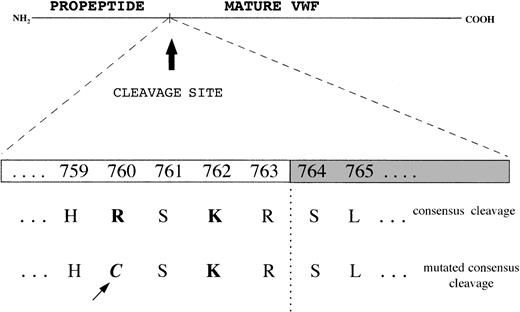
To investigate the cause of pro-VWF persistence, exon 17, which encodes the COOH-terminal portion of the propeptide, and exon 18, which includes the Arg763-Ser764 cleavage site, were amplified and sequenced. In both the proband and his father a Cys2527Thr transition at the heterozygous level was found in exon 17, which results in a Cys → Arg substitution at position 760 in the pro-VWF; this mutation lies 4 amino acids before the cleavage site for pro-VWF (at position −4), in a consensus sequence for the furin processing enzyme, where a Lys at position −2 and an Arg at position −4 play major roles (Figure5). To elucidate the origin of the FVIII binding defect in the mother, and the more pronounced defect in the son than in the father, exons 18, 19, and 20 encoding the NH2-terminal region of mature VWF and mainly involved in FVIII binding function of VWF were amplified and sequenced. The Arg91Gln mutation was demonstrated in exon 20 at the heterozygous level in both the mother and the son; in the father, no mutations were found in exon 20 or in exons 18 or 19.
Consensus sequence for the cleavage site of propeptide from mature VWF by the processing enzyme PACE-furin, characterized by a functionally important Lys (K) at position −2 and Arg (R) at position −4 upstream of the cleavage site (R-S).
The Cys2527Thr mutation falls at position −4 and changes the Arg to a Cys.
Consensus sequence for the cleavage site of propeptide from mature VWF by the processing enzyme PACE-furin, characterized by a functionally important Lys (K) at position −2 and Arg (R) at position −4 upstream of the cleavage site (R-S).
The Cys2527Thr mutation falls at position −4 and changes the Arg to a Cys.
Recombinant VWF
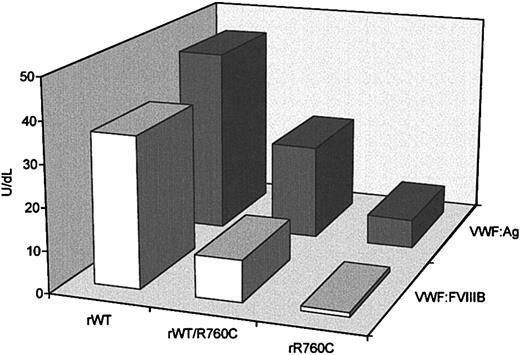
To investigate the synthesis and secretion of VWF containing the missense mutation Cys2527Thr, plasmids expressing wild-type VWF (WTVWF) and the R760C-mutated form (R760CVWF) were transiently transfected into FUR4BHK cells. The cells were cotransfected with equal amounts of wild-type and mutated cDNA to mimic the heterozygous state, in addition to wild-type and mutated cDNAs alone. Both intracellular rVWF, obtained from cell lysates, and rVWF, secreted in the conditioned medium, were quantified by an ELISA method, and the mean values from 5 separate experiments were calculated. Intracellular rVWF values were normalized to 1 × 106 cells, to abolish the difference in cell number between different flasks. The VWF:Ag concentration of rWTVWF in the culture media was 46.3 ± 5.8 U/dL, versus 22.1 ± 4.3 U/dL, for the hybrid rWT/R760CVWF and 6.7 ± 1.8 U/dL for rR760CVWF (Figure6). No significant differences in intracellular levels of rWTVWF or rWT/R760CVWF were observed (33.3 ± 2.75 U/dL and 33.9 ± 2.33 U/dL, respectively) or in rR760CVWF, which was 30.2 ± 1.06 U/dL.
VWF:Ag and VWF:FVIIIB values in conditioned media obtained from FUR4BHK cells transfected with a plasmid expressing recombinant wild-type VWF, mutant rR760CVWF, or both rWTVWF and rR760CVWF.
rWT indicates recombinant wild type; rR760C, mutant rR760CVWF; and rWT/R760C, both rWTVWF and rR760CVWF. For each sample 2 dilutions were performed, and the results are expressed as the means of 5 different experiments.
VWF:Ag and VWF:FVIIIB values in conditioned media obtained from FUR4BHK cells transfected with a plasmid expressing recombinant wild-type VWF, mutant rR760CVWF, or both rWTVWF and rR760CVWF.
rWT indicates recombinant wild type; rR760C, mutant rR760CVWF; and rWT/R760C, both rWTVWF and rR760CVWF. For each sample 2 dilutions were performed, and the results are expressed as the means of 5 different experiments.
The capability of rVWF to bind FVIII was evaluated by an ELISA method (Figure 6). rWTVWF showed a VWF:FVIIIB value of 36 U/dL with a 0.8 ratio; the binding capacity of the hybrid rWT/R760CVWF was 16 U/dL with a 0.4 ratio, whereas rR760CVWF showed undetectable FVIII binding capacity (< 1 U/dL) (Figure 6).
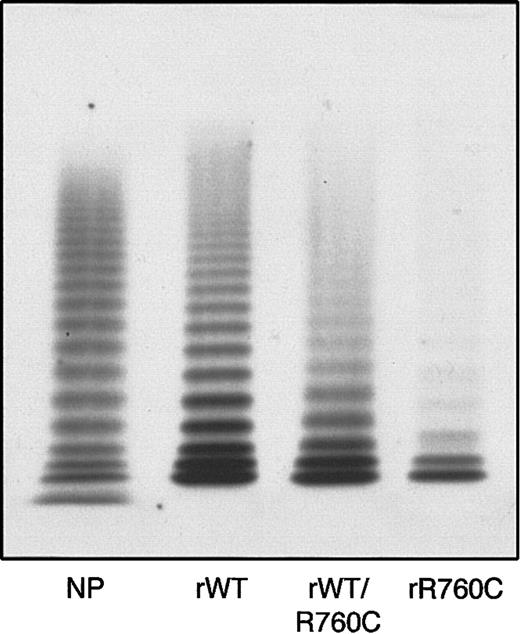
The multimer organization of recombinant VWF was characterized by the presence of multimers in both rWT and hybrid rWT/R760CVWF, but with a decrease in all components present in the hybrid, and a more pronounced decrease in rR760CVWF (Figure 7).
Multimer pattern of rWTVWF, hybrid rWTVWF/R760C VWF, and homozygous rR760CVWF obtained by 1.2% agarose gel electrophoresis.
rWT indicates rWTVWF; rWT/R760C, hybrid rWTVWF/R760C VWF; rR760C, homozygous rR760CVWF; and NP, normal plasma.
Multimer pattern of rWTVWF, hybrid rWTVWF/R760C VWF, and homozygous rR760CVWF obtained by 1.2% agarose gel electrophoresis.
rWT indicates rWTVWF; rWT/R760C, hybrid rWTVWF/R760C VWF; rR760C, homozygous rR760CVWF; and NP, normal plasma.
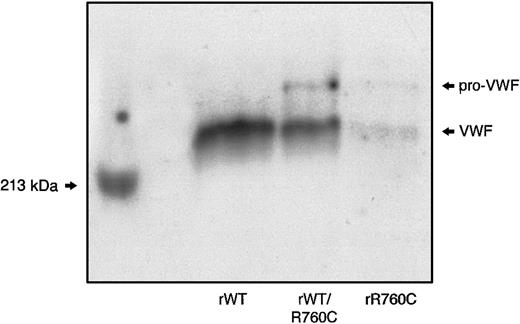
SDS-PAGE of the hybrid rWT/R760CVWF demonstrated the presence of both unprocessed and mature VWF, with the unprocessed form less represented than the mature component,8 as observed in the proband's plasma and rR760CVWF. In contrast, only the mature VWF band was detectable in rWTVWF (Figure 8).
SDS-PAGE of rWT, hybrid rWT/R760C, and homozygous rR760C.
Myosin, having a molecular weight of 213 kDa, was used as a marker.
SDS-PAGE of rWT, hybrid rWT/R760C, and homozygous rR760C.
Myosin, having a molecular weight of 213 kDa, was used as a marker.
Discussion
We describe a new VWD variant characterized by the persistence of pro-VWF because of an Arg→Cys mutation at position 760 of pre–pro-VWF in a consensus sequence for the propeptide cleavage site of VWF. Our patients's phenotype was characterized by a mild decrease in plasma FVIII and VWF levels and a normal platelet VWF content in association with decreased FVIII binding capacity of VWF. The defect appeared to be transmitted as a dominant trait. Our findings suggest that an Arg must be present 4 amino acids upstream of the cleavage site of pro-VWF to guarantee appropriate substrate recognition by the processing enzyme PACE-furin, and they also confirm that the removal of the VWF propeptide is necessary for a normal FVIII-VWF interaction. Indeed, the persistence of the VWF propeptide appears to be a new cause of type 2N VWD.
Pro-VWF is a 741–amino acid peptide that plays a key role in the process of multimeric assembly and targeting of VWF in Weibel-Palade bodies.7,13,32 Prior to release of VWF from endothelial cells, most of the propeptide is cleaved from the mature subunit, even though some subunits retain the propeptide after their secretion.33 The pro-VWF cleavage site is located between the pair of dibasic amino acids (Cys-Arg) at residues 763 to 764 of pre–pro-VWF.9,11,34 The proteolytic activity for this process is preferentially expressed by the enzyme PACE/furin, a calcium-dependent serine protease, or related subtilisinlike processing proteases.8,32,35 In addition to the paired dibasic residue at the propeptide cleavage site, PACE-furin also requires an upstream consensus sequence to express full activity, mainly involving the Arg and Lys at positions 4 and 2, respectively.34However, the persistence of pro-VWF obtained in vitro by mutation of Arg763Gly does not seem to compromise normal VWF multimerization.8
The family described in this report demonstrates that the persistence of pro-VWF does not compromise VWF synthesis or storage in both endothelial cell Weibel-Palade bodies and platelet α-granules, because platelet VWF content and DDAVP-induced release of VWF from Weibel-Palade bodies were normal18; however, it reduces circulating levels of VWF, albeit to a mild degree. This last finding suggests a defect in the constitutive release of VWF by endothelial cells, which was confirmed by the demonstration of reduced secretion of mutated recombinant VWF expressed in BHK cells. Indeed, the secretion of hybrid rWT/R760CVWF, which mimics the heterozygous state of our patients, appeared reduced by 50% compared with the wild type. In contrast, the intracellular rVWF content of both hybrid rWT/R760CVWF and homozygous rR760CVWF appeared to be normal. Taken together, these findings establish that the major outcome of pro-VWF persistence because of the Arg760Cys mutation is compromised VWF secretion, the degree of which depends on the heterozygous or homozygous state. That the defect in VWF release mainly involves pro-VWF is based on the finding that, in plasma, unprocessed VWF was less represented than the mature subunit, similarly to the observation made with the recombinant hybrid VWF secreted by BHK cells. Our results agree with the in vitro observation that recombinant VWF carrying the conservative Arg→ Lys substitution at position 4 upstream cleavage site yielded 80% mature VWF and only 20% of unprocessed VWF, whereas the nonconservative Arg → Ala substitution at the same position yielded 65% mature VWF.11 Instead, mutations involving the Cys at position 2 upstream of the cleavage site result in a more pronounced defect in VWF processing, suggesting that this position plays a major role in cleavage activity.11 The persistence of pro-VWF does not compromise VWF multimer composition, because all oligomers were present in the patients studied, even though they were less represented. Other multimer abnormalities, such as the presence of the smear between contiguous oligomers and the unusually large VWF multimers may be explained by the introduction of an additional Cys,36 known to have a key role in multimer organization.
Arg760Cys VWF also displayed a decrease in FVIII binding capacity, as previously observed in an in vitro model of unprocessed VWF.37 This finding might be explained by the fact that persistence of pro-VWF makes the FVIII binding sites, which are located in the amino-terminal portion of mature VWF, less accessible to the FVIII molecule because of steric hindrance. Alternatively, it may be advanced that after proteolytic cleavage, VWF allows a correct folding for the development of high-affinity binding domains. In any case, the patient's FVIII binding capacity was only mildly compromised, in agreement with the observation that circulating pro-VWF appeared relatively less represented than the mature subunit. Indeed, the entity of decrease in FVIII binding capacity of VWF carrying the Arg760Cys mutation was similar to that observed in heterozygous type 2N VWD; however, when a second type 2N mutation was also present, as in the proband who also carried the Arg91Gln mutation, the FVIII binding defect became significantly more pronounced.
This family study also suggests that the persistence of pro-VWF induces an abnormal VWF and platelet GPIb interaction, as documented by the impaired or absent RIPA and the decrease in VWF:RCo that was more pronounced than expected from the VWF:Ag levels. This latter finding does not seem explainable by a slight decrease in large VWF multimers because VWF:CB, which is very sensitive to large VWF multimer representation,38 was reduced in both affected patients, almost proportionally to the decrease in VWF:Ag. As already advanced for the FVIII binding defect, it is possible that the persistence of pro-VWF may also compromise the availability of the A1 domain of VWF for platelet GPIb.
This family represents a new model for type 2N VWD, in addition to the classic type involving mutations in the amino-terminal region of mature VWF. As the etiopathogenesis of this VWD variant, which presents both quantitative and type 2N defects, differs from the others identified to date, we suggest the designation type 2P, based on the persistence of pro-VWF.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-04-1046.
Supported by grants from Telethon Foundation and Ministero Dell' Universita' e della Ricerca Scientifica e Technologia (MURST) (60%), Rome, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alessandra Casonato, Department of Medical and Surgical Sciences, University of Padua, Via Ospedale Civile 105, Padova, Italy; e-mail: sandra.casonato@unipd.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal