Abstract
Risk assessment in acute myeloid leukemia (AML) using pretreatment characteristics may be improved by incorporating parameters of early response to therapy. In the 1992 trial of the German AML Cooperative Group (AMLCG), the amount of residual leukemic blasts in bone marrow was assessed one week after the first induction course (day 16 blasts). A total of 449 patients 16 to 76 years of age (median, 53 years) with de novo AML entered the trial and were evaluable. Treatment included TAD/HAM (thioguanine, cytosine arabinoside, and daunorubicin/high-dose cytosine arabinoside and mitoxantrone) double induction, TAD consolidation, and randomly either maintenance therapy or S-HAM consolidation. Cytogenetics were favorable, intermediate, unfavorable and not available in 10.0%, 48.3%, 13.1%, and 28.5%, respectively. Day 16 blasts ranged from 0% to 100% (median, 5%, mean ± SD, 18.6 ± 28.5%). Complete remission (CR) rate was 72.6%, 17.6% had persistent leukemia (PL), and 9.8% succumbed to hypoplastic death. Median overall survival (OS), event-free survival (EFS), and relapse-free survival (RFS) were 18, 9, and 15 months with 28.4%, 21.6%, and 30.1% at 5 years, respectively. As a continuous variable, day 16 blasts were related to CR rate (P < 0.0001), PL rate (P < 0.0001), OS (P < 0.0001), EFS (P < 0.0001), and RFS (P = 0.0049). Multivariate analyses identified the following parameters to be associated with the respective end points. CR rate: day 16 blasts (P < .0001), age (P = .0036), and LDH (P = .0072); OS: unfavorable cytogenetics (P < .0001), day 16 blasts (P < .0001), age (P < .0001), and LDH (P = .0040); EFS: unfavorable cytogenetics (P < .0001), LDH (P < .0001), day 16 blasts (P < .0001), and age (P = .0061); RFS: unfavorable cytogenetics (P < .0001), LDH (P < .0001), and day 16 blasts (P = .0359). The prognostic significance of day 16 blasts is independent of pretherapeutic parameters and predicts outcome even in patients achieving a CR.
Introduction
Treatment of patients with newly diagnosed acute myeloid leukemia (AML) has improved during the past decades due to the intensification of induction and postremission chemotherapies and due to the incorporation of autologous and allogeneic transplantation procedures into the first-line management of the disease. Long-term remissions, however, are achieved in a quarter of patients only.1 The prognosis of patients with AML can be estimated based on several patient-specific and disease-related factors among which karyotype abnormalities have the most important independent impact.2 3 Thus, most patients with CBF leukemias including AML associated with t(8;21) and with inv(16)/t(16;16) achieve long-lasting remissions, while in cases with abnormalities of chromosomes 5 and 7 and with complex aberrant karyotypes in particular, the median survival amounts to a few months only. Despite the use of additional prognostic factors such as age and history of preceding hematologic diseases for stratification models, the prognosis of patients within the respective subgroups remains quite heterogeneous and, thus, the prognosis of an individual patient cannot yet be estimated accurately.
To this end a vigorous assessment of treatment effects may further help to define the prognosis of the individual patient and to possibly adapt the intensity of the antileukemic therapy to be applied. Thus, the early quantification of therapy-induced cytoreduction in leukemic bone marrow has been shown to highly correlate with the response to induction therapy in a cohort of patients with newly diagnosed AML.4 Trying to further define the prognosis in individual patients, the quantification of minimal residual disease (MRD) by molecular markers was assessed. However, this approach is limited to cases of AML associated with specific genetic changes such as the translocation PML/RARα in acute promyelocytic leukemia. In these cases, a persisting or recurring positivity for the transcript during remission and the detection of a distinct level of the transcript following consolidation therapy, respectively, are associated with an increased risk of relapse.5-12 Similar approaches are being evaluated for other subgroups of AML, all of which focus on the quantification of the level of disease after patients have achieved a remission.13-17
In contrast, the early assessment of treatment response of AML has not yet been studied in larger series of AML patients. In childhood acute lymphoblastic leukemia, a rapid decline of leukemic blasts was identified as the most important prognostic factor.18 In AML, however, parameters of responsiveness identified to have a major importance were restricted mainly to the rapidity of achievement of remission19,20 or the achievement of remission by 1 course only,21 while early response to therapy as assessed by residual leukemic bone marrow blasts during aplasia has been shown to have major prognostic impact in 2 reports only.2 22 Thus, the current analysis was aimed at defining the impact of the level of bone marrow blasts 1 week after the end of the first course of induction therapy on the prognosis of patients with de novo AML of all ages treated within the 1992 trial of the German AML Cooperative Group.
Patients and methods
Patients
The current analysis is based on patients with newly diagnosed de novo AML who were treated within the prospective randomized multicenter 1992 trial of the German AML Cooperative Group. Patients older than 16 years with newly diagnosed de novo AML were eligible for this trial. Patients with acute promyelocytic leukemia were treated in a separate trial.23 Patients with prior antileukemic treatment, AML secondary to prior chemotherapy, and with AML developing from an antecedent hematologic malignancy were excluded, as were patients with severe comorbidity precluding the initiation of intensive induction chemotherapy (ie, severe uncontrolled infections, coronary heart disease World Health Organization (WHO) grades III°/IV°, congestive heart failure WHO grades III°/IV°, severe hyperbilirubinemia WHO grades III°/IV°, or severe creatinine elevation WHO grades III°/IV° unless due to leukemia). Only patients with both central cytomorphologic review including an evaluation of myelodysplastic features and assessment of residual bone marrow blast cells on day 16 are included in the present analyses.
Antileukemic therapy
Induction.
For remission induction, patients were treated according to the double induction strategy as previously published, with the second course starting on day 21 irrespective of response of the disease to the first course.2 The first course consisted of the TAD combination with standard-dose cytosine arabinoside 100 mg/m2/d continuous infusion on days 1 and 2, 100 mg/m2every 12 hours intravenously as a 1-hour infusion on days 3 to 8, daunorubicin 60 mg/m2 intravenously as a 1-hour infusion on days 3 to 5, and oral thioguanine 100 mg/m2 every 12 hours on days 3 to 9.24 The second course was HAM with high-dose AraC 3 g/m2 (1 g/m2 in patients aged 60 years and older) every 12 hours intravenously as a 3-hour infusion on days 1 to 3 and mitoxantrone 10 mg/m2 intravenously as a 1-hour infusion on days 3 to 5.25 The HAM course was scheduled to be started on day 21 unless patients had severe life-threatening nonhematologic toxicity, in case of which chemotherapy was postponed until resolution of toxicity. The second course of the double induction therapy was applied to patients older than 60 years only if they had residual leukemic blasts of 5% or more in the bone marrow on day 16 (ie, 1 week after completion of the first course).
Consolidation.
Consolidation therapy consisted of 1 course of TAD, which was applied 2 to 4 weeks after achievement of complete remission. Patients with HLA-identical sibling donors subsequently underwent allogeneic bone marrow or peripheral blood stem cell transplantation. All other patients received further treatment according to the randomization performed at study entry. Patients were randomized up-front to 3 years of myelosuppressive maintenance therapy or to a second course of intensive consolidation therapy following TAD consolidation, respectively.
Maintenance.
Maintenance therapy was applied every 4 weeks and consisted of AraC 100 mg/m2 every 12 hours subcutaneously on days 1 to 5 in combination with either daunorubicin 45 mg/m2 on days 2 and 3 (courses 1, 5, 9, etc), thioguanine 100 mg/m2 every 12 hours on days 1 to 5 (courses 2, 4, 6, etc), or cyclophosphamide 1 g/m2 on day 3 (courses 3, 7, 11, etc).20 26Treatment was delayed and doses were reduced for hematologic toxicity according to predefined criteria. Upon achievement of a cumulative dose of daunorubicin of 540 mg/m2, daunorubicin was replaced by thioguanine.
Second course of consolidation.
The second course of consolidation therapy consisted of the sequential high-dose AraC and mitoxantrone (S-HAM) combination27 and was applied 4 to 6 weeks after recovery from hematologic toxicity following TAD consolidation. S-HAM consisted of high-dose AraC (HDAraC) as a 3-hour infusion every 12 hours on days 1, 2, 8, and 9. The dose per application of HDAraC was 1 g/m2 in patients younger than 60 years and 500 mg/m2 in older patients. Mitoxantrone at 10 mg/m2 was applied as a 1-hour infusion on days 3, 4, 10, and 11.
Diagnostics
Cytomorphology.
Cytomorphologic assessment was based on May-Grünwald-Giemsa stains, myeloperoxidase reaction, nonspecific esterase using α-naphtyl-acetate, and chloroacetate-esterase stains. All stainings were performed centrally according to standard procedures.28 AML was diagnosed cytomorphologically according to the criteria of the French-American-British (FAB) classification.29-31 Classification of dysplastic features was performed as described previously in detail.32 The percentage of residual leukemic blasts in the bone marrow was assessed cytomorphologically on day 16 at the respective local institutions (ie, 1 week after completion of the first course of induction therapy).
Cytogenetics.
Cytogenetic analyses were performed centrally according to standard protocols. Cytogenetic data were classified according to the International System for Human Cytogenetic Nomenclature (ISCN).33 Patients were classified into 3 subgroups based on cytogenetics: the group associated with a favorable prognosis included AML with t(8;21), inv(16), or t(16;16); the unfavorable-prognosis group contained AML with aberrations of chromosomes 5 or 7, aberrations of 11q23 or 17p, inv(3), t(3;3), or with a complex aberrant karyotype; the group associated with an intermediate prognosis included AML with other karyotype aberrations as well as AML with a normal karyotype.
Study parameters
Bone marrow examinations were carried out on day 16 (ie, 1 week after the end of chemotherapy) and upon full recovery of peripheral blood counts. Response to therapy was assessed according to Cancer and Leukemia Group B (CALGB) criteria.2 34 Complete remission (CR) was defined by a bone marrow with normal hematopoiesis of all cell lines, fewer than 5% blast cells, and a peripheral blood with at least 1500/μL (1.5 × 109/L) neutrophils and 100 000/μL (100 × 109/L) platelets. Therapeutic failures were classified as persistent leukemia, death fewer than 7 days after completion of the first induction therapy course (early death), and death during treatment-induced bone marrow hypoplasia, irrespective of the time after chemotherapy (hypoplastic death). Cases with early death (death before day 16) were excluded from the present analyses. Relapse was defined as reinfiltration of the bone marrow by 25% or more leukemic blasts or a proven leukemic infiltration at any other site.
Survival was measured by the time from inclusion into the AML Cooperative Group (AMLCG) 1992 study to death, and event-free survival (EFS) was measured by the time from inclusion into the study to death, documentation of persistent leukemia, or relapse, respectively. Relapse-free survival (RFS) was measured by the time from achievement of CR to relapse or death during CR. Freedom from relapse was measured by the time from achievement of CR to relapse. Estimates of time-dependent variables were calculated by the Kaplan-Meier method.35 Patients receiving bone marrow transplantation were censored at the time of transplantation.
Statistics
Univariate and multivariate analyses were performed to evaluate the dependence of the variables CR, persistent leukemia, survival, EFS, and RFS on day 16 blasts as a continuous variable as well as on pretreatment factors that were previously shown to have independent prognostic significance on a similar population (favorable/intermediate/unfavorable cytogenetics as dichotomous covariates; age and lactate dehydrogenase [LDH] as continuous covariates).32
Univariate and multivariate analyses were performed for time-dependent variables by a proportional hazards model and for dichotomous variables by a logistic regression model using SAS 6.12.36 AllP values reported are 2-sided.
Because the application of the second course of double induction therapy to patients older than 60 years was dependent on achievement of fewer than 5% day 16 blasts, these analyses were performed for the total study population as well as for patients younger than 60 years only to prove the significance of the results in a most homogeneously treated group.
Study conduct
Prior to therapy all patients gave their informed consent for participation in the current evaluation after having been advised about the purpose and investigational nature of the study as well as of potential risks. The study design adhered to the declaration of Helsinki and was approved by the ethics committees of the participating institutions prior to its initiation.
Results
Patients
A total of 787 patients with AML were entered into the German AML Cooperative Group 1992 Trial between December 1992 and May 1999, 449 of whom are fully evaluable for the present analysis. In 152 patients the day 16 marrow was not available due to early death (n = 50) or due to lack of assessment because of reasons not specified (n = 102). In a further 186 patients the bone marrow evaluation at diagnosis had not been centrally reviewed, and these cases are not included in the present analysis. The patients' ages ranged from 16 to 76 years (median, 53 years), and the ratio of male-to-female was 1.02:1.00 (Table 1). Cytogenetic data were available from 321 of 449 patients (71.5%) and were rated favorable in 45 (10.0%) cases, prognostically intermediate in 217 (48.3%), unfavorable in 59 (13.1%), and were not available in 128 (28.5%). The amount of residual leukemic blasts in the bone marrow on day 16 blasts ranged from 0% to 100% (median, 5%; mean ± SD, 18.6% ± 28.5%). The distribution of the percentages of day 16 blasts is shown in Figure 1. AML subtypes according to the FAB classification are listed in Table 1. LDH in serum ranged from 98 U/L to 5220 U/L (median, 422 U/L).
Patient characteristics
| Characteristics . | n . |
|---|---|
| Sex, M/F | 227/222 |
| Median age, y (range) | 53 (16-76) |
| Cytogenetics | |
| Favorable | 45 (10.0%) |
| Intermediate | 217 (48.3%) |
| Unfavorable | 59 (13.1%) |
| NA | 128 (28.5%) |
| FAB subtype | |
| M0 | 17 (3.8%) |
| M1 | 95 (21.2%) |
| M2 | 151 (33.6%) |
| M4 | 77 (17.1%) |
| M4Eo | 31 (6.9%) |
| M5a | 24 (5.3%) |
| M5b | 33 (7.3%) |
| M6 | 16 (3.6%) |
| M7 | 2 (0.4%) |
| NA | 3 (0.7%) |
| Median LDH, U/L (range) | 422 (98-5220) |
| Median bone marrow blasts day 16, % (range) | 5% (0%-100%) |
| Characteristics . | n . |
|---|---|
| Sex, M/F | 227/222 |
| Median age, y (range) | 53 (16-76) |
| Cytogenetics | |
| Favorable | 45 (10.0%) |
| Intermediate | 217 (48.3%) |
| Unfavorable | 59 (13.1%) |
| NA | 128 (28.5%) |
| FAB subtype | |
| M0 | 17 (3.8%) |
| M1 | 95 (21.2%) |
| M2 | 151 (33.6%) |
| M4 | 77 (17.1%) |
| M4Eo | 31 (6.9%) |
| M5a | 24 (5.3%) |
| M5b | 33 (7.3%) |
| M6 | 16 (3.6%) |
| M7 | 2 (0.4%) |
| NA | 3 (0.7%) |
| Median LDH, U/L (range) | 422 (98-5220) |
| Median bone marrow blasts day 16, % (range) | 5% (0%-100%) |
NA indicates not available.
Treatment outcome
Of all 449 patients, 326 (72.6%) achieved CR, 79 (17.6%) had persistent leukemia, and 44 (9.8%) died from hypoplastic deaths. The median overall survival (OS) was 18 months (28.4% at 5 years), the median EFS was 9 months (21.6% at 5 years), and the median RFS was 15 months (30.1% at 5 years).
Prognostic impact of day 16 bone marrow blasts
Univariate analyses.
For the total study population, the percentage of day 16 blasts as a continuous variable significantly influenced both response rates and long-term outcome (Table 2). Even in patients having achieved CR, the percentage of day 16 blasts had impact on the prognosis and was significantly associated with the durations of RFS (P = .0049) and of OS (P = .0068).
Association of day 16 residual leukemic bone marrow blasts with response to therapy and long-term outcome
| End point . | P . |
|---|---|
| Complete remission | < .0001 |
| Persistent leukemia | < .0001 |
| Overall survival | < .0001 |
| Event-free survival | < .0001 |
| Relapse-free survival | .0049 |
| End point . | P . |
|---|---|
| Complete remission | < .0001 |
| Persistent leukemia | < .0001 |
| Overall survival | < .0001 |
| Event-free survival | < .0001 |
| Relapse-free survival | .0049 |
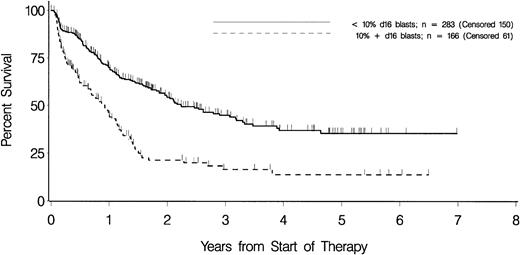
Separation of patients into 2 groups according to day 16 blasts was performed for cutoff values of 5%, 10%, 15%, 20%, and 40%. In all analyses the respective subgroups differed significantly in all end points assessed (data not shown). Separation according to a cutoff of 10% day 16 blasts resulted in a balanced distribution between both subgroups (283 vs 166). The subgroups with fewer than 10% and with 10% or more day 16 blasts had significant differences in response rates and long-term outcome (Table 3 and Figure 2). Also, in cases having achieved CR, the separation of patients according to a cutoff of 10% day 16 blasts resulted in a significantly different long-term outcome. The day 16 blasts had prognostic impact within patients receiving either maintenance therapy or S-HAM as second course of consolidation therapy. Thus, in both study arms day 16 blasts were significantly related to EFS (P < .0001 and P < .0001, respectively), OS (P < .0001 and P < .0001, respectively), and RFS (P = .0994 andP = .0454, respectively). Furthermore, there is no indication that there were differences between patients with fewer than 10% day 16 blasts and those with 10% or more day 16 blasts in the types of second-line therapy (specified in 85.9% vs 78.2%), which possibly could have affected overall survival. Thus, regimens applied included high-dose cytosine arabinoside plus anthracycline with or without fludarabine (51.2% vs 51.4%), standard-dose cytosine arabinoside plus anthracycline with or without etoposide (21.1% vs 21.6%), anthracycline with or without etoposide (12.7% vs 10.8%), and supportive therapy only without antileukemic therapy (15.1% vs 16.2%).
Outcome of patients separated according to a cutoff level of 10% day 16 blasts
| . | Patients with fewer than 10% day 16 blasts . | Patients with 10% or more day 16 blasts . | P . |
|---|---|---|---|
| Complete remission | 83.75% | 53.61% | < .0001 |
| Persistent leukemia | 2.83% | 32.53% | < .0001 |
| Overall survival | |||
| Median, mo | 27 | 11 | < .0001 |
| 5-y survival | 35.4% | 13.7% | |
| Event-free survival | |||
| Median, mo | 14 | 3 | < .0001 |
| 5-y EFS | 27.4% | 10.9% | |
| Overall survival in patients with CR | |||
| Median, mo | 37 | 18 | .01972 |
| 5-y survival | 40.6% | 25.4% | |
| Relapse-free survival in patients with CR | |||
| Median, mo | 19 | 10 | .01035 |
| 5-y RFS | 32.9% | 20.8% | |
| Freedom from relapse in patients with CR | |||
| Median, mo | 21 | 10 | .01523 |
| 5-y FR | 37.1% | 27.4% |
| . | Patients with fewer than 10% day 16 blasts . | Patients with 10% or more day 16 blasts . | P . |
|---|---|---|---|
| Complete remission | 83.75% | 53.61% | < .0001 |
| Persistent leukemia | 2.83% | 32.53% | < .0001 |
| Overall survival | |||
| Median, mo | 27 | 11 | < .0001 |
| 5-y survival | 35.4% | 13.7% | |
| Event-free survival | |||
| Median, mo | 14 | 3 | < .0001 |
| 5-y EFS | 27.4% | 10.9% | |
| Overall survival in patients with CR | |||
| Median, mo | 37 | 18 | .01972 |
| 5-y survival | 40.6% | 25.4% | |
| Relapse-free survival in patients with CR | |||
| Median, mo | 19 | 10 | .01035 |
| 5-y RFS | 32.9% | 20.8% | |
| Freedom from relapse in patients with CR | |||
| Median, mo | 21 | 10 | .01523 |
| 5-y FR | 37.1% | 27.4% |
Overall survival in patients with fewer than 10% and 10% or more day 16 blasts.
Tick marks indicate patients who were alive at last follow-up. Patients undergoing allogeneic transplantation are censored at the time of transplantation.
Overall survival in patients with fewer than 10% and 10% or more day 16 blasts.
Tick marks indicate patients who were alive at last follow-up. Patients undergoing allogeneic transplantation are censored at the time of transplantation.
Multivariate analyses.
The day 16 blasts were entered into a multivariate model in addition to previously defined independent prognostic factors—that is, unfavorable cytogenetics, intermediate cytogenetics, favorable cytogenetics, age (continuous variable), and LDH (continuous variable).32The day 16 blasts were independently associated with all analyzed end points (Table 4). The day 16 blasts were the parameter resulting in the strongest association with the CR rate (P < .0001). The day 16 blasts had independent prognostic significance even in patients achieving a CR—that is, the day 16 blasts were an independent prognostic parameter for RFS (P = .0359).
Multivariate analysis of associations between prognostic parameters and outcome
| End point . | Parameter . | P . |
|---|---|---|
| Complete remission | ||
| Day 16 blasts | < .0001 | |
| Age | .0036 | |
| LDH | .0072 | |
| Overall survival | ||
| Unfavorable cytogenetics | < .0001 | |
| Day 16 blasts | < .0001 | |
| Age | < .0001 | |
| LDH | .0040 | |
| Event-free survival | ||
| Unfavorable cytogenetics | < .0001 | |
| LDH | < .0001 | |
| Day 16 blasts | < .0001 | |
| Age | .0061 | |
| Relapse-free survival | ||
| Unfavorable cytogenetics | < .0001 | |
| LDH | < .0001 | |
| Day 16 blasts | .0359 |
| End point . | Parameter . | P . |
|---|---|---|
| Complete remission | ||
| Day 16 blasts | < .0001 | |
| Age | .0036 | |
| LDH | .0072 | |
| Overall survival | ||
| Unfavorable cytogenetics | < .0001 | |
| Day 16 blasts | < .0001 | |
| Age | < .0001 | |
| LDH | .0040 | |
| Event-free survival | ||
| Unfavorable cytogenetics | < .0001 | |
| LDH | < .0001 | |
| Day 16 blasts | < .0001 | |
| Age | .0061 | |
| Relapse-free survival | ||
| Unfavorable cytogenetics | < .0001 | |
| LDH | < .0001 | |
| Day 16 blasts | .0359 |
Only independent parameters are shown; parameters are sorted in order of significance.
Analyses in cytogenetically defined subgroups.
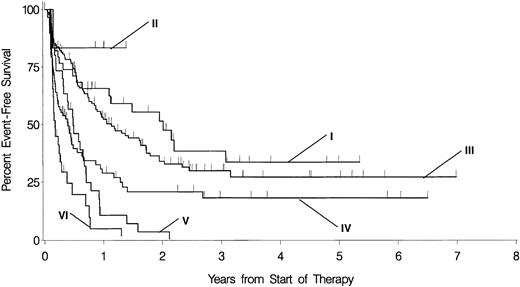
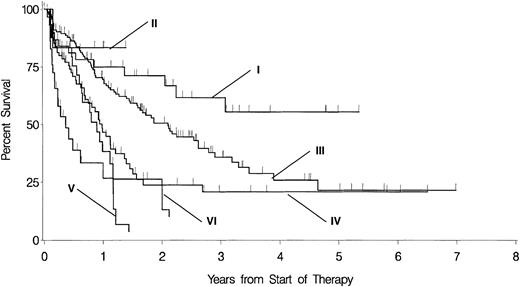
Within the cytogenetically defined subgroups of patients with prognostically intermediate and unfavorable karyotypes, respectively, day 16 blasts as a continuous variable were significantly associated with CR rate, rate of persistent leukemia, OS, and EFS (Table5). There were no associations between the respective end points and the day 16 blasts within the group of patients with favorable cytogenetics. As depicted in Figures3 and 4, 81 of 217 patients out of the group with prognostically intermediate karyotypes have 10% or more day 16 blasts. The prognosis of these patients is most similar to those cases with prognostically unfavorable karyotypes and fewer than 10% day 16 blasts. In addition, 29 of 59 patients out of the group with prognostically unfavorable karyotypes have 10% or more day 16 blasts and are prone to a particularly dismal prognosis.
Association of day 16 residual leukemic bone marrow blasts with response to therapy and long-term outcome in cytogenetically defined subgroups
| End point . | Cytogenetics . | ||
|---|---|---|---|
| Favorable (n = 45) . | Intermediate (n = 217) . | Unfavorable (n = 59) . | |
| Complete remission | NS | P < .0001 | P = .0034 |
| Event-free survival | NS | P < .0001 | P = .0061 |
| Overall survival | NS | P = .0002 | P = .0418 |
| Median event-free survival, fewer than 10% versus 10% or more day 16 blasts | 25 mo vs NR, NS | 14 mo vs 5 mo, P = .00031 | 6 mo vs 2 mo, P = .00768 |
| Median overall survival, fewer than 10% versus 10% or more day 16 blasts | NR vs NR, NS | 26 mo vs 12 mo, P = .00263 | 11 mo vs 4 mo, P = .02095 |
| End point . | Cytogenetics . | ||
|---|---|---|---|
| Favorable (n = 45) . | Intermediate (n = 217) . | Unfavorable (n = 59) . | |
| Complete remission | NS | P < .0001 | P = .0034 |
| Event-free survival | NS | P < .0001 | P = .0061 |
| Overall survival | NS | P = .0002 | P = .0418 |
| Median event-free survival, fewer than 10% versus 10% or more day 16 blasts | 25 mo vs NR, NS | 14 mo vs 5 mo, P = .00031 | 6 mo vs 2 mo, P = .00768 |
| Median overall survival, fewer than 10% versus 10% or more day 16 blasts | NR vs NR, NS | 26 mo vs 12 mo, P = .00263 | 11 mo vs 4 mo, P = .02095 |
Univarite analyses for correlation of day 16 blasts as a continuous variable with the respective end points were performed within the respective groups with favorable, prognostically intermediate, and unfavorable cytogenetics. In addition, median event-free survival and median overall survival are shown for the respective cyogenetically defined risk groups as separated according to fewer than 10% versus 10% or more day 16 blasts. NS indicates not significant; NR, not reached.
EFS in patients with the respective cytogenetically defined risk group separated according to day 16 blasts fewer than 10% versus 10% or more.
I indicates favorable cytogenetics and day 16 blasts fewer than 10% (n = 39, censored = 18; median, 25 months); II, favorable cytogenetics and day 16 blasts 10% or more (n = 6, censored = 5; median, not reached); III, prognostically intermediate cytogenetics and day 16 blasts fewer than 10% (n = 136, censored = 56; median, 14 months); IV, prognostically intermediate cytogenetics and day 16 blasts 10% or more (n = 81, censored = 24; median, 5 months); V, unfavorable cytogenetics and day 16 blasts fewer than 10% (n = 30, censored = 1; median, 6 months); VI, unfavorable cytogenetics and day 16 blasts 10% or more (n = 29, censored = 3; median, 2 months). The level of significance of the respective differences according to fewer than 10% versus 10% or more day 16 blasts within the groups with favorable, prognostically intermediate, and unfavorable cytogenetics are nonsignificant (ns),P = .00031 and P = .00768, respectively.
EFS in patients with the respective cytogenetically defined risk group separated according to day 16 blasts fewer than 10% versus 10% or more.
I indicates favorable cytogenetics and day 16 blasts fewer than 10% (n = 39, censored = 18; median, 25 months); II, favorable cytogenetics and day 16 blasts 10% or more (n = 6, censored = 5; median, not reached); III, prognostically intermediate cytogenetics and day 16 blasts fewer than 10% (n = 136, censored = 56; median, 14 months); IV, prognostically intermediate cytogenetics and day 16 blasts 10% or more (n = 81, censored = 24; median, 5 months); V, unfavorable cytogenetics and day 16 blasts fewer than 10% (n = 30, censored = 1; median, 6 months); VI, unfavorable cytogenetics and day 16 blasts 10% or more (n = 29, censored = 3; median, 2 months). The level of significance of the respective differences according to fewer than 10% versus 10% or more day 16 blasts within the groups with favorable, prognostically intermediate, and unfavorable cytogenetics are nonsignificant (ns),P = .00031 and P = .00768, respectively.
OS in patients with the respective cytogenetically defined risk group separated according to day 16 blasts less than 10% versus 10% or more.
I indicates favorable cytogenetics and day 16 blasts fewer than 10% (n = 39, censored = 26; median, nr); II, favorable cytogenetics and day 16 blasts 10% or more (n = 6, censored = 5; median, nr); III, prognostically intermediate cytogenetics and day 16 blasts fewer than 10% (n = 136, censored = 64; median, 26 months); IV, prognostically intermediate cytogenetics and day 16 blasts 10% or more (n = 81, censored = 33; median, 12 months); V, unfavorable cytogenetics and day 16 blasts fewer than 10% (n = 30, censored = 10; median, 11 months); VI, unfavorable cytogenetics and day 16 blasts 10% or more (n = 29, censored = 7; median, 4 months). The level of significance of the respective differences according to fewer than 10% versus 10% or more day 16 blasts within the groups with favorable, prognostically intermediate, and unfavorable cytogenetics are ns,P = .00263 and P = .02095, respectively.
OS in patients with the respective cytogenetically defined risk group separated according to day 16 blasts less than 10% versus 10% or more.
I indicates favorable cytogenetics and day 16 blasts fewer than 10% (n = 39, censored = 26; median, nr); II, favorable cytogenetics and day 16 blasts 10% or more (n = 6, censored = 5; median, nr); III, prognostically intermediate cytogenetics and day 16 blasts fewer than 10% (n = 136, censored = 64; median, 26 months); IV, prognostically intermediate cytogenetics and day 16 blasts 10% or more (n = 81, censored = 33; median, 12 months); V, unfavorable cytogenetics and day 16 blasts fewer than 10% (n = 30, censored = 10; median, 11 months); VI, unfavorable cytogenetics and day 16 blasts 10% or more (n = 29, censored = 7; median, 4 months). The level of significance of the respective differences according to fewer than 10% versus 10% or more day 16 blasts within the groups with favorable, prognostically intermediate, and unfavorable cytogenetics are ns,P = .00263 and P = .02095, respectively.
Analysis of patients younger than 60 years only.
In patients younger than 60 years who were treated with both courses of double induction therapy independent of the percentage of day 16 blasts, the prognostic significance of day 16 blasts was proved. Thus, the day 16 blasts as a continuous variable were highly correlated with response to therapy as well as long-term outcome in univariate analyses (Table 6) and prove to be of independent and major prognostic significance for all end points in multivariate analyses (Table 7).
Association of day 16 residual leukemic bone marrow blasts with response to therapy and long-term outcome in patients younger than 60 years only
| End point . | P . |
|---|---|
| Complete remission | < .0001 |
| Persistent leukemia | < .0001 |
| Overall survival | < .0001 |
| Event-free survival | < .0001 |
| Relapse-free survival | .0029 |
| End point . | P . |
|---|---|
| Complete remission | < .0001 |
| Persistent leukemia | < .0001 |
| Overall survival | < .0001 |
| Event-free survival | < .0001 |
| Relapse-free survival | .0029 |
Multivariate analysis of associations between prognostic parameters and outcome in patients younger than 60 years only
| End point . | Parameter . | P . |
|---|---|---|
| Complete remission | ||
| Day 16 blasts | < .0001 | |
| Age | .0096 | |
| Overall survival | ||
| Day 16 blasts | .0016 | |
| Age | .0030 | |
| Unfavorable cytogenetics | .0037 | |
| LDH | .0064 | |
| Event-free survival | ||
| Unfavorable cytogenetics | < .0001 | |
| Day 16 blasts | < .0001 | |
| LDH | .0002 | |
| Relapse-free survival | ||
| Unfavorable cytogenetics | < .0001 | |
| LDH | < .0001 | |
| Day 16 blasts | .0416 |
| End point . | Parameter . | P . |
|---|---|---|
| Complete remission | ||
| Day 16 blasts | < .0001 | |
| Age | .0096 | |
| Overall survival | ||
| Day 16 blasts | .0016 | |
| Age | .0030 | |
| Unfavorable cytogenetics | .0037 | |
| LDH | .0064 | |
| Event-free survival | ||
| Unfavorable cytogenetics | < .0001 | |
| Day 16 blasts | < .0001 | |
| LDH | .0002 | |
| Relapse-free survival | ||
| Unfavorable cytogenetics | < .0001 | |
| LDH | < .0001 | |
| Day 16 blasts | .0416 |
Only independent parameters are shown; parameters are sorted in order of significance.
Discussion
Despite the use of stratification models for the treatment of patients with AML that are based mainly on pretherapeutic prognostic parameters such as cytogenetics and age, the prognosis of patients within the respective groups remains heterogeneous. In contrast, the early assessment of response to therapy represents an in vivo assessment of chemosensitivity and may be a powerful tool to delineate the prognosis in individual patients, as has been demonstrated for childhood acute lymphoblastic leukemias and osteosarcomas.18,37 As a consequence, this measure may be implemented into treatment decision strategies. Accordingly, to improve the stratification models used in AML, the current study aimed at defining therapy-dependent prognostic parameters. Along this line, the amount of residual leukemic blasts 1 week after the end of the first course of induction therapy (ie, on day 16) proved to be of major prognostic significance with regard to all analyzed end points independently of previously defined pretherapeutic prognostic parameters. Thus, highly significant correlations exist between day 16 blasts and CR rate, rate of persistent leukemia, OS, EFS, and RFS. As might have been anticipated, day 16 blasts were the factor having the strongest association with the CR rate and with the rate of persistent leukemia. In contrast, with regard to end points reflecting the long-term outcome (ie, OS, EFS, and RFS), unfavorable cytogenetics was the most important factor. However, day 16 blasts still had independent prognostic significance. In particular, the influence of day 16 blasts was not limited to the initial treatment phase but was also demonstrated for the long-term outcome. Thus, besides the influence on OS and EFS, both being closely connected with the CR rate, day 16 blasts also affected the outcome of patients having achieved a CR as demonstrated by the independent impact on the RFS. Furthermore, univariate and multivariate analyses limited to patients younger than 60 years who were uniformly treated by 2 courses of double induction therapy irrespective of response to the first course proved the significant correlation of day 16 blasts with all end points analyzed as well as the independent prognostic significance of day 16 blasts. These results are in accordance with analyses performed during a previous trial of the German AMLCG demonstrating an independent prognostic significance of day 16 blasts on OS and on RFS in patients 16 to 60 years of age.2
The present analyses are based on a large study population with no upper age limit (median age, 53 years). Besides the diagnosis of de novo AML there were no further limitations to the eligibility of the patients. Thus, the distribution of cytogenetically defined subgroups rather reflects the pattern observed in population-based analyses38 39 and suggests that the prognostic significance of day 16 blasts applies generally to patients with de novo AML. In fact, analyses within cytogenetically defined subgroups confirm the importance of day 16 blasts. The day 16 blasts had no impact on the outcome of patients with favorable karyotype abnormalities; however, due to the overall superior outcome of these patients, the identification of an additional prognostic parameter is rather unlikely. Underlining the importance for the patients with de novo AML in general, the day 16 blasts were significantly associated with all analyzed end points within both the prognostically intermediate and the prognostically unfavorable subgroups. Thus, even in the group of 59 patients with unfavorable cytogenetics the prognosis was heterogeneous and was related to day 16 blasts not only with regard to the CR rate (P = .0034) but also with regard to OS and EFS (P = .0418 and P = .0061, respectively).
Previous analyses dealing with the leukemic cell mass have focused on the prognostic relevance of the white blood cell (WBC) count at presentation of the patients. However, only limited efforts were made to characterize the dynamics of their elimination. Thus, a hyperleukocytosis has been identified to be associated with a higher early death rate and an inferior OS.40-44 These associations have been confirmed in some multivariate analyses.45-48
In contrast, there are only a few studies addressing issues similar to those in the present analysis. The rapidity of achievement of CR has been identified to influence the patients' outcome in 2 studies. Thus, it was demonstrated that patients achieving a CR within 30 days of the start of antileukemic therapy had a superior remission duration compared with other patients (n = 156;P = .017).20 Similarly, there was a strong inverse correlation between time to achievement of CR and RFS (n = 1101; P < .001), and a duration to achievement of CR of more than 50 days was associated with a long-term outcome resembling that of patients with resistant disease, while other patients had a strikingly superior outcome (n = 1101).19
The present data are in agreement with those reported from the Medical Research Council (MRC) AML 10 trial49 where the response to the first course of induction therapy has been identified as an independent prognostic factor in a large study population of 1711 patients up to 55 years of age. The MRC therefore incorporated early response together with cytogenetics into a prognostic test discriminating 3 subgroups with highly differing outcomes.21 In the MRC AML 10 trial, the response had been categorized into 3 groups according to bone marrow blast counts of fewer than 5%, 5% to 15%, and more than 15% as assessed 2 weeks after completion of induction therapy. As in the present analysis, these categories did not influence the outcome of patients with prognostically favorable cytogenetics but affected the outcome of patients within both the intermediate and unfavorable groups.
Based on the current data, the day 16 blasts represent a highly independent and sensitive prognostic factor and may be used for the stratification of treatment early enough before the second course of a double induction regimen. Clearly, this parameter allows the refinement but not the replacement of the most important system for a prognostically based classification of patients with AML (ie, the grouping according to karyotype abnormalities).3,33,50 The monitoring of early reduction of the leukemic cell burden may be further improved by methods more sensitive and reproducible than cytomorphology, such as immunopenotyping using multiparameter flow cytometry.51 52 Both methods are applied in parallel within the ongoing trial of the German AMLCG to accomplish a comparative analysis.
Appendix: Centers participating in the German AML Cooperative Group
University Hospital Aachen: T. H. Ittel; University Hospital Berlin-Buch: W. D. Ludwig; University Hospital Berlin-Steglitz: E. Thiel; Krankenhaus Neukölln, Berlin: A. Grüneisen; Franziskus-Hospital Bielefeld: H. J. Weh; Zentralkrankenhaus St. Jürgens, Bremen: U. Kubica; Krankenhaus Düren: J. Karow; University Hospital Düsseldorf: C. Aul; St.-Antonius-Hospital Eschweiler: R. Fuchs; University Hospital Göttingen: W. Hiddemann; Städtisches Krankenhaus Gütersloh: R. Depenbusch; Katholisches Krankenhaus Hagen: H. Eimermacher; Städtisches Krankenhaus Martha-Maria, Halle: U. Haak; Allgemeines Krankenhaus Altona, Hamburg: D. Braumann; Evangelisches Krankenhaus Hamm: A. Grote-Metke; Kreiskrankenhaus Herford: U. Schmitz-Hübner; Städtische Kliniken Kassel: W. D. Hirschmann; University Hospital Kiel: H. Löffler; University Hospital Köln: P. Staib; Städtische Krankenanstalten Krefeld: M. Planker; Dreifaltigkeits-Hospital Lippstadt: K. A. Jost; Städtisches Krankenhaus Süd, Lübeck: H. Bartels; Klinikum der Stadt Ludwigshafen: M. Baldus; University Hospital Mannheim: E. Lengfelder; Krankenhaus Maria Hilf, Mönchengladbach: H. E. Reis; University Hospital Münster: T. Büchner; Paracelsusklinik Osnabrück: O. M. Koch; Städtisches Krankenhaus Osnabrück: T. Hegge; University Hospital Regensburg: A. Reichle; Klinikum Landeshauptstadt Wiesbaden: H. G. Fuhr; St-Willehad-Hospital Wilhelmshaven W. Augener; Heinrich-Braun-Krankenhaus Zwickau: G. Schott.
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2002-02-0532.
A complete list of the members of the German AML Cooperative Group appears in the “Appendix.”
W.K. and T.H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Wolfgang Kern, Ludwig-Maximilians-University, University Hospital Grosshadern, Department of Internal Medicine III, 81366 Muenchen, Germany; e-mail:wolfgang.kern@med3.med.uni-muenchen.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal