Abstract
Interleukin-2 (IL-2) prevents cell apoptosis and promotes survival, but the involved mechanisms have not been completely defined. Although phosphatidylinositide 3-kinase (PI 3-kinase) has been implicated in IL-2–mediated survival mechanisms, none of the 3 chains of the IL-2 receptor (IL-2R) expresses a binding site for PI 3-kinase. However, IL-2Rβ does express a Syk-binding motif. By using an IL-2–dependent natural killer (NK) cell line, followed by validation of the results in fresh human NK cells, we identified Syk as a critical effector essential for IL-2–mediated prosurvival signaling in NK cells. Down-regulation of Syk by piceatannol treatment impaired NK cellular viability and induced prominent apoptosis as effectively as suppression of PI 3-kinase function by LY294002. Expression of kinase-deficient Syk or pretreatment with piceatannol markedly suppressed IL-2–stimulated activation of PI 3-kinase and Akt, demonstrating that Syk is upstream of PI 3-kinase and Akt. However, constitutively active PI 3-kinase reversed this loss of Akt function caused by kinase-deficient Syk or piceatannol. Thus, Syk appears to regulate PI 3-kinase, which controls Akt activity during IL-2 stimulation. More important, we observed Rac1 activation by IL-2 and found that it mediated PI 3-kinase activation of Akt. This conclusion came from experiments in which dominant-negative Rac1 significantly decreased IL-2–induced Akt activation, whereas constitutively active Rac1 reelevated Akt activity not only in Syk-impaired but also in PI 3-kinase–impaired NK cells. These results constitute the first report of a Syk → PI3K → Rac1 → Akt signal cascade controlled by IL-2 that mediates NK cell survival.
Introduction
Interleukin-2 (IL-2) plays a critical role in the regulation of immune responses. It functions as a growth factor for natural killer (NK) and T cells and promotes cell survival, thus affecting the amplification of an immune response against an antigen. The IL-2 receptor (IL-2R) comprises a heterotrimeric complex consisting of a high-affinity cytokine-binding subunit, IL-2Rα, which associates with IL-2Rβ, and the common subunit IL-2Rγ, which is shared by other cytokine receptors including IL-4, IL-7, IL-9, and IL-15.1 The IL-2–IL-2R interaction triggers a multitude of signaling molecules, beginning with protein tyrosine kinases.1 The Src family kinases Lck, Fyn, and Lyn and the Janus kinases (Jaks) closely associate with IL-2R.2-6 Jaks have been well documented for initiating signaling of IL-2. The current concept is that IL-2–activated Jaks recruit critical Src homology 2 (SH2)–containing signaling mediators, leading to several cascades—including signal transducer and activator of transcription (STAT), Ras/Raf/MEK/mitogen-activated protein kinase (MAPK), and PI 3-kinase.7-10 These events then regulate the function of key transcription factors and regulators implicated in cell proliferation and differentiation.1,11 12
Despite the insight into the IL-2 signaling pathways that trigger activation, those that promote cell survival in lymphocytes have not been completely elucidated. The PI 3-kinase/Akt pathway promoting survival has been implicated in IL-2–activated T cells and BAF/3 cells.13,14 There is no direct binding site for PI 3-kinase in the IL-2R, yet PI 3-kinase is readily activated by IL-2.15 Thus, the activation of PI 3-kinase must rely on other upstream effector(s) that associate with the IL-2R. In searching for molecules that would fit this function, we narrowed in on Syk, which has long been known to directly bind IL-2Rβ.16,17 Although much investigation regarding Syk has been in B cells, monocytes, and mast cells, primarily delineating its key role in activating the phospholipase C γ (PLC-γ) and the Ras/MAPK pathways,18-20 some reports do suggest that Syk is involved in PI 3-kinase activation—for example, in oxidative stress- and insulin-induced cell survival21,22and αIIbβ3 integrin signaling in platelet/megakaryocyte cells.23 To critically address whether a specific Syk-directed pathway essentially dictates IL-2 survival signaling, we used an IL-2–dependent NK cell line and freshly isolated human large granule lymphocytes (LGLs) and examined whether Syk links IL-2 to cell survival. We also attempted to identify what the downstream signal components might be. Here we demonstrated that Syk and PI 3-kinase are both required for IL-2–mediated NK survival and made the important discovery that Syk is one major upstream effector for PI 3-kinase/Akt in IL-2 signaling. Another important finding is the critical role of Rac1 in mediating PI 3-kinase activation of Akt.
Materials and methods
Cell culture, antibodies, and reagents
NK92 cells, provided by Dr H. G. Klingeman (Terry Fox Laboratory, Vancouver, BC, Canada), were maintained in α-minimum essential medium (MEM) supplemented with 20% fetal calf serum, 100 U/mL IL-2, and 5 × 10−5 M 2-mercaptoethanol. LGLs with high NK activity were freshly isolated from healthy donor blood by Percoll gradient centrifugation and were cultured in IL-2 as described.24 Highly enriched LGLs of 85% to 90% purity were routinely obtained.
Mouse monoclonal antibodies (mAbs) to Syk and PI 3-kinase were from Upstate Biotechnology (Lake Placid, NY). Rabbit antibodies to Syk and PI 3-kinase were from Santa Cruz Biochemicals (Santa Cruz, CA). Rabbit antiphospho-Akt (Ser473) and pan-Akt were from Cell Signaling Technology (Beverly, MA). Piceatannol, wortmannin, and LY294002 were purchased from Calbiochem (La Jolla, CA).
Apoptosis assay
NK92 and LGL cells were cultured in complete medium containing IL-2 at a density of 3 × 105 cells/mL in the absence or presence of 25 μM piceatannol or LY294002. The cells were collected at 0, 12, 24, and 48 hours, washed in wash buffer, and stained with annexin V–fluorescein isothiocyanate (FITC) in combination with propidium iodide according to the manufacturer's recommendation (PharMingen, San Diego, CA). Cells stained positively by flow cytometry for annexin V–FITC were considered apoptotic. These experiments were performed in triplicate.
Assessment of apoptotic cell morphology and DNA fragmentation
NK cells, cultured in complete medium containing IL-2, were treated with 25 μM piceatannol or LY294002 for 24 or 48 hours. Cells were resuspended at a concentration of 2 × 105 cells/0.1 mL, and cytospins were made in triplicate. The slides were then stained with modified Wright-Giemsa. Cells demonstrating nuclear condensation or nuclear bodies were considered apoptotic. Two investigators, one of whom was masked to the study, examined each slide. For DNA fragmentation, cellular DNA was prepared, analyzed on 1.4% agarose gel, and visualized by ethidium bromide staining for the presence of DNA ladders.
Vaccinia virus construction and gene delivery
The plasmid containing p85(DN), which is a dominant-negative form of the regulatory subunit of PI 3-kinase, was kindly provided by Dr Masato Kasuga (Kobe University School of Medicine, Japan). Myc-tagged p110* mutant, which acts as a constitutively active component of PI 3-kinase, was kindly provided by Dr Anke Klippel (Chiron, Emeryville, CA).25 Recombinant vaccinia virus encoding dominant-negative p85 (p85[DN]) or Myc-p110* was constructed in the pSC11 vector. Vaccinia virus expressing kinase-deficient SykT, encoding a truncated kinase domain, with a molecular weight of approximately 50 kDa, was kindly provided by Dr Andrew M. Scharenberg (Harvard Medical School, Boston, MA).26
The procedure of vaccinia virus infection has been described.27 28 Briefly, cells were incubated with recombinant vaccinia for 2 hours at 37°C at a multiplicity of infection of 5, then washed 3 times and cultured in serum-free medium containing 0.5% bovine serum albumin (BSA) for another 2 hours before activation assay. For experiments combining pharmaceutical inhibitors and viral infection, cells were pretreated for 30 minutes at 37°C with 25 μM inhibitor before infection, as described, except that the inhibitor was added back for the last 2 hours of infection before the evaluation of survival function. The virally transferred protein expression of SykT, Myc-p110, and p85(DN) was examined in the infected cells by Western blotting with specific antibodies and was detected by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ).
Immunoprecipitation and immunoblotting
Cells were lysed in a lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride, 1.5 μg/mL each aprotinin and leupeptin, 10 mM NaF, 3 mM sodium vanadate, and 15 mM glycerol phosphate. Lysates were centrifuged at 13 000g for 10 minutes at 4°C before preclearance and immunoprecipitation or Western blotting. Equal amounts of the lysates were analyzed for protein phosphorylation and enzyme activity. For immunoprecipitation, lysates were incubated with the antibody in the presence of 100 μL protein A–protein G 1:1 agarose beads (Sigma, St Louis, MO) overnight at 4°C. Immunoprecipitates were then washed 4 times with lysis buffer before activity analysis. Protein phosphorylation was examined by Western blot analysis with phosphospecific antibodies.
In vitro protein kinase assay
Kinase assays were performed as previously described.28 Briefly, after immunoprecipitation, the reaction was carried out in the presence of 10 μCi (0.37 MBq) γ-32P–adenosine triphosphate (ATP) and 5 μM unlabeled ATP in 40 μL reaction buffer containing 20 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 10 mM MnCl2, and 1 mM dithiothreitol. After incubation at room temperature for 20 minutes, the reaction was stopped and the mixture was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Relative amounts of incorporated radioactivity were determined by autoradiography and quantitated with a PhosphorImager (Molecular Dynamics, Amersham Pharmacia Biotech, Piscataway, NJ). The amount of protein immunoprecipitates in each reaction was also examined by Western blot analysis to ensure equal loading.
Detection of PI 3-kinase activities
Cells were lysed and immunoprecipitated with anti-p85 antibody overnight and incubated with protein A–protein G 1:1 agarose beads for 2 hours at 4°C. Immunoprecipitates were washed 4 times with lysis buffer and twice with kinase reaction buffer, and then they were divided into 2 equal aliquots. One aliquot was used to check for equal loading, and the other aliquot was analyzed for PI 3-kinase activity by incubating the p85 immunoprecipitates with reaction buffer containing 100 μM ATP, 10 μCi (0.37 MBq) γ-32p]–ATP, 20 μg L-phosphatidylinositol-4,5-bisphosphate (PI4,5P2), 25 mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid) (pH 7.4), and 15 mM MgCl2 for 25 minutes at 25°C. The reaction was stopped by adding 200 μL 1 M HCl. The reaction mixture was extracted with CHCl3-MeOH. Phosphorylated inositol was differentiated by thin-layer chromatography as described.29 The conversion of phosphatidylinositol (PI) 4,5-trisphosphate (PI4,5P2) to PI3,4,5P3 was detected by autoradiography and quantitated with a PhosphorImager (Molecular Dynamics).
Detection of Rac1 activity by PAK1 PBD affinity assay
Cells were lysed with Mg2+ lysis/wash buffer provided with the Rac activation kit (Upstate Biotechnology). The active form of Rac1, Rac1-guanosine triphosphate (GTP), was affinity-precipitated from the lysates by incubation with 15 μg PAK1 p21-binding protein (PBD)–linked agarose, washed 3 times with the wash buffer provided, and subjected to 12.5% SDS-PAGE. The activated Rac1 that bound with PAK1 PBD was examined by Western blotting with anti-Rac provided in the kit and was detected by enhanced chemiluminescence.
Results
Requirement for Syk in IL-2–dependent survival
Syk has been reported to associate with IL-2R in peripheral blood lymphocytes, implicating its role in c-myc gene activation and cellular proliferation.16 PI 3-kinase/Akt is also known to be involved in IL-2 antiapoptotic signaling in T and BAF/3 cells.13,14 We therefore examined whether IL-2 maintains survival through Syk or PI 3-kinase in an IL-2–dependent NK cell line. NK92 cells, cultured in complete medium containing IL-2, were treated with 25 μM piceatannol (Pic), 25 μM LY294002 (LY), or dimethyl sulfoxide (DMSO) (used as the solvent). Piceatannol is a naturally occurring plant polyphenol (trans-3,4,3,5-tetrahydroxystilbene, also known as 3-hydroxyresveratol) with tyrosine kinase inhibitory activity that selectively targets Syk.30 LY294002, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-1, is a derivative of quercetin, which specifically abolishes PI 3-kinase activity but not PI 4-kinase or other protein and lipid kinases.31 Cell viability and survival was evaluated by trypan blue exclusion every 24 hours. Medium and DMSO control cells had high viability that ranged from 94% to 99% at all time points. In contrast, interference with either Syk or PI 3-kinase significantly impaired cellular viability, which was reduced to 66%, 33%, and 19% for piceatannol and to 73%, 43%, and 27% for LY294002 at 24, 48, and 72 hours, respectively (Figure 1A).
Inhibition of IL-2–mediated survival by inactivating Syk and PI 3-kinase.
(A) NK92 cells, cultured in complete medium containing 100 U/mL IL-2, were incubated with 25 μM piceatannol or 25 μM LY294002 or DMSO vehicle control and were examined for cell viability by trypan blue exclusion at 0, 24, 48, and 72 hours. Experiments were performed in triplicate. The percentage of viable cells calculated per experiment is shown (P < .05). (B) NK92 cells, obtained from cultures described in panel A, were examined for morphologic changes at 24 hours. Experiments were performed in triplicate. The percentage of apoptotic cells and total number of cells counted per experiment are shown at the bottom of each panel. Statistical analysis using a χ2 test revealed that piceatannol and LY294002 significantly induced apoptotic morphology in NK cells (P < .05). Nuclear condensation and nuclear body (arrow) are indicated. (C) NK92 cells, obtained from cultures described in panel A, were examined for apoptosis by annexin V–FITC binding and PI uptake. Cells were collected at 24 and 48 hours in each experiment, washed in sample wash buffer, and stained with annexin V–FITC in combination with PI. Annexin V–FITC binding in piceatannol-, LY294002-, or DMSO-treated and untreated NK92 cells is shown.
Inhibition of IL-2–mediated survival by inactivating Syk and PI 3-kinase.
(A) NK92 cells, cultured in complete medium containing 100 U/mL IL-2, were incubated with 25 μM piceatannol or 25 μM LY294002 or DMSO vehicle control and were examined for cell viability by trypan blue exclusion at 0, 24, 48, and 72 hours. Experiments were performed in triplicate. The percentage of viable cells calculated per experiment is shown (P < .05). (B) NK92 cells, obtained from cultures described in panel A, were examined for morphologic changes at 24 hours. Experiments were performed in triplicate. The percentage of apoptotic cells and total number of cells counted per experiment are shown at the bottom of each panel. Statistical analysis using a χ2 test revealed that piceatannol and LY294002 significantly induced apoptotic morphology in NK cells (P < .05). Nuclear condensation and nuclear body (arrow) are indicated. (C) NK92 cells, obtained from cultures described in panel A, were examined for apoptosis by annexin V–FITC binding and PI uptake. Cells were collected at 24 and 48 hours in each experiment, washed in sample wash buffer, and stained with annexin V–FITC in combination with PI. Annexin V–FITC binding in piceatannol-, LY294002-, or DMSO-treated and untreated NK92 cells is shown.
To determine whether these cells underwent apoptosis, we examined their morphologic changes. Two typical features of apoptosis, the condensation of nuclei and the formation of nuclear bodies, were assessed. In medium and DMSO controls, only 2% to 4% of cells appeared to be apoptotic (Figure 1B). In contrast, a robust increase of nuclei condensation and nuclear bodies was observed after piceatannol or LY294002 treatment, amounting to 43% and 65%, or 38% and 57%, at 24-hour and 48-hour intervals (Figure 1B). In addition, analysis of nuclear DNA also revealed that piceatannol and LY294002 treatment induced significant internucleosomal DNA fragmentation, another indicator of apoptosis (data not shown). These data suggest that Syk and PI 3-kinase are required for sustaining NK viability.
Inactivation of Syk induces apoptosis
One of the earliest markers of apoptosis is the appearance of phosphoserine on the cell surface before DNA degradation. The role of Syk and PI 3-kinase in IL-2–mediated prosurvival and antiapoptotic signaling was thus further evaluated by flow cytometric analysis using FITC-labeled annexin V that binds phosphoserine and PI, which detect fragmented DNA. A rapid increase in the percentage of apoptotic cells was seen with piceatannol or LY294002 treatment in comparison with controls (Figure 1C). At 12, 24, and 48 hours, respectively, 29%, 47%, and 79% of IL-2–cultured NK cells showed apoptosis when treated with piceatannol, whereas 26%, 43%, and 71% of IL-2–cultured and LY294002-treated cells were apoptotic (P < .05). The slight difference between these annexin V results and those from morphologic examination (Figure 1B) might stem from the fact that morphologic detection of phosphoserine has a higher sensitivity than microscopic observation. These data strongly support the involvement of Syk and PI 3-kinase in IL-2–mediated cell survival and delay of apoptosis.
Inhibition of Syk activation by piceatannol and SykT
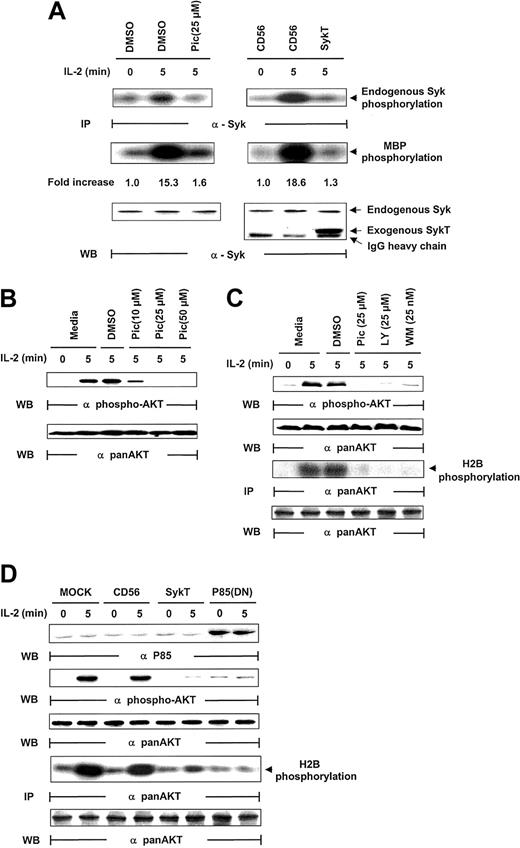
Given that piceatannol reduced NK survival and induced apoptosis, we analyzed whether IL-2 activated Syk in NK cells. Following IL-2 stimulation (100 U/mL), Syk was immunoprecipitated from NK cells and evaluated for kinase activity through analyses of Syk autophosphorylation and Syk phosphorylation of myelin basic protein (MBP) as the in vitro kinase assay substrate. Syk was activated within 5 minutes of IL-2 exposure, as demonstrated by Syk phosphorylation of MBP and of Syk itself, which was markedly suppressed by piceatannol treatment (Figure 2A). For further proof that Syk is activated by IL-2, we introduced kinase-deficient Syk, SykT,26 or an irrelevant control, CD56, into NK92 cells by way of vaccinia virus–mediated gene transfer. SykT, but not CD56, significantly suppressed Syk activation by IL-2 (Figure 2A). Western blotting of the same filter showed an equal amount of endogenous Syk immunoprecipitated from each sample and a significant level of virally expressed truncated SykT26 at a molecular weight of approximately 50 kDa (Figure 2A, bottom).
Inhibition of IL-2–induced Akt function by inactivating Syk.
(A) IL-2–starved NK92 cells were treated with piceatannol or DMSO or infected with recombinant vaccinia virus encoding SykT or CD56 irrelevant control gene. Cells were then stimulated with IL-2 and analyzed for Syk protein kinase activation. The same membrane was probed with anti-Syk to check for equal loading. (B) NK92 cells were treated with 10, 25, or 50 μM piceatannol or DMSO were stimulated with IL-2 and analyzed for Akt phosphorylation with Ser473-specific antibody. The same membrane was stripped and reprobed with anti–pan-Akt to check for equal loading. (C) NK92 cells treated with piceatannol, LY294002, wortmannin, or DMSO were stimulated with IL-2 before Western blot analysis of Akt phosphorylation Akt kinase activation with histone H2B as the substrate. The same membrane was probed with anti–pan-Akt to check for equal loading. (D) NK92 cells infected with recombinant vaccinia virus encoding SykT, p85(DN), or CD56 irrelevant control gene were stimulated with IL-2 and analyzed for Akt phosphorylation and Akt kinase activation. The same membrane was stripped and reprobed with anti–pan-Akt. Results are representative of 1 of 4 independent experiments.
Inhibition of IL-2–induced Akt function by inactivating Syk.
(A) IL-2–starved NK92 cells were treated with piceatannol or DMSO or infected with recombinant vaccinia virus encoding SykT or CD56 irrelevant control gene. Cells were then stimulated with IL-2 and analyzed for Syk protein kinase activation. The same membrane was probed with anti-Syk to check for equal loading. (B) NK92 cells were treated with 10, 25, or 50 μM piceatannol or DMSO were stimulated with IL-2 and analyzed for Akt phosphorylation with Ser473-specific antibody. The same membrane was stripped and reprobed with anti–pan-Akt to check for equal loading. (C) NK92 cells treated with piceatannol, LY294002, wortmannin, or DMSO were stimulated with IL-2 before Western blot analysis of Akt phosphorylation Akt kinase activation with histone H2B as the substrate. The same membrane was probed with anti–pan-Akt to check for equal loading. (D) NK92 cells infected with recombinant vaccinia virus encoding SykT, p85(DN), or CD56 irrelevant control gene were stimulated with IL-2 and analyzed for Akt phosphorylation and Akt kinase activation. The same membrane was stripped and reprobed with anti–pan-Akt. Results are representative of 1 of 4 independent experiments.
Suppressive effects of inactivation of Syk on IL-2–induced Akt function
The PI 3-kinase/Akt pathway is activated by IL-2R triggering in lymphocytes, despite the fact that IL-2R has no binding capacity for them.7,8,14,32 33 We thus investigated whether Syk, which does directly bind IL-2Rβ, could be involved in PI3-kinase/Akt activation by IL-2. In analyzing Akt activation by its phosphorylation at Ser437, we found that piceatannol pretreatment for 30 minutes sharply reduced Akt phosphorylation in IL-2–triggered NK92 cells, even with the lowest concentration of piceatannol at 10 μM (Figure 2B). These data suggest that Syk is involved in the IL-2 regulation of Akt.
Similar effects of Syk and PI 3-kinase inhibitors on Akt
We next examined whether LY294002 and wortmannin had the same effects as piceatannol on Akt activation in IL-2–triggered NK cells. Wortmannin is a fungal metabolite that specifically inactivates PI 3-kinase by modifying Lys802, a residue required in the phosphate transfer reaction in the ATP-binding site.34 Inhibitors of Syk and PI 3-kinase were equally effective in suppressing IL-2–induced Akt phosphorylation (Figure 2C). To check that Akt function, and not simply Akt phosphorylation, was affected, the same lysates were immunoprecipitated with anti–pan-Akt and were analyzed for kinase activity using histone 2B (H2B) as a substrate. The results verified that Akt kinase function was also lost by piceatannol, LY294002, or wortmannin treatment in IL-2–stimulated NK92 cells (Figure 2C). These results strongly support our proposal that Syk regulates Akt in IL-2 signaling.
Inhibition of IL-2–induced Akt activation by function-deficient Syk
To further explore the contribution of Syk to IL-2–induced Akt activation, we introduced function-deficient SykT or p85(DN) into NK92 cells before IL-2 stimulation. SykT and p85(DN) remarkably suppressed IL-2–induced Akt phosphorylation (Figure 2D). Correspondingly, in vitro kinase assays showed similar impairment of IL-2–stimulated Akt kinase by SykT and p85(DN) (Figure 2D).
Syk control of IL-2–stimulated PI 3-kinase activation
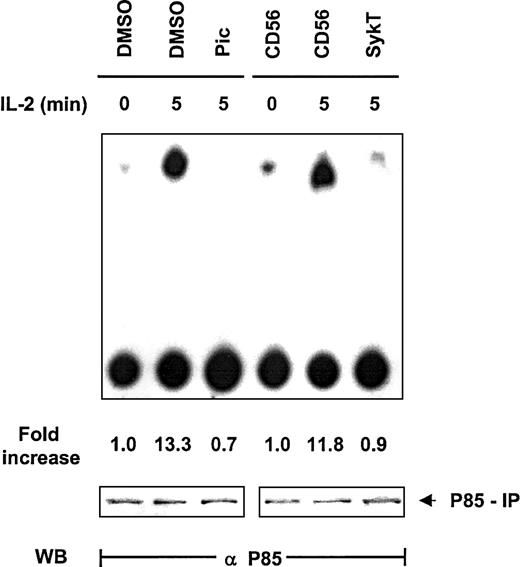
Thus far, our data clearly revealed that Syk and PI 3-kinase are the critical modulators located upstream of Akt in IL-2 survival signaling but gave no clue to whether they operated within the same pathway to control Akt. Based on information from other systems in which Syk regulates PI 3-kinase, we explored the impact of Syk on PI 3-kinase in IL-2 signaling. NK92 cells were evaluated for PI 3-kinase–mediated inositol kinase activity by in vitro kinase assay with phosphatidylinositol3 4 diphosphate (PI3,4P2) as the substrate and by thin-layer chromatography. Within 5 minutes of IL-2 stimulation, PI 3-kinase immunoprecipitated by anti-p85 from NK92 cells displayed high capacity to produce PI3,4,5P3 (Figure3). Piceatannol pretreatment substantially reduced this capacity, as did the expression of SykT. In contrast, CD56 expression, as a control, did not interfere with IL-2–stimulated PI 3-kinase activation (Figure 3). Western blot analysis of the p85 immunoprecipitates indicated equal loading of all samples. Thus, Syk appeared to control PI 3-kinase activation in IL-2 signaling.
Suppression of IL-2–induced PI 3-kinase activation by inhibiting Syk.
IL-2–starved NK92 cells, treated with 25 μM piceatannol or infected with recombinant vaccinia virus encoding SykT or CD56 irrelevant control gene, were stimulated with IL-2 (100 U/mL) for 5 minutes at 37°C. Cells were then analyzed for PI 3-kinase activity by in vitro kinase assay and thin-layer chromatography analysis with PI3,4P2 as the substrate. Results are representative of 1 of 3 independent experiments.
Suppression of IL-2–induced PI 3-kinase activation by inhibiting Syk.
IL-2–starved NK92 cells, treated with 25 μM piceatannol or infected with recombinant vaccinia virus encoding SykT or CD56 irrelevant control gene, were stimulated with IL-2 (100 U/mL) for 5 minutes at 37°C. Cells were then analyzed for PI 3-kinase activity by in vitro kinase assay and thin-layer chromatography analysis with PI3,4P2 as the substrate. Results are representative of 1 of 3 independent experiments.
Reversal of Syk inhibition of Akt by constitutively active PI 3-kinase
As additional proof that Syk regulates PI 3-kinase/Akt, we resorted to Myc-p110*, a constitutively active mutant of the p110 subunit of PI 3-kinase.25 We reasoned that if PI 3-kinase lies downstream of Syk for Akt activation, p110* expression in Syk-inactivated cells should restore Akt activation triggered by IL-2. We therefore constructed the vaccinia virus encoding Myc-p110* and examined whether piceatannol-mediated Akt suppression could be overcome by vaccinia virus–transferred p110*. NK92 cells were pretreated with 25 μM piceatannol and then infected with vaccinia virus encoding Myc-p110* or CD56 before IL-2 engagement. Virally transduced Myc-p110* was readily expressed in the cells (Figure4A, top panel). IL-2 elicited Akt phosphorylation in medium and DMSO + CD56 control groups. As noted, piceatannol impaired IL-2–induced Akt activation. However, this impairment was markedly reversed by the expression of Myc-p110*, whereas Myc-p110* also elevated the basal level of Akt phosphorylation (Figure 4A, middle panel). Western blot analysis confirmed the equal loading of Akt (Figure 4A, bottom panel).
Rescue of IL-2–induced Akt activation in Syk-impaired cells by constitutively active PI 3-kinase.
(A) IL-2–starved NK92 cells, treated with 25 μM piceatannol or DMSO, were infected with recombinant vaccinia virus encoding P110*, the constitutively active catalytic subunit of PI 3-kinase, or CD56 irrelevant control gene and then were stimulated with IL-2 (100 U/mL) for 5 minutes at 37°C. Cells were analyzed for Akt activation by Western blot analysis with Ser473-specific antibodies. (B) Cells were treated similarly to those in panel A, and whole-cell lysates were analyzed by in vitro kinase assays for Akt activation with H2B as the substrate. The same membranes were stripped and reprobed with anti–pan-Akt. Results are representative of 1 of 4 independent experiments.
Rescue of IL-2–induced Akt activation in Syk-impaired cells by constitutively active PI 3-kinase.
(A) IL-2–starved NK92 cells, treated with 25 μM piceatannol or DMSO, were infected with recombinant vaccinia virus encoding P110*, the constitutively active catalytic subunit of PI 3-kinase, or CD56 irrelevant control gene and then were stimulated with IL-2 (100 U/mL) for 5 minutes at 37°C. Cells were analyzed for Akt activation by Western blot analysis with Ser473-specific antibodies. (B) Cells were treated similarly to those in panel A, and whole-cell lysates were analyzed by in vitro kinase assays for Akt activation with H2B as the substrate. The same membranes were stripped and reprobed with anti–pan-Akt. Results are representative of 1 of 4 independent experiments.
Analyses of Akt kinase activity, with anti-Akt immunoprecipitates using H2B as a substrate, demonstrated a similar pattern. IL-2 sharply induced Akt function in all control groups—medium, DMSO, and CD56 (Figure 4B). SykT effectively blocked Akt kinase function, as expected. Piceatannol + CD56-treated cells showed similarly reduced Akt activation; however, piceatannol + Myc-p110*-treated cells showed high Akt kinase activity, comparable to those of the control groups (Figure 4B), indicating that Myc-p110* overcomes the inhibition by piceatannol. These results reconfirm that PI 3-kinase and Akt are downstream of Syk in IL-2 signaling.
Regulation of IL-2–induced Akt activation by Rac1
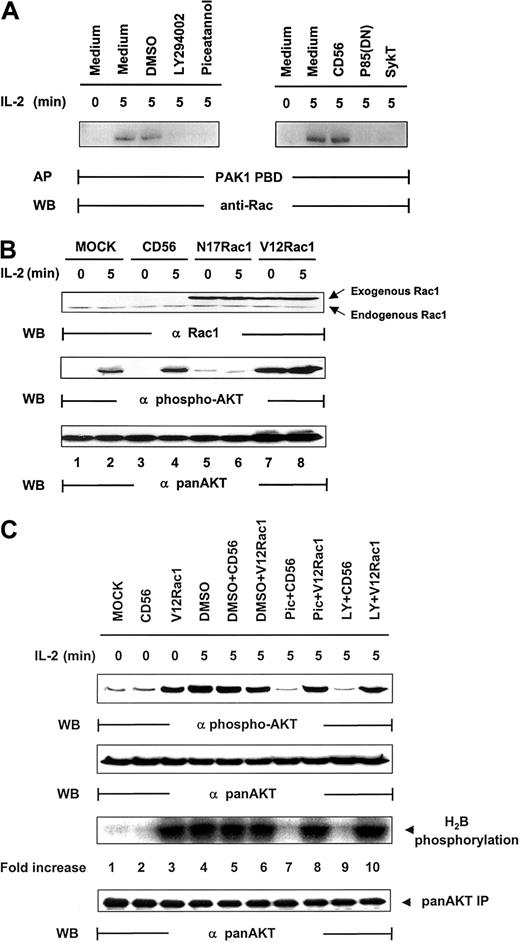
Rac1 has been coupled to the mediation of PI 3-kinase signaling in various functions, including cell mobility, actin rearrangement, and membrane ruffling.35-37 Upstream of PI 3-kinase, it has also been implicated in the regulation of Akt activation after T-cell receptor (TCR) engagement.38 We therefore sought to determine whether Rac1 was involved in Akt activation in IL-2 signaling. Rac1 was significantly activated by IL-2, and this activation could be blocked by SykT or piceatannol or by p85(DN) or LY294002 (Figure 5A). Thus, Syk and PI 3-kinase appear to control IL-2–stimulated Rac1 activation. We then expressed mutant Rac1 in the cells and examined their effects on IL-2–induced Akt activation. When dominant-negative Rac1, N17Rac1, was introduced into NK92 cells, IL-2 could no longer activate Akt phosphorylation (Figure 5B). In contrast, constitutively active Rac1, V12Rac1, markedly elevated Akt phosphorylation, even in the absence of IL-2. Thus, Rac1, like Syk and PI 3-kinase, is required for IL-2–induced Akt function.
Regulation of IL-2–mediated Akt activation by Rac1.
(A) IL-2–starved NK92 cells, pretreated by LY294002 (25 μM), piceatannol (25 μM), or DMSO control or infected with recombinant vaccinia virus encoding p85(DN), kinase-deficient Syk (SykT), or CD56 irrelevant control gene, were stimulated with IL-2 (100 U/mL) for 5 minutes at 37°C and analyzed for IL-2–triggered Rac1 activation before and after the impairment of Syk function. Rac1 was immunoprecipitated from NK92 cell lysates and examined for activity by affinity precipitation (AP) with PAK1 PBD, which binds only to activated Rac1-guanosine triphosphate (GTP) but not to inactivated Rac1-guanosine diphosphate (GDP). The IL-2–activated Rac1, Rac1-GTP, was precipitated by PAK1 PBD agarose, then resolved by 12.5% SDS-PAGE and examined by anti-Rac monoclonal antibody provided in the kit. (B) IL-2–starved NK92 cells, infected with recombinant vaccinia virus encoding dominant-negative Rac1 (N17Rac1), constitutively active Rac1 (V12Rac1), or CD56 irrelevant control gene, were stimulated with IL-2 (100 U/mL) for 5 minutes at 37°C. Cells were analyzed for Akt activation by Western blot (WB) analysis. (C) IL-2–starved NK92 cells, treated with 25 μM piceatannol or 25 μM LY294002 or DMSO, were infected with recombinant vaccinia virus encoding constitutively active V12Rac1 or CD56 irrelevant control gene, then stimulated with IL-2 (100 U/mL) for 5 minutes at 37°C. Cells were analyzed for Akt activation by Western blot analysis with Ser473-specific antibody. The same membranes were stripped and reprobed with anti–pan-Akt. In parallel, these NK cells were also analyzed by in vitro kinase assays for Akt activation with H2B as the substrate. Results are representative of 1 of 4 independent experiments.
Regulation of IL-2–mediated Akt activation by Rac1.
(A) IL-2–starved NK92 cells, pretreated by LY294002 (25 μM), piceatannol (25 μM), or DMSO control or infected with recombinant vaccinia virus encoding p85(DN), kinase-deficient Syk (SykT), or CD56 irrelevant control gene, were stimulated with IL-2 (100 U/mL) for 5 minutes at 37°C and analyzed for IL-2–triggered Rac1 activation before and after the impairment of Syk function. Rac1 was immunoprecipitated from NK92 cell lysates and examined for activity by affinity precipitation (AP) with PAK1 PBD, which binds only to activated Rac1-guanosine triphosphate (GTP) but not to inactivated Rac1-guanosine diphosphate (GDP). The IL-2–activated Rac1, Rac1-GTP, was precipitated by PAK1 PBD agarose, then resolved by 12.5% SDS-PAGE and examined by anti-Rac monoclonal antibody provided in the kit. (B) IL-2–starved NK92 cells, infected with recombinant vaccinia virus encoding dominant-negative Rac1 (N17Rac1), constitutively active Rac1 (V12Rac1), or CD56 irrelevant control gene, were stimulated with IL-2 (100 U/mL) for 5 minutes at 37°C. Cells were analyzed for Akt activation by Western blot (WB) analysis. (C) IL-2–starved NK92 cells, treated with 25 μM piceatannol or 25 μM LY294002 or DMSO, were infected with recombinant vaccinia virus encoding constitutively active V12Rac1 or CD56 irrelevant control gene, then stimulated with IL-2 (100 U/mL) for 5 minutes at 37°C. Cells were analyzed for Akt activation by Western blot analysis with Ser473-specific antibody. The same membranes were stripped and reprobed with anti–pan-Akt. In parallel, these NK cells were also analyzed by in vitro kinase assays for Akt activation with H2B as the substrate. Results are representative of 1 of 4 independent experiments.
Counteracting the inactivation of Syk by constitutively active Rac1
Several possibilities could have occurred. One was that Rac1 and Syk acted independently on PI 3-kinase in parallel, nonintersecting pathways to result in Akt activation. A second was that Rac1 and Syk were within the same pathway to modulate PI 3-kinase. If this were the case, it would be essential to define whether Syk is upstream of Rac1 or vice versa and to identify the specific sequence of this signaling cascade. With the combination of V12Rac1, piceatannol, and LY294002, we were able to address this issue. NK92 cells, pretreated with piceatannol, LY294002, or DMSO, were infected with vaccinia virus encoding V12Rac1. These cells were then stimulated with IL-2 for 5 minutes. Although DMSO, DMSO + CD56, and DMSO + V12Rac1 controls all exhibited a high level of Akt activity, piceatannol and LY294002 abrogated this activation (Figure 5C). However, V12Rac1 remarkably counteracted the inhibitory effect of piceatannol to restore Akt function (Figure 5C). Moreover, V12Rac1 also restored Akt activation lost in LY294002-treated NK cells, demonstrating that Rac1 was downstream of Syk and PI 3-kinase (Figure 5C). Taken together, these data clearly suggest that a specific signaling cascade is triggered by IL-2 that involves the sequential activation of Syk, PI 3-kinase, and Rac1 that leads to Akt activation.
Validation of this Syk-dependent IL-2 signaling in human NK cells
To confirm that the same pathway is physiologically relevant and not isolated to the NK92 cell line, we extended our observations to fresh LGL cells from healthy donors cultured in IL-2–containing medium. Again, we found a marked induction of apoptosis in piceatannol- or LY294002-treated LGLs, demonstrated by annexin V labeling (Figure6A). Internuclear DNA fragmentation showed the same results (data not shown).
Confirmation of the critical role of Syk in IL-2 maintained LGL survival.
(A) Human fresh NK (LGL) cells, cultured in complete medium containing 100 U/mL IL-2, were incubated with 25 μM piceatannol, 25 μM LY294002, or DMSO vehicle control and were examined for cellular viability by annexin V–FITC binding and PI uptake. LGL cells were collected at 24 and 48 hours, washed in sample wash buffer, and stained with annexin V–FITC in combination with PI. Annexin V–FITC binding in piceatannol-, LY294002-, or DMSO-treated and untreated NK92 cells is shown. (B) Inhibition of IL-2–induced Akt function by inactivating Syk and the rescue of this inhibition by constitutively active PI 3-kinase. IL-2–starved LGL cells, treated with 25 μM piceatannol or DMSO, were infected with recombinant vaccinia virus encoding the constitutively active catalytic subunit of PI 3-kinase, P110*, or CD56 irrelevant control gene and then were stimulated with IL-2 (100 U/mL) for 5 minutes at 37°C. Cells were analyzed for Akt activation by Western blot analysis with Ser473-specific antibodies. (C) LGL cells were treated as described in panel B, and whole-cell lysates were analyzed by in vitro kinase assays for Akt activation with H2B as the substrate. The same membranes were stripped and reprobed with anti–pan-Akt. Results are representative of 1 of 4 independent experiments.
Confirmation of the critical role of Syk in IL-2 maintained LGL survival.
(A) Human fresh NK (LGL) cells, cultured in complete medium containing 100 U/mL IL-2, were incubated with 25 μM piceatannol, 25 μM LY294002, or DMSO vehicle control and were examined for cellular viability by annexin V–FITC binding and PI uptake. LGL cells were collected at 24 and 48 hours, washed in sample wash buffer, and stained with annexin V–FITC in combination with PI. Annexin V–FITC binding in piceatannol-, LY294002-, or DMSO-treated and untreated NK92 cells is shown. (B) Inhibition of IL-2–induced Akt function by inactivating Syk and the rescue of this inhibition by constitutively active PI 3-kinase. IL-2–starved LGL cells, treated with 25 μM piceatannol or DMSO, were infected with recombinant vaccinia virus encoding the constitutively active catalytic subunit of PI 3-kinase, P110*, or CD56 irrelevant control gene and then were stimulated with IL-2 (100 U/mL) for 5 minutes at 37°C. Cells were analyzed for Akt activation by Western blot analysis with Ser473-specific antibodies. (C) LGL cells were treated as described in panel B, and whole-cell lysates were analyzed by in vitro kinase assays for Akt activation with H2B as the substrate. The same membranes were stripped and reprobed with anti–pan-Akt. Results are representative of 1 of 4 independent experiments.
Inspection of Akt phosphorylation and kinase function correspondingly showed results similar to those obtained from NK92 cells. Piceatannol notably suppressed IL-2–induced Akt phosphorylation and kinase function in LGLs, which were significantly reelevated by the expression of V12Rac1 (Figure 6B-C). Taken together, these results demonstrated that this Syk-directed IL-2 signaling pathway also operates in healthy human NK cells, where PI 3-kinase, Rac1, and Akt interact and function downstream of Syk.
Discussion
The key role of Syk in IL-2–maintained NK survival has, to date, not been addressed, though it has been documented that Syk physically associates with IL-2R in peripheral lymphocytes.16,17 IL-2 modulates multiple cellular processes, including lymphocyte maturation, proliferation, survival, homeostasis, and lytic function.1 8 Although multiple signaling and effector pathways have been implicated in IL-2 signaling in independent observations, the contribution of each of them to guide a specific biologic function is still incompletely understood. Before our work, little was known about the important role of Syk in sustaining NK survival; neither the correlation between Syk and IL-2–directed PI 3-kinase/Akt cascade nor that between Syk and NK survival has been reported.
We chose to focus on deciphering the IL-2 signaling code that controls survival and apoptosis and uncovered a novel, specific, Syk-regulated pathway that controls PI 3-kinase/Akt and cell survival. First, we demonstrated that intact function of Syk and PI 3-kinase is essential for IL-2–regulated NK survival; down-regulation of either of them affected cell viability and resulted in apoptosis. Second, we discovered that Syk regulates IL-2–dependent Akt activation by its critical control over PI 3-kinase. Syk inhibition by dominant-negative SykT expression or piceatannol pretreatment abrogated PI 3-kinase and Akt activities in IL-2–stimulated NK cells. Impaired Akt function in Syk-inhibited NK cells can be restored by constitutively active PI 3-kinase, indicating that PI 3-kinase is the intermediate signal molecule between Syk and Akt. These findings suggest that Syk, which associates with IL-2R, is critical for PI 3-kinase–dependent Akt activation. Previous studies with PI 3-kinase in IL-2 function have implicated numerous pathways regulated downstream of PI 3-kinase. These include MEK/MAPK activation,39 p70S6K, E2F induction, T-lymphocyte proliferation,9,10,40 cytoskeleton alterations,36 and survival.9,10,13,14 No information has been available on what signal component could act upstream of PI 3-kinase. Our results represent the first report on Syk linkage to PI 3-kinase in IL-2 signaling for NK cell survival. Of note, Syk-deficient mice, and, in fact, Syk−/−Zap70−/− mice have been reported to express normal NK cell development and function.41,42 It is known that NK cell development and function are complex and depend on a combination of cytokines, including c-kit ligand, Flt-3 ligand, IL-7, IL-12, and IL-15 in addition to IL-2.43-46 The likely explanation for normal NK cell development and function in Syk−/− and Syk−/−Zap70−/−mice is that some of these other cytokines can use signals independent of Syk or Zap70 and thus provide redundant mechanisms for growth and survival.
The mechanism by which Syk activates PI 3-kinase has not yet been studied, but several possibilities come to mind. As a direct substrate of Syk, Shc appears to be a good candidate that can recruit either the Ras/MAPK cascade through Grb2/SOS or the PI 3-kinase pathway.47 In BAF3 cells, engagement of the granulocyte macrophage–colony-stimulating factor (GM-CSF) or IL-3 receptor leads to Shc phosphorylation, which leads to Gab2 tyrosine phosphorylation and its binding with the p85 subunit of PI 3-kinase.48Additionally, gene delivery of Syk into 293T cells results in Gab2 phosphorylation.49 Thus, Syk can activate Shc, which phosphorylates Gab2, creating a binding site for PI 3-kinase, resulting in its activation. IL-2 also has been reported to activate Gab2 through Shc.48,49 In addition, the cross-linking of CD2 and CD16 in NK3.3 cells induced Syk and PI 3-kinase activation, with the latter correlating with the tyrosine phosphorylation of Shc.50Other systems may also exist that link Syk to PI 3-kinase. It has been reported that p85 binds Jak1,51 and this association is suggested to be another means by which PI 3-kinase becomes activated. A novel B-cell adaptor for PI 3-kinase, termed BCAP—which triggers B-cell receptor (BCR)–associated protein tyrosine kinase-induced PI 3-kinase activation—has been implicated in BCR-induced PI 3-kinase signaling, whose phosphorylation, mediated by Syk and Btk, provides binding site(s) for p85.52 In T cells, the p36/38 linker protein also provides a bridge to the recruitment/activation of PI 3-kinase through its phosphorylation by Syk or a Syk-regulated kinase after TCR-triggering.53 It remains to be seen which pathway mediates Syk activation of PI 3-kinase in IL-2 signaling, but it seems likely that Gab2 is responsible through Shc, particularly because of reports that IL-2 induces Gab2 activation.48 49
Akt is conventionally considered to be activated in a PI 3-kinase–dependent manner.54-56 Our findings indicate that Akt activation by IL-2 is also Syk dependent. This observation is supported in other systems. BCR-induced Akt activation requires Syk, whereas Lyn acts as an endogenous antagonist for this activation, implying a mechanism for the delicate adjustment on BCR-triggered Akt.57 Syk is also required for the activation of the PI 3-kinase/Akt survival pathway induced by oxidative stress in B cells.21 Additionally, Syk regulates Akt activation in platelet/megakaryocyte-specific αIIbβ3 integrin signaling in fibrinogen-adherent cells.23
Although Akt dependence on Syk is becoming known, its reliance on Rac1 is a novel finding. This conclusion derived from experiments in this study whereby dominant-negative Rac1 suppressed Akt activation in IL-2–treated NK cells. In addition, constitutively active Rac1 could induce Akt activation in piceatannol- or LY294002-treated NK cells, indicating that Rac1 can work downstream of Syk and PI 3-kinase. Rac1 is particularly noted for its role in cytoskeletal organization, membrane trafficking, and cellular adhesion.36,37,58,59Our data suggest that Rac1 is also critical for cell survival through the activation of Akt. Despite the well-accepted concept that Akt activation requires 3-phosphoinosilide–dependent protein kinase–1 (PDK1), integrin-linked kinase (ILK), or both, we now have supportive data to indicate that Akt activation, at least in IL-2 signaling, requires Rac1. It is possible that the Rac1 pathway for Akt activation may be restricted to cells of the immune system because 2 recent reports have also demonstrated Akt as a downstream target of Rac1 in TCR signaling and FcεRI stimulation.38,60 It should be noted that Rac1 can also apparently act upstream of PI 3-kinase to activate Akt in certain cell systems.38 Some systems, such as membrane ruffling, appear to use a pathway similar ours in that Rac1 worked downstream of PI 3-kinase.61Interestingly, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a tumor-suppressor with phosphatase activity on PI3,4,5P3, a product of PI 3-kinase, which decreases PDK1 and Akt activation, remarkably down-regulates Rac1 and Cdc42 function to negatively control cell motility, providing another important indication that Rac1 might mediate PI 3-kinase effects on Akt.62 63 We do not know yet how Rac1 can function on either side of PI 3-kinase. It is conceivable that the divergence between our observation and that of other groups might stem from the different signaling components inherent in the systems of choice. It is also possible that the variations in the respective functions between different systems influenced the upstream signaling that regulates Akt.
We recently reported that Rac1, acting downstream of Syk and PI 3-kinase, controls PAK1, MEK, and extracellular regulatory kinase (ERK) function in human NK cells, which resulted in the mobilization of lytic granules toward the engaged tumor cell.24,28,64 Thus, Rac1 may have multiple downstream effectors, depending on the kinases that are ultimately regulated. At least, in NK cells, we were able to dissect 2 distinct pathways using Rac1, depending on the receptor. NK receptor engagement by tumor cells triggered Rac1-dependent ERK, which drives lytic function,65 whereas IL-2R engagement elicited Rac1-dependent Akt activation, which controls survival. Of note is that NK receptor engagement and IL-2R engagement triggered Syk activation, leading initially to the activation of PI 3-kinase, followed by Rac1. However, substrates downstream of Rac1 differ, depending on lytic function or cell survival.
This is the first report documenting a novel linkage between Syk and Rac1 in the control of IL-2–mediated NK survival. These data have provided functional and biochemical evidence pertaining to the sequential and specific interaction of Syk, PI 3-kinase, Rac1, and Akt in NK cells to prevent apoptosis; the cytotoxic function of human NK cells also requires the sequential interaction of these molecules.64 65
We thank the staffs of the Analytical Microscopy Core and the Molecular Imaging Core facilities of the H. Lee Moffitt Cancer and Research Institute for their technical assistance.
Prepublished online as Blood First Edition Paper, August 29, 2002; DOI 10.1182/blood-2002-04-1251.
Supported by National Institutes of Health grant CA83146 (J.Y.D.), and American Heart Association grant AHA9701715 (S.W.).
K.J. and B.Z. contributed equally to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Julie Y. Djeu, Immunology Program, H. Lee Moffitt Cancer Center and Research Institute, Department of Interdisciplinary Oncology, University of South Florida College of Medicine, 12902 Magnolia Dr, Tampa, FL 33612; e-mail:djeu@moffitt.usf.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal