Abstract
Hepatitis C virus (HCV) is predominantly a hepatotropic virus. Nonetheless, there is mounting evidence that hematopoietic cells may support HCV replication. The HCV 5′ untranslated region (5′UTR), responsible for initiation of viral translation, via an internal ribosome entry site (IRES), has been previously described to contain specific nucleotide substitutions when cultured in infected lymphoid cells. Our purpose was to establish whether the 5′UTR polymorphism of quasispecies from 3 cell compartments (liver, peripheral blood mononuclear cells [PBMG], and monocyte-derived dendritic cells [DCs]) of a patient chronically infected with HCV1b affects the corresponding translational efficiencies and thus the capacity for replication. The 5′UTR polymorphism was characterized by identification of changes at 3 crucial sites as compared with the reference nucleotide (nt) sequence: a G insertion between positions 19 and 20, a C>A substitution at position 204 and a G>A substitution at position 243. The quasispecies detected in DCs was unique and differed from those present in the liver, suggesting a particular tropism of HCV quasispecies for DCs. Moreover, its translational activity was significantly impaired when compared with those from liver and PBMCs in different cell lines. This impairment was thoroughly confirmed in primary cultures of both human hepatocytes and monocyte-derived DCs. Taken together, our data lend support both to a specific location and impaired replication of HCV quasispecies in DCs, which could be related to viral persistence and perturbation of DC function in chronically infected patients.
Introduction
Hepatitis C virus (HCV) is a positive strand RNA virus that establishes a chronic infection in more than 80% of cases. Chronicity frequently leads to liver cirrhosis and hepatocellular carcinoma,1 making HCV infection the first indication for liver transplantation. Poor fidelity of the viral RNA–dependent RNA polymerase results in the coexistence of closely related but distinct variants called quasispecies, which are likely involved in viral persistence.2 3
There is mounting evidence for an extrahepatic replication based on the frequent association of hepatitis C infection with lymphoproliferative disorders such as cryoglobulinemia and non-Hodgkin lymphomas (reviewed by Dammacco et al4), and the possibility of experimental transmission of non-A non-B hepatitis to a chimpanzee by mononuclear leucocytes from a chronically infected patient.5Supporting the results of Hellings et al,6 severe combined immunodeficiency (SCID) mice have been shown to be susceptible to persistent HCV infection after injection of human HCV-positive peripheral blood mononuclear cells (PBMCs). In addition, tissue compartmentalization of the HCV genome has been described during HCV infection7,8 and coinfection with HIV.8Finally, the detection of HCV RNA-negative strand as the theoretical intermediate of replication, although somewhat controversial,9,10 has been well documented in PBMCs. Indeed, negative- strand RNA has been reported in mononuclear cells such as B and T lymphocytes and monocytes,11-15 in polynuclear lymphocytes,16,17 and even in hematopoietic progenitor cells.18 Moreover, the viral genomic RNA has been detected more frequently in PBMCs from nonresponders19 to interferon (IFN) treatment. Interestingly, considering viral dynamics such as virus half-life, the suppressing effect on viral replication observed through treatment appears consistently slower in PBMCs than in serum.20 On the basis of these studies, one can argue that a specific replicative dynamics of HCV exists in PBMCs.
The relationship between quasispecies and replication in PBMCs is unclear. In a previous study we demonstrated, in the serum of a patient chronically infected with HCV,21 the coexistence of internal ribosome entry site (IRES) quasispecies segregating according to their respective translation efficiencies in lymphoid and nonlymphoid optimal IRES. The HCV IRES responsible for the cap-independent initiation of viral RNA translation22,23comprises most of the 5′ untranslated region (5′UTR) and extends to a short stretch (12-30 nucleotides [nt]) downstream of the initiator codon.24 The IRES element is highly organized in secondary and tertiary structures, which have been shown to be crucial for its functionality (reviewed in Rijnbrand and Lemon25). By analogy with pestiviruses (belonging like HCV to the Flaviviridae family) and with Picornaviridae, the HCV 5′UTR is expected to be implicated in regulation of the viral life cycle by playing a key role in both initiation of translation and replication of viral RNA. Moreover, this structure, albeit genetically stable across different genotypes, might be implicated, as shown for viruses from Picornaviridae family,26-28 in promoting HCV replication in particular cells such as hematopoietic cells, depending on specific changes in its sequence. Many groups have described several nucleotide positions within the IRES structure associated with lymphoid replication.29-33 Therefore, the presence of HCV RNA in lymphoid cells, which is more frequently observed in the setting of immune suppression,8 34 can be related to viral persistence.
The exact mechanisms responsible for immune disorders caused by extrahepatic infection are still poorly understood.35Studies have highlighted (1) an impaired allostimulatory function of monocyte-derived dendritic cells (DCs) in chronic HCV carriers33,36,37 and (2) a low stimulatory capacity of murine DCs expressing HCV proteins.38 Moreover, the detection of HCV RNA-negative strand has been described in human DCs, suggesting a viral replication in these cells.39
As DCs are known to play a crucial role in the induction of both innate and adaptive immune responses,40 the purpose of the current work was to investigate the genetic variability of HCV IRES, its quasispecies distribution, and corresponding translational efficiencies in DCs, in an attempt to gain insight into their involvement in the pathogenesis of HCV infection. With this aim, the relative translational efficiencies of HCV IRES quasispecies retrieved from different tissue compartments of a patient chronically infected with HCV were monitored after transfection of various cell lines, using a bicistronic-based assay vector.21 This was achieved both in cell lines of hepatic or lymphoid origin, as well as in human cells in primary culture, such as hepatocytes and dendritic cells.
Materials and methods
Clinical samples
Heterogeneity within HCV 5′UTR was investigated in a 64-year-old woman chronically infected with HCV genotype 1b (Inno-Lipa HCV II test; Eurogentec, Seraing, Belgium), who did not respond to previous interferon alpha (IFNα) treatment and was under ribavirin antiviral therapy at sampling time. The alanine aminotransferase level was 109 IU/mL and viral load was 3.8 MEq/mL (Quantiplex, HCV RNA 2.0 Assay, bDNA; Chiron, Emeryville, CA). Fibrosis status was evaluated as A3F3 using Metavir scoring on liver biopsy. Infection either with HIV or hepatitis B virus (HBV) was excluded by enzyme-linked immunosorbent assays.
Two different samples were studied: a liver biopsy and a blood sample. Mononuclear cells were obtained from blood collected in EDTA (ethylenediaminetetraacetic acid)–treated tubes and isolated by standard Ficoll-Hypaque (Pharmacia, St Quentin en Yvelines, France) density gradient centrifugation and were resuspended in complete RPMI 1640 medium (Invitrogen, Cergy-Pontoise, France) with a view to adhesion in 24-well plates for further derivation into dendritic cells. Adherent monocytes were cultured in RPMI in the presence of 100 ng/mL recombinant human (rh) granulocyte-macrophage colony-stimulating factor (GM-CSF) and 500 U/mL rh-interleukin 4 (rhIL-4) as described before.33 After a 6-day culture, cells (DCs) were centrifuged and resuspended in complete medium containing 2.5 ng/mL rh-tumor necrosis factor (TNF)–α.
HCV 5′UTR amplification
Total RNA was extracted from PBMC and DC cellular pellets (104-5 × 104 cells/pellet) using a 2-step extraction with phenol/guanidium thiocyanate and chloroform, followed by a precipitation with ethanol. For the liver biopsy, total RNA was extracted from 20 mg of liver by using TRI-reagent according to the recommended procedures (Sigma-Aldrich-France).
Complementary DNAs (cDNAs) were synthesized as already described41 by using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen) for 1 hour at 37°C with antisense primer 1HVR3 (nt 1679-1700, ATGCGCTCTGGGCACCCGGAC). They were further amplified by heminested polymerase chain reaction (PCR), with the high fidelity Arrow Taq DNA polymerase (Stratagene) (sense primer Quasi5′, nt 1-18: GCCAGCCCCTGTTGGGGG and antisense primer Quasi3′, nt 409-426: AGTTCCCCGGGTGGCGGTC for the first round; sense primer IRES5′, nt 1-15: CGCCGGATCCGCCAGCCCCCTGATG and antisense primer IRES3′, nt 353-371: GCGCCCTGCAGTTTTCTTTGAGGTTTAGG for the second round). PCR reactions involved 30 cycles (94°C for 20 seconds, 50°C for 1 minute, and 72°C for 1 minute, followed by a final elongation step at 72°C for 7 minutes).
Cloning and sequencing
PCR products were digested with BamHI andPstI enzymes, then purified by using the QIAquick PCR purification kit (Qiagen) and cloned into theBamHI/PstI-digested pIRF vector, previously described.21 Briefly, this vector comprises the T7 promoter for transcription, the firefly luciferase (FLuc) gene, followed by the HCV 5′UTR sequence and the renilla luciferase (RLuc) gene. In this system the upstream (control) reporter FLuc is translated in a cap-dependent fashion, whereas the downstream (assay) reporter RLuc is under HCV IRES control.
Thirty clones were generated per sample origin and then sequenced in both directions by using an ABI 377 automated DNA sequencer (Applied Biosystems) and the Dye Dideoxy TM Terminator Cycle Sequencing kit (Applied Biosystems). When detected, mutations were considered to be representative of a quasispecies distribution only when observed in at least 5 independent clones. This precaution was respected to exclude any polymerase artifact because of nucleotide misincorporations, which cannot be totally ruled out even when using a high fidelity polymerase with proofreading activity (Malet I., unpublished results, 2002).
Cell cultures
Five human cell lines were used: HepG2 and HuH7 of hepatocellular carcinoma origin, Jurkat a T-cell line derived from lymphoblastic leukemia, Daudi a B-cell line from B-lymphoma, and KG1 a cell line of myeloblastic origin. Adherent cells (HepG2 and HuH7) were maintained in Dulbecco-modified Eagle medium (DMEM; Invitrogen) and nonadherent cells in RPMI 1640 medium at 37°C in a 5% CO2 atmosphere. All media were supplemented with glutamax, 10% fetal calf serum, and 1% penicillin-streptomycin (Invitrogen). One day before transfection, 2 × 105 adherent cells (HepG2, HuH7) or 5 × 105 nonadherent cells (Jurkat, Daudi, and KG1) were seeded in 24-well plates.
Hepatocytes in primary culture were prepared from liver lobectomy segments taken from adult patients for medical purposes unrelated to our research program. All patients tested negative for HCV (IMX, Abbott), as well as HBV and HIV (VIDAS, Biomérieux) antibodies. Hepatocytes were isolated and plated at confluence in 24-well plates precoated with collagen and cultured for 2 days according to the previously published procedure,42 before transfection.
Preparation of monocyte-induced DCs for transfection experiments was carried out as described above from blood samples tested negative for HCV, HBV, and HIV antibodies. After a 6-day culture, DCs were harvested and plated in 24-well plates (106 cells/mL per well) and transfected the next day.
DNA transfections
Cells lines were infected with the vTF7-3 recombinant vaccinia virus expressing T7 RNA polymerase (kindly provided by B. Moss, National Institutes of Health, Bethesda, MD) at 5 plaque-forming units (PFU)/cell in 300 μL serum-free medium Opti-MEM (Invitrogen) for 1 hour at 37°C. They were then transfected in triplicate with respective constructs, using 1 μg plasmid DNA mixed with 5 μg DAC-30 (Eurogentec) in 300 μL Opti-MEM. At 18 hours after transfection, cells were harvested and lysates were stored at −20°C until use.
Cells in primary culture were infected with the vTF7-3 recombinant vaccinia virus at 3 PFU/cell in 300 μL serum-free medium DMEM for 1 hour at 37°C. The cells were then transfected in triplicate with respective constructs, using 2 μg plasmid DNA mixed with 6 μg Fugene 6 (Roche, Meylan, France) in 400 μL DMEM, incubated for 5 hours at 37°C, and complete medium was added. At 18 hours after transfection, cells were harvested and lysates stored at −20°C.
Luciferase assays
Both renilla and firefly luciferases were measured for their respective luminometric activity from cell lysates by using the Dual Luciferase kit assay (Promega). IRES relative translational activity is represented by the RLuc/FLuc ratio.
Statistical analysis
Variations between the mean translational efficiencies were analyzed by means of methods of analysis of variance. The tests were performed by using Excel software (Ritme Informatique, Paris, France). The significance of observed differences was assessed by using the Snedecor F test.43 Multiple comparisons between individual variations were performed by using the Scheffétest.44 The Scheffé test is a procedure that permits to test unplanned comparisons among means without inflating the type I error rate. Differences were considered to be significant whenP < .05.
Results
Characterization of HCV 5′UTR quasispecies
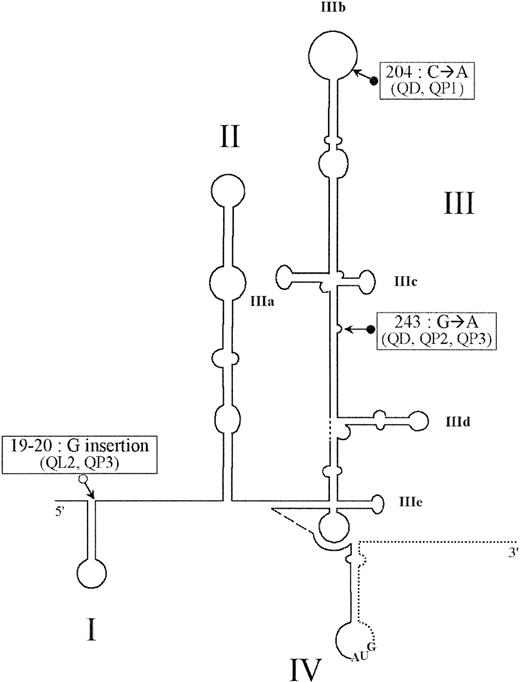
A 371-nt region corresponding to the HCV IRES was amplified from a patient chronically infected with genotype 1b HCV. Quasispecies was characterized in 3 tissue samples of different origins: a liver biopsy, PBMCs from whole blood, and DCs derived from blood monocytes. The amplified products were cloned into the pIRF vector, and 30 clones per tissue origin were sequenced. More than 90 clones were isolated from the 3 samples; 3 principal sites reflected a quasispecies distribution after comparison with the 5′UTR sequence of HCV genotype 1b45: a G insertion between nucleotides 19 and 20, a C>A substitution at position 204, and a G>A substitution at position 243. The combination of nucleotide substitutions at these 3 crucial positions varied depending on the sample origin and allowed a segregation of clones studied into 6 independent quasispecies: QD, QL1, QL2, QP1, QP2, and QP3, described hereafter, and represented in Table1 and Figure1. QD was the unique sequence isolated in the DC sample, and QL1 and QL2 were both representative of the liver sample. All these quasispecies were detected in the PBMCs, with 3 additional sequences (QP1, QP2, and QP3) exclusively detected in that tissue sample. Of note QL1 was also predominantly represented in the serum33 and exhibited a complete homology with the HCV 1b reference. Therefore, it was considered as the consensus sequence. Two substitutions were found to be associated in the DC sample: a C>A at position 204 and a G>A at position 243 (QD). These mutations were detected in the PBMC sample, either associated in most clones (QD, 20 of 30 clones), or unique at position 204 (QP1, 2 of 30) or 243 (QP2 and QP3, 1 of 30). The G insertion between nt 19 and 20 was predominantly observed in the liver compartment (QL2, 10 of 30) and was also detected, albeit at low frequency, in the PBMC sample (QL2 and QP3, 1 of 30), whereas it was absent in the DCs.
Mutations observed from HCV IRES quasispecies depending on the cell origin
| Origin . | Clone identification . | Nucleotide at base position . | Clone frequency† . | ||
|---|---|---|---|---|---|
| 19-20* . | 204 . | 243 . | |||
| Reference‡ | NA | None | C | G | NA |
| Liver | QL1 | None | C | G | 20/30 |
| QL2 | G | C | G | 10/30 | |
| DC | QD | None | A | A | 30/30 |
| PBMC | QD | None | A | A | 20/30 |
| QL1 | None | C | G | 5/30 | |
| QP1 | None | A | G | 2/30 | |
| QP2 | None | C | A | 1/30 | |
| QP3 | G | C | A | 1/30 | |
| QL2 | G | C | G | 1/30 | |
| Origin . | Clone identification . | Nucleotide at base position . | Clone frequency† . | ||
|---|---|---|---|---|---|
| 19-20* . | 204 . | 243 . | |||
| Reference‡ | NA | None | C | G | NA |
| Liver | QL1 | None | C | G | 20/30 |
| QL2 | G | C | G | 10/30 | |
| DC | QD | None | A | A | 30/30 |
| PBMC | QD | None | A | A | 20/30 |
| QL1 | None | C | G | 5/30 | |
| QP1 | None | A | G | 2/30 | |
| QP2 | None | C | A | 1/30 | |
| QP3 | G | C | A | 1/30 | |
| QL2 | G | C | G | 1/30 | |
NA indicates not applicable.
Nucleotide insertion between positions 19 and 20.
Number of clones with the indicated sequence/number of all clones recovered and sequenced from the indicated source.
According to the HCV 1b sequence.
Locations of the nucleotide mutations in the HCV IRES.
Scheme of the secondary structure of the HCV IRES, showing the locations of the nucleotide mutations in IRES elements of sequenced quasispecies studied. The secondary structure prediction and loop numbering are based on those of Honda et al46; the coding sequence is represented by a dotted line, and the initiator AUG codon is represented in stem-loop IV. All quasispecies mutations are depicted by arrows preceded by ● for substitution, and ○ for insertion. Clone identifications are shown in parentheses.
Locations of the nucleotide mutations in the HCV IRES.
Scheme of the secondary structure of the HCV IRES, showing the locations of the nucleotide mutations in IRES elements of sequenced quasispecies studied. The secondary structure prediction and loop numbering are based on those of Honda et al46; the coding sequence is represented by a dotted line, and the initiator AUG codon is represented in stem-loop IV. All quasispecies mutations are depicted by arrows preceded by ● for substitution, and ○ for insertion. Clone identifications are shown in parentheses.
The 5′UTR IRES quasispecies translational efficiencies in cell lines
To establish whether the observed mutations could be related to a functional variability, we performed translational luciferase assays following transfection of 5 cell lines as described in “Materials and methods.” The pIRF bicistronic system used in that work has the advantage of bypassing the possible differences in transfection efficiency and susceptibility occurring in each cell line. Indeed, during the transfection procedure, the upstream FLuctranslation product serves as an internal control, whereas the secondRLuc product depends on the HCV IRES activity.
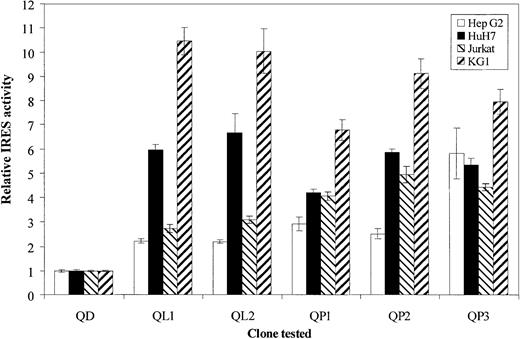
A great heterogeneity, with a highly significant difference (P < .001), was observed between the IRES efficiencies, depending on the cell line used (Figure2). In different experiments no signal could be obtained from the Daudi cells. To facilitate interpretation of the results, QD was chosen for normalization of relative translational efficiency with an arbitrary value of 1 in each cell line. On independent experiments performed in triplicate, QD clearly appeared to be the least efficient IRES in all cell lines (Scheffe test,P < .05). Consequently, relative activity of quasispecies isolated from the other compartments was enhanced 2- to 6-fold in HepG2 and HuH7 cells and 3- to more than 10-fold in Jurkat and KG1 cells. Interestingly, comparison between QD efficiency and that from QP1 and QP2 quasispecies revealed a dramatic decrease for QD: from 2.5- to 9-fold (HepG2 versus KG1 cell line). QD contains substitutions C>A at nt 204 and G>A at nt 243, compared with QP1 and QP2, each of them possessing only one of these mutations at nt 204 and 243, respectively. This finding strongly suggests that the restricted association of both mutations, as is the case in QD, is deleterious for the virus, impairing its initiation translation step.
Translation efficiencies of identified HCV 5′UTR quasispecies in cell lines.
Four cell lines (HepG2, HuH7, Jurkat, and KG1, respectively, represented by ■, ▪, ▧, and ▨) were seeded in 24-well plates at 2 × 105 cells/well for adherent cells and 5 × 105 cells/well for nonadherent cells. The next day, they were infected with vTF7-3 recombinant vaccinia virus at 5 PFU/cell for 1 hour at 37°C and then transfected in triplicate as described in “Materials and methods” with respective bicistronic constructs, containing each of the identified HCV quasispecies. At 18 hours after transfection cells were lysed and assayed for luciferases activities. For each quasispecies (QD, QL1, QL2, QP1, QP2, and QP3) IRES relative activities were given as RLuc/FLuc ratios normalized to the QD (originated from DCs) RLuc/FLuc ratio. The data bars and error bars represent the means and SDs of 3 independent triplicate transfections.
Translation efficiencies of identified HCV 5′UTR quasispecies in cell lines.
Four cell lines (HepG2, HuH7, Jurkat, and KG1, respectively, represented by ■, ▪, ▧, and ▨) were seeded in 24-well plates at 2 × 105 cells/well for adherent cells and 5 × 105 cells/well for nonadherent cells. The next day, they were infected with vTF7-3 recombinant vaccinia virus at 5 PFU/cell for 1 hour at 37°C and then transfected in triplicate as described in “Materials and methods” with respective bicistronic constructs, containing each of the identified HCV quasispecies. At 18 hours after transfection cells were lysed and assayed for luciferases activities. For each quasispecies (QD, QL1, QL2, QP1, QP2, and QP3) IRES relative activities were given as RLuc/FLuc ratios normalized to the QD (originated from DCs) RLuc/FLuc ratio. The data bars and error bars represent the means and SDs of 3 independent triplicate transfections.
Although not statistically significant, opposite trends in the translation mechanism were noted in cell lines of the same origin, depending on the mutation involved. Indeed, concerning hepatocellular cell lines, an increase in IRES activity was observed in HepG2 cells and a decrease in HuH7 cells, whether A substitution occurred at nt 204 (QP1 versus QL1) or at nt 243 (QP3 versus QL2). Similarly, for lymphoid cell lines, an increase in IRES efficiency appeared in Jurkat cells and a decrease in KG1 cells, whether A substitution was detected at nt 204 (QP2 versus QL1) or at nt 243 (QP1 versus QL1 and QP3 versus QL2). It appeared from these observations that cell lines, albeit of a common origin, react independently to the same viral pressure, perhaps because of the presence in each of them, of specific factors able to modulate the IRES activity, as already suggested in other studies.
The 5′UTR quasispecies translational efficiencies in primary cells
To strengthen our data obtained with cell lines, we performed the same experiments using primary cell cultures that were assumed to mimic more closely the conditions of natural infection.
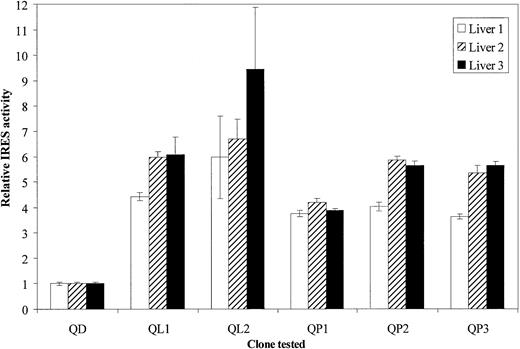
Hepatocytes in primary culture were isolated from 3 different human livers to exclude any host factor selection bias. After seeding in 24-well plates, they were transfected with the bicistronic constructs containing any of the IRES quasispecies and assayed by luminometry. As represented in Figure 3, the values obtained from 3 independent experiments were highly reproducible and closely related for each clone tested. The differences between the quasispecies activities were statistically significant (P < .05) in the 3 assays. Importantly, it can be noted that the luciferase levels obtained in primary hepatocytes were of the same magnitude as that obtained with the cell lines. QD was again significantly less efficient than the other quasispecies (P < .05, donor 2), with a difference of at least 3.7-fold for QD versus QP3 and 9.4-fold for QD versus QL2.
Translation efficiencies of identified HCV 5′UTR quasispecies in hepatocytes in primary culture.
Hepatocytes from 3 different donors were used, respectively represented by ■, ▨, and ▪. Approximately 2 × 105 cells were seeded in 24-well plates coated with collagen. After a 3-day culture, they were infected with vTF7-3 recombinant vaccinia virus at 5 PFU/cell for 1 hour at 37°C and then transfected in triplicate as described in “Materials and methods” with respective bicistronic constructs, containing each of the identified HCV quasispecies. At 18 hours after transfection cells were lysed and assayed for luciferases activities. For each quasispecies (QD, QL1, QL2, QP1, QP2, and QP3) IRES relative activities were given as RLuc/FLuc ratios normalized to the QD (originated from DCs) RLuc/FLuc ratio. The data bars and error bars represent the means and SDs of 3 independent triplicate transfections.
Translation efficiencies of identified HCV 5′UTR quasispecies in hepatocytes in primary culture.
Hepatocytes from 3 different donors were used, respectively represented by ■, ▨, and ▪. Approximately 2 × 105 cells were seeded in 24-well plates coated with collagen. After a 3-day culture, they were infected with vTF7-3 recombinant vaccinia virus at 5 PFU/cell for 1 hour at 37°C and then transfected in triplicate as described in “Materials and methods” with respective bicistronic constructs, containing each of the identified HCV quasispecies. At 18 hours after transfection cells were lysed and assayed for luciferases activities. For each quasispecies (QD, QL1, QL2, QP1, QP2, and QP3) IRES relative activities were given as RLuc/FLuc ratios normalized to the QD (originated from DCs) RLuc/FLuc ratio. The data bars and error bars represent the means and SDs of 3 independent triplicate transfections.
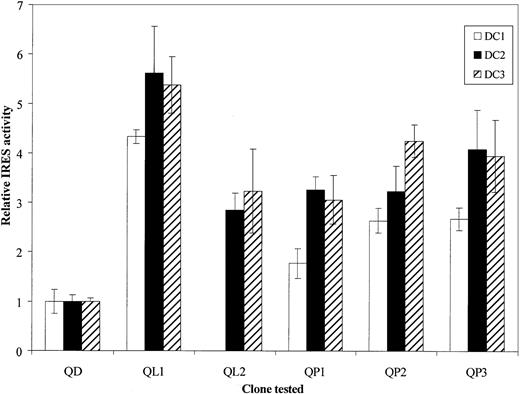
Furthermore, the transfection experiment was performed in DCs derived from blood monocytes (Figure 4). Despite the poor transfection efficiency observed, probably because of high cell mortality and slight contamination with other PBMC populations. QD still significantly appeared the least efficient of the quasispecies (first and second assays, P < .01; third assay,P < .05).
5′UTR functionality in monocyte-derived DCs.
PBMCs were collected from 3 different blood donors (respectively represented by ■, ▪, and ▨), and monocytes were derived to DCs as described in “Materials and methods.” After seeding in 24-well at 1 × 106 cells/mL per well, DCs were infected with vTF7-3 recombinant vaccinia virus and then transfected in triplicate with respective bicistronic constructs, containing each of the identified HCV quasispecies. At 18 hours after transfection cells were lysed and assayed for luciferases activities. For each quasispecies (QD, QL1, QL2, QP1, QP2, and QP3) IRES relative activities were given asRLuc/FLuc ratios normalized to the QD (originated from DCs)RLuc/FLuc ratio. The data bars and error bars represent the means and SDs of 3 independent triplicate transfections.
5′UTR functionality in monocyte-derived DCs.
PBMCs were collected from 3 different blood donors (respectively represented by ■, ▪, and ▨), and monocytes were derived to DCs as described in “Materials and methods.” After seeding in 24-well at 1 × 106 cells/mL per well, DCs were infected with vTF7-3 recombinant vaccinia virus and then transfected in triplicate with respective bicistronic constructs, containing each of the identified HCV quasispecies. At 18 hours after transfection cells were lysed and assayed for luciferases activities. For each quasispecies (QD, QL1, QL2, QP1, QP2, and QP3) IRES relative activities were given asRLuc/FLuc ratios normalized to the QD (originated from DCs)RLuc/FLuc ratio. The data bars and error bars represent the means and SDs of 3 independent triplicate transfections.
Our results clearly indicated that the unique IRES sequence present in DCs was less efficient than the other sequences detected in liver and PBMCs. As expected, according to the above results, the combination of the 2 substitutions at nt 204 and 243 seems required to impair IRES activity.
Discussion
Quasispecies polymorphism is associated in many RNA viruses with viral persistence, and several reports have demonstrated that among Picornaviridae, such as poliovirus26 and HAV,27 the 5′UTR determines cell type–specific replication. The current study was undertaken to determine whether variability occurring in HCV 5′UTR could confer a putative replication advantage in cells of lymphoid origin. We present data that support that hypothesis with the identification of a particular HCV quasispecies in one subpopulation of DCs, the monocyte-derived DC, as defined by a unique sequence within 5′UTR.
Among IRES quasispecies amplified from 3 cell compartments of the same individual, 3 mutations were found to be representative of a polymorphism within the HCV 5′UTR: a Gly insertion between nt 19 and 20, a C>A substitution at position 204, and a G>A substitution at position 243. The 5′UTR originated from the DCs (QD) differed from the sequences from the liver and was predominantly recovered from the PBMC sample. The hypothesis of a passive adsorption onto DCs or PBMCs of viral particles released from the liver into the blood stream seems unlikely for several reasons. (1) Although the liver is considered as the major virus-producing compartment in an infected host, quasispecies retrieved from the liver are recovered at a low frequency from the PBMCs (QL1, 5 of 30; and QL2, 1 of 30) and never from the DCs. (2) Substitutions at positions 204 (C>A) and 243 (G>A) observed in the DCs and in the PBMCs have already been described as lymphoid specific, either after selection during passage of HCV in lymphoblastic B and T cells,29 or more recently in PBMCs of a chronically infected patient.33 (3) The possibility that human PBMCs could concentrate a subset of the circulating viral population differing in the IRES area has been considered in a study, and thus excluded by adsorption assays.8
Our work provides original observations further supporting the concept of HCV replication in PBMCs. They consist of the identification of different mutation patterns observed for quasispecies of lymphoid origin (DCs and PBMCs) compared with that recovered from the liver. Most of the published reports have described the coexistence of positive and negative strands of HCV RNA in the PBMCs of infected patients.11,12,14,17,47 Although convincing, because of the use of assays carefully optimized for strand specificity, these observations must be considered with caution. It should be kept in mind that the presence of a nuclease-resistant form of HCV-negative strand has been reported in the serum.13 Thus, it cannot be totally excluded that the basis for the negative strand detection in these cells resulted from phagocytosis, such as internalization either of fragments of replicating liver cells or of protected negative strand RNA from serum.
One originality of this work is the use of primary human cells to monitor the IRES translational efficiency. The human hepatocyte is the primary target for HCV replication and has been shown to be metabolically active for at least 35 days48-50 and able to support in vitro HCV replication as described.42 Although quasispecies retrieved from the liver (QL1 and QL2) was, as expected, the most efficient in primary hepatocytes, QD, the unique quasispecies from the DCs, exhibited a dramatic low translational activity in 3 independent assays using primary hepatocytes prepared from 3 different donors. This result was not surprising, as QD variant derived from DCs was supposed to be adapted to the lymphoid rather than to the liver compartment. However, a similar unexpected reduced activity was observed in DCs induced from blood monocyte primary culture, the same cell subset from which QD was recovered. Nonetheless, such an impaired IRES activity observed in DC cultures cannot rule out the possibility of an unadapted culture system, lacking some indispensable factors. Alternatively, if QD reflects an adaptation to lymphotropism, this would imply that such a variant might have a lower fitness than the wild type for DCs, because of its low translational activity, thus making DCs a potent viral reservoir accounting for viral latency.
In general 2 mechanisms are proposed to justify the viral persistence in a host, beside the possibility of integration into the cell genome, which is excluded for HCV infection: (1) the disruption of immune system and/or (2) the alteration of replication. As reviewed in Klagge and Schneider-Schaulies51 for chronic retroviral infection and acute measles infections, and in Akbar et al52 for chronic viral hepatites, viruses can modulate DC functions and hence interfere with the immune response. Together with 2 reports,33 36 our results fit such mechanisms. Indeed, the impaired activity of DC quasispecies could be explained by persistent maintenance of HCV in DCs either at a low level and/or at a small percentage within the cell population. In addition, it is consistent with the attribution to DCs, of a role in disseminating the virus via lymph nodes, therefore contributing to the pathogenesis of HCV infection.
In conclusion, our study provides another strong argument in favor of extrahepatic replication of HCV taking the advantage of human primary cells for testing the assayed IRES activity. The unique IRES variant from the DCs was clearly impaired in translational efficiency, suggesting that viral adaptation to these cells leads to a low replication phenotype. DC infection with HCV may explain HCV persistence in the host, even after treatment or orthotopic liver transplantation,34,53 54 and it corroborates the hypothesis that lymphoid cells are an alternative reservoir for the virus. Nonetheless, further studies are necessary to assess the incidence of this phenomenon on immune disorders associated with HCV infection, such as cryoglobulinemia and non-Hodgkin lymphoma, and whether it could be related to the progression to cirrhosis or resistance to treatment.
We thank Nadège Goutagny for her help in harvesting and preparing the dendritic cell cultures.
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2002-03-0818.
Supported in part by the Ministère de l'Education Nationale, de la Recherche et de la Technologie (MENRT): programme de Recherche Fondamentale en Microbiologie et Maladies Infectieuses et Parasitaires (réseau Hépatite C), the Association pour la Recherche contre le Cancer, and the Association Claude Bernard. J. L. is supported by a doctoral grant from the MENRT No. 99623.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Annie Cahour, Laboratoire de Virologie, C.E.R.V.I., UPRES EA 2387, Hôpital Pitié-Salpêtrière, 83 Bd de l'hôpital, 75651 Paris Cedex 13, France; e-mail: cahour@idf.ext.jussieu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal