Abstract
Despite the frequency of HIV-specific CD8 T cells, most HIV-infected patients do not control viral replication without antiviral drugs. Although CD8 T cells are important in containing acute HIV and simian immunodeficiency virus (SIV) infection, CD8 T-cell functions are compromised in chronic infection. To investigate whether functional deficits are specific to HIV, the phenotypic and functional properties of HIV, Epstein-Barr virus (EBV), and cytomegalovirus (CMV)–specific CD8 T cells, labeled with HLA A2.1 or B8 tetramers, were compared in 35 HIV-infected and 9 healthy donors. Cytotoxic T lymphocytes express the cytolytic molecules perforin and granzymes, and are thought to be CD45RA+CD27−. Although most HIV- specific cells are antigen experienced and express granzyme A (median, 85%), few express high levels of perforin (median, 10%) or CD45RA (median, 14%) or have down-modulated CD27 (median, 12%). Perforin expression by HIV-specific cells is not significantly different from that of EBV- or CMV-specific cells in the same donors or in healthy donors. EBV- and CMV-specific cells, like HIV-specific cells, are often not cytotoxic when tested directly ex vivo. HIV-specific T-cell expression of other phenotypic markers is similar to that of EBV- and CMV-specific CD8 T cells in healthy donors. However, CMV-specific cells (and, to a lesser extent, EBV-specific cells) in HIV-infected donors are more likely to be CD27−, CD45RA+, and GzmA+. These results suggest that the chance to eradicate an infection by T-cell–mediated lysis may be undermined once an infection becomes chronic. Impaired antiviral cytotoxicity during chronic infection is not specific to HIV but likely represents the immune response to chronic antigenic exposure.
Introduction
Antigen-specific CD8 T cells suppress viral replication in vitro by direct cytotoxicity and by secretion of soluble factors.1-3 There is compelling evidence that antiviral CD8 T cells are important in containing HIV and simian immunodeficiency virus (SIV) replication during acute infection. Acute HIV-1 viremia resolves with the appearance of HIV-specific cytotoxic T lymphocytes (CTLs).4,5 Moreover, in rhesus macaques, the elimination of CD8 T cells results in a dramatic increase in SIV viral load.6,7 The role CD8 T cells play during chronic infection is less clear. Cytotoxicity and interferon-γ (IFN-γ) production by HIV-specific CD8 T cells in response to HIV-infected CD4 T cells or to target cells expressing HIV antigens are often impaired in the absence of exogenous costimulatory molecules such as interleukin-2 (IL-2).8 Moreover, no clear-cut differences in CD8 T-cell phenotype or cytolytic function have been identified in HIV-infected donors with a favorable disease course.
CTL lysis of HIV-infected cells occurs primarily through granule exocytosis, which requires perforin to facilitate the entry of apoptosis-inducing serine protease granzymes into the cytosol of infected target cells.9 However, perforin expression at the site of infection in lymphoid tissues during chronic HIV infection is rare, despite a high frequency of antigen-experienced CD8 T cells expressing granzyme A (GzmA).10 After recent infection, perforin expression in the lymph nodes is greater, but still substantially less, than GzmA expression.11 In addition, though a large proportion of circulating CD8 T cells are perforin positive (34% ± 20% in HIV-infected donors compared with 6% ± 2% in healthy donors in one study12), circulating tetramer-stained HIV-specific CD8 T cells generally lack perforin expression.13 Some studies have suggested that effector CTLs in healthy donors are primarily CD27−CD45RA+CD57+, but this phenotypic association may not hold in patients with persistent infections.14-18 However, HIV tetramer-positive CD8 T cells are largely CD27+CD45RA−CD57−.12,13,19Indeed, specific cytotoxicity by freshly isolated CD8 T cells against targets presenting HIV antigens is impaired.13,19,20However, CD8 T cells with cytolytic potential are present in large numbers and can be readily activated for cytotoxicity by short-term in vitro exposure to costimulatory signals ordinarily delivered by CD4 T cells or antigen-presenting cells, such as IL-2, IL-12, or CD40L trimer.19-21
Because HIV infection specifically targets the critical CD4 T-helper compartment and also infects CD4dim professional antigen-presenting cells, including macrophages and dendritic cells, impaired cytotoxic function by HIV-specific CD8 T cells could be a special property of HIV infection. In fact, in a few subjects in one study, impaired cytotoxicity and low perforin expression were more pronounced in response to HIV than to CMV, suggesting that CD8 T-cell dysfunction is a special feature of HIV-specific cells.13Immune responses are tightly regulated, however, in patients with chronic antigenic exposure to prevent autoimmune reactions to self-antigens. This has been studied mostly for CD4 T cells, but similar mechanisms likely regulate CD8 responses. Therefore, it would not be surprising if CD8 T cells repeatedly exposed to antigen during other chronic infections also have reduced cytotoxicity. To address this question, we compared the functional and phenotypic properties of HIV-specific CD8 T cells, identified by tetramer staining, with those of EBV- and CMV-specific cells in 35 HIV-infected donors and 9 healthy donors expressing HLA A2.1 or B8.
Patients, materials, and methods
Study population
This work was carried out on a cross-section of HLA-A2+ and HLA-B8+ HIV-infected donors (n = 35) and 9 healthy volunteers (Table1). HLA-A2– and HLA-B8–expressing subjects were identified by serology or by flow cytometric analysis with HLA-A2.1–specific monoclonal antibody (mAb) PA2.1 (a kind gift from Herman Eisen, Massachusetts Institute of Technology, Cambridge) or HLA-B8 mAb from One Lambda (Canoga Park, CA). The study was approved by the Center for Blood Research and University Hospitals of Cleveland Institutional Review Committees. Blood was drawn after informed consent was obtained, and PBMCs were isolated by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) density-gradient centrifugation. Samples were either freshly obtained or cryopreserved. Flow cytometry results obtained from thawed cells were comparable to those from freshly isolated cells.
Clinical characteristics of patients in this study
| Patient . | Stage . | CD4 count cells/mm3 . | Viral load copies/ mL . | A2.1/B8 . | Rx . |
|---|---|---|---|---|---|
| 136 | B2 | 410 | 4 338 | A2 | AZT |
| 146 | A2 | 280 | ND | A2, B8 | None |
| 203 | A2 | 490 | < 500 | A2, B8 | AZT, ddI |
| 204 | B3 | 185 | 8 300 | A2, B8 | ddC |
| 215 | B3 | 150 | 46 322 | A2 | AZT, ddC |
| 219 | A2 | 370 | 1 512 | A2 | AZT |
| 224 | A3 | 180 | ND | B8 | AZT, ddC |
| 225 | B3 | 124 | ND | B8 | ddI |
| 226 | C3 | 130 | 65 438 | B8 | None |
| 230 | A2 | 250 | 132 500 | B8 | AZT, 3TC |
| 237 | C2 | 260 | 3 430 | A2 | AZT, 3TC |
| 307 | B2 | 283 | 8 633 | A2 | None |
| 350 | A1 | 1005 | 70 | A2 | AZT, ddC, 3TC |
| 501 | B2 | 307 | < 50 | A2 | HAART |
| 606 | A2 | 615 | < 50 | A2 | HAART |
| 701 | B3 | 71 | < 50 | A2 | HAART |
| 703 | A1 | 529 | < 50 | A2 | ddI, d4T |
| 705 | A2 | 650 | 13 000 | A2 | ddI, d4T, efavirenz |
| CW1 | C3 | 348 | 68 618 | B8 | None |
| CW3 | B1 | 518 | 175 135 | B8 | None |
| CW4 | B3 | 38 | 52 363 | A2, B8 | 3TC, d4T, RTV, SQV |
| CW5 | A1 | 590 | 23 565 | A2 | None, LTNP |
| CW6 | C3 | 739 | < 50 | A2 | 3TC, d4T, ABV, efavirenz |
| CW7 | A2 | 437 | < 50 | A2 | None, LTNP |
| CW9 | B3 | 477 | < 100 | A2 | AZT, 3TC, RTV |
| CW10 | C3 | 280 | < 100 | B8 | AZT, 3TC, RTV |
| CW11 | B2 | 574 | < 50 | B8 | 3TC, d4T, NFV |
| CW14 | A1 | 670 | 54 000 | B8 | None, LTNP |
| CW16 | A1 | 694 | 549 | A2 | None, LTNP |
| CW18 | A1 | 599 | < 50 | A2 | None, LTNP |
| CW19 | A2 | 286 | 746 | A2 | AZT, 3TC, ± efavirenz, ± NFV |
| CW20 | A2 | 467 | 8 400 | B8 | None, LTNP |
| CW24 | B1 | 718 | 103 | A2 | AZT, 3TC, RTV, SQV |
| CW28 | C3 | 539 | < 50 | A2 | 3TC, d4T, Kaletra |
| CW29 | B3 | 817 | < 50 | B8 | AZT, ddI, IDV |
| Patient . | Stage . | CD4 count cells/mm3 . | Viral load copies/ mL . | A2.1/B8 . | Rx . |
|---|---|---|---|---|---|
| 136 | B2 | 410 | 4 338 | A2 | AZT |
| 146 | A2 | 280 | ND | A2, B8 | None |
| 203 | A2 | 490 | < 500 | A2, B8 | AZT, ddI |
| 204 | B3 | 185 | 8 300 | A2, B8 | ddC |
| 215 | B3 | 150 | 46 322 | A2 | AZT, ddC |
| 219 | A2 | 370 | 1 512 | A2 | AZT |
| 224 | A3 | 180 | ND | B8 | AZT, ddC |
| 225 | B3 | 124 | ND | B8 | ddI |
| 226 | C3 | 130 | 65 438 | B8 | None |
| 230 | A2 | 250 | 132 500 | B8 | AZT, 3TC |
| 237 | C2 | 260 | 3 430 | A2 | AZT, 3TC |
| 307 | B2 | 283 | 8 633 | A2 | None |
| 350 | A1 | 1005 | 70 | A2 | AZT, ddC, 3TC |
| 501 | B2 | 307 | < 50 | A2 | HAART |
| 606 | A2 | 615 | < 50 | A2 | HAART |
| 701 | B3 | 71 | < 50 | A2 | HAART |
| 703 | A1 | 529 | < 50 | A2 | ddI, d4T |
| 705 | A2 | 650 | 13 000 | A2 | ddI, d4T, efavirenz |
| CW1 | C3 | 348 | 68 618 | B8 | None |
| CW3 | B1 | 518 | 175 135 | B8 | None |
| CW4 | B3 | 38 | 52 363 | A2, B8 | 3TC, d4T, RTV, SQV |
| CW5 | A1 | 590 | 23 565 | A2 | None, LTNP |
| CW6 | C3 | 739 | < 50 | A2 | 3TC, d4T, ABV, efavirenz |
| CW7 | A2 | 437 | < 50 | A2 | None, LTNP |
| CW9 | B3 | 477 | < 100 | A2 | AZT, 3TC, RTV |
| CW10 | C3 | 280 | < 100 | B8 | AZT, 3TC, RTV |
| CW11 | B2 | 574 | < 50 | B8 | 3TC, d4T, NFV |
| CW14 | A1 | 670 | 54 000 | B8 | None, LTNP |
| CW16 | A1 | 694 | 549 | A2 | None, LTNP |
| CW18 | A1 | 599 | < 50 | A2 | None, LTNP |
| CW19 | A2 | 286 | 746 | A2 | AZT, 3TC, ± efavirenz, ± NFV |
| CW20 | A2 | 467 | 8 400 | B8 | None, LTNP |
| CW24 | B1 | 718 | 103 | A2 | AZT, 3TC, RTV, SQV |
| CW28 | C3 | 539 | < 50 | A2 | 3TC, d4T, Kaletra |
| CW29 | B3 | 817 | < 50 | B8 | AZT, ddI, IDV |
Disease staging was determined by the lowest CD4 count and in accordance with the CDC classification system. ND indicates not done; AZT, zidovidine; ddI, dideoxyinosine; ddC, dideoxycytosine; 3TC, lamivudine; d4T, stavudine; RTV, ritonavir; SQV, saquinavir; NFV, nelfinavir; IDV, indinavir; ABV, abacavir; Kaletra, lopinavir/ritonavir; LTNP, long-term nonprogressor.
Tetramers
Tetramers specific for A2.1- or B8-restricted epitopes in HIV, EBV, or CMV were produced as described19 or were obtained from the National Institute of Allergy and Infectious Diseases Major Histocompatibility Complex Tetramer Core Facility (Yerkes Regional Primate Center, Atlanta, GA).22 Tetramers used in this study are shown in Table 2. Before use, the tetrameric complexes were titrated to minimize background staining. Specificity of staining was confirmed for each tetrameric complex with peptide-specific CTL lines. The sensitivity of detection of the assay was 0.01%. For phenotypic analysis, streptavidin–phycoerythrin (PE) tetramer-stained populations were required to be well separated on flow cytometric dot plots of tightly gated lymphocytes costained for tetramers and CD8-Cy5 (mAb B9.11; Immunotech, Westbrook, ME) and to represent at least 0.05% of CD8 T cells in the sample. The median and range of frequencies of CD8 T cells recognizing each tetramer for both sets of donors is given in Table 2. Of the 71 tetramer-positive populations analyzed, only 3 had low frequencies of 0.05% to 0.10%. Therefore, the phenotypic analysis was unambiguous.
Tetramers used for this study
| Virus . | Protein . | Amino acids . | Sequence . | Restricting element . | %tetramer-positive CD8 T cells [median, (range)] . | Reference . | |
|---|---|---|---|---|---|---|---|
| Healthy donor . | HIV seropositive . | ||||||
| HIV | gag | 77-85 | SLYNTVATL | A0201 | — | 0.53 (0.14, 1.85) | 22-24 |
| HIV | RT | 309-317 | ILKEPVHGV | A0201 | — | 0.49 (0.27, 0.95) | 22,24,25 |
| HIV | RT | 293-302 | KYTAFTIPSI | A0201 | — | 0.64 (0.60, 0.81) | 26 |
| HIV | env | 593-600 | YLKDQQLL | B8 | — | 0.11 (0.05, 0.13) | 27 |
| CMV | pp65 | 495-503 | NLVPMVATV | A0201 | 0.48 (0.25, 4.24) | 1.89 (0.30, 11.0) | 28-30 |
| EBV | BMLF1 | 280-288 | GLCTLVAML | A0201 | 0.15 (0.08, 0.43) | 0.46 (0.16, 2.23) | 31,32 |
| EBV | BZLF1 | 190-197 | RAKFKQLL | B8 | 0.74 (0.37, 1.02) | 1.04 (0.18, 3.46) | 31,32 |
| Virus . | Protein . | Amino acids . | Sequence . | Restricting element . | %tetramer-positive CD8 T cells [median, (range)] . | Reference . | |
|---|---|---|---|---|---|---|---|
| Healthy donor . | HIV seropositive . | ||||||
| HIV | gag | 77-85 | SLYNTVATL | A0201 | — | 0.53 (0.14, 1.85) | 22-24 |
| HIV | RT | 309-317 | ILKEPVHGV | A0201 | — | 0.49 (0.27, 0.95) | 22,24,25 |
| HIV | RT | 293-302 | KYTAFTIPSI | A0201 | — | 0.64 (0.60, 0.81) | 26 |
| HIV | env | 593-600 | YLKDQQLL | B8 | — | 0.11 (0.05, 0.13) | 27 |
| CMV | pp65 | 495-503 | NLVPMVATV | A0201 | 0.48 (0.25, 4.24) | 1.89 (0.30, 11.0) | 28-30 |
| EBV | BMLF1 | 280-288 | GLCTLVAML | A0201 | 0.15 (0.08, 0.43) | 0.46 (0.16, 2.23) | 31,32 |
| EBV | BZLF1 | 190-197 | RAKFKQLL | B8 | 0.74 (0.37, 1.02) | 1.04 (0.18, 3.46) | 31,32 |
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were resuspended in 500 μL fluorescence-activated cell sorter (FACS) blocking buffer (Hanks balanced salt solution [HBSS] with 10% human AB serum, 0.5% human immunoglobulin G [IgG], 5 mM EDTA [ethylenediaminetetraacetic acid]) for 15 minutes at 4°C and then incubated with streptavidin-PE–conjugated tetramer for an additional 40 minutes at 4°C. For external staining, the cells were washed and resuspended in FACS buffer, and aliquots of the suspension were stained with 2 μL fluorescein isothiocyanate (FITC)–conjugated mAbs to CD27 or CD45RA and Cy5-conjugated CD8 mAb or with IgG-FITC and PE and Cy5 isotype-matched controls (Immunotech). After incubation for 30 minutes at 4°C, cells were washed and resuspended in FACS buffer with 1% formaldehyde for analysis. For internal staining with GzmA mAb CB9 or perforin mAb δG9 (PharMingen, San Diego, CA), tetramer-stained cells were resuspended in 50 μL FACS buffer and were permeabilized using the Caltag Laboratories (Burlingame, CA) Fix and Perm kit according to the manufacturer's protocol. Fixed cells were incubated for 15 minutes at room temperature with 2 μL respective antibodies conjugated to FITC, washed, and resuspended in 50 μL FACS buffer. Cells were then stained with CD8-Cy5 for 15 minutes and fixed in FACS buffer with 1% formaldehyde. All samples were analyzed on a FACScalibur with Cell Quest software (Becton Dickinson, San Jose, CA) on a lymphocyte-gated population. In a subset of samples, instead of tetramer staining, PBMCs were costained for CD8-Cy5, CD27-PE, or CD45RA-PE and were stained for perforin-FITC or GzmA-FITC, as above.
Modulation of perforin and GzmA expression by agents that block granule exocytosis, lysosomal acidification, protein secretion, or proteolysis
Peptide-specific CD8 T-cell lines were generated by incubating PBMCs from HLA A2.1- or B8-expressing healthy donors with 1 μg/mL epitopic peptide and adding recombinant human (rh) IL-2 (1-100 IU/mL) 1 to 2 days later. Cultures were fed biweekly to maintain a cell density of 5 × 105 cells/mL and were used in experiments 5 to 10 days after stimulation. Cell lines were incubated for 2 hours at 37°C with the following agents, alone or in combination, at the stated final concentration: cathepsin B inhibitors (CI), 50 μM CA074me and 50 μM z-FA-fmk (CalBiochem, San Diego, CA); proteasome inhibitors (PI), 50 μM MG-132 (z-Leu-Leu-Leu-CHO) and 50 μM PSI (z-Ile-Glu(OtBu)-Ala-Leu-CHO) (CalBiochem); 400 μM chloroquine (Sigma, St Louis, MO); 50 μg/mL Brefeldin A (Sigma); 2 mM EGTA (ethyleneglycoltetraacetic acid; Sigma); and 50 μg/mL cycloheximide (Sigma). Cells were stained and analyzed for tetramer, CD8, and perforin or GzmA as above. Modifying agents were added to the FACS blocking buffer and were maintained throughout the staining procedure.
Immunomagnetic enrichment of tetramer-positive population
PBMCs, stained with PE-labeled HLA-A2 or -B8 tetramers in sterile phosphate-buffered saline (PBS) with 2% fetal calf serum (FCS) for 40 minutes in the cold, were washed and incubated with α-PE Miltenyi (Miltenyi Biotech, Bergisch Gladbach, Germany) beads for another 15 minutes. Tetramer-PE cells that bound the beads were immunomagnetically selected on a Miltenyi column following the manufacturer's instructions. An aliquot of selected cells was costained with α-CD8–Cy5 to ascertain the levels of enrichment. Usually, more than 100-fold enrichment of the tetramer-positive population could be obtained.
Cytotoxicity assay
Log-phase autologous B lymphoblastoid cell line (BLCL) target cells were labeled with 100 μCi (3.7 MBq) chromium Cr 51 for 1 hour, washed 3 times in RPMI 1640 medium with 10% FCS, and resuspended at 105/mL as described.33 Labeled target cells (104) were added to triplicate U-bottom microtiter wells in the presence or absence of relevant antigenic peptides. Effector cells were prepared from freshly isolated, density-separated PBMCs used directly or after enrichment for tetramer-positive cells as above. After incubating target cells with peptides (1 μg/mL) for 1 hour at 37°C, effector cells suspended at indicated E/T ratios in 100 μL were added to the wells, and the plates were incubated at 37°C over CO2 for 6 hours. Supernatants (35 μL) were counted on a Top Count (Packard, Meriden, CT) microplate reader, and the percentage of specific cytotoxicity was calculated from the average cpm as [(average cpm − spontaneous release)/(total release − spontaneous release) × 100]. The spontaneous release for all experiments was less than 20%. Peptide-specific cytotoxicity was calculated as the difference between percentage specific cytotoxicity against peptide-loaded targets and targets incubated with medium.
Statistical analysis
Comparisons between groups were analyzed by 2-sided Wilcoxon rank sum test. Results were compared for percentage tetramer-positive cells expressing the indicated markers analyzed for each HIV tetramer and for HIV tetramer-positive cells versus EBV and/or CMV tetramer-positive cells in HIV-infected donors and for each virus between HIV-seropositive samples and healthy donor samples. Data were expressed as medians and ranges. Differences of expression in the HIV-seropositive samples were also tested for correlation with CDC stage, CD4 count (fewer than 500 cells/mm3, 500 or more cells/mm3) and for differences depending on whether plasma viremia was suppressed (fewer than 100 copies/mL, 100 or more copies/mL). Given the exploratory nature of the analysis,P values were not adjusted for multiple comparisons.
Results
Despite persistent infection, HIV-specific tetramer-positive CD8 T cells have the phenotype of memory cells and lack perforin required for cytolysis
It has been hypothesized that antigen-primed CD8 T cells segregate into a memory population that expresses the RO isoform of CD45 and is CD27+ and an effector CTL population that expresses the RA isoform of CD45 and is CD27−.14 However, this simple phenotypic picture may not be true in patients with persistent infection.15-18 A few studies have shown that HIV tetramer-binding cells from most HIV-infected subjects are of the memory subtype because they are CD27+ and CD45RA− and express high levels of bcl-2.13,19 Other properties (GzmA+, CD28−, CCR7−, and CD62L−), however, may not be typical of either naive or memory cells.12,13,19,34 Although the tetramer-positive cells express GzmA, they generally do not stain for perforin13and thus may be incapable of lysing HIV-infected target cells by granule exocytosis.9 These results were confirmed in the present analysis of HIV tetramer-positive CD8 T cells from 35 HIV-infected subjects stained with 3 HLA A2.1 tetramers produced with gag or RT peptides and 1 HLA B8 env tetramer (Table 2). The subjects represent a cross-section of disease stages (Table 1), but nearly half (16 of 35 or 46%) are stage A subjects with no history of HIV-related symptoms. The CD4 counts of the group were also relatively well preserved (median, 437 cells/mm3), but 7 patients had CD4 counts lower than 200/mm3, and the range of CD4 counts was wide (range, 38-1005/mm3). Approximately one third of these patients were receiving highly active antiretroviral therapy (HAART), but one third of the samples were obtained before HAART was available, and approximately one third of the subjects were not receiving any antiretroviral drugs because of stable disease without drugs, drug intolerance, or individual preference. Among the latter group were 6 long-term asymptomatic subjects (CW5, CW7, CW14, CW16, CW18, CW20) who had been infected for more than 5 years and maintained CD4 counts greater than 450/mm3 without antiviral drug therapy. Approximately one third of all subjects had plasma viremia below the levels of detection.
The frequencies of CD8 T cells reacting with each of the 3 HLA A2.1-restricted gag and RT tetramers were similar, but the HLA B8 env epitope was less commonly recognized. The mean proportion of CD8 T cells that recognized gag SLYNTVATL was 0.67% (range, 0.18%-1.85%; n = 11); that recognized RT epitope ILKEPVHGV was 0.53% (range, 0.27%-0.95%; n = 6); and that recognized RT epitope KYTAFTIPSI was 0.64% (range, 0.60%-0.81%; n = 5). Only an average of 0.09% CD8 T cells in 5 B8+ donors recognized the env epitope YLKDQQLL (range, 0.05%-0.13%).
Despite the fact that most of the subjects had uncontrolled viral production, HIV tetramer-positive cells did not have the phenotype of effector CTLs. Although a median of 85% (range, 26%-95%) HIV tetramer-positive cells stained for GzmA, only a median of 10% stained for perforin. However, perforin expression in HIV-reactive cells was heterogeneous in HIV-infected persons (range, less than 1%-70%). Surprisingly fewer HIV tetramer-positive cells stained for perforin than did CD8 T cells as a whole (median, 27%; range, 8%-85%). Moreover, few HIV tetramer-positive cells had other characteristics attributed to effector CTLs: only 12% (range, 0%-23%) down-modulated CD27, and 14% (range, 0%-59%) expressed CD45RA. Representative flow cytometry dot plots are shown in Figure1A for a long-term asymptomatic donor. Figure 2 and Table3 show the phenotypic profile for all subjects in aggregate. The properties of the tetramer-positive cells recognizing each of the HIV epitopes were statistically indistinguishable from each other.
Representative dot plots of HIV tetramer–positive CD8 T cells stained for markers associated with cytotoxic function.
Samples from HIV-infected, long-term asymptomatic donor CW5 (A) and healthy donors (B-C) were stained for CD8, indicated tetramer, and perforin, GzmA, CD27, or CD45RA. The left panel of each row depicts the dot plot after gating tightly on lymphocytes; all other panels are gated on CD8brightlymphocytes. In the HIV-infected donor, HIV-specific cells are CD27+ and CD45RA−, whereas the EBV- and CMV-specific cells in the same donor are more heterogeneous in their expression of these markers. The sample in panel B from a healthy donor was one of the few samples with a high frequency of perforin-staining, tetramer-positive cells.
Representative dot plots of HIV tetramer–positive CD8 T cells stained for markers associated with cytotoxic function.
Samples from HIV-infected, long-term asymptomatic donor CW5 (A) and healthy donors (B-C) were stained for CD8, indicated tetramer, and perforin, GzmA, CD27, or CD45RA. The left panel of each row depicts the dot plot after gating tightly on lymphocytes; all other panels are gated on CD8brightlymphocytes. In the HIV-infected donor, HIV-specific cells are CD27+ and CD45RA−, whereas the EBV- and CMV-specific cells in the same donor are more heterogeneous in their expression of these markers. The sample in panel B from a healthy donor was one of the few samples with a high frequency of perforin-staining, tetramer-positive cells.
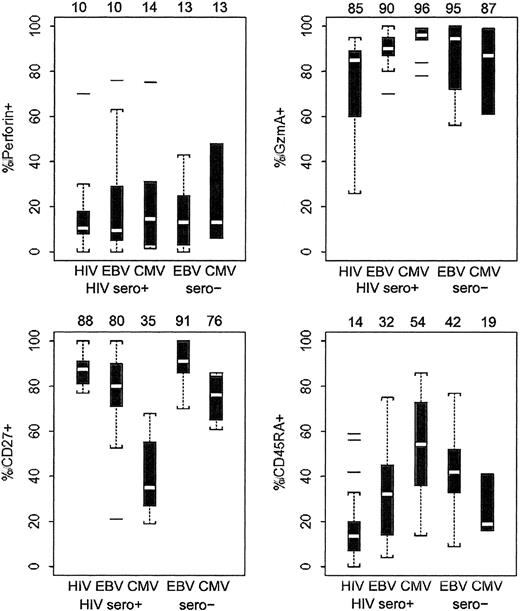
Phenotypic analysis of CD8 T cells that stain with HIV, EBV, and CMV tetramers in 35 HIV-infected patients and 9 healthy donors.
Data are represented by dot plots depicting median (white bar) and interquartile distances (25th-75th percentiles, indicated by box). Dotted lines represent 1.5 interquartile distances. Any outlying data points outside the dotted lines are shown. Median values for each set of samples are given above the graphs. The statistical analysis of these data by 2-sided Wilcoxon rank sum test were tabulated and are indicated in Table3. Only a minority of tetramer-positive cells stained for perforin in most samples. There were no significant differences in perforin staining between HIV-specific and CMV- or EBV-specific cells in HIV-seropositive or healthy donors. However, differences in expression of the other markers are significant.
Phenotypic analysis of CD8 T cells that stain with HIV, EBV, and CMV tetramers in 35 HIV-infected patients and 9 healthy donors.
Data are represented by dot plots depicting median (white bar) and interquartile distances (25th-75th percentiles, indicated by box). Dotted lines represent 1.5 interquartile distances. Any outlying data points outside the dotted lines are shown. Median values for each set of samples are given above the graphs. The statistical analysis of these data by 2-sided Wilcoxon rank sum test were tabulated and are indicated in Table3. Only a minority of tetramer-positive cells stained for perforin in most samples. There were no significant differences in perforin staining between HIV-specific and CMV- or EBV-specific cells in HIV-seropositive or healthy donors. However, differences in expression of the other markers are significant.
Statistical analysis by Wilcoxon rank sum test for phenotypic data of CD8 T cells that stain with HIV, EBV, and CMV tetramers in 35 HIV-infected patients and 9 healthy donors
| Comparison . | Parameter . | |||
|---|---|---|---|---|
| Perforin . | GzmA . | CD27 . | CD45RA . | |
| Within HIV sero positive samples | ||||
| HIV vs EBV | .77 | .0053-150 | .0173-150 | .0063-150 |
| HIV vs CMV | .92 | .0023-150 | < .0013-150 | .0013-150 |
| EBV vs CMV | About 1 | .0763-150 | < .0013-150 | .0303-150 |
| Between HIV and healthy donor samples | ||||
| EBV tetramer-positive | .87 | .60 | .0503-150 | .31 |
| CMV tetramer-positive | .68 | .50 | .0213-150 | .13 |
| EBV and CMV tetramer-positive | .95 | .86 | .0193-150 | .86 |
| Comparison . | Parameter . | |||
|---|---|---|---|---|
| Perforin . | GzmA . | CD27 . | CD45RA . | |
| Within HIV sero positive samples | ||||
| HIV vs EBV | .77 | .0053-150 | .0173-150 | .0063-150 |
| HIV vs CMV | .92 | .0023-150 | < .0013-150 | .0013-150 |
| EBV vs CMV | About 1 | .0763-150 | < .0013-150 | .0303-150 |
| Between HIV and healthy donor samples | ||||
| EBV tetramer-positive | .87 | .60 | .0503-150 | .31 |
| CMV tetramer-positive | .68 | .50 | .0213-150 | .13 |
| EBV and CMV tetramer-positive | .95 | .86 | .0193-150 | .86 |
See Figure 2 for data analysis.
Statistically significant values.
The phenotypic properties of the HIV tetramer-positive cells did not correlate significantly with clinical disease parameters (Table4). The 20 HIV tetramer-positive samples were grouped by Centers for Disease Control (CDC) stage, CD4 count (fewer than 500 cells/mm3 or 500 or more cells/mm3), or plasma viral load (fewer than 100 copies/mL or 100 or more copies/mL). HIV-specific cells did not vary significantly with any of these parameters and were mostly CD27+CD45RA−perforin−GzmA+in all clinical subgroups. Therefore, any disease-related variations in the phenotype of HIV-specific CD8 T cells across clinical groups, if they exist, are subtle.
Phenotype of HIV tetramer-positive CD8 T cells does not vary significantly with HIV disease status
| . | % of HIV tetramer-positive CD8 T-cells that express . | |||
|---|---|---|---|---|
| CD27 . | CD45RA . | Perforin . | GzmA . | |
| Clinical stage | ||||
| A, n = 11 | 90 | 10 | 13 | 84 |
| B, n = 6 | 82 | 13 | 10 | 86 |
| C, n = 3 | 80 | 46 | 5 | 87 |
| CD4 count, cells/mm3 | ||||
| 500 or more, n = 10 | 87 | 17 | 10 | 88 |
| Less than 500, n = 10 | 88 | 11 | 17 | 67 |
| Plasma viral load, copies/mL | ||||
| Less than 100, n = 7 | 83 | 20 | 5 | 84 |
| 100 or more, n = 12 | 90 | 11 | 11 | 86 |
| . | % of HIV tetramer-positive CD8 T-cells that express . | |||
|---|---|---|---|---|
| CD27 . | CD45RA . | Perforin . | GzmA . | |
| Clinical stage | ||||
| A, n = 11 | 90 | 10 | 13 | 84 |
| B, n = 6 | 82 | 13 | 10 | 86 |
| C, n = 3 | 80 | 46 | 5 | 87 |
| CD4 count, cells/mm3 | ||||
| 500 or more, n = 10 | 87 | 17 | 10 | 88 |
| Less than 500, n = 10 | 88 | 11 | 17 | 67 |
| Plasma viral load, copies/mL | ||||
| Less than 100, n = 7 | 83 | 20 | 5 | 84 |
| 100 or more, n = 12 | 90 | 11 | 11 | 86 |
Median values for the percentages of HIV tetramer-positive cells that stain positively for each of the phenotypic markers are shown for samples classified by CDC disease stage, CD4 count, and plasma viral load. None of the differences between any of the groups were statistically significant. Three donors recognized more than one tetramer; each tetramer-positive population was counted separately in the analysis.
Lack of perforin staining is not attributed to degranulation in culture or low sensitivity of detection
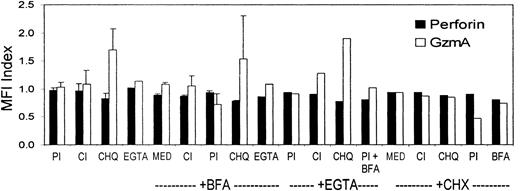
Because of the discrepancy between staining for GzmA and perforin in HIV-specific CD8 T cells, we performed experiments to verify that low perforin staining was not caused by degranulation during freezing, thawing, or staining or by low sensitivity from a short perforin half-life within CD8 T cells. Parallel analysis of fresh samples and thawed samples gave comparable results (data not shown). Perforin is stored in acidic cytotoxic granules, which are specialized secretory lysosomes. We determined whether agents that block the acidification of the granules or that inhibit proteolysis by granule cathepsins, which process granzymes into their active form, enhance perforin protein levels in cells. The FACS buffer used for tetramer staining contains 5 mM EDTA, which chelates Ca++ and blocks perforin polymerization and granule exocytosis. Therefore, decreased perforin staining because of Ca++-dependent degranulation is unlikely. Because the composition of the Caltag fixation and permeabilization reagents are proprietary, however, we also compared staining using these reagents in the presence of an additional 2 mM EGTA (Figure 3). For these experiments, tetramer-positive cell lines were generated through the stimulation of PBMCs from an HLA B8-expressing donor with the EBV B8-restricted peptide (Table 2). Cells were preincubated for 2 hours at 37°C and were costained for the B8 EBV tetramer and for perforin and GzmA in the presence of agents that block granule exocytosis (2 mM EGTA), protein secretion (25 μg/mL Brefeldin A), lysosomal acidification (400 μM chloroquine), or proteolysis. Protease inhibitors that were tested included a cocktail of cathepsin inhibitors (z-FA-fmk and CA-074me) and a cocktail of proteosome inhibitors (MG-132 and PSI). These inhibitors were tested individually and in combination. Data from 4 experiments are shown in Figure 3. Although incubation with chloroquine, which blocks endosome and lysosome acidification, enhanced GzmA mean fluorescence intensity, it had no effect on perforin staining. In fact none of the reagents enhanced perforin mean fluorescence intensity or percentage of perforin-staining cells (not shown). Therefore, the lack of perforin detection seems unlikely to have been secondary to artifacts caused by degranulation or by rapid degradation or secretion of perforin during processing and staining.
Perforin staining is not enhanced by preventing degranulation, preventing acidification of cytotoxic granules, blocking secretion, or inhibiting proteolysis.
A T-cell line from a healthy donor specific for an HLA B8-restricted EBV peptide was incubated for 2 hours at 37°C with the indicated agents before staining for CD8, the EBV tetramer, and perforin or GzmA. Chloroquine (CHQ), which blocks lysosomal acidification, enhanced GzmA staining but not perforin staining. The ratio of mean fluorescence intensity (MFI) of perforin or GzmA expression of treated cells to mock-treated cells is shown. The agents tested were protease inhibitors (PI), cathepsin inhibitors (CI), CHQ, EGTA, Brefeldin A (BFA), or cycloheximide (CHX) at concentrations given in “Patients, materials, and methods.” Med indicates medium.
Perforin staining is not enhanced by preventing degranulation, preventing acidification of cytotoxic granules, blocking secretion, or inhibiting proteolysis.
A T-cell line from a healthy donor specific for an HLA B8-restricted EBV peptide was incubated for 2 hours at 37°C with the indicated agents before staining for CD8, the EBV tetramer, and perforin or GzmA. Chloroquine (CHQ), which blocks lysosomal acidification, enhanced GzmA staining but not perforin staining. The ratio of mean fluorescence intensity (MFI) of perforin or GzmA expression of treated cells to mock-treated cells is shown. The agents tested were protease inhibitors (PI), cathepsin inhibitors (CI), CHQ, EGTA, Brefeldin A (BFA), or cycloheximide (CHX) at concentrations given in “Patients, materials, and methods.” Med indicates medium.
In addition, cycloheximide, an inhibitor of protein synthesis, had little effect on perforin levels but did reduce GzmA somewhat. These results taken together suggest that GzmA may have a relatively short half-life in cells because of lysosomal degradation and that it is constantly synthesized to maintain the cellular pool. However, de novo synthesis of perforin does not occur in these cells. These preliminary results must be verified by more formal studies of perforin and GzmA protein synthesis and degradation, which are outside the scope of this study.
CD8 T cells specific for EBV and CMV in HIV-infected donors and healthy donors are also mostly perforin negative
To determine whether the properties of HIV-specific CD8 T cells are similar to those of CD8 T cells directed at other chronic infections, we costained EBV- and CMV-specific CD8 T cells with HLA A2.1 and HLA B8 tetramers and with perforin and GzmA in the same donors and in healthy donors. In HIV-infected patients, the proportions of EBV and CMV tetramer-positive cells that stained for perforin were similar to the staining pattern of HIV tetramer-positive cells. Most cells specific for these chronic infections are also perforin negative (Figures 1, 2). Only 14% (range, 1.4%-75%) of CMV tetramer-positive cells (n = 10) and 10% (range, 0%-76%) of EBV tetramer-positive cells (n = 23) were perforin positive (P = .92 and P = .77, respectively) compared with HIV tetramer-positive cells. These results differ from those reported by Appay et al,13 who found that perforin was preferentially down-modulated in HIV-specific CD8 T cells compared with CMV-specific CD8 T cells. As for HIV-specific cells, in an occasional donor, a high proportion of EBV or CMV tetramer-positive CD8 T cells was also perforin positive. However, donors with high proportions of perforin staining cells recognizing one virus did not have a high proportion of perforin-positive cells recognizing other viruses. These rare instances may correspond to recent infections or flares in viral production to which an effective CD8 T-cell response is generated. Plasma samples were unavailable to examine this. We also found no significant difference by 2-sided Wilcoxon rank sum test in perforin expression in EBV and CMV tetramer-positive cells in healthy donors compared with HIV-infected donors: 13% (0%-43%) of EBV-specific cells (n = 7) and 13% (range, 6%-48%) of CMV-specific cells (n = 4) from healthy donors were perforin positive (P = .87 and P = .68, respectively, compared with HIV-specific cells) (Figure 2; Table 3).
EBV and CMV tetramer-positive cells in HIV-infected donors are more often GzmA+, CD27−, and CD45RA+, phenotypic properties attributed to effector CTLs, than are HIV tetramer-positive cells
Although the low expression of perforin in HIV tetramer-positive cells was not distinctive, we also looked at 3 other markers of CD8 T-cell differentiation: expression of GzmA and CD45RA and down-modulation of CD27. In HIV-infected donors these postulated markers of terminally differentiated CTLs were more frequent on EBV- and CMV-specific cells than on HIV-specific cells in the same infected donors or on EBV- and CMV-specific cells in healthy donors (Figure 2; Table 3). For these parameters, the EBV tetramer-positive cells had properties intermediate between those of the HIV- and CMV-specific cells.
In HIV-infected donors, EBV- and CMV-specific cells were more uniformly GzmA+ than were HIV-specific cells (Figure 2; Table 3). GzmA was expressed by 85% (range, 26%-95%) of HIV tetramer-positive cells compared with 90% (range, 70%-100%) of EBV tetramer-positive cells and 96% (range, 78%-99%) of CMV tetramer-positive cells in HIV-infected donors (P = .005 and P = .002, respectively). GzmA expression in EBV- and CMV-specific cells from healthy donors was indistinguishable from that in EBV- and CMV-specific cells in HIV-infected donors (P = .60 and P = .50, respectively), with most of these circulating cells expressing GzmA.
Although 88% (range, 77%-100%) of HIV tetramer-positive cells express CD27, 80% (range, 21%-100%) of EBV tetramer-positive cells and 35% (range, 19%-68%) of CMV tetramer-positive cells express CD27 in HIV-infected donors (P = .02 andP < .001, respectively, compared with HIV). The difference in CD27 expression between EBV- and CMV-specific cells in the HIV-infected donors was also highly significant (P < .001). Similarly, though few HIV-specific cells express CD45RA (14%; range, 0%-59%), in the same infected donors more EBV-specific cells (32%; range, 0%-79%, P = .006) and still more CMV-specific cells (54%; range, 14%-86%;P = .001) express this marker. Again, the difference between EBV- and CMV-specific cell expression of CD45RA was significant (P = .030).
By CD27 expression, EBV- and CMV-specific CD8 T cells in healthy donors looked more like memory cells than they did in HIV-infected donors. Although 91% (range, 70%-100%) of healthy donor EBV tetramer-positive cells are CD27+, only 80% (range, 21%-100%) of HIV-seropositive donor EBV-specific cells are CD27+ (P = .050). Similarly, for CMV tetramer-positive cells, 76% (range, 61%-86%) of healthy donor and 35% (range, 19%-68%) of HIV-infected donor cells expressed CD27 (P = .021). However, CD45RA expression was statistically indistinguishable on EBV- and CMV-specific cells in healthy and HIV-infected donors (P = .31 and P = .13, respectively). The lack of clear differences in CD45RA expression may reflect the fact that CD45RA does not correlate as well as CD27 down-modulation with CTL effector status (see “Most perforin+ CD8 T cells are also CD27−”).
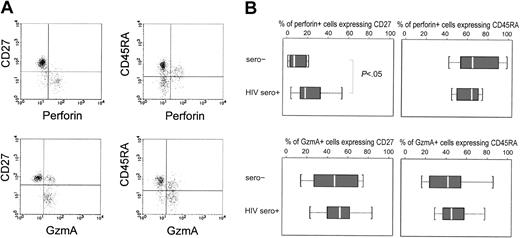
Most perforin+ CD8 T cells are also CD27−
Because we were limited to 3-color staining in this study, we were unable to costain for CD8 and tetramer and more than one other marker to look at the correlation of other phenotypic markers with perforin expression. Therefore, in a subset of subjects (10 HIV-seropositive and 8 healthy donors), we costained for CD8, perforin or GzmA, and CD27 or CD45RA (Figure 4). The only significant difference in the costaining profiles between healthy donors and HIV-seropositive donors was for CD27 expression. Virtually all perforin+ cells have down-modulated CD27; only 7% (range, 1%-21%) of perforin+ CD8 T cells expressed CD27 in healthy donors, compared with 18% (range, 12%-33%) in HIV-seropositive samples (P = .049). Hence, though CD27 down-modulation coincided with perforin expression in healthy donors, in patients with HIV approximately 1 in 5 perforin+ cells still expressed CD27. Although most perforin+ CD8 T cells were CD45RA+, staining for CD45RA was diverse—a median of 65% of perforin+ cells is CD45RA+ in healthy donor or HIV-seropositive samples. CD27 down-modulation correlated better with perforin expression than did CD45RA, but neither was a perfect indicator of potential CTL function in HIV-infected donors. Neither marker was useful in identifying the GzmA+ subset of CD8 T cells.
CD27 down-modulation correlates well with perforin expression in CD8 T cells, but CD45RA expression is more variable.
PBMCs from healthy and HIV-infected donors were costained for CD8, CD27, or CD45RA, and perforin or GzmA. Cells in a CD8+lymphocyte gate were analyzed. Typical dot plots are shown for long-term asymptomatic HIV-infected donor CW18 in panel A. The percentages of perforin+ and GzmA+ CD8 T cells that stain for CD27 or CD45RA for healthy donor and HIV-seropositive samples are depicted by box plots in panel B. In the box plots, the median value is indicated by a white bar, and interquartile differences (25th-75th percentile) by the box. Dotted lines represent 1.5 interquartile differences. There were no outlying data points. Perforin+ cells are uniformly CD27− in healthy donors, but a significant proportion of perforin+ cells in HIV-infected donors still express CD27.
CD27 down-modulation correlates well with perforin expression in CD8 T cells, but CD45RA expression is more variable.
PBMCs from healthy and HIV-infected donors were costained for CD8, CD27, or CD45RA, and perforin or GzmA. Cells in a CD8+lymphocyte gate were analyzed. Typical dot plots are shown for long-term asymptomatic HIV-infected donor CW18 in panel A. The percentages of perforin+ and GzmA+ CD8 T cells that stain for CD27 or CD45RA for healthy donor and HIV-seropositive samples are depicted by box plots in panel B. In the box plots, the median value is indicated by a white bar, and interquartile differences (25th-75th percentile) by the box. Dotted lines represent 1.5 interquartile differences. There were no outlying data points. Perforin+ cells are uniformly CD27− in healthy donors, but a significant proportion of perforin+ cells in HIV-infected donors still express CD27.
Perforin expression is required for cytotoxicity by freshly tested virus-specific cells in healthy donors and HIV-infected donors
To verify that the low levels of perforin expression in CMV and EBV tetramer-positive cells signified impaired cytotoxicity, we tested cytotoxicity by using freshly isolated healthy donor (n = 3) and HIV-seropositive donor (n = 4) PBMCs against autologous BLCLs incubated with EBV or CMV peptides (Tables5, 6). Although cell lines in which the frequency of perforin expression on tetramer-positive cells was greater than 25% demonstrated peptide-specific cytotoxicity (range, 12%-23%) above background at E/T ratios of 100:1 to 200:1, PBMCs with low numbers of perforin+ tetramer-positive cells did not. However, EBV-specific cells from one donor (CW16) did not have cytotoxic activity above background, even though the frequency of tetramer-positive cells was reasonably high (0.76%) and most tetramer-positive cells stained for perforin.
EBV and CMV peptide-specific cytotoxicity in HIV-infected and healthy donors requires perforin expression on tetramer-positive CD8 T cells: direct testing of whole PBMCs
| Subject . | HIV status . | Tetramer . | Tetramer-positive cells . | Specific cytotoxicity at E/T ratio, % . | ||||
|---|---|---|---|---|---|---|---|---|
| CD8 T cells, % . | Perforin+, % . | 200:1 . | 100:1 . | 50:1 . | 25:1 . | |||
| 307 | Seropositive | A2 CMV | 0.96 | 25.0 | 7 | 3 | 0 | — |
| 501 | Seropositive | A2 CMV | 2.47 | 35.0 | — | 30* | 11* | 7* |
| CW16 | Seropositive | A2 EBV | 0.76 | 71.0 | — | 2 | 2 | 0 |
| CW20 | Seropositive | B8 EBV | 1.04 | 0.0 | 0 | 0 | 2 | 0 |
| HD1 | Seronegative | A2 CMV | 4.1 | 82.0 | 25* | 12* | 7* | — |
| HD2 | Seronegative | A2 CMV | 0.59 | 2.5 | 0.3 | 0 | 0 | — |
| HD3 | Seronegative | A2 EBV | 0.05 | 0.0 | 0 | 0 | 0 | — |
| Subject . | HIV status . | Tetramer . | Tetramer-positive cells . | Specific cytotoxicity at E/T ratio, % . | ||||
|---|---|---|---|---|---|---|---|---|
| CD8 T cells, % . | Perforin+, % . | 200:1 . | 100:1 . | 50:1 . | 25:1 . | |||
| 307 | Seropositive | A2 CMV | 0.96 | 25.0 | 7 | 3 | 0 | — |
| 501 | Seropositive | A2 CMV | 2.47 | 35.0 | — | 30* | 11* | 7* |
| CW16 | Seropositive | A2 EBV | 0.76 | 71.0 | — | 2 | 2 | 0 |
| CW20 | Seropositive | B8 EBV | 1.04 | 0.0 | 0 | 0 | 2 | 0 |
| HD1 | Seronegative | A2 CMV | 4.1 | 82.0 | 25* | 12* | 7* | — |
| HD2 | Seronegative | A2 CMV | 0.59 | 2.5 | 0.3 | 0 | 0 | — |
| HD3 | Seronegative | A2 EBV | 0.05 | 0.0 | 0 | 0 | 0 | — |
PBMCs were tested directly for cytotoxicity against autologous B-cell lines incubated with tetrameric peptide or media. The frequencies of tetramer-positive cells before and after selection are shown, together with the percentages of tetramer-positive cells that stain for perforin. *Percent specific cytotoxicity is greater than 5%.
EBV and CMV peptide-specific cytotoxicity in HIV-infected and healthy donors requires perforin expression on tetramer-positive CD8 T cells: testing of immunomagnetically enriched tetramer-positive cells
| Subject . | HIV status . | Tetramer . | Tetramer-positive CD8 T cells, % . | Perforin+, % . | Specific cytotoxicity after selection . | |||
|---|---|---|---|---|---|---|---|---|
| Before selection . | After selection . | E/T ratio . | Tetramer E/T ratio . | % . | ||||
| 307 | Seropositive | A2 CMV | 0.96 | 79.6 | 25 | 2:1 | 1.6:1 | 15* |
| 501 | Seropositive | A2 CMV | 2.47 | 61.2 | 35 | 4.5:1 | 2.8:1 | 16* |
| CW16 | Seropositive | A2 EBV | 0.76 | 16.2 | 71 | 10:1 | 1.6:1 | 1.6 |
| HD2 | Seronegative | A2 CMV | 0.59 | 18.0 | 2.5 | 10:1 | 1.8:1 | 2.9 |
| HD3 | Seronegative | A2 EBV | 0.05 | 59.9 | 0 | 0.6:1 | 0.4:1 | 2.8 |
| Subject . | HIV status . | Tetramer . | Tetramer-positive CD8 T cells, % . | Perforin+, % . | Specific cytotoxicity after selection . | |||
|---|---|---|---|---|---|---|---|---|
| Before selection . | After selection . | E/T ratio . | Tetramer E/T ratio . | % . | ||||
| 307 | Seropositive | A2 CMV | 0.96 | 79.6 | 25 | 2:1 | 1.6:1 | 15* |
| 501 | Seropositive | A2 CMV | 2.47 | 61.2 | 35 | 4.5:1 | 2.8:1 | 16* |
| CW16 | Seropositive | A2 EBV | 0.76 | 16.2 | 71 | 10:1 | 1.6:1 | 1.6 |
| HD2 | Seronegative | A2 CMV | 0.59 | 18.0 | 2.5 | 10:1 | 1.8:1 | 2.9 |
| HD3 | Seronegative | A2 EBV | 0.05 | 59.9 | 0 | 0.6:1 | 0.4:1 | 2.8 |
PBMCs were tested after immunomagnetic enrichment for tetramer-positive cells for cytotoxicity against autologous B-cell lines incubated with tetrameric peptide or media. The frequencies of tetramer-positive cells before and after selection are shown, together with the percentages of tetramer-positive cells that stain for perforin. Tetramer E/T ratio gives the ratio of peptide-specific cells to peptide-loaded targets, using the frequency of tetramer-positive cells after enrichment. *Percent specific cytotoxicity is greater than 5%.
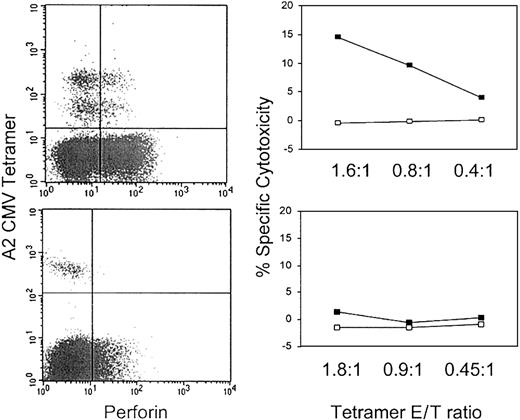
Because the frequency of tetramer-positive cells was low (0.05%-4.1% of total PBMCs), we also verified these results by testing cytotoxicity after immunomagnetic enrichment of tetramer-positive cells in 5 samples (Tables 5, 6). Representative flow cytometry plots and cytotoxicity assays for one sample with perforin-staining tetramer cells and one sample without are shown in Figure5. After enrichment, 16% to 80% of the cells were tetramer positive. Results for total PBMCs were verified in the samples enriched for tetramer-positive cells tested at lower E/T ratios. Only samples with at least 25% of tetramer cells expressing perforin were capable of significant levels of antigen-specific cytotoxicity. The sample from CW16 was again unable to lyse specific targets. Therefore, as expected, perforin is required for cytotoxic function. Results for sample CW16, however, suggest that perforin staining may not be sufficient for cytotoxicity.
Perforin is required for specific cytotoxicity.
Representative perforin and CMV A2 tetramer dot plots and cytotoxicity assays against CMV A2-restricted peptide (NLVPMVATL)–loaded cells in samples enriched for tetramer-positive cells. In the top row, 25% of CMV tetramer-positive cells from HIV-seropositive donor 307 PBMCs stain for perforin, whereas in the bottom row only 2.5% of cells reacting with the same tetramer from healthy donor HD2 are perforin positive. On the right, cytotoxicity assays were performed after PBMCs were enriched for tetramer-positive cells (Tables 4-5). The ratio of tetramer-positive cells to target cells is shown on the x-axis of the cytotoxicity graphs. Only the sample with perforin-staining, tetramer-positive cells has significant cytotoxic activity against peptide-loaded cells (▪) compared with control cells (■).
Perforin is required for specific cytotoxicity.
Representative perforin and CMV A2 tetramer dot plots and cytotoxicity assays against CMV A2-restricted peptide (NLVPMVATL)–loaded cells in samples enriched for tetramer-positive cells. In the top row, 25% of CMV tetramer-positive cells from HIV-seropositive donor 307 PBMCs stain for perforin, whereas in the bottom row only 2.5% of cells reacting with the same tetramer from healthy donor HD2 are perforin positive. On the right, cytotoxicity assays were performed after PBMCs were enriched for tetramer-positive cells (Tables 4-5). The ratio of tetramer-positive cells to target cells is shown on the x-axis of the cytotoxicity graphs. Only the sample with perforin-staining, tetramer-positive cells has significant cytotoxic activity against peptide-loaded cells (▪) compared with control cells (■).
Discussion
In this study most cells specific for the chronic viruses HIV, EBV, and CMV in nearly all HIV-infected donors and healthy donors do not express high levels of perforin, the key determinant of immediate cytotoxic function. The low frequency of high perforin expression by chronic virus-specific CD8 T cells is especially striking because approximately one quarter of CD8 T cells are perforin positive in HIV-infected patients across the disease spectrum.12 We investigated whether low perforin expression might be an artifact of protein secretion or cytotoxic granule exocytosis during cell processing and staining or whether high protein turnover caused by proteolytic degradation might account for the low levels of perforin staining. We did not find any of these to be the case. Further, ex vivo cytotoxicity correlates with high perforin expression. When more than 25% of tetramer-positive cells express perforin, specific cytotoxicity is readily detectable; when the frequency is lower, as it is in most samples, specific cytotoxicity is not much above background, even when assays are performed on populations enriched for tetramer reactivity. Thus, low perforin expression by EBV- and CMV-specific CD8 T cells from most healthy and HIV-seropositive persons suggests that, as has been shown for HIV-specific CD8 T cells, the cytotoxic function of EBV- and CMV-specific cells tested directly without in vitro culture is also limited in many donors. These results imply that in patients with chronic antigen exposure, CD8 T-cell cytotoxicity, and possibly other functions, is tightly regulated so that most cells that have seen antigen repeatedly are not cytotoxic. This may protect from damage that could arise by CTL recognition and destruction of uninfected host cells displaying weakly reacting self-peptides. Therefore, lack of specific cytotoxicity is not peculiar to HIV-specific cells and is not restricted to HIV-infected donors.
Our results differ from those of Appay et al,13 who found that HIV-specific CD8 T cells were specifically impaired in perforin expression and cytotoxic function compared with CMV-specific cells. However, a more recent paper17 by this group, published after this submission, suggests that perforin expression among CD8 T cells responding to 4 persistent infections (EBV, CMV, HCV, and HIV) may not be that dissimilar. Apparent differences between studies might be explained by the inclusion of a few patients with recent infection, which might be missed because the primary infection with these viruses can be subclinical. This might explain the occasional donor with a high frequency of perforin-staining antiviral cells. Future studies of viral antibody status may determine whether this is the case. Alternatively, occasional patients with exceptional control of viral production may respond to a burst in viral replication, as they do to a cleared infection. Understanding the reasons for this wide variation in perforin staining will be important for understanding what regulates cytotoxic function.
These results suggest that the best chance for controlling an infection is early, presumably when CD8 T cells highly express perforin and can eliminate infected cells. During that time, CD8 T cells contribute substantially to controlling the total body viral burden, as has been demonstrated conclusively in SIV-infected macaques.6,7 Our data support the notion that later, during the chronic phase, their ability to control viral production is more limited. This is also supported by a study of perforin expression in lymphoid tissues of HIV-infected patients in which the proportion of CD8 T cells expressing perforin within 4 to 5 months of symptomatic primary HIV infection was significantly higher than in chronic infection (0.3%-1.5% of all lymphoid cells in recent infection vs mean of less than 0.1% in chronic infection).10 In another study in macaques infected with pathogenic SIV, most SIV tetramer-positive cells produced IFN-γ after infection, but not 6 months later.35 Mouse models also suggest that CD8 T cells do not sustain effector CTL function within a few weeks of persistent infection.36-38 These results underline the importance of the early immune response in defining the viral set-point and the eventual course of infection. In fact, in patients with recent acute infection, the expansion of activated CD8 T cells that are CD57+ or CD45RA+CD62L− correlates inversely with the viral set-point.39
The low frequency of perforin expression is unlikely to be secondary to recent degranulation because one would then expect both perforin and GzmA to be depleted. However, GzmA expression is high. In fact our studies of perforin and GzmA staining after blocking protein synthesis suggest that GzmA is continuously synthesized and degraded in CTLs. Nonetheless, it remains possible that perforin expression might be so tightly regulated, in a manner analogous to cytokines,40that constitutive expression is low and cells require antigenic stimulation for it to be readily detectable.
When interpreting these data, it is also important to bear in mind that, at least in murine models, low levels of perforin expression are adequate for CTL cytotoxicity.41 Although in this study lack of cytotoxicity correlates with low expression of perforin, sufficient perforin for cytotoxic function may still be expressed in tetramer-positive cells but may be below the sensitivity level of flow cytometry detection. In that case, the lack of cytotoxic function in perforin low cells might be caused by other factors, such as defective signaling of cytotoxicity by the T cell.20 42
Because EBV- and CMV-specific CD8 T cells in healthy donors also generally lack perforin and are not cytotoxic, CD4 T-cell depletion may not be the underlying reason for CD8 T-cell dysfunction during chronic HIV infection. However, this does not mean that CD4 T cells are not important. Although CD4 cell proliferative responses to CMV can be measured in many chronically infected healthy donors, many antigen-specific CD4 T cells may still be partially anergized. Many CMV-reactive CD4 cells in HIV infection are activated to produce IFN-γ, yet they fail to produce IL-2, which is required to induce perforin expression.43,44 CD8 HIV- or EBV-specific cytotoxicity in samples from patients with less advanced disease or from healthy donors can be restored in vitro after overnight exposure to high, but not low, concentrations of IL-2 or other costimulatory signals.19-21,45,46 This is presumably because of well-described signaling defects in these cells that result in an absence of high affinity IL-2 receptor (CD25) induction on activation.46 Based on preliminary in vitro evidence (D.Z. and J.L., unpublished observation, 2000), we speculate that a costimulatory signal induced by IL-2 receptor engagement is required for high perforin expression. This implies that restimulation of CD8 T cells to become effector CTLs may require high local concentrations of IL-2—only present if an antigen-specific, nonanergized CD4 T cell is also at the site of re-encounter with antigen on an infected cell.
CD4 lymphoproliferative responses to HIV p24 (which correlate with IL-2 production) have been suggested to be associated with improved disease prognosis primarily because they lead to an increase in CD8 T-cell function.47-50 Although this is an attractive hypothesis, data presented here suggest that this must be examined more closely. Because most HAART-treated patients and healthy donors have lymphoproliferative responses to CMV antigens, one would anticipate that their CMV-specific CD8 T cells would have higher levels of perforin. However, this is not the case. Moreover, the CD8 T cells from the long-term asymptomatic HIV-infected subjects in this study did not have higher proportions of perforin expression (data not shown). Looking at perforin expression and CTL function in long-term nonprogressors (LTNPs) and patients with CD4 proliferative responses to p24 merits further study.
Perforin expression is the defining feature of terminally differentiated effector CD8 T cells. Although other phenotypic changes, such as CD27 down-modulation and expression of GzmA and CD45RA, may often accompany differentiation into effector CTLs, our findings here suggest that antigenic stimulation conditions for different viral infections may alter the differentiation program. EBV- and CMV-specific CD8 T cells in HIV-infected donors are more likely than HIV-specific cells to be CD27−, CD45RA+, and GzmA+. This may indicate the existence of subpopulations of CD8 T cells at different stages of readiness to become perforin-expressing CTLs. This is consistent with the heterogeneous expression of CD45RA and the lymph-node–homing receptor CCR7 by antigen-experienced CD8 T cells.12,17 34 Our results suggest that in patients with chronic infection, the memory pool of antiviral CD8 T cells is not homogeneous but contains subpopulations with distinct phenotypes and functional capabilities that reflect each cell's history of exposure to antigenic, costimulatory, and inhibitory signals.
In this study, CD27 down-modulation was the phenotypic change that correlated most closely with perforin expression. However, in HIV-infected donors, one fifth of perforin-positive cells were CD27+. CD45RA expression was not a good indicator of perforin expression in healthy donors or HIV-seropositive donors. Phenotypic properties of effector CTLs in healthy donors or in patients with cleared infection cannot be extrapolated to the more complex state of persistent infection.
Differences in expression of CD8 differentiation molecules in cells responding to different viruses or between HIV-infected and healthy donors (Figure 2; Table 3) may also reflect differences in the likelihood of recent encounters with cells actively replicating virus. For the markers we studied, the EBV-specific cells in HIV-infected donors are intermediate in activation phenotype between the HIV- and CMV-tetramer populations. This suggests that CMV replication may be more active than EBV replication in HIV-infected donors. In healthy donors, most EBV- and CMV-specific cells have a memory phenotype, suggesting that they have not recently encountered antigen. In HIV-infected donors, higher proportions of EBV- and CMV-specific cells have down-modulated CD27, and there is also a trend toward more expression of GzmA and CD45RA. Therefore, the cells specific for these viruses appear to have been more recently activated in HIV-infected patients than in healthy donors. This is probably because they have more recently encountered antigen, as is implied by higher frequencies of cells infected with these herpesviruses in immunosuppressed persons.51 52
The decrease in CD27 down-modulation and CD45RA re-expression on HIV tetramer-positive CD8 T cells compared with EBV- and CMV-specific cells, even in patients with uncontrolled viral replication, could indicate that HIV antigen presentation or T-cell recognition may be particularly impaired in HIV infection.13 HIV-specific CD8 T cells may look like memory cells because they have not recently recognized, or been stimulated by, an infected cell. This may be because of viral mutation or nef-mediated down-modulation of major histocompatibility complex class 1 or because of other unknown viral effects on antigen presentation or of impaired signaling by the T cell itself.20,42,53 54
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-03-0791.
Supported by National Institutes of Health (NIH) grants AI-42519 and AI-45406 (J.L.); and the NIH-funded Dana Farber Cancer Institute–Beth Israel Deaconess Medical Center–Children's Hospital Center for AIDS Research (AI28691) and Case Western Reserve University Center for AIDS Research (AI36219).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Judy Lieberman, Center for Blood Research, Harvard Medical School, 800 Huntington Ave, Boston, MA 02115; e-mail: lieberman@cbr.med.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal