Abstract
Human CD133 (AC133)+CD34+ stem and progenitor cells derived from fetal liver and from bone marrow and blood incorporate a functional population of circulating endothelial precursor cells. Vascular endothelial growth factor receptor 3 (VEGFR-3) regulates cardiovascular development and physiological and pathological lymphangiogenesis and angiogenesis. However, the origin of VEGFR-3+ endothelial cells (ECs) and the mechanisms by which these cells contribute to postnatal physiological processes are not known, and the possible existence of VEGFR-3+ lymphatic or vascular EC progenitors has not been studied. Using monoclonal antibodies to the extracellular domain of VEGFR-3, we show that 11% ± 1% of CD34+ cells isolated from human fetal liver, 1.9% ± 0.8% CD34+ cells from human cord blood, and 0.2% ± 0.1% of CD34+ cells from healthy adult blood donors are positive for VEGFR-3. CD34+VEGFR-3+ cells from fetal liver coexpress the stem/precursor cell marker CD133 (AC133). Because mature ECs do not express CD133, coexpression of VEGFR-3 and CD133 on CD34+cells identifies a unique population of stem and progenitor cells. Incubation of isolated CD34+VEGFR-3+ cells in EC growth medium resulted in a strong proliferation (40-fold in 2 weeks) of nonadherent VEGFR-3+ cells. Plating of these cells resulted in the formation of adherent VEGFR-3+Ac-LDL+ (Ac-LDL = acetylated low-density lipoprotein) EC monolayers expressing various vascular and lymphatic endothelial-specific surface markers, including CD34, VE-cadherin, CD51/61, CD105, LYVE-1, and podoplanin. These data demonstrate that human CD34+CD133+ cells expressing VEGFR-3 constitute a phenotypically and functionally distinct population of endothelial stem and precursor cells that may play a role in postnatal lymphangiogenesis and/or angiogenesis.
Introduction
Members of the vascular endothelial growth factor (VEGF) family, including VEGF, VEGF-B, VEGF-C, and VEGF-D, regulate various aspects of blood vascular and lymphatic growth and function. VEGF acts through the tyrosine kinase receptors VEGFR-2 (KDR, Flk-1) and VEGFR-1 (Flt-1), which convey signals that are essential for embryonic angiogenesis and hematopoiesis.1VEGF-C and VEGF-D, in turn, bind to and mediate signals to the blood vascular and lymphatic endothelium through VEGFR-2 and VEGFR-3. The resulting signals regulate cardiovascular development, angiogenesis, and lymphangiogenesis.2,3 VEGF-C, which is expressed by various human cancers,4-6 has a potent angiogenic effect in vivo7 and also induces lymphangiogenesis, which in tumors can promote the dissemination of tumor cells to regional lymph nodes.8-14
The expression of the VEGFR-3 gene starts during mouse embryonic day 8.5 in developing blood vessels, and VEGFR-3–deficient embryos die at midgestation owing to defects in the remodeling of primary vascular networks.15 In adult tissues, VEGFR-3 expression occurs mainly in the lymphatic endothelia.15-18 In healing wounds, VEGFR-3–positive lymphatic vessels appear in the wound concurrently with blood vessels but regress quickly.19However, VEGFR-3–expressing endothelial cells (ECs) can also be found in fenestrated capillaries of several adult organs, including bone marrow, splenic and hepatic sinusoids, kidney glomeruli, and endocrine glands.18 Notably, in human cancer VEGFR-3–expressing vascular ECs are detected in angiogenic capillaries,5,20and in nude mice inactivation of VEGFR-3 signaling by blocking antibodies suppresses tumor growth by inhibiting the angiogenesis.21
Recent evience suggests that in adult humans circulating endothelial precursor (CEP) cells may be recruited from the stem-cell reservoirs to the peripheral circulation, and that they play a role in postnatal angiogenesis associated with vascular trauma or tumor growth.22-31 Recent data also demonstrate that lymphangiogenesis, facilitated by VEGFR-3 signaling, contributes to cancer dissemination.9,10,12,13,32 33 However, the origin of lymphatic ECs (LECs) is not known. It is conceivable that a lymphangiogenic switch promotes the recruitment of LECs from neighboring pre-existing lymphatic vessels or recruits LEC precursors from the bone marrow. However, the possible existence of LEC progenitors has not been studied. Here we demonstrate that a subset of CD34+ cells coexpress VEGFR-3 and CD133. These cells are functionally a unique population of progenitor cells with the capacity to differentiate into lymphatic or vascular ECs. They could contribute to postnatal lympangiogenesis and/or angiogenesis.
Materials and methods
Positive selection of human CD34+ cells by magnetic cell sorting
Human fetal liver (FL) samples from 12- to 18-week abortus and human cord blood (CB) samples were obtained in conjunction with ethical and biohazard guidelines set by the institution. CD34+ cells were purified by magnetic activated cell sorting columns (MACS; Miltenyi Biotech, Auburn, CA) using microbead-conjugated antibodies. Two purification cycles on separate columns were performed. The purity of each batch of CD34-selected cells was determined by flow cytometry using fluorescein isothiocyanate (FITC; green fluorescence)–labeled anti-CD34 monoclonal antibodies (mAbs; Miltenyi Biotech).
Circulating CD34+ cells were also obtained from peripheral blood samples from growth factor–treated patients and from healthy blood donors. In brief, mononuclear cell fraction was separated with Ficoll-Hypaque (Amersham Pharmacia, Uppsala, Sweden) centrifugation and CD34 purification was done with a Clinimacs CD34-separation kit (Miltenyi Biotech). The purity of CD34-selected cells was determined by flow cytometry using FITC-labeled anti-CD34 mAbs (Pharmingen, San Diego, CA). Approval was provided by the institutional review boards of Weill Medical College of Cornell University and Helsinki University Central Hospital for these studies. Informed consent was provided according to the Declaration of Helsinki.
Flow cytometry
For identification of VEGFR-3+ cells, isolated CD34+ cells were incubated with high-affinity mAbs against the extracellular domain of VEGFR-3 (clone 9d95,17). The mAbs against VEGFR-3 were labeled with phycoerythrin34(PE, red fluorescence) or cyanine 2 (Cy2, green fluorescence; Amersham Pharmacia Biotech, Piscataway, NJ); labeling was performed according to the instructions of the manufacturer. PE-labeled or FITC-labeled anti-CD34 mAbs and PE-labeled anti-CD133 mAbs were purchased from Miltenyi Biotech. Other PE-, FITC- or Cy2-labeled mAbs used in flow cytometry were anti-CD105 (Serotec, Raleigh, NC), anti-CD51/61 (Pharmingen), anti-CD11b (Pharmingen), anti-CD14 (Pharmingen), anti–VE-cadherin (clone BV6; ImClone Systems, New York, NY), anti–VEGFR-1 (clone FB5; ImClone Systems), and anti–VEGFR-2 (clone 6.64; ImClone Systems). In the stainings, mAbs (5 μL of each) were incubated in different combinations with CD34+ cells for 20 minutes at 4°C. Subsequently, the cells were washed twice and fixed with 2% paraformaldehyde. The number of positive cells was compared with the number of cells positive in staining with the immunoglobulin G isotype controls (BD Pharmingen) and determined with a Coulter Elite flow cytometer (Coulter, Hialeah, FL) or FACSort flow cytometer (BD Biosciences, San Jose, CA).
Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of VEGFR-3 mRNA expression in isolated CD34+ and CD34− cells
Total RNA was extracted from isolated fetal liver CD34+ and CD34− cells by guanidine isothiocyanate lysis and silica gel membrane purification (RNeasy RNA extraction kit, Qiagen, Hilden, Germany) according to the manufacturer's protocol and was quantitated spectrophotometrically. Primers specific for exons 7 and 8 of the human VEGFR-3 gene were used (the design of the primers was based on published sequences,35,36 GenBank accession number X68203; sense, 5′-catccagctgttgcccagg-3′, and antisense, 5′-gagccactcgacgctgatgaa-3′). For RT-PCR analysis, 1.0 μg of total RNA from each sample was amplified with an intron-spanning primer pair for the human GAPDH gene37 and another 1.0 μg was amplified with the VEGFR-3 primer pair. The OneStep RT-PCR kit (Qiagen) was used. After a 30-minute reverse transcription reaction at 54°C and an initial denaturation at 95°C for 15 minutes, a 40-cycle PCR reaction was carried out, using the following amplification profile: denaturation at 94°C for 45 seconds, annealing at 65°C for 45 seconds, extension at 72°C for 45 seconds. For the GAPDH primers, a 35-cycle amplification was performed with annealing at 63°C. The PCR products were then electrophoresed on 1.5% agarose gel, stained with 0.5 μg/mL ethidium bromide, visualized under ultraviolet (UV) light, and photographed.
Liquid cultures of human CD34+ cells
Freshly isolated CD34+ cells from human FL were analyzed for the presence of CD133+VEGFR-3+ cells. Subsequently, 105 CD34+ cells were cultured in EC medium containing medium 199 (M199; GIBCO-BRL, Gaithersburg, MD) supplemented with 18% fetal bovine serum (HyClone, Logan, UT), 2% human serum, VEGF at 1 ng/mL (R&D Systems, Minneapolis, MN), bFGF at 2.5 ng/mL (Sigma, St Louis, MO), heparin at 5 U/mL, and VEGF-C at 60 ng/mL. The recombinant human VEGF-C was produced and purified as described earlier.38-40 As a control, the same experiments were performed in parallel with the freshly isolated CD34−cells from human FL. The number of viable nonadherent cells in the cultures was determined at 3, 5, 7, and 14 days by means of a bright-line counting chamber. The incubation resulted in a strong proliferation of the CD34-selected nonadherent cells. To prepare the cells for flow cytometry, the nonadherent cells were collected, washed with phosphate-buffered saline (PBS), and stained with PE- or FITC-labeled mAbs.
Generation of EC monolayers from human CD34+ cells in vitro
Freshly isolated CD34+ cells from FL were analyzed for the presence of CD133+VEGFR-3+cells. Subsequently, the cells were cultured in EC medium. After 3 days, nonadherent cells were transferred to plastic dishes and grown in the same medium for 2 to 4 weeks. This incubation resulted in attachment and proliferation of the CD34-selected cells into monolayers of mature ECs. After removal of the nonadherent cells, the adherent cell population was studied by 2-color flow cytometry. In addition, the ECs were quantified by means of low-density lipoprotein (LDL) incorporation. Dil-conjugated acetylated LDL (1 μg/mL; Intracel, Rockville, MD) was added to the media for 4 hours, and EC-specific labeling was assessed by flow cytometry.
Immunostaining of ECs
To further characterize EC monolayers generated from FL CD34+ cells in vitro, cells cultured in the chamber slides were fixed for 2 minutes in acetone and subjected to double immunostaining analyses using mouse monoclonal antibodies against human VEGFR-3 (clone 9d9) and rabbit antisera against human LYVE-141 (kindly provided by David G. Jackson, Oxford, and by Pirjo Laakkonen and Erkki Ruoslahti, La Jolla, CA) or affinity-purified rabbit antihuman podoplanin.42 The double staining procedure was essentially as described earlier.43 The slides were first rehydrated and incubated for 30 minutes in 4% normal goat serum at room temperature. The slides were incubated for 1 hour at room temperature with the rabbit polyclonal anti–LYVE-1 antiserum at a dilution of 1:100 or with the rabbit polyclonal antipodoplanin at a dilution of 1:300. A subsequent incubation for 30 minutes in biotinylated antirabbit serum was followed by a 30-minute incubation using reagents of the Vectastain Elite ABC Peroxidase antirabbit IgG kit (Vector Laboratories, Burlingame, CA). Peroxidase activity was developed with NovaRED substrate (Vector Laboratories) for 10 minutes. Subsequently, the slides were rehydrated and incubated for 30 minutes in 4% in normal horse serum at room temperature. The slides were then incubated for 1 hour with mouse monoclonal anti–VEGFR-3 antibodies at a concentration of 2.0 μg/mL. A subsequent incubation for 30 minutes in biotinylated antimouse serum was followed by a 30-minute incubation using reagents of the Vectastain ABC Alkaline phosphatase antimouse IgG kit (Vector Laboratories). Alkaline phosphatase activity was developed with Vector Blue alkaline phosphate substrate (Vector Laboratories) for 20 minutes. Negative controls were performed by omitting one or both of the primary antibodies or by using irrelevant primary antibodies.
Preparation of human umbilical vein EC (HUVEC) monolayers
HUVECs were isolated from freshly obtained umbilical cords by collagenase treatment44 and grown in EC medium. Confluent monolayers from passages 2 to 4 were studied by flow cytometry.
Statistical analysis
Data are expressed as mean ± standard error (SE) of at least 3 independent experiments.
Results
Identification of a population of circulating CD34+VEGFR-3+ cells
VEGFR-3 cell-surface expression was analyzed by 2-color flow cytometry on MACS-selected CD34+ and CD34−cells isolated from human FL. The purity of the CD34+ cells was routinely 85% to 95%. Using PE-labeled mAbs against the extracellular domain of VEGFR-3, we found that 11% ± 1% (n = 6) of CD34+ cells from FL were VEGFR-3+ (Figure1). No VEGFR-3+ cells could be detected in the CD34− cell fractions (Figure 1). This indicates that VEGFR-3+ cells are a small subpopulation of CD34+ cells.
VEGFR-3 cell-surface expression in human CD34+ cells.
After immunomagnetic separation of CD34+ cells from human fetal liver, the CD34+ and CD34− cell fractions were stained with FITC-labeled anti-CD34 and PE-labeled anti–VEGFR-3 mAbs and analyzed by 2-color flow cytometry. The results indicate that VEGFR-3+ cells are a small subpopulation of CD34+ cells.
VEGFR-3 cell-surface expression in human CD34+ cells.
After immunomagnetic separation of CD34+ cells from human fetal liver, the CD34+ and CD34− cell fractions were stained with FITC-labeled anti-CD34 and PE-labeled anti–VEGFR-3 mAbs and analyzed by 2-color flow cytometry. The results indicate that VEGFR-3+ cells are a small subpopulation of CD34+ cells.
VEGFR-3 cell-surface expression was also studied on purified CB CD34+ cells (n = 3), on purified peripheral blood CD34+ cells from adult patients treated with cyclophosphamide and granulocyte colony-stimulating factor (G-CSF; n = 16), and on CD34+ cells from healthy adult blood donors (n = 6). VEGFR-3 cell-surface expression was detected in 1.9% ± 0.8% of CD34+ cells from CB. Similarly, the number of VEGFR-3+ cells was generally found to be lower in adult patients (0.8% ± 0.2% of CD34+cells) and in healthy adult blood donors (0.2% ± 0.1% of CD34+ cells) than in the FL samples.
VEGFR-3 mRNA is expressed in CD34+ cells but not in CD34− cells from fetal liver
Amplification of cDNA from CD34+ cells isolated from FL gave rise to a band of 322 bp (Figure2). This is the size predicted for the VEGFR-3 transcript on the basis of the published cDNA sequence.35 36 In contrast, the FL CD34−cells were negative for VEGFR-3 mRNA. These data are consistent with the detection of VEGFR-3 cell-surface expression on FL CD34+ and CD34− cells.
RT-PCR for VEGFR-3 on human CD34+ and CD34− cells.
CD34+ and CD34− cells derived from human fetal liver were analyzed for the expression of VEGFR-3 and GAPDH mRNAs. One μg of total RNA from each sample was amplified by intron-spanning primer pairs. The results indicate that VEGFR-3 mRNA is expressed in CD34+ cells but not in CD34− cells.
RT-PCR for VEGFR-3 on human CD34+ and CD34− cells.
CD34+ and CD34− cells derived from human fetal liver were analyzed for the expression of VEGFR-3 and GAPDH mRNAs. One μg of total RNA from each sample was amplified by intron-spanning primer pairs. The results indicate that VEGFR-3 mRNA is expressed in CD34+ cells but not in CD34− cells.
Phenotypic characterization of CD34+VEGFR-3+ cells identifies a population of putative endothelial progenitor cells
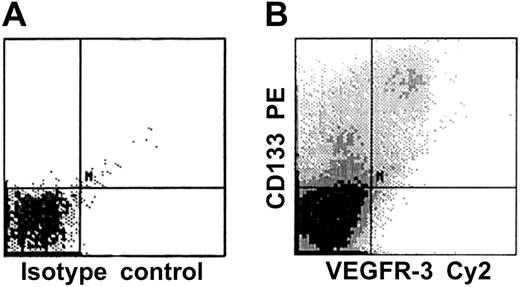
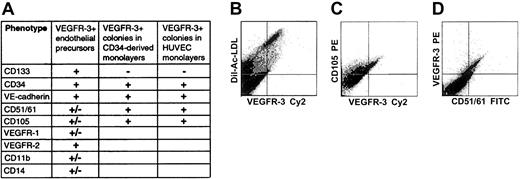
Coexpression of VEGFR-3 and defined stem/progenitor cell and EC markers on CD34+ cells isolated from FL or CB was analyzed by 2-color flow cytometry. The CD34+VEGFR-3+cells could represent a heterogeneous population of mature and immature ECs, since CD34 is expressed on both mature and immature ECs. Therefore, CD34+ cells were first analyzed by using PE-conjugated mAbs against the stem-cell marker CD133 (AC133) and Cy2-conjugated anti–VEGFR-3 mAbs. We found that almost all CD34+VEGFR-3+ cells were positive for CD133 (Figure 3). Because mature ECs do not express CD133, coexpression of VEGFR-3 and CD133 on CD34+cells identifies a unique population of stem and progenitor cells. We also found that about half of the CD34+VEGFR3+cells also express VE-cadherin or VEGFR-2, markers that have already previously been found on circulating endothelial precursors (Figure5).27,28,31 45 Only inconsistent, low-level, or nonexistent cell-surface expression of the endothelial-specific markers integrin αvβ3 heterodimeric complex (CD51/CD61), endoglin (CD105), and VEGFR-1 could be observed on isolated CD34+ cells coexpressing VEGFR-3 (Figure 5). Similarly, no significant cell-surface expression of CD11b or CD14 could be detected on the CD34+VEGFR-3+ cells (Figure 5).
CD133, an early stem and precursor cell marker, is expressed on VEGFR-3+ cells.
CD34+ cells derived from human fetal liver were analyzed for the expression of CD133 and VEGFR-3 (panel B); isotype control is shown in panel A. The results demonstrate that almost all CD34+VEGFR-3+ cells are positive for CD133.
CD133, an early stem and precursor cell marker, is expressed on VEGFR-3+ cells.
CD34+ cells derived from human fetal liver were analyzed for the expression of CD133 and VEGFR-3 (panel B); isotype control is shown in panel A. The results demonstrate that almost all CD34+VEGFR-3+ cells are positive for CD133.
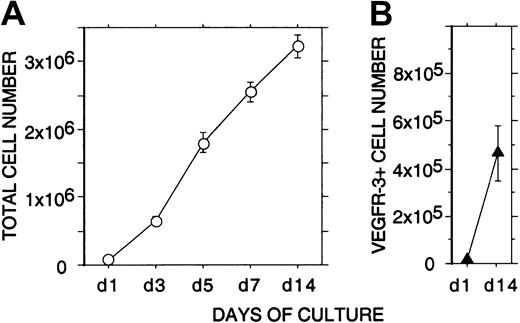
Expansion of VEGFR-3+ cells in liquid culture
Incubation of 105 freshly isolated CD34+cells from human FL for 2 weeks in EC growth medium containing VEGF, VEGF-C, and bFGF resulted in a strong proliferation (more than 30-fold) of nonadherent cells (Figure 4). Flow cytometry indicated that during the 2-week culture the mean number of nonadherent VEGFR-3+ cells had expanded more than 40-fold (from 11 × 103 to 47 × 104 cells, 11% ± 2% and 14% ± 4% of the total nonadherent cells, respectively; Figure 4).
Expansion of nonadherent VEGFR-3+ cells in liquid culture.
A quantity of 1 × 105 freshly isolated CD34+cells from human fetal liver were incubated for 2 weeks in EC growth medium. (A) The total number of viable nonadherent cells (○) in the cultures was determined at 1, 3, 5, 7, and 14 days. (B) The number of nonadherent VEGFR-3+ cells (▴) in the cultures was determined at 1 and 14 days by flow cytometry. The results are given as means ± SEs of 3 independent experiments. During the 2-week culture, a strong proliferation (more than 40-fold) of nonadherent VEGFR-3+ cells can be seen.
Expansion of nonadherent VEGFR-3+ cells in liquid culture.
A quantity of 1 × 105 freshly isolated CD34+cells from human fetal liver were incubated for 2 weeks in EC growth medium. (A) The total number of viable nonadherent cells (○) in the cultures was determined at 1, 3, 5, 7, and 14 days. (B) The number of nonadherent VEGFR-3+ cells (▴) in the cultures was determined at 1 and 14 days by flow cytometry. The results are given as means ± SEs of 3 independent experiments. During the 2-week culture, a strong proliferation (more than 40-fold) of nonadherent VEGFR-3+ cells can be seen.
Generation of ECs from CD34+ cells is associated with expression of VEGFR-3 on the cell surface
Incubation of freshly isolated, nonadherent CD34+cells for 3 to 5 weeks in EC medium resulted in their differentiation into adherent monolayers of ECs. Flow cytometry using PE-labeled anti–VEGFR-3 mAbs indicated that 10% ± 2% of the cells in the monolayers expressed VEGFR-3. All adherent VEGFR-3+ cells incorporated Ac-LDL, confirming that they were ECs (Figure 5). Adherent VEGFR-3+ cells coexpressing CD34, VE-cadherin, CD51/61, or CD105 were also detected (Figure 5). The monolayer cells did not express CD133, thus supporting the notion that CD133+CD34+VEGFR-3+ cells may be considered nonadherent EC precursors with progenitor potential. In double immunostainings, adherent ECs were found to coexpress VEGFR-3+ and lymphatic EC markers LYVE-1 or podoplanin (Figure 6). In cultured confluent HUVECs of passages 2 to 4 we found cell-surface expression of VEGFR-3 on 49% ± 6% of the cells. The surface-marker expression pattern of VEGFR-3+ HUVEC colonies, when analyzed by flow cytometry (CD133-VEGFR-3+CD34+VE-cadherin+CD51/61+CD105+), was similar to that of VEGFR-3+ EC colonies found in the monolayers generated from FL CD34+ cells (Figure5).
Flow cytometric analysis of surface marker expression on VEGFR-3+ cells in isolated CD34+ cells, in monolayers generated from isolated CD34+ cells, and in HUVEC monolayers.
Isolated cells coexpress VEGFR-3+ and stem/progenitor cell markers CD133 and CD34 (A). All adherent VEGFR-3+ cells in monolayers derived from isolated CD34+ cells incorporate Ac-LDL, confirming that they are ECs (B). Adherent VEGFR-3+cells expressing endothelial markers CD105 (C) or CD51/61 (D) are also detected. VEGFR-3+ cells found in HUVEC monolayers have a surface marker expression pattern similar to that of adherent VEGFR-3+ ECs derived from CD34+ cells (panel A).
Flow cytometric analysis of surface marker expression on VEGFR-3+ cells in isolated CD34+ cells, in monolayers generated from isolated CD34+ cells, and in HUVEC monolayers.
Isolated cells coexpress VEGFR-3+ and stem/progenitor cell markers CD133 and CD34 (A). All adherent VEGFR-3+ cells in monolayers derived from isolated CD34+ cells incorporate Ac-LDL, confirming that they are ECs (B). Adherent VEGFR-3+cells expressing endothelial markers CD105 (C) or CD51/61 (D) are also detected. VEGFR-3+ cells found in HUVEC monolayers have a surface marker expression pattern similar to that of adherent VEGFR-3+ ECs derived from CD34+ cells (panel A).
Expression of lymphatic EC markers LYVE-1 and podoplanin on VEGFR-3+ cells in monolayers generated from isolated CD34+ cells.
Double immunostaining of EC monolayers derived from isolated fetal liver CD34+ cells detected adherent VEGFR-3+cells (blue; panels A and B) coexpressing lymphatic EC markers LYVE-1 (red; panel A) or podoplanin (red; panel B). Original magnification × 500.
Expression of lymphatic EC markers LYVE-1 and podoplanin on VEGFR-3+ cells in monolayers generated from isolated CD34+ cells.
Double immunostaining of EC monolayers derived from isolated fetal liver CD34+ cells detected adherent VEGFR-3+cells (blue; panels A and B) coexpressing lymphatic EC markers LYVE-1 (red; panel A) or podoplanin (red; panel B). Original magnification × 500.
Discussion
Bone marrow is a rich reservoir of organ-specific pluripotent and committed stem cells. Hematopoietic, endothelial, and mesenchymal stem cells have been shown to reside in the bone marrow, with the potential to be recruited to the peripheral circulation. They have also been shown to contribute to tumor angiogenesis and wound healing. Here, we have demonstrated that CD133+CD34+ cells coexpressing VEGFR-3 are functionally nonadherent EC precursors that have the capacity to differentiate into mature adherent VEGFR-3+ ECs. Both endothelial precursor cells and mature ECs may express similar endothelial-specific markers. In contrast, the expression of CD133 is rapidly down-regulated as progenitors and stem cells differentiate into more mature postmitotic cells.27,46 Mature endothelial cells do not express CD133.27 47 Therefore, CD34+ cells coexpressing VEGFR-3 and CD133 represent a phenotypic and functional subset of stem and progenitor cells. Although the number of circulating CD34+VEGFR-3+ cells was very small in healthy adult blood donors, these cells could be mobilized to the peripheral circulation in pathological conditions, such as tumor growth and wound healing.
The adherent CD133−VEGFR3+Ac-LDL+cells coexpressing CD34, VE-cadherin, D51/61, and CD105 that we observed after plating of nonadherent CD34+VEGFR-3+ cells could represent either lymphatic or vascular ECs or both.48-52 Therefore, circulating CD133+CD34+VEGFR-3+cells may contain subpopulations of both vascular and lymphatic EC precursors. VEGFR-3–expressing vascular ECs can be observed in blood vessels during tumor growth and wound healing,5 19 and circulating VEGFR-3+ vascular endothelial precursors could play a role in trauma- or tumor-induced blood vessel growth.
We also observed CD34+ cell–derived ECs coexpressing VEGFR-3 and the lymphatic EC markers LYVE-141 and podoplanin42 (Figure 6). Interestingly, Makinen et al have recently shown that up to 50% of freshly isolated human ECs (HUVECs and dermal microvascular ECs) were positive for VEGFR-3, and that the number of VEGFR-3+ ECs then decreased upon repeated subculture in vascular EC growth medium.49 The authors concluded that the cultured HUVECs and microvascular ECs consisted of distinct populations of vascular ECs and lymphatic ECs, and that the majority of VEGFR-3+ ECs were of lymphatic endothelial origin. In agreement with these conclusions, when we studied HUVECs of passages 2 to 4, we found cell-surface expression of VEGFR-3 on about half of the cells. These VEGFR-3+ HUVECs had a phenotype comparable to that of VEGFR-3+ ECs in CD34+ cell–derived monolayers. In CD34+cell–derived monolayers the VEGFR-3+ ECs were more scarce than in HUVEC monolayers, constituting about 10% of the ECs. Taken together, these observations support the concept that a subset of VEGFR-3+ cells on cultured CD34+ cell–derived monolayers are true lymphatic ECs that have differentiated from circulating CD133+CD34+VEGFR-3+ LEC precursors. Mobilization of these LEC precursors might play a role in lymphangiogenesis associated with wound healing or tumor growth.
In summary, we have described the developmental potential of human VEGFR-3+ progenitor cells, showing that when cultured in the presence of EC growth factors they give rise to EC monolayers expressing surface markers typical of both vascular and lymphatic ECs. Further identification and characterization of the VEGFR-3+precursor cell subsets with vascular endothelial potential should define their role in physiological and pathological blood vessel growth. Further studies should also provide a unique approach to prove or disprove the existence of circulating lymphatic EC precursors in adults and their possible role in postnatal lymphatic vessel formation.
Prepublished online as Blood First Edition Paper, August 15, 2002; DOI 10.1182/blood-2002-03-0755.
Supported by grants from the Sigrid Juselius Foundation, the Finnish Medical Foundation, the Maud Kuistila Memorial Foundation, the Academy of Finland; and Helsinki University Hospital Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Petri Salven, Division of Hematology-Oncology, Weill Medical College of Cornell University, 1300 York Ave, Room C-606, New York, NY 10021; e-mail:pjs2004@med.cornell.edu; petri.salven@helsinki.fl.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal