Abstract
T-lymphocyte depletion of bone marrow grafts compromises engraftment, suggesting a facilitating mechanism provided by the T cells that has been shown to associate with CD8+ but not CD4+ T cells. Explanations for this phenomenon have focused on immune targeting of residual host cells or cytokine production. We provide evidence for an alternative mechanism based on cooperative effects on cell motility. We observed that engraftment of CD34+ cells in a β2-microglobulin–deficient nonobese diabetic/severe combined immunodeficiency (β2m−/− NOD/SCID) mouse model paralleled clinical observations in humans, with an enhancing effect noted from the addition of CD8+ cells but not CD4+ cells. This correlated with CD8+ augmentation of CD34+cell homing to the bone marrow in vivo and CD8+cell–associated increases of CD34+ cell transmigration through a bone marrow endothelial cell line in vitro. The cooperative interaction was not sensitive to brefeldin A inhibition of protein secretion. However, cytochalasin D–induced inhibition of CD8+ cytoskeletal rearrangements abrogated CD34+ transendothelial migration and impaired CD34+ cell homing in vivo. CD8+ cells did not migrate in tandem with CD34+ cells or alter endothelial barrier integrity; rather, they affected phosphotyrosine-mediated signaling in CD34+ cells in response to the chemokine stromal derived factor-1α (SDF-1α). These data demonstrate cell-cell cooperativity between different cell types in mediating chemotactic events and provide one potential explanation for the clinically observed effect of CD8+ cells on bone marrow transplantation. This modification of cell migration by neighboring cells provides broad possibilities for combinatorial effects between cells of different types to influence cell localization.
Introduction
Bone marrow grafts are depleted of T cells to reduce graft-versus- host disease (GVHD), as a by-product of tumor cell purging or as a result of hematopoietic stem/progenitor cell selection for the introduction of genetic constructs.1,2 However, when compared with unmanipulated grafts, T cell–depleted autologous and allogeneic transplants result in lower levels of engraftment during the first 6 months after transplantation.3,4 This implies that T cells facilitate optimal engraftment of CD34+ cells and has been supported by clinical trials in humans demonstrating that the presence of CD8+ cells but not CD4+ cells results in an increased level of engraftment in the allogeneic transplant setting.5 Studies of allogeneic transplants in mice have also shown that CD8+cells facilitate engraftment6-8; however, the exact phenotype of these cells remains controversial. The mechanism of action by which CD8+ cells facilitate engraftment is generally attributed to the elimination of residual immune cells of the recipient.9 However, stem cells transplanted into multi-immunodeficient hosts similarly benefit from accessory cells, suggesting the possibility of other roles often assumed to be that of cytokine production.10 11
The mechanisms by which hematopoietic stem cells home and engraft have been defined to a limited extent. Mice engineered to be deficient in either the chemokine receptor CXCR4 or its ligand, stromal derived factor-1α (SDF-1α), die perinatally with severe defects in stem cell trafficking from fetal liver to establish hematopoiesis in the bone marrow.12-15 However, transplanted CXCR4−/− fetal liver cells successfully engraft in the bone marrow of wild-type mice and only exhibit defects in the retention of B-lymphoid and granulocytic lineage precursors in the bone marrow.16,17 Therefore, SDF-1α–CXCR4 interactions act in conjunction with other molecular mediators of stem cell homing and engraftment. Nevertheless, SDF-1α is a known chemoattractant for candidate human stem cells in vitro.18,19 This action has been suggested to be important for homing in vivo,20 and treatment of primitive cells with anti-CXCR4 antibodies abolishes the engraftment capability of those cells in animal models.21SDF-1α is also known to alter cell motility and up-regulate surface adhesion molecules stimulating arrest of CD34+ cells on and transmigration through vascular endothelium.22,23 The association of SDF-1α–mediated cell migration and stem cell engraftment has recently been further solidified with human clinical data that correlate in vitro chemotaxis with in vivo blood count recovery after bone marrow transplantation.24
Using in vivo and in vitro model systems, we assessed the interaction of CD8+ and CD34+ cells resulting in the clinically observed facilitating effect of the lymphoid population. We demonstrate correlation between in vivo and in vitro systems to define a CD8+ cell population capable of augmenting CD34+ engraftment. We define that a secreted product of CD8+ cells is not required; rather, altered CXCR4 signaling in CD34+ cells mediated by CD8+ cells associates with augmentation of CD34+ migration, homing, and engraftment. These data demonstrate a unique cell-cell cooperativity affecting chemotaxis and provide a novel mechanism for lymphoid augmentation of bone marrow transplantation.
Materials and methods
Cell purification
Umbilical cord blood samples were obtained from the Pediatric Research Institute, University of St Louis, MO, according to guidelines established by the Human Investigation Committee. Mononuclear cells were isolated from umbilical cord blood by density gradient centrifugation over Ficoll (Pharmacia Biotech, Piscataway, NJ). CD34+ cells were then selected using the immunomagnetic MiniMACS system to a purity of 94% to 99% (Miltenyi Biotec, Auburn, CA). CD4+ and CD8+ cells were subsequently selected from the CD34− cell fraction at a purity of 94% to 99%. These cells were all cultured overnight in Iscove medium supplemented with 10% fetal calf serum, penicillin and streptomycin, and l-glutamine (all from Cellgro, Herndon, VA) at 37°C/5% CO2 in a humidified atmosphere.
Flow cytometry
Cells were stained with fluorescently labeled (allophycocyanin [APC], fluorescein isothiocyanate [FITC], phycoerythrin [PE], or peridinin chlorophyll protein [PerCP]) anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD34, or anti-CD45 (all from Becton Dickinson, San Jose, CA). Following staining, cells were washed with phosphate-buffered saline (PBS) and fixed in 1% paraformaldehyde. Cells were analyzed on a FACSCalibur cytometer using CellQuest software (Becton Dickinson).
Engraftment of β2m−/− NOD/SCID mice
Mice were obtained and used in accordance with the Subcommittee on Research Animal Care of the Massachusetts General Hospital guidelines. Mice were housed in sterilized microisolator cages and received autoclaved food and water ad libitum. For the engraftment experiments, selected CD34+ cells (3 × 104) were incubated alone or with either selected CD4+ or CD8+ in a 1:1 ratio for 2 to 3 hours. Cells from the same umbilical cord blood (UCB) collection were then injected into the tail vein of 6- to 8-week-old female littermate β2-microglobulin–deficient nonobese diabetic/severe combined immunodeficient (β2m−/−NOD/SCID) mice (Jackson Laboratories, Bar Harbor, ME), which had been sublethally irradiated (3.5 Gy) 24 hours previously. After 6 to 8 weeks, the mice were killed with CO2, and the bone marrow and spleen were removed. The bone marrow was flushed with fully supplemented Iscove medium, and the spleen was mechanically disaggregated. The level of engraftment of the mice was calculated by flow cytometry using antibodies toward human CD45, CD3, CD19, CD33, and CD34.
In vivo homing
Selected CD34+ cells (1 × 105 to 4 × 105) were labeled with 0.1 μM carboxyfluorescein diacetate (CFDA)–succinimidyl ester (SE) (Molecular Probes, Eugene, OR) according to the manufacturer's instructions and incubated either alone or with an equal number of CD4+ or CD8+ cells for 2 to 3 hours. Cells were then injected into the tail vein of 6- to 8-week-old female littermate β2m−/− NOD/SCID mice or SCID mice (bred at Massachusetts General Hospital), which had been sublethally irradiated (3.5 Gy) 24 hours previously. Mice were then killed after 9 hours, and the levels of human cells were measured in the bone marrow and spleen through the detection of CFDA-SE+ cells by flow cytometry.
Chemotaxis assay
Chemotaxis assays were performed by plating 3 × 104 bone marrow endothelial cells (BMECs) (kindly provided by Dr Joao Ascensao, University of Nevada School of Medicine, Reno) in a Transwell (5-μm pore size) (Corning-Costar, New York, NY) and incubating at 37°C for 3 days or until confluent. Purified CD34+ cells (1 × 104 to 2 × 104) with or without an equal number of purified CD4+ or CD8+ cells in fully supplemented Iscove medium were then added to the upper well. Chemotaxis toward 300 ng/mL SDF-1α (PeproTech, Rocky Hill, NY) was allowed to continue for 4 hours at 37°C/5% CO2 in a humidified atmosphere. Cells were harvested from the lower well, counted with a hemocytometer, and the relative numbers of the different cell types calculated by flow cytometry. To block secretion of factors from or movement of the CD8+ cells, they were treated with 3.5 μM brefeldin A (Sigma Chemical, St Louis, MO) for 4 hours at 37°C or 10 μg/mL cytochalasin D (Sigma) for 15 minutes at 37°C. To assess the integrity of the endothelial barrier, we used a labeled protein as a surrogate as has been described by others.25-27 FITC-conjugated albumin (Molecular Probes) was added to the upper well at a concentration of 0.2 mM, and chemotaxis assays were performed as described above. The level of fluorescence was then measured in the lower well using a CytoFluor II plate reader (Perspective Biosystems, Framingham, MA).
Time-lapse video microscopy
BMEC cells were plated onto 12-well plates and allowed to grow until confluent. CD34+ and CFDA-SE–labeled CD8+ cells were then added on the cell line and incubated for 30 minutes at 37°C to allow the cells to settle upon the monolayer. Images (both light and fluorescence) were acquired every minute using an inverted microscope with a Spot RT Color (version 3.0.3) camera (Diagnostic Instruments, Sterling Heights, MI) and UniBlitz shutter driver (model VMM-D1) (Vincent Associates, Rochester, NY). Images were first acquired using IPLab software (version 3.5.2) (Scanalytics, Fairfax, VA) and then converted into time-lapse sequences with Scion Image software (Scion, Frederick, MD).
Calcium flux
Selected CD34+ cells were incubated alone or with either CD4+ or CD8+ cells for 2 to 3 hours following which they were incubated with 3 μg/mL Indo-1 (Molecular Probes) for 45 minutes at 37°C. Cells were then stained for CD34, CD4, or CD8 for 15 minutes on ice. Calcium flux was measured by a ratio of 400:40 (short) to 510:20 (long) wavelengths with UV light from a He-Cad laser (325 nm) on an LSR cytometer (Becton Dickinson) after the addition of 1 μg/mL SDF-1α. Calcium flux was measured specifically in the CD34+ cell populations using FlowJo software (Tree Star, Stanford, CA).
Measurement of tyrosine phosphorylation
Selected CD34+ cells were incubated alone or with either CD4+ or CD8+ cells for 2 to 3 hours. SDF-1α was then added to the cells at a concentration of 1 μg/mL. At 0, 1, 2, and 3 minutes following the addition of SDF-1α, the cells were permeabilized with Fix & Perm Cell Permeabilization Kit (Caltag Laboratories, Burlingame, CA) and then stained for CD34, CD4, CD8, and antiphosphotyrosine (Upstate Biotechnology, Lake Placid, NY) or phospho-FAK (Becton Dickinson). Cells were then analyzed on a FACSCalibur cytometer using CellQuest software.
Statistical analysis
Paired comparisons were carried out using the Studentt test.
Results
CD8+ cells enhance the engraftment of CD34+cells in β2m−/− NOD/SCID mice
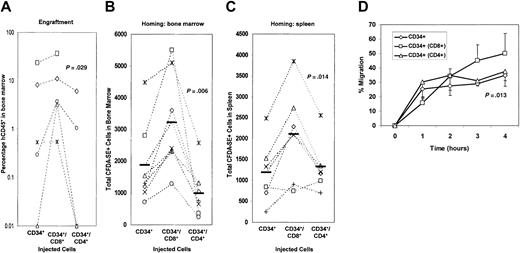
The effect of T cells upon engraftment of CD34+ cells was studied in vivo in the β2m−/− NOD/SCID mouse model. To assess the impact of mixed cell populations, we titrated down the dose of infused cells reasoning that above a certain threshold no further augmentation would be observable. Rather than the customary 3 × 105 CD34+ cells, we employed 3 × 104 CD34+ cells preincubated and undergoing transplantation with either no cells or autologous CD4+ or CD8+ cells at a 1:1 ratio into sublethally irradiated mice and evaluated for engraftment after 6 to 8 weeks. Stem cells admixed with CD4+ cells consistently engrafted less well than those admixed with CD8+ cells, and those admixed with CD8+ cells engrafted with a mean 10-fold increase over control CD34+ cells (P = .029) (Figure 1A).
Engraftment of β2m−/− NOD/SCID mice and in vivo homing by human CD34+ cells is enhanced by CD8+ cells.
(A) Mice were injected with CD34+ alone, CD34+ and CD8+, or CD34+ and CD4+ cells. Measurements of the level of human CD45+ cells in the bone marrow were then measured by flow cytometry at 6 weeks after injection. Cells from an individual cord blood source were divided and transplanted, and the outcome of engraftment of CD34+ alone was compared to that of CD34+ and CD8+ and to that of CD34+and CD4+ cells. Transplantations were performed in duplicate; experiments using cells from the same donor source are linked by dashed lines. (B-C) Fluorescently labeled CD34+ cells, alone or mixed with CD8+ or CD4+ cells from the same donor, were injected intravenously into mice from the same litter. Nine hours following the injection, the mice were killed and the numbers of labeled cells in the bone marrow (B) or spleen (C) were measured by flow cytometry. Each point represents a single mouse. The solid bars represent mean values. (D) Transmigration of CD34+ cells alone, CD8+or CD4+ alone, or CD34+ cells in the presence of CD8+ or CD4+ cells was measured by cell counting and flow cytometry. A BMEC-coated Transwell with SDF-1α as the chemotactic agent was used. Data are represented as the means of 4 independent experiments; error bars represent SEMs. See theBlood website for Figures S1, supplemental to panel B, and S2, supplemental to panel A; go to the Supplemental Materials link at the top of the online article.
Engraftment of β2m−/− NOD/SCID mice and in vivo homing by human CD34+ cells is enhanced by CD8+ cells.
(A) Mice were injected with CD34+ alone, CD34+ and CD8+, or CD34+ and CD4+ cells. Measurements of the level of human CD45+ cells in the bone marrow were then measured by flow cytometry at 6 weeks after injection. Cells from an individual cord blood source were divided and transplanted, and the outcome of engraftment of CD34+ alone was compared to that of CD34+ and CD8+ and to that of CD34+and CD4+ cells. Transplantations were performed in duplicate; experiments using cells from the same donor source are linked by dashed lines. (B-C) Fluorescently labeled CD34+ cells, alone or mixed with CD8+ or CD4+ cells from the same donor, were injected intravenously into mice from the same litter. Nine hours following the injection, the mice were killed and the numbers of labeled cells in the bone marrow (B) or spleen (C) were measured by flow cytometry. Each point represents a single mouse. The solid bars represent mean values. (D) Transmigration of CD34+ cells alone, CD8+or CD4+ alone, or CD34+ cells in the presence of CD8+ or CD4+ cells was measured by cell counting and flow cytometry. A BMEC-coated Transwell with SDF-1α as the chemotactic agent was used. Data are represented as the means of 4 independent experiments; error bars represent SEMs. See theBlood website for Figures S1, supplemental to panel B, and S2, supplemental to panel A; go to the Supplemental Materials link at the top of the online article.
CD8+ cells enhance the in vivo homing of CD34+ cells to the bone marrow
To assess the mechanism of enhancement of engraftment by CD8+ cells, homing to the bone marrow of CD34+cells mixed with CD4+ or CD8+ cells was studied using direct examination of fluorescently labeled cells in vivo. CD8+ cells significantly enhanced the homing to the bone marrow of CFDA-SE–labeled CD34+ cells (P = .006) (Figure 1B) compared with control CD34+ cells alone. This augmentation of homing was specific to the CD8+ subset of cells. Incubation of CD34+ cells with CD4+ cells led to a decrease in bone marrow homing (P = .045). To determine if this enhancement of CD34+ localization was distinct for the bone marrow environment, homing to the spleen was evaluated (Figure 1C). Consistent with that seen in marrow, increased CD34+ homing was noted when CD8+ cells were added (P = .014), though no inhibition or a trend toward increased homing was observed with addition of CD4+ cells (P = .255).
CD8+ cells enhance the transmigration of CD34+ cells
To study the mechanism of the enhancement of in vivo homing, an in vitro transmigration model of homing was used. In these experiments, an SDF-1α concentration of 300 ng/mL was used as the chemotactic agent. This concentration of SDF-1α has been originally defined by Aiuti and colleagues as a strong stimulus for CD34+ cell chemotaxis18 and within the range of 200 ng/mL to 600 ng/mL as reported by others.28-30 To better mimic the cellular context of the bone marrow, transmigrations were carried out with adherent bone marrow endothelial cells. The immortalized primary BMEC line was used to standardize the cell environment. This cell line is derived from adult human bone marrow endothelial cells yet retains the characteristics of primary cells.31 Due to concerns about potential effects of immune activation in the mixed cell experiments, we used autologous cells only and did not mix cord blood–derived cells from different donors, thereby constraining the number of cells possible to use in each well. Although small numbers of cells (1 × 104 to 2 × 104 cells per well) were used, these numbers are now commonly used by others in chemotaxis analyses.32 33 In this assay system, minimal random migration (chemokinesis) was observed either with or without the addition of T cells (approximately 5% of the CD34+ cells transmigrating to the lower chamber). Chemotaxis of CD34+cells over a 4-hour period is shown in Figure 1D. The kinetics of transmigration demonstrated that the addition of CD8+ cells increased the transmigration of CD34+ cells at the 3- and 4-hour time points. The addition of CD4+ cells to the transmigration assay had no effect on CD34+ cell transmigration. The kinetics of transmigration of CD34+plus CD8+ or CD4+ cells paralleled the kinetics of transmigration for either lymphocyte subset alone (data not shown), suggesting that lymphoid migration kinetics dictated the effect on CD34+ cells.
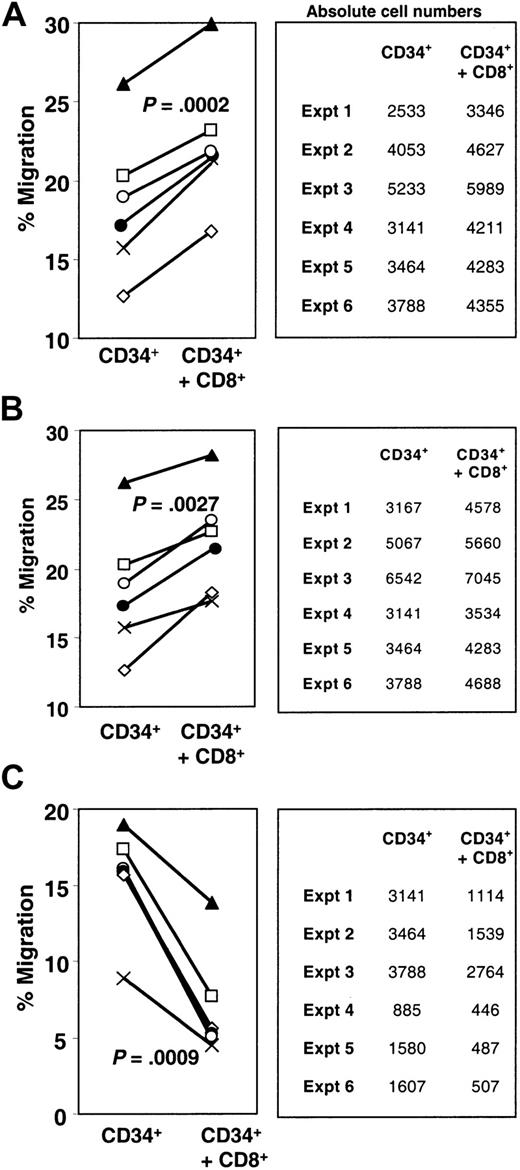
To test whether the enhancement of CD34+ transmigration was due to a factor secreted from the CD8+ cells, CD8+-conditioned medium was added to the upper well of the transmigration chamber. This had no effect on the CD34+ cell transmigration (data not shown). However, because the direct interaction of the cells could lead to the secretion of a factor from the cells, we next pretreated CD8+ cells with the secretory pathway inhibitor brefeldin A and tested their effect on CD34+ cell transmigration. In these experiments, significant enhancement of transmigration of the CD34+cells was observed upon addition of CD8+ cells (P = .0002) (Figure 2A). Treatment of the same cells with brefeldin A did not result in any discernable alteration of the enhancing effect (P = .0027) (Figure 2B). There was no significant difference between the levels of enhancement of CD34+ cell transmigration shown by untreated CD8+ cells or those that had been pretreated with brefeldin A (P = .64), arguing against a secreted factor from the CD8+ cells contributing to CD34+ cell localization.
Enhancement of transmigration is not due to a secreted factor but is dependent upon cytoskeletal rearrangements with CD8+ cells.
Transmigration of CD34+ cells in the presence of untreated CD8+ cells (A), brefeldin A–treated CD8+ cells (B), or CD8+ cells pretreated with cytochalasin D (C) was measured as previously described. Solid lines link shared donor cell sources. The absolute numbers of cells that transmigrated are indicated in the tables beside the graphs. For all graphs, ⋄ represents experiment (expt) 1; ■, expt 2; ▴, expt 3; ×, expt 4; ●, expt 5; and ○, expt 6.
Enhancement of transmigration is not due to a secreted factor but is dependent upon cytoskeletal rearrangements with CD8+ cells.
Transmigration of CD34+ cells in the presence of untreated CD8+ cells (A), brefeldin A–treated CD8+ cells (B), or CD8+ cells pretreated with cytochalasin D (C) was measured as previously described. Solid lines link shared donor cell sources. The absolute numbers of cells that transmigrated are indicated in the tables beside the graphs. For all graphs, ⋄ represents experiment (expt) 1; ■, expt 2; ▴, expt 3; ×, expt 4; ●, expt 5; and ○, expt 6.
Enhancement of CD34+ cell transmigration is dependent upon cytoskeletal rearrangement in CD8+ cells
To test whether cytoskeletal rearrangements within the CD8+ population of cells was a necessary component for the enhancement of transmigration, the CD8+ cells were pretreated with cytochalasin D, an inhibitor of actin polymerization. Cytochalasin D is well defined as an inhibitor of the cytoskeletal relationships within a cell necessary for chemotaxis34 and indeed diminished chemotaxis of the CD8+ cells to a level of 12.8% as compared with the untreated CD8+ cells (data not shown). Cytochalasin D–induced inhibition of CD8+ cell chemotaxis abrogated their enhancing effect on CD34+ cell transmigration and actually decreased CD34+ cell transmigration (P = .0009) (Figure2C). To rule out the possibility that the cytochalasin D may have leached out of the CD8+ cells and inhibited CD34+ cells directly, we also treated CD4+cells with cytochalasin D. This treatment had no effect on the level of transmigration of the CD34+ cells (data not shown). An alternative method of impairing cytoskeletal-mediated events is irradiation.35 We observed decreased CD8+ cell chemotaxis (to 8%) and abrogation of the CD8+ cell effect on CD34+ migration (migration of CD34+ cells alone vs migration of CD34+ cells plus irradiated CD8+ cells, P = .636) following pretreatment of CD8+ cells (data not shown). Together, these data demonstrate that CD8+ cell cytoskeletal rearrangements were required for the enhancement of CD34+ cell transmigration; the basis for the more profound effects of cytochalasin D is not clear but may reflect inhibitory products released by cytochalasin D–treated CD8+ cells.
Cytoskeletal rearrangements within CD8+ cells are required for the enhancement of CD34+ cell homing to the bone marrow in vivo
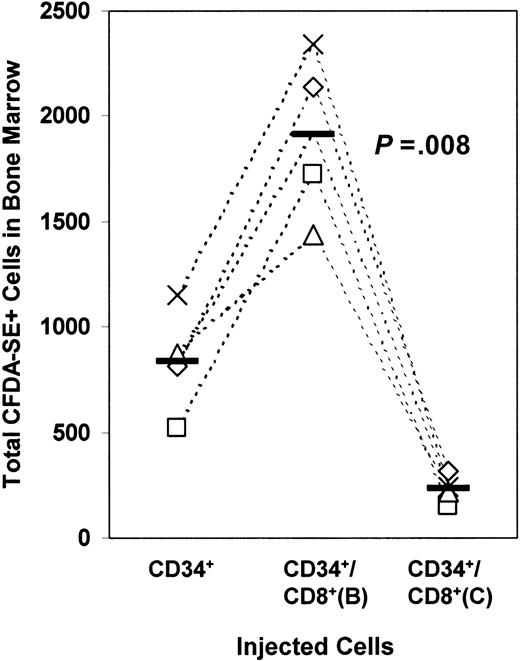
To determine whether the same mechanism by which CD8+cells enhanced in vitro CD34+ cell transmigration also applied in the in vivo setting, we treated the CD8+ cells with brefeldin A or cytochalasin D before performing in vivo homing assays. Treatment of the CD8+ cells with brefeldin A continued to result in an augmentation of CD34+ cell homing to the bone marrow (P = .008) (Figure3). Treatment of CD8+ cells with cytochalasin D led to a reduction in the number of CD34+ cells homing to the bone marrow (P = .014). These results parallel the in vitro transmigration setting, suggesting that similar mechanisms are involved in both systems.
The requirements for the enhancement of transmigration in vitro also apply in vivo.
Fluorescently labeled CD34+ cells mixed with CD8+ cells that had been pretreated with brefeldin A or cytochalasin D were injected intravenously. Homing to the bone marrow was evaluated by flow cytometry. The solid bars represent mean values. Each data point represents results from an individual mouse. Experiments using cells from the same donor source are linked by dashed lines.
The requirements for the enhancement of transmigration in vitro also apply in vivo.
Fluorescently labeled CD34+ cells mixed with CD8+ cells that had been pretreated with brefeldin A or cytochalasin D were injected intravenously. Homing to the bone marrow was evaluated by flow cytometry. The solid bars represent mean values. Each data point represents results from an individual mouse. Experiments using cells from the same donor source are linked by dashed lines.
CD8+ cells do not alter permeability of the endothelial barrier
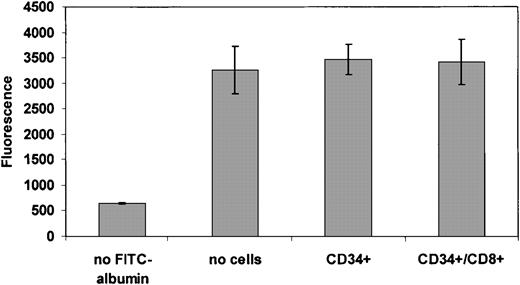
Because treatment of CD8+ cells abrogated any chemotactic ability of these cells, we investigated the possibility that CD8+ cell transmigration may alter the endothelial barrier, leading to enhancement of CD34+ cell transmigration. We first tested whether the CD8+ cells disrupted the integrity of the endothelial layer by placing FITC-conjugated albumin in the upper chamber of the Transwell. We did not observe any significant differences in the level of protein that passed across the BMEC cells in the setting of CD34+ cells with or without CD8+ cells. These data argue against a fundamental change in the endothelial barrier that may allow increased transmigration of the CD34+ cells (Figure4). Using time-lapse video microscopy over a period of 2 to 4 hours, we then observed mixtures of differentially fluorescently labeled CD34+ and CD8+ cells on the BMEC cell line. Both CD34+and CD8+ cells transmigrated through the BMEC layer but did not comigrate in tandem across the endothelial layer (Videos 1-3 in the online supplemental materials). Therefore, enhancement of CD34+ transmigration does not require cell-cell contact during the migration process itself.
CD8+ cells do not alter the integrity of the endothelial barrier.
Chemotaxis assays were performed as described with the addition of fluorescently labeled albumin. Movement of labeled albumin into the bottom of the Transwell was used as a surrogate for the integrity of the BMEC layer measuring the ability of albumin to pass through the cell-coated Transwell in the presence or absence of the indicated nonadherent cells. The data are represented as the means of 3 independent experiments; error bars represent the SEMs.
CD8+ cells do not alter the integrity of the endothelial barrier.
Chemotaxis assays were performed as described with the addition of fluorescently labeled albumin. Movement of labeled albumin into the bottom of the Transwell was used as a surrogate for the integrity of the BMEC layer measuring the ability of albumin to pass through the cell-coated Transwell in the presence or absence of the indicated nonadherent cells. The data are represented as the means of 3 independent experiments; error bars represent the SEMs.
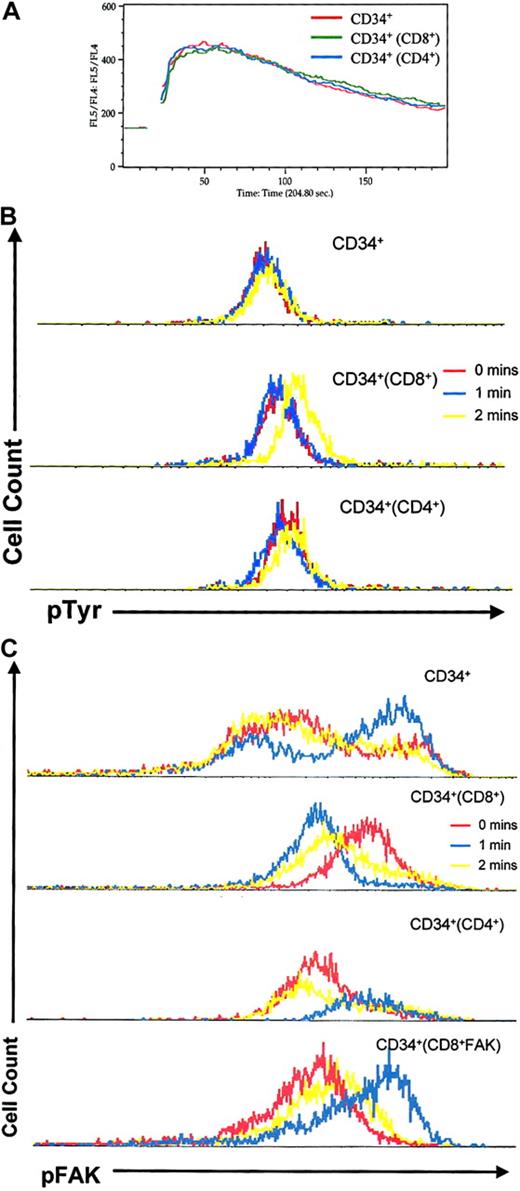
CD8+ cells affect SDF-1α–mediated downstream signaling events in CD34+ cells
Given the role of CXCR4 in stem cell homing to the bone marrow, we sought to determine if the effect of CD8+ cells on CD34+ cell homing was mediated by altered CXCR4 expression or signaling. Incubation of CD34+ cells with CD8+ or CD4+ cells did not lead to any alteration of CXCR4 expression (data not shown). To address whether signaling was affected, we incubated CD34+ cells with CD4+ or CD8+ cells and measured CXCR4 signaling of the CD34+ cells by Ca++ flux in response to SDF-1α. The Ca++ flux response for CD34+cells was unaltered whether incubated alone or with either CD4+ or CD8+ cells (Figure5A). Therefore, neither CD4+nor CD8+ cells appear to affect the intracellular calcium shifts downstream of SDF-1α interaction with CXCR4. However, measurements of the level of phosphorylated tyrosine residues within the CD34+ cells following SDF-1α stimulation demonstrated an increase only in those cells that had been incubated with CD8+ cells (Figure 5B). Examination of a specific component of the CXCR4 signaling pathway that may be envisioned to alter transmigration, focal adhesion kinase,36 37 demonstrated differences in the level of phosphorylation of this protein (increase in mean fluorescence intensity from 50.69 to 63.77) (Figure 5C). Baseline phospho-FAK mean fluorescence intensity levels were increased by the coincubation of CD34+ and CD8+ cells (from 427 to 537), indicating altered FAK activation in CD34+ cells by the presence of CD8+ cells. The level of phospho-FAK decreased with SDF-1α stimulation in the setting of CD34+ cells coincubated with CD8+ cells in contrast to CD34+ cells alone where phospho-FAK increased. These differences indicate alteration of the constitutive regulation of FAK and in the CXCR4 signaling cascade in CD34+ cells induced by the presence of CD8+ cells. The specific biochemical basis for the changes in protein phosphorylation was not further defined.
CD8+ cells alter CXCR4 signaling events in CD34+ cells.
CD34+ cells were incubated alone (red) or with CD8+ (green) or CD4+ (blue) cells. Intracellular signaling events were then measured specifically in CD34+ cells in response to SDF-1α stimulation. (A) Calcium flux. (B) Intracellular levels of phosphotyrosine as measured by staining with anti-TyrP FITC. (C) Intracellular levels of phosphorylated FAK as measured by staining with phospho-FAK goat anti-mouse (GAM) PE at the indicated intervals after addition of SDF-1α. Representative results of 3 independent experiments with consistent results are shown.
CD8+ cells alter CXCR4 signaling events in CD34+ cells.
CD34+ cells were incubated alone (red) or with CD8+ (green) or CD4+ (blue) cells. Intracellular signaling events were then measured specifically in CD34+ cells in response to SDF-1α stimulation. (A) Calcium flux. (B) Intracellular levels of phosphotyrosine as measured by staining with anti-TyrP FITC. (C) Intracellular levels of phosphorylated FAK as measured by staining with phospho-FAK goat anti-mouse (GAM) PE at the indicated intervals after addition of SDF-1α. Representative results of 3 independent experiments with consistent results are shown.
Discussion
It is known that lymphocytes augment the recovery of hematopoiesis following stem cell transplantation, yet the contribution by T cells to stem cell function has remained an area of controversy.3,4Leading hypotheses have focused on infused immune effector cells abrogating residual host rejection phenomena5 or providing cytokines that augment stem cell proliferation.10 11 The data presented here provide an alternative model in which CD8+ T cells contribute to stem/progenitor cell localization through augmentation of the entry to the bone marrow microenvironment, an essential part of establishing bone marrow hematopoiesis. The model we propose is one in which events between cotransplanted cells influence the efficiency of localization and engraftment in hematopoietic tissues. However, these early events after transplantation do not preclude later, distinct immunologically mediated phenomena. Indeed, collaborative data generated in the setting of cotransplanted T cells lacking cytotoxic effector mechanisms suggest an additional role for cytolytic interactions (G.B.A. and D.T.S., manuscript submitted).
The relationship of cell movement to hematopoiesis was first suggested by Aiuti and colleagues, who identified the chemokine receptor CXCR4 on the surface of CD34+ cells and demonstrated the ability of the cognate ligand, SDF-1α, to induce CD34+ cell transmigration.18 When mice were engineered to be deficient in either the receptor or its single known ligand, the phenotype of bone marrow hematopoiesis confirmed a relationship of chemokine-induced cell motility to stem cell localization.12-15 Recent reports correlating transmigration in vitro with engraftment kinetics in vivo in humans provided further support for the model of stem cell migration being a critical step in the successful establishment of hematopoiesis.24 These studies do not exclude other contributory, possibly linked, phenomena of adhesion and activation but argue for a causative link of migration to engraftment.
We explored the relationship of engraftment and homing using in vivo chimeric models of human hematopoiesis. The presence of CD8+ cells led to an increase in the engraftment of the CD34+ cells, recapitulating previously published reports on the effects of CD8+ cells on engraftment.5-8Analysis of the mechanism of this enhancement demonstrated that CD8+ cells but not CD4+ cells enhanced the homing of CD34+ cells to the bone marrow. Comparison of this effect in another hematopoietic organ, the spleen, also showed an enhancing effect mediated by CD8+ cells. However, in this case the CD4+ cells actually had no effect on the level of homing. These results are not entirely comparable with the effect seen in the bone marrow and may imply that differing mechanisms exist for the homing of cells to the bone marrow and spleen.38 39
Homing involves at least 3 steps, including loose or rolling interaction of cells with vascular endothelium, firm adhesion to the vessel or sinus, and diapedesis through the endothelial layer. Although any of these processes may affect in vivo homing, we noted reasonable correlation between our in vivo observations and in vitro transmigration in which only limited adhesive interactions may be necessary. To at least approximate cellular interactions in the low flow marrow sinus, we used an endothelial-coated Transwell system. Whether this system requires anything beyond cell crawling phenomena is not defined, but based on the videomicroscopic imaging of cell movement in culture (Video 1) it appears that adhesive interactions between CD34+ cells and endothelial cells are ongoing and dynamic.
The stimulus we used for cell migration was SDF-1α, known to be produced by marrow stroma. The concentration of SDF-1α selected could arguably be outside the physiologic range but is one that we found optimized CD34+ cell migration and has been used by others in either exact18 or similar concentration.28-30 Due to the affinity of SDF-1α for matrix glycoproteins, it is very difficult to accurately assess local concentrations cells encounter in the homing process, and therefore a concentration was selected based on precedent and reasonable approximation. In addition, we recognize that the cell dose we chose to assess (1:1 ratios) is arbitrary and may or may not reflect the physiologic context in which cells reside. Further, we tested only umbilical cord blood–derived CD34+ cells; other tissue sources of CD34+ may yield differing results. Recognizing these limitations to our experimental systems, we did note qualitatively consistent effects in both in vivo and in vitro contexts and did observe phenomena in vivo mimicking that seen in the setting of human allogeneic bone marrow transplantation. Whether there is a role for T cells in autologous transplantation in humans has not been defined.
The basis for a CD8+ cell effect on CD34+ cells was unexpectedly not due to a secreted cell product but, rather, required cell-cell contact and a dynamic cytoskeleton of the CD8+ cells. Given that the interaction was cytoskeleton- and time-sensitive (preincubation of CD8+ and CD34+ cells was necessary), it may be that cell crosstalk involved clustering of cell surface proteins on the CD8+cells to enable the changes in CD34+ cell function. That CD34+ cell physiology was altered is best evident in the changed profile of FAK phosphorylation under resting conditions when preincubated with CD8+ cells. Basal levels of phospho-FAK were increased following cell-cell contact, and less pronounced phosphotyrosine increases were seen after cell-cell contact and SDF-1α stimulation. What specific molecular interactions between CD8+ cells and CD34+ cells induced these changes is unknown, but subfractionation of the CD8+ cells that accomplished this change and comparing them molecularly with CD8+ cells that do not induce the effect and CD4+ cells may provide insight.
Whether the CD8+ cells affecting CD34+function bear the phenotype of the “facilitator cell” defined by various groups was not tested here. Those investigators have identified that CD8+ (T-cell receptor–negative or –positive), CD2+, CD5+, and CD3ε+ cells were able to enhance engraftment without inducing GVHD.6-8 What role such cells play in immunity and why they may have a particular effect on CD34+ cells is unknown. That effector cells of the immune response could influence primitive cells has multiple precedents in the early-acting cytokines known to be produced by mature hematopoietic cells.11 If primitive cells have the broader regenerative capability proposed by many, localization of primitive cells by immune effector cells to sites of injury could be hypothesized, though multiple other scenarios may be active.
The data presented here demonstrate cell-cell cooperativity in chemotaxis where one cell type modulates the responsiveness of a different cell type to a chemokinetic stimulus. This adds an additional dimension to chemokine activity where the cellular context in which a chemokine stimulus is received influences the phenotype of response. Depending upon the cellular milieu, a highly specific effect may be envisioned that may be physiologically significant if the model proposed here for stem cell engraftment is representative. Varying the composition of the cellular context may be a mechanism for achieving highly modulable, combinatorial effects in vivo. Understanding the role of chemotaxis in physiologic settings may therefore require analysis of chemokines on complex mixtures of cells as well as individual cell types. The potential to exploit such interactions therapeutically is evident in the bone marrow transplantation setting and may be instructive for future efforts to enhance cell targeting in vivo to bone marrow and other tissue types.
The authors thank Cory Johnson and Dr Donna Wall of St Louis University for the invaluable provision of umbilical cord blood. We also thank Drs Richard H. Evans, Tao Cheng, and Kenneth S. Cohen for their valued input.
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-02-0486.
Supported by the National Institutes of Health (D.T.S., M.C.P.), the Richard Saltonstall Charitable Foundation (D.T.S.), and the American Foundation for AIDS Research (M.C.P.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David T. Scadden, Massachusetts General Hospital, Harvard Medical School, Building 149, 13th St (Room 5212), Boston, MA 02129; e-mail:scadden.david@mgh.harvard.edu.
Supplemental data
CD34+ alone.
CD8+ alone.
CD34+ and CD8+.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal