Abstract
Human monocytes differentiate into dendritic cells (DCs) or macrophages according to the nature of environmental signals. Monocytes stimulated with granulocyte-macrophage colony-stimulating factor (GM-CSF) plus interleukin 4 (IL-4) yield DCs. We tested here whether interferon-γ (IFN-γ), a potent activator of macrophages, may modulate monocyte differentiation. Addition of IFN-γ to IL-4 plus GM-CSF–stimulated monocytes switches their differentiation from DCs to CD14−CD64+ macrophages. IFN-γ increases macrophage colony-stimulating factor (M-CSF) and IL-6 production by IL-4 plus GM-CSF–stimulated monocytes by acting at the transcriptional level and acts together with IL-4 to up-regulate M-CSF but not IL-6 production. IFN-γ also increases M-CSF receptor internalization. Results from neutralizing experiments show that both M-CSF and IL-6 are involved in the ability of IFN-γ to skew monocyte differentiation from DCs to macrophages. Finally, this effect of IFN-γ is limited to early stages of differentiation. When added to immature DCs, IFN-γ up-regulates IL-6 but not M-CSF production and does not convert them to macrophages, even in the presence of exogenous M-CSF. In conclusion, IFN-γ shifts monocyte differentiation to macrophages rather than DCs through autocrine M-CSF and IL-6 production. These data show that IFN-γ controls the differentiation of antigen-presenting cells and thereby reveals a new mechanism by which IFN-γ orchestrates the outcome of specific immune responses.
Introduction
Peripheral blood monocytes can differentiate into dendritic cells (DCs) or macrophages depending on environmental factors encountered during their migration from blood to peripheral tissues.1-4 Transendothelial trafficking5 and culture in the presence of serum from systemic lupus erythematosus (through the presence of interferon α [IFN-α]) induce monocyte differentiation into immature DCs.6 On contact with interleukin 4 (IL-4) plus granulocyte-macrophage colony-stimulating factor (GM-CSF; cytokines that could be produced by tissue mast cells), monocytes also differentiate into immature DCs.2-4Addition of transforming growth factor β (TGF-β) or exposition of monocytes to GM-CSF plus IL-15 led to DCs with features of Langerhans cells.7,8 In contrast, macrophage colony-stimulating factor (M-CSF) is a potent macrophage differentiation factor.1 IL-69,10 and IL-1011also shift monocyte differentiation from DCs to macrophages. Tumor cells produce IL-6 and M-CSF that shift the differentiation of CD34+ progenitors from DCs to macrophages.9Fibroblasts, via IL-6 production, up-regulate functional M-CSF receptor (CD115) expression and autocrine M-CSF consummation by monocytes, thereby switching their differentiation from DCs to macrophages.10
DCs are the most potent antigen-presenting cells (APCs).12 In the periphery, immature DCs capture antigens and, on contact with stress factors (such as microbial components), migrate to the lymphoid organs and undergo a maturation process. They express high levels of costimulatory and accessory molecules, up-regulate major histocompatibility complex (MHC) class I and II molecules, and neoexpress CD83. In the lymph nodes, mature DCs prime naive antigen-specific T cells.12 In contrast to DCs, macrophages are effector cells that produce various mediators and have evolved to ingest as many pathogens as possible. Although they present antigens, macrophages are less efficient than DCs and unable to prime naive T cells.13
IFN-γ, released during early and late stages of the immune response by natural killer (NK) cells and activated T cells, respectively, regulates several aspects of the immune response.14 In addition to direct antiviral activity, IFN-γ orchestrates leukocyte-endothelium interaction14and plays a crucial role in vivo in “cancer immunosurveillance.”15 IFN-γ is a potent activator of macrophages. On contact with IFN-γ, monocyte-macrophages undergo biochemical and morphologic modifications that allow them to perform their functional activities.16 INF-γ stimulates macrophage antimicrobial and tumoricidal activities and accessory cell functions and modulates proteasome gene expression.16IFN-γ also acts on uncommitted myeloid immature DCs to polarize them into TH1 cell–promoting effector cells that produce high levels of IL-12 on stimulation.17 We tested here whether IFN-γ could be involved in monocyte differentiation and report that IFN-γ switches monocyte differentiation from DCs to macrophages, at least partly via an autocrine production of M-CSF and IL-6.
Materials and methods
Cytokines
All human and murine recombinant cytokines were from R & D Systems (Abingdon, United Kingdom).
Human monocyte differentiation
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque (Life Technologies, Cergy Pontoise, France) density gradient centrifugation. Monocytes were purified from PBMCs by positive selection using a magnetic cell separator (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany). Purity, assessed by fluorescence-activated cell sorting (FACS) analysis using a fluorescein isothiocyanate (FITC)–labeled anti-CD13 monoclonal antibody (mAb), was more than 98%. Monocytes were differentiated into DCs by 5 days of culture in complete medium (CM) consisting of RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and 0.1 mM nonessential amino acids (all from Life Technologies) at 5 × 106 cells/5 mL/well in 6-well tissue culture plates (Corning Costar, Cambridge, MA) with 20 ng/mL IL-4 and 20 ng/mL GM-CSF. Macrophages were obtained by culturing monocytes for 5 days in CM with 2 ng/mL GM-CSF and 20 ng/mL M-CSF.18 In some experiments, monocytes were cultured for 5 days in CM with 20 ng/mL GM-CSF. All these cultures were also performed in the presence of different concentrations of IFN-γ (2-50 ng/mL) added at different time points, from day 0 to day 5. In neutralization experiments, monocytes in GM-CSF plus IL-4 containing or not 25 ng/mL IFN-γ were treated at day 0 and day 2 with 30 μg/mL neutralizing antihuman M-CSF mAb (R & D Systems) plus 30 μg/mL neutralizing antihuman IL-6 mAb (Diaclone, Besancon, France) or with 30 μg/mL mouse isotype control mAbs (BD Pharmingen, San Diego, CA). In other experiments, day 5 immature DCs were recultured in GM-CSF plus IL-4 without or with 25 ng/mL IFN-γ alone, 25 ng/mL IFN-γ plus 100 ng/mL M-CSF, or 100 ng/mL M-CSF plus 100 ng/mL IL-6 or were recultured in CM without cytokine. Finally, in some experiments cells were stimulated with 20 ng/mL lipopolysaccharide (LPS; Sigma, St Louis, MO).
MLRs with human cells
DCs and macrophages, obtained as described above, were washed, irradiated (3000 rad), and cultured in quintuplicate at 4 × 102, 2 × 103, or 2 × 104 cells/200 μL/well in 96-well flat-bottomed plates with 105 allogenic T cells purified from PBMCs by rosetting with sheep red blood cells (the purity, assessed by FACS analysis using a FITC-labeled anti-CD3 mAb, was > 95%). In some experiments, day-5 immature DCs were treated or not with 25 ng/mL IFN-γ. After 48 hours, cells were or were not stimulated with 2 ng/mL LPS for 24 hours and used in mixed lymphocyte reaction (MLR) assays at 2 × 104 cells/mL with 106 allogenic purified T cells. After 5 days, cells were pulsed during the last 16 hours with 0.25 μCi/well (0.00925 MBq) 3H-thymidine (Amersham, Uppsala, Sweden). Thymidine incorporation was measured by standard liquid scintillation counting. Results are expressed in counts per minute (mean of quintuplicate values).
Murine cells
C57BL/6 (IAb) and Balb/c (IAd) mice were from Harlan (Gannat, France). Murine DCs were generated as described19 by culturing bone marrow cells from C57BL/6 mice in CM supplemented with 50 μM β-mercaptoethanol (β-ME) and containing 3 ng/mL GM-CSF. In some experiments, 25 ng/mL murine IFN-γ was added at the beginning of the culture. After 5 days, the phenotype of the cells was analyzed by FACS and MLRs were performed as follows. Briefly, allogenic CD4+ T cells from Balb/c mice were purified by incubation with a FITC-labeled antimurine CD4 mAb (BD Pharmingen) followed by positive selection using anti-FITC mAb-coated microbeads (Miltenyi Biotec). After 5 days of culture in GM-CSF, myeloid APCs were depleted in Gr1+ cells by incubation with an anti-Gr1 mAb (Caltag, Burlingame, CA) followed by antimouse Ig mAb-coated beads (Dynal, Oslo, Norway). In 96-well flat-bottomed culture plates (Corning Costar), 4 × 105CD4+ T cells plus 5 × 104 APCs were cultured in triplicate for 72 hours. During the last 16 hours,3H-thymidine was added. Results are expressed in counts per minute × 10−3 as mean ± SD (n = 3).
FACS analysis
The phenotype of cells was analyzed by cytofluorometry using a FACSvantage cytofluorometer (BD Biosciences, Erembodegem, Belgium). For human cells, the following mAbs were used: FITC-labeled anti-CD1a (Immunoquality Products, Groningen, The Netherlands), anti-CD14 (Dako, Glostrup, Denmark), anti-CD64 (Caltag), anti-CD86, and anti–HLA-DR (both from BD Pharmingen) mAbs; unlabeled anti–mannose receptor (MR; Research Diagnostic, Flanders, NJ), -MHC I, -CD83 (both from Beckman Coulter, Villepinte, France) revealed by FITC-labeled antimouse IgG antibody (Silenus, Melbourne, Australia) and goat anti-CD115 antibody (R & D Systems) revealed by FITC-labeled antigoat IgG antibody (Silenus). To analyze intracellular expression of CD115 and RFD7, cells were fixed and permeabilized using the Intrastain kit (Dako) before staining with anti-CD115 or anti-RFD7 mAbs (Serotec, Oxford, United Kingdom) revealed by FITC-labeled antigoat IgG antibody or antimouse IgG antibody, respectively. Murine cells were phenotyped using FITC-labeled anti-CD11b, anti-CD11c, anti-CD86, anti-IAb(all from BD Pharmingen), biotin-labeled anti-F4/80 mAb (Caltag) revealed by FITC-labeled streptavidin (Molecular Probes, Eugene, OR) and phycoerythrin (PE)–labeled anti-CD11c (BD Pharmingen) and anti-Gr1 mAbs. Isotype control mAbs were from BD Pharmingen. Results are expressed as a percentage of positive cells or in mean fluorescence intensity (MFI) values after subtraction of the MFI obtained with the control mAb.
Analysis of mRNA expression by RT-PCR
In freshly purified human monocytes and in monocytes cultured in GM-CSF plus IL-4 in the absence or presence of 25 ng/mL IFN-γ for 2, 4, 8, or 16 hours, the expression of the mRNA encoding IL-6, IL-10, M-CSF, and CD115 was determined by reverse transcription–polymerase chain reaction (RT-PCR). Briefly, RNA was extracted using Trizol reagent (Life Technologies) and the single-strand cDNA was synthesized using 2 μg total RNA by reverse transcription using an oligo-dT primer (Amersham). PCRs were performed with cDNA corresponding to 50 ng total RNA and primers designed to amplify the coding sequence of the cytokines and cytokine receptor. PCR was as follows: 94°C for 5 minutes, 30 cycles 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 minute followed by a final extension at 72°C for 5 minutes. RNA integrity and cDNA synthesis were verified by amplifying glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA. The amplified fragments were size-separated on a 1% agarose gel and visualized by ethidium bromide.
Quantification of human cytokines and soluble IL-6 receptor
IL-6, M-CSF, and glycoprotein 80 (gp80; soluble IL-6 receptor) were quantified in the cell-free culture supernatants by enzyme-linked immunosorbent assay (ELISA; R & D Systems; sensitivity of 0.7, 9, and 6.5 pg/mL, respectively). Results are expressed in nanograms per milliliter or micrograms per milliliter (as mean ± SD, n = 4).
Phagocytosis and cytochemistry
To analyze endocytic properties, cells were incubated for 20 minutes at 37°C with FITC-labeled dextran (40 000 molecular weight),Staphylococcus aureus, or latex beads (2 μm diameter; all from Molecular Probes). After extensive washings, cells were analyzed by FACS. Results are expressed in MFI or as a percentage of fluorescent cells. Nonspecific esterase activity was analyzed using the α naphthyl acetate esterase kit (Sigma) following the manufacturer's instructions. Cell staining was analyzed by light microscopy.
Results
IFN-γ shifts monocyte differentiation from DCs to activated macrophages
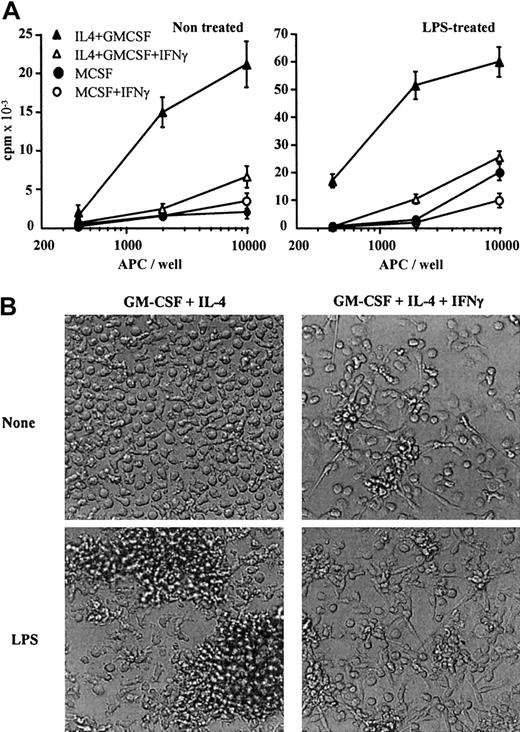
CD1a−CD14+ monocytes cultured with GM-CSF plus IL-4 differentiate after 5 days into CD1a+CD14− immature DCs2 3 (Table1). When cultured in GM-CSF plus IL-4 containing IFN-γ, monocytes differentiate into CD1alowCD14− cells (Table 1). Compared to monocyte-derived immature DCs, cells treated with IFN-γ express CD64 and CD86, express higher levels of MHC class I and class II molecules, express lower levels of MR (Table 1), and present reduced T-cell costimulatory properties (Figure 1A, left panel). Microscopic observation shows that, whereas immature DCs are mostly nonadherent round cells, IFN-γ–treated cells form a network of adherent elongated cells (Table 1 and Figure 1B). In contrast to macrophages, LPS-stimulated immature DCs undergo a maturation process. They present morphologic changes associated with veils (Table 1 and Figure 1B); neoexpress CD83 (Table 1); up-regulate MHC class II, CD40, CD80, and CD86 expression (data not shown); and acquire potent costimulatory properties (Figure 1A, right panel). On LPS stimulation, IFN-γ–treated cells are adherent, do not present veils (Table 1 and Figure 1B), and do not acquire CD83 expression (Table 1); in addition, the expression of MHC class II and costimulatory molecules (CD40, CD80, and CD86) is not modulated (data not shown). Moreover, these cells have limited costimulatory properties compared to mature DCs (Figure 1A, right panel). Lastly, in response to 2 ng/mL LPS, IFN-γ–treated cells produce undetectable levels of bioactive IL-12, in contrast to immature DCs (835 ± 160 pg/mL; data not shown). IFN-γ–treated cells do not express Langerhans cell–associated markers (langerhin and E-cadherin; data not shown). Together, these data suggest that GM-CSF plus IL-4 plus IFN-γ–treated monocytes present a macrophage phenotype.
Characteristics of monocytes cultured for 5 days with GM-CSF plus IL-4, GM-CSF, or M-CSF in the absence or presence of IFN-γ
| . | GM-CSF + IL-4 . | GM-CSF . | M-CSF . | |||
|---|---|---|---|---|---|---|
| − IFN-γ . | + IFN-γ . | − IFN-γ . | + IFN-γ . | − IFN-γ . | + IFN-γ . | |
| Markers | ||||||
| CD1a | 320 | 70 | 36 | 16 | 27 | 18 |
| CD14 | 5 | 8 | 590 | 43 | 370 | 89 |
| CD64 | 4 | 112 | 58 | 309 | 13 | 33 |
| CD86 | 8 | 76 | 18 | 24 | 12 | 22 |
| CD83 | 3 | 3 | 3 | 3 | 3 | 3 |
| CD83* | 130 | 3 | 3 | 3 | 3 | 3 |
| MHC-I | 335 | 1112 | 744 | 856 | 137 | 250 |
| HLA-DR | 539 | 1115 | 319 | 502 | 186 | 302 |
| RFD7 | 3 | 3 | 290 | 37 | 39 | 3 |
| MR | 271 | 175 | 160 | 51 | 320 | 100 |
| Endocytic activity | ||||||
| Dextran | 395 | 142 | 131 | 22 | 80 | 13 |
| Staphylococcus aureus | 34% | 7% | ND | ND | 90% | 10% |
| Latex beads | 0% | 3% | ND | ND | 73% | 13% |
| Enzymatic activity | ||||||
| Nonspecific esterase | − | +/− | ND | ND | ++ | +/− |
| Morphology | ||||||
| Nonadherent round/ovoid cells | + | − | + | − | + | − |
| Network of elongated and adherent cells | − | + | − | + | − | + |
| Nonadherent round/ovoid cells* | − | − | + | − | + | − |
| Network of elongated and adherent cells* | − | ++ | − | + | − | + |
| Ovoid and veils* | + | − | − | − | − | − |
| . | GM-CSF + IL-4 . | GM-CSF . | M-CSF . | |||
|---|---|---|---|---|---|---|
| − IFN-γ . | + IFN-γ . | − IFN-γ . | + IFN-γ . | − IFN-γ . | + IFN-γ . | |
| Markers | ||||||
| CD1a | 320 | 70 | 36 | 16 | 27 | 18 |
| CD14 | 5 | 8 | 590 | 43 | 370 | 89 |
| CD64 | 4 | 112 | 58 | 309 | 13 | 33 |
| CD86 | 8 | 76 | 18 | 24 | 12 | 22 |
| CD83 | 3 | 3 | 3 | 3 | 3 | 3 |
| CD83* | 130 | 3 | 3 | 3 | 3 | 3 |
| MHC-I | 335 | 1112 | 744 | 856 | 137 | 250 |
| HLA-DR | 539 | 1115 | 319 | 502 | 186 | 302 |
| RFD7 | 3 | 3 | 290 | 37 | 39 | 3 |
| MR | 271 | 175 | 160 | 51 | 320 | 100 |
| Endocytic activity | ||||||
| Dextran | 395 | 142 | 131 | 22 | 80 | 13 |
| Staphylococcus aureus | 34% | 7% | ND | ND | 90% | 10% |
| Latex beads | 0% | 3% | ND | ND | 73% | 13% |
| Enzymatic activity | ||||||
| Nonspecific esterase | − | +/− | ND | ND | ++ | +/− |
| Morphology | ||||||
| Nonadherent round/ovoid cells | + | − | + | − | + | − |
| Network of elongated and adherent cells | − | + | − | + | − | + |
| Nonadherent round/ovoid cells* | − | − | + | − | + | − |
| Network of elongated and adherent cells* | − | ++ | − | + | − | + |
| Ovoid and veils* | + | − | − | − | − | − |
Monocytes were cultured in medium containing GM-CSF plus IL-4, GM-CSF, or M-CSF in the absence or presence of 25 ng/mL IFN-γ. After 5 days, the phenotype and the uptake of FITC-labeled dextran, S aureus or latex beads was analyzed by FACS and cell morphology was determined by microscopy. Results are expressed in MFI values excepted the uptake of S aureus and latex beads expressed in percentage of positive cells. Results are representative of 1 of 5 experiments.
ND indicates not done; −, +/−, +, and ++, respectively, indicate absent, slightly detectable, detectable, and strongly evidenced.
CD83 expression and cell morphology were analyzed after 24 hours' incubation with 20 ng/mL LPS.
IFN-γ shifts monocyte differentiation from DCs to macrophages.
(A) Monocytes were cultured in medium containing M-CSF (circles) or GM-CSF plus IL-4 (triangles) in the absence (filled symbols) or presence (open symbols) of 25 ng/mL IFN-γ. After 5 days, cells were (right panel) or were not (left panel) stimulated for 24 hours with 20 ng/mL LPS. Cells were then irradiated and used to stimulate allogenic T cells. Results are expressed in counts per minute × 10−3as means ± SDs of quintuplicate values. Results shown are of 1 experiment representative of 3. Results show mean SD of quintuplicate values of 1 experiment representative of 3. (B) Monocytes were cultured in GM-CSF plus IL-4 in the absence (left) or presence of 25 ng/mL IFN-γ (right). After 5 days, cells were (top) or were not (bottom) stimulated for 24 hours with 20 ng/mL LPS and observed by microscopy (original magnification, × 100).
IFN-γ shifts monocyte differentiation from DCs to macrophages.
(A) Monocytes were cultured in medium containing M-CSF (circles) or GM-CSF plus IL-4 (triangles) in the absence (filled symbols) or presence (open symbols) of 25 ng/mL IFN-γ. After 5 days, cells were (right panel) or were not (left panel) stimulated for 24 hours with 20 ng/mL LPS. Cells were then irradiated and used to stimulate allogenic T cells. Results are expressed in counts per minute × 10−3as means ± SDs of quintuplicate values. Results shown are of 1 experiment representative of 3. Results show mean SD of quintuplicate values of 1 experiment representative of 3. (B) Monocytes were cultured in GM-CSF plus IL-4 in the absence (left) or presence of 25 ng/mL IFN-γ (right). After 5 days, cells were (top) or were not (bottom) stimulated for 24 hours with 20 ng/mL LPS and observed by microscopy (original magnification, × 100).
Some macrophage subsets, such as monocytes differentiated into macrophages in the presence of M-CSF, are characterized by CD14, CD64, RFD7, and MR expression and by potent phagocytic and nonspecific esterase activities (Table 1). IFN-γ modulates macrophage phenotype and functions; it up-regulates MHC class I, HLA-DR, and CD64 expression20 and down-regulates CD14,21RFD7,22 and MR23 expression as well as phagocytic properties14,24 and nonspecific esterase activity (Table 1). In agreement with these data, monocytes cultured in GM-CSF plus IL-4 plus IFN-γ express CD86 and CD64 but not RFD7 and CD14, and present reduced endocytic and nonspecific esterase activities (Table 1). In parallel, IFN-γ poorly modulates macrophage accessory cell function25 (Figure 1A, right panel). Together, these data show that IFN-γ skews monocyte differentiation from immature DCs to IFN-γ–stimulated macrophages.
By comparing the respective properties of IL-4 and IFN-γ on GM-CSF–treated monocytes (Table 1, left and middle panels), it appears that IL-4 and IFN-γ have an additive effect on the up-regulation of MHC class I and MHC class II molecule expression.26 This additive effect was observed at any time point analyzed during the differentiation process (from day 1 to day 5; data not shown). Moreover, IL-4 and IFN-γ have also an additive effect in up-regulating CD64 and CD86 expression on day 5–differentiated macrophages. Together, these observations suggest a more complex dialogue between IL-4 and IFN-γ than just a decrease in monocyte-differentiating cell sensitivity to IL-4 mediated by IFN-γ.27
IFN-γ prevents murine bone marrow progenitor differentiation into DCs
We therefore tested whether the ability of IFN-γ to skew myeloid cell differentiation from DCs to macrophages could be extended to an IL-4–independent model of DC generation. In the presence of GM-CSF, murine bone marrow–derived progenitors differentiate into DCs.19 Bone marrow progenitors were incubated for 5 days with GM-CSF in the absence or presence of IFN-γ. Under the aegis of GM-CSF, MHC class II− progenitors give rise to a major population of DCs (75%-90% of the cells according to the experiments, n = 5) characterized by MHC class II, CD11c, CD86, and F4/80 expression and potent T-cell stimulatory properties (Table2). In addition to DCs, macrophages (adherent, CD11c−, Gr1−, CD11b+, and low or no MHC class II) and granulocytes (nonadherent, Gr1+ and MHC class II−) are also present19 (data not shown).
IFN-γ prevents murine progenitor differentiation into DCs
| . | None . | IFN-γ . |
|---|---|---|
| Surface markers | ||
| CD11b | 857 ± 75 | 320 ± 95 |
| CD11c | 536 ± 45 | 4 ± 0.2 |
| CD86 | 136 ± 23 | 33 ± 9 |
| F4/80 | 140 ± 19 | 60 ± 10 |
| IAb | 49% ± 10, 978 ± 83* | 8 ± 1.2 |
| 61% ± 22, 59 ± 13* | ||
| Allogenic MLR | 41 ± 9 | 4 ± 0.8 |
| . | None . | IFN-γ . |
|---|---|---|
| Surface markers | ||
| CD11b | 857 ± 75 | 320 ± 95 |
| CD11c | 536 ± 45 | 4 ± 0.2 |
| CD86 | 136 ± 23 | 33 ± 9 |
| F4/80 | 140 ± 19 | 60 ± 10 |
| IAb | 49% ± 10, 978 ± 83* | 8 ± 1.2 |
| 61% ± 22, 59 ± 13* | ||
| Allogenic MLR | 41 ± 9 | 4 ± 0.8 |
Bone marrow cells from C57BL/6 mice were cultured in CM containing 5 ng/mL GM-CSF in the absence or presence of 25 ng/mL IFN-γ. After 5 days, phenotype was analyzed by FACS. In the absence or presence of IFN-γ, cells were gated on CD11c+or on Gr1− cells, respectively, as described in “Materials and methods.” For all the markers, excepted for IAb, homogeneous populations were obtained and results are expressed in MFI values (mean ± SD, n = 4).
For IAb, two populations expressing high and low levels of IAb were observed. The percentage of cells in each population (left values; mean percent ± SD, n = 4) and the MFI values of each population (right values; mean ± SD, n = 4) are mentioned. At day 5, cells were used as stimulatory cells in primary allogenic MLRs. Results are expressed in counts per minute × 10−3 as mean ± SD of quadruplicate values and are representative of 1 of 3 experiments.
When progenitors are cultured in the presence of GM-CSF plus IFN-γ for 5 days, no CD11c+ cells are generated (Table 2). In addition to a minor proportion of Gr1+ granulocytes (15%-32%), the Gr1− and CD11c− cells express low or undetectable levels of MHC class II, but express CD86, F4/80, and CD11b (Table 2). They do not express B, T, or NK cell markers (data not shown). Finally, in contrast to DCs, these CD11c−CD11b+ cells present poor MLR-stimulating activity (Table 2). These data show that IFN-γ skews murine bone marrow progenitor differentiation from DCs to macrophagelike cells. They also support a direct effect of IFN-γ on DC precursors.
IFN-γ up-regulates M-CSF production by monocytes
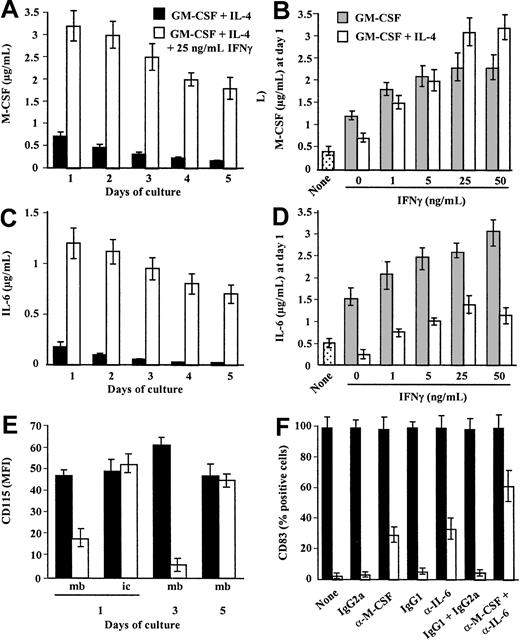
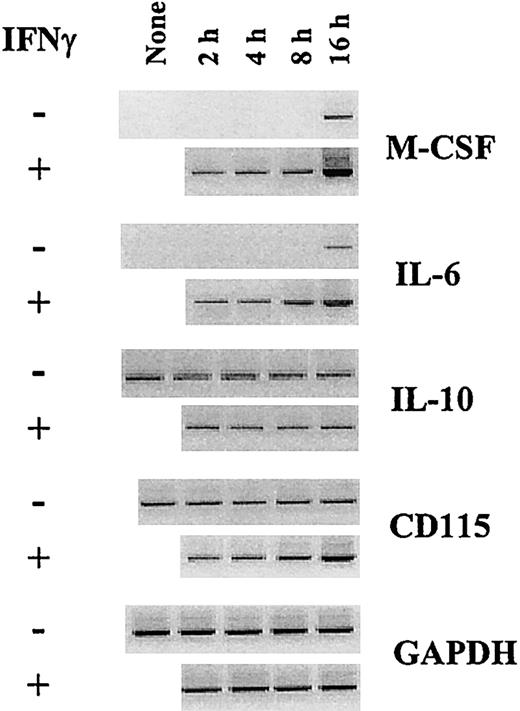
M-CSF is the most potent macrophage differentiation factor described.1 We then tested whether IFN-γ may affect M-CSF production by monocytes. Human monocytes were cultured in GM-CSF plus IL-4 containing or not IFN-γ, and M-CSF was quantified in the supernatants at different time points during the differentiation process. Monocytes cultured in GM-CSF plus IL-4 produce M-CSF28 (Figure 2A). M-CSF production is maximal at day 1 and declines during the 5-day period of culture (Figure 2A). Surprisingly, the presence of IFN-γ together with GM-CSF plus IL-4 results in a sustained (Figure 2A) and dose-dependent up-regulation of M-CSF production (maximal increase of 300% ± 42% using 25 ng/mL IFN-γ; mean ± SD, n = 4; Figure 2B). M-CSF mRNA expression is induced by culturing monocytes in GM-CSF plus IL-428 and is up-regulated by IFN-γ as early as 2 hours after stimulation (Figure3).
IFN-γ up-regulates M-CSF and IL-6 production by IL-4 plus GM-CSF–stimulated monocytes.
(A,C) Monocytes in GM-CSF plus IL-4 were (■) or were not (▪) stimulated with 25 ng/mL IFN-γ at day 0 and M-CSF (A) and IL-6 (C) were quantified in the cell-free supernatants from day 1 to day 5. (B,D) Monocytes were cultured in GM-CSF (░) or GM-CSF plus IL-4 (■) in the absence or presence of 1 to 50 ng/mL IFN-γ added at day 0 and M-CSF (B) and IL-6 (D) were quantified in the 24-hour supernatants (day 1). As control, monocytes were cultured in medium without cytokine (dotted bars). (A-D) Results are expressed in micrograms per milliliter as means ± SDs of 4 experiments. (E) Monocytes in GM-CSF plus IL-4 were (■) or were not (▪) stimulated with 25 ng/mL IFN-γ. At days 1, 3, and 5, membrane CD115 expression was analyzed by FACS. At day 1, intracellular CD115 expression was analyzed after cell permeabilization. Results are expressed in MFI (after subtraction of the MFI obtained with the control mAb) as means ± SDs of 4 separate experiments. (F) Monocytes in GM-CSF plus IL-4 were not (▪) or were (■) stimulated with 25 ng/mL IFN-γ in the absence or presence of neutralizing anti–M-CSF plus anti–IL-6 mAbs or of isotype control mAbs. After 5 days, cells were stimulated with 20 ng/mL LPS and CD83 expression was analyzed by FACS. Results are expressed in MFI values as means ± SDs, n = 3.
IFN-γ up-regulates M-CSF and IL-6 production by IL-4 plus GM-CSF–stimulated monocytes.
(A,C) Monocytes in GM-CSF plus IL-4 were (■) or were not (▪) stimulated with 25 ng/mL IFN-γ at day 0 and M-CSF (A) and IL-6 (C) were quantified in the cell-free supernatants from day 1 to day 5. (B,D) Monocytes were cultured in GM-CSF (░) or GM-CSF plus IL-4 (■) in the absence or presence of 1 to 50 ng/mL IFN-γ added at day 0 and M-CSF (B) and IL-6 (D) were quantified in the 24-hour supernatants (day 1). As control, monocytes were cultured in medium without cytokine (dotted bars). (A-D) Results are expressed in micrograms per milliliter as means ± SDs of 4 experiments. (E) Monocytes in GM-CSF plus IL-4 were (■) or were not (▪) stimulated with 25 ng/mL IFN-γ. At days 1, 3, and 5, membrane CD115 expression was analyzed by FACS. At day 1, intracellular CD115 expression was analyzed after cell permeabilization. Results are expressed in MFI (after subtraction of the MFI obtained with the control mAb) as means ± SDs of 4 separate experiments. (F) Monocytes in GM-CSF plus IL-4 were not (▪) or were (■) stimulated with 25 ng/mL IFN-γ in the absence or presence of neutralizing anti–M-CSF plus anti–IL-6 mAbs or of isotype control mAbs. After 5 days, cells were stimulated with 20 ng/mL LPS and CD83 expression was analyzed by FACS. Results are expressed in MFI values as means ± SDs, n = 3.
IFN-γ enhances M-CSF and IL-6 mRNA expression by monocytes in GM-CSF plus IL-4.
Freshly isolated monocytes were not (none) or were cultured in GM-CSF plus IL-4 in the absence or presence of 25 ng/mL IFN-γ. After 2, 4, 8, and 16 hours, the expression of the mRNA encoding M-CSF, IL-6, IL-10, CD115, and GAPDH was analyzed by RT-PCR.
IFN-γ enhances M-CSF and IL-6 mRNA expression by monocytes in GM-CSF plus IL-4.
Freshly isolated monocytes were not (none) or were cultured in GM-CSF plus IL-4 in the absence or presence of 25 ng/mL IFN-γ. After 2, 4, 8, and 16 hours, the expression of the mRNA encoding M-CSF, IL-6, IL-10, CD115, and GAPDH was analyzed by RT-PCR.
We next evaluated the respective roles of IL-4 and IFN-γ on M-CSF production (Figure 2B). Monocytes were exposed to GM-CSF in the absence or presence of IL-4 and different concentrations of IFN-γ. M-CSF was quantified in the 24-hour supernatants. GM-CSF induces M-CSF production28 (Figure 2B) in a dose-dependent manner (data not shown). IL-4 and IFN-γ have antagonistic effects on GM-CSF–induced M-CSF production as they down-regulate28and up-regulate M-CSF production, respectively (Figure 2B). Surprisingly, when added together, IFN-γ and IL-4 have an additive effect on the up-regulation of M-CSF production (Figure 2B). Monocytes in GM-CSF produce higher levels of M-CSF when stimulated with IFN-γ plus IL-4 than with IFN-γ alone (increase of 300% ± 42% and 80% ± 19% using 25 ng/mL IFN-γ, respectively; mean ± SD, n = 4; Figure 2B). Finally, IFN-γ also up-regulates murine M-CSF production by bone marrow progenitors (data not shown). Thus, we report for the first time that IFN-γ is a potent inducer of M-CSF production by human and murine myeloid cells.
IFN-γ up-regulates IL-6 production by monocytes
IL-6 shifts monocyte differentiation from DCs to macrophages by enhancing M-CSF consummation.9,10 We then evaluated whether IFN-γ may control the production of IL-6 by monocyte-differentiating DCs. Human monocytes were maintained in GM-CSF plus IL-4 containing or not IFN-γ, and IL-6 was quantified in the supernatants during the differentiation process. Monocytes in GM-CSF plus IL-4 produce IL-6 (Figure 2C). Maximal IL-6 levels are obtained at day 1 and then decline time dependently (Figure 2C). Addition of IFN-γ up-regulates IL-6 production in a time- (Figure 2C) and dose-dependent manner (with a maximum obtained using 50 ng/mL; Figure2D). IL-6 mRNA expression is induced by culturing monocytes in GM-CSF plus IL-4 and is up-regulated by IFN-γ (with an effect detectable 2 hours after stimulation; Figure 3). Analysis of the respective roles of IL-4 and IFN-γ on IL-6 production shows that GM-CSF induces IL-6 production by monocytes and that IL-4 and IFN-γ down- and up-regulate GM-CSF–induced IL-6 production, respectively (Figure 2D). In contrast to M-CSF, no additive effect of IL-4 plus IFN-γ on IL-6 production is observed as IL-4 partly prevents IFN-γ–induced IL-6 production (Figure 2D). A previous study reported that soluble IL-6 receptor (gp80) cooperates with IL-6 and M-CSF in switching monocyte differentiation to macrophages.10 We observed that IFN-γ only slightly up-regulated gp80 production with a maximum occurring at day 3 (0.26 ± 0.05 and 0.35 ± 0.06 ng/mL, in the absence or presence of IFN-γ, respectively; mean ± SD, n = 4). Lastly, IL-10 has also been shown to shift monocyte differentiation into macrophages.11 We failed in detecting IL-10 production (data not shown) or an up-regulation of IL-10 mRNA expression on treatment with IFN-γ (Figure 3).
Together, these data show that IFN-γ up-regulates IL-6 production by monocytes in GM-CSF plus IL-4 by acting, at least partly, at the transcriptional level.
IFN-γ down-regulates CD115 cell surface expression on monocytes cultured in GM-CSF plus IL-4
M-CSF consummation is associated with CD115 internalization.29 We then evaluated whether the addition of IFN-γ to monocytes cultured in GM-CSF plus IL-4 results in a modulation of CD115 expression. Monocytes in GM-CSF plus IL-4 express CD115 and this expression remains stable during the differentiation process10 28 (Figure 2E). The presence of IFN-γ in GM-CSF plus IL-4 results in a decrease in membrane CD115 expression, observed at day 1 and maximal at day 3 (Figure 2E). CD115 expression returns to basal level at day 5 (Figure 2E). Analysis of CD115 expression in permeabilized cells shows similar levels of expression on monocytes cultured in GM-CSF plus IL-4 with or without IFN-γ (Figure 2E), thereby suggesting an internalization of CD115 by IFN-γ–treated cells. In accordance with these data, a decrease in M-CSF concentrations in the day 3 supernatants of monocytes in GM-CSF plus IL-4 plus IFN-γ is observed (Figure 2A). Moreover, IFN-γ slightly enhances CD115 mRNA expression on monocytes in GM-CSF plus IL-4 (Figure3). Together, these data suggest that the up-regulation of M-CSF and IL-6 production by monocytes induced by IFN-γ is associated to an autocrine enhancement of M-CSF consummation.
Both M-CSF and IL-6 are involved in the effect of IFN-γ on monocyte differentiation into DCs
In neutralizing experiments, we evaluated whether autocrine IL-6 and M-CSF production induced by IFN-γ was involved in its ability to skew monocyte differentiation from DCs to macrophages. Monocytes in GM-CSF plus IL-4 were treated or not with IFN-γ in the absence or presence of neutralizing anti–M-CSF or anti–IL-6 mAbs. At day 5, cells were stimulated with LPS for 24 hours before to analyze CD83 expression. Results show that IFN-γ–treated cells partly recover the ability to acquire CD83 expression in the presence of neutralizing mAbs (61% ± 10%; mean ± SD, n = 4; Figure 2F). When anti–M-CSF mAb or anti–IL-6 mAb was used alone, CD83 expression was also slightly recovered (35% ± 8% and 29% ± 5%, respectively; Figure 2F), thereby suggesting that both cytokines participate to the effect of IFN-γ on monocyte differentiation. No significant effect was observed with control mAbs (Figure 2F). As control, the neutralizing mAbs do not affect IL-4 plus GM-CSF–induced monocyte differentiation into DCs (Figure 2F). Together, these data show that IFN-γ shifts differentiating DCs toward macrophages at least partly by up-regulating autocrine consummation of M-CSF and IL-6.
Immature DCs do not produce M-CSF nor differentiate into macrophages in response to IFN-γ
We then evaluated whether IFN-γ may reconvert immature DCs toward macrophages. IFN-γ has been reported to polarize immature DCs into DC1s that produce high levels of IL-12 and present potent costimulatory properties.17 In accordance with these data, we show that immature DCs exposed to IFN-γ retain a phenotype of CD1a+CD14− DC (Table3). Moreover, on LPS stimulation, immature DCs exposed to IFN-γ acquire veils (data not shown), neoexpress CD83, and present potent T-cell costimulatory properties (Table 3).
IFN-γ does not shift immature DCs toward macrophages
| . | No cytokine . | GM-CSF + IL-4 . | |||
|---|---|---|---|---|---|
| None . | IFN-γ . | IFN-γ + M-CSF . | M-CSF + IL-6 . | ||
| CD1153-150 | ND | 76 ± 10 | 83 ± 13 | 10 ± 3 | 15 ± 2 |
| CD1a3-151 | 11 ± 2 | 310 ± 35 | 298 ± 29 | 358 ± 42 | 399 ± 50 |
| CD143-151 | 56 ± 9 | < 5 | < 5 | < 5 | < 5 |
| CD833-152 | < 5% | 99% | 98% | 99% | 99% |
| MLR3-153 | ND | 2 ± 0.1 | 5 ± 0.8 | ND | ND |
| MLR3-155 | ND | 22 ± 2 | 24 ± 3 | ND | ND |
| . | No cytokine . | GM-CSF + IL-4 . | |||
|---|---|---|---|---|---|
| None . | IFN-γ . | IFN-γ + M-CSF . | M-CSF + IL-6 . | ||
| CD1153-150 | ND | 76 ± 10 | 83 ± 13 | 10 ± 3 | 15 ± 2 |
| CD1a3-151 | 11 ± 2 | 310 ± 35 | 298 ± 29 | 358 ± 42 | 399 ± 50 |
| CD143-151 | 56 ± 9 | < 5 | < 5 | < 5 | < 5 |
| CD833-152 | < 5% | 99% | 98% | 99% | 99% |
| MLR3-153 | ND | 2 ± 0.1 | 5 ± 0.8 | ND | ND |
| MLR3-155 | ND | 22 ± 2 | 24 ± 3 | ND | ND |
Day 5 immature DCs were cultured in the absence of cytokine or in GM-CSF plus IL-4 in the absence or presence of 25 ng/mL IFN-γ, 25 ng/mL IFN-γ plus 100 ng/mL M-CSF, or 100 ng/mL M-CSF plus 100 ng/mL IL-6.
ND indicates not done.
After 1 day, membrane CD115 expression was analyzed by FACS.
After 3 days, CD1a and CD14 expression were analyzed by FACS. For CD1a, CD14, and CD115, results are expressed in MFI ± values, mean SD of 4 separate experiments.
After 3 days, cells were further stimulated for 24 hours with LPS, and CD83 was analyzed by FACS. Results are expressed in percentage of positive cells.
After 2 days, cells were used as stimulatory cells in primary allogeneic MLRs.
After 2 days, cells were stimulated for 24 hours with LPS before being used as stimulatory cells in primary allogeneic MLRs. Results are expressed in counts per minute × 10−3 as mean ± SD of quadruplicate values and are representative of 1 of 3 experiments.
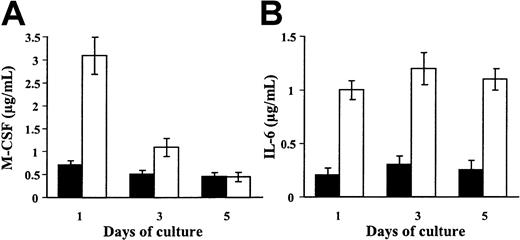
We tested whether IFN-γ may control M-CSF and IL-6 production by immature DCs. IFN-γ does not modulate M-CSF production (Figure4A) nor mRNA expression (data not shown) by immature DCs. Kinetic experiments show that the ability of monocytes cultured in GM-CSF plus IL-4 to produce M-CSF in response to IFN-γ is maximal at day 1 and decreases time dependently during the differentiation process (Figure 4A). In contrast, immature DCs retain the ability to produce IL-6 in response to IFN-γ (Figure 4B). These data show that the regulation of M-CSF production in response to IFN-γ is tightly regulated during the differentiation process from monocytes to DCs.
IFN-γ does not modulate M-CSF production by immature DCs.
(A-B) Day 5 immature DCs were maintained in GM-CSF plus IL-4 and 25 ng/mL IFN-γ was not (▪) or was (■) added at day 0, 2, or 4. After 24 hours, M-CSF (A) and IL-6 (B) were quantified in the supernatants. Results are expressed in micrograms per milliliter (means ± SDs, n = 4).
IFN-γ does not modulate M-CSF production by immature DCs.
(A-B) Day 5 immature DCs were maintained in GM-CSF plus IL-4 and 25 ng/mL IFN-γ was not (▪) or was (■) added at day 0, 2, or 4. After 24 hours, M-CSF (A) and IL-6 (B) were quantified in the supernatants. Results are expressed in micrograms per milliliter (means ± SDs, n = 4).
We evaluated whether IFN-γ may reconvert immature DCs to macrophages when exogenous M-CSF is added. In the presence of a high concentration of M-CSF (100 ng/mL), IFN-γ–treated immature DCs in GM-CSF plus IL-4 retain a DC phenotype; they express CD1a but not CD14 (Table 3), present a morphology of immature DCs (data not shown), and, on LPS stimulation, acquire CD83 expression (Table 3) and veils (data not shown). In agreement with others,10 they also present a decrease in cell surface CD115 expression (Table 3), thereby suggesting a consummation of M-CSF by immature DCs. Finally, even in the presence of exogenous M-CSF and IL-6, immature DCs in GM-CSF plus IL-4 retain a DC phenotype (Table 3). As a positive control,30 on GM-CSF and IL-4 removal, immature DCs acquire CD14 expression and convert toward adherent macrophages (Table 3). Thus, these data suggest that, in the presence of GM-CSF plus IL-4, immature DCs may express functional CD115 but are resistant to IFN-γ–, IL-6–, and M-CSF–induced macrophage differentiation.
Discussion
IFN-γ produced by NK or activated T cells participates in the control of the innate and adaptive phases of the immune response.14 IFN-γ exerts most of its effects on APCs; it activates macrophages and polarizes immature DCs into Th1 cell–promoting effector DCs.14,16 17 We show here for the first time that IFN-γ also participates to the control of APC differentiation; IFN-γ switches monocyte differentiation to activated macrophages at least in part via autocrine activation by IL-6 and M-CSF.
M-CSF is a major macrophage differentiation factor1 that skews CD34+ progenitor and monocyte differentiation from DCs to macrophages.9,10,30 By enhancing functional M-CSF receptor expression and M-CSF consummation,10 IL-6 cooperates with M-CSF in this process.9 10 In agreement with these data, we report that IFN-γ switches monocyte differentiation into macrophages via an up-regulation of autocrine IL-6 and M-CSF production.
GM-CSF induces M-CSF production by monocytes10,28 in a dose-dependent manner with a maximum at 100 ng/mL (data not shown). In our experimental conditions, the concentrations of M-CSF present in the supernatants of monocytes in GM-CSF plus IL-4 were lower than those reported by others.10 28 The use of 20 ng/mL instead of 100 ng/mL GM-CSF in our study may explain this difference.
We also show that monocytes treated with IL-4 plus GM-CSF produce IL-6. Levels of IL-6 induced by IL-4 plus GM-CSF were lower than those induced by GM-CSF alone. Thus, it appears that IL-4 inhibits GM-CSF–induced M-CSF28 and IL-6 production. It is therefore tempting to speculate that the inhibitory effect of IL-4 on M-CSF and IL-6 production may contribute to explain its DC differentiation factor property.
Although IFN-γ antagonizes many IL-4–mediated responses,27 it has been also previously observed that IL-4 and IFN-γ act synergistically to enhance MR-dependent phagocytosis31 and CD23 expression on macrophages.32 We report that whereas IFN-γ up-regulates IL-6 and M-CSF production, IL-4 down-regulates their production. However, when used together, they mutually inhibit their respective effects on IL-6 production and have an additive effect on the up-regulation of M-CSF production. Together, these observations suggest a complex dialogue between IL-4 and IFN-γ not limited to antagonistic effects.
IFN-γ antagonizes many physiologic responses mediated by IL-4, by acting at posttranscriptional27 or transcriptional levels. IFN-γ and IL-4 activate signal transducer and activator of transcription (Stat)1 and Stat6, respectively.33,34IFN-γ inhibits IL-4–mediated Stat6 activation by inducing the expression of suppressors of cytokine signaling (SOCS)1.35The antagonist effect of IFN-γ on IL-4–induced inhibition of IL-6 production could be related to the SOCS1-mediated Stat6 inhibition. Moreover, expression of IL6 gene is under the control of transcription factors, including AP-1, nuclear factor-κB (NF-κB), and NF–IL-6.36 Whereas NF–IL-6 is strongly inhibited by IL-4,37 it is poorly affected by IFN-γ.38 Consequently, the ability of IL-4 to down-regulate IFN-γ–induced IL-6 production could be related to the strong inhibition of NF–IL-6 which is mediated by IL-4 and not counteracted by IFN-γ.
In accordance with data showing a differential regulation of M-CSF and IL-6 gene expression in monocytes,39 we report that IFN-γ plus IL-4 have an additive effect of M-CSF production. The role of members of the Stat family in the regulation of M-CSF expression is poorly documented. Nevertheless, we could hypothesize that the down-regulation of IL-4–induced activation of some members of the Stat family mediated by IFN-γ may contribute to explain this effect. In addition, the increase of M-CSF production induced by IL-4 plus IFN-γ could be also indirect. M-CSF increases M-CSF mRNA expression by GM-CSF–activated monocytes.40 M-CSF receptor gene expression is controlled by transcription factors including NF-κB, AP-1, and PU-1. PU-1 is activated by IFN-γ41 and interacts with the interferon regulatory factor 4 (IRF4) which expression is increased by IL-4.42 Thus, the additive effect of IFN-γ and IL-4 on M-CSF production could be consecutive to an increase of M-CSF receptor transcription. Additional experiments are required to determine the effects of IL-4 plus IFN-γ on the expression of the transcription factors involved in IL-6 and M-CSF production.
DCs and macrophages have different roles in the immune response. Whereas DCs initiate specific immune responses, macrophages and especially IFN-γ–activated macrophages exhibit potent bactericidal and antitumoral activities.14 When added to differentiated uncommitted immature DCs, IFN-γ polarizes them into Th1 cell–promoting effector DCs.17 Th1 cells produce IFN-γ, IL-2, and tumor necrosis factor β (TNF-β) and evoke cell-mediated immunity and phagocyte-dependent inflammation.
In addition to acting on mature APC functions, IFN-γ may also act on DC/macrophage precursors to favor their differentiation into macrophages. Interestingly, IFN-γ acts mainly at the initial stages of monocyte differentiation. The ability of cells to produce M-CSF in response to IFN-γ varies with the status of differentiation. In contrast to monocytes, immature DCs express CD115 but do not revert to macrophages on contact with IL-6, M-CSF, or IFN-γ. The mechanisms underlying this resistance remain to be identified. These data also suggest that the effects of IFN-γ are tightly controlled according to the status of cell differentiation. Lastly, in agreement with data showing that environmental cytokines tightly control monocyte differentiation into immature DCs and macrophages and the interconversion into one another,4,30 we observed that the removal of IFN-γ from the culture at the early stages of differentiation partly prevents its blocking effect on DC differentiation. IL-10 also shifts monocyte differentiation from DCs to macrophages.11 IL-10 and IFN-γ give rise to macrophagelike cells with divergent antigen presentation and endocytosis properties.43 These observations suggest that monocyte differentiation and the function of the cells generated are also tightly controlled by the nature of cytokines encountered.
DCs are the only APCs that prime naive T cells and initiate specific immune responses.12 Consequently, they have a central role in vaccine strategies and especially in antitumor immunotherapies.44,45 Tumor-specific vaccination using antigen-loaded autologous DCs has reached the stage of human clinical trials. These studies have been made possible by the development of methods for obtaining large numbers of DCs. Most of these studies have been carried out with ex vivo generated monocyte-derived DCs.44 45 Thus, to identify factors that control monocyte differentiation is crucial in order to optimize DC generation. Our results show that IFN-γ skews monocyte differentiation from DCs to macrophages, suggesting that the presence of IFN-γ–producing cells together with monocytes could interfere with the differentiation process into DCs. Finally, in agreement with others, we report that IFN-γ–stimulated macrophages present undetectable or low levels of markers that are usually considered as macrophage markers (RFD7, CD14, and MR). This observation points out that the expression of CD1a or CD14 (or both) is sometimes not sufficient to clearly distinguish between monocyte-derived macrophages or DCs and that the study of additive morphologic, phenotypic, and functional parameters is required.
In conclusion, our data show that, in addition to a direct effect on APC functions, IFN-γ skews monocyte differentiation from DCs to macrophages and thereby reveals a new mechanism by which IFN-γ may control the outcome of the immune response.
We sincerely acknowledge Ms R. Vellaidom for manuscript handling.
Prepublished online as Blood First Edition Paper, August 15, 2002; DOI 10.1182/blood-2002-04-1164.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Pascale Jeannin, Centre d'Immunologie Pierre Fabre, 5, Avenue Napoléon III, F-74160 Saint-Julien en Genevois, France; e-mail:pascale.jeannin@pierre-fabre.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal