Abstract
Fifty patients with Philadelphia chromosome–positive (Ph+) chronic myelogenous leukemia (CML) in early chronic phase received imatinib mesylate, 400 mg orally daily. After a median follow-up of 9 months, 49 patients (98%) achieved a complete hematologic response and 45 patients (90%) achieved a major cytogenetic response, complete in 36 patients (72%). Compared with similar patients who received interferon-α with or without hydroxyurea or other interferon-α combination regimens, those receiving imatinib mesylate had higher incidences of complete and major (Ph < 35%) cytogenetic responses at 3 months (34% and 74% versus 1%-4% and 9%-24%, respectively), 6 months (52% and 80% versus 3%-7% and 11%-28%, respectively), and 9 months (60% and 77% versus 5%-11% and 14%-30%, respectively; P < .001). Competitive quantitative polymerase chain reaction (QPCR) studies at 9 months showed a median QPCR value (ratio of BCR-ABL/ABL transcripts × 100) of 0.59% overall and of 0.24% (range, 0.001%-29.5%) for complete cytogenetic response.
Introduction
The causal association between the Philadelphia chromosome (Ph) and BCR-ABL molecular events and the pathophysiology of chronic myelogenous leukemia (CML) has focused research on strategies that suppress the Ph+ cells or the expression of BCR-ABL.1-4 These have included allogeneic stem cell transplantation (SCT), interferon α (IFN-α), cytosine arabinoside, and others.5-13 Achieving minimal residual disease (major or complete cytogenetic response) has been independently associated with survival prolongation and has become the therapeutic research goal. Imatinib mesylate (Gleevec, STI571), a selective Bcr-Abl tyrosine kinase inhibitor, has shown activity in all CML phases (blastic, accelerated, chronic after failure of IFN-α).14-20 Herein, we summarize our results, previously reported in abstract form,21 in patients with newly diagnosed CML treated with imatinib mesylate 400 mg orally daily, demonstrating high early rates of major and complete cytogenetic responses.
Study design
Study group
Patients with Ph+ CML in early chronic phase (diagnosis to therapy < 12 months) were treated; informed consent was obtained according to institutional guidelines. Eligibility criteria were age 15 years or older; Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2; adequate renal (creatinine less than twice upper limit of normal), hepatic (bilirubin less than twice upper limit of normal), and cardiac functions (New York Heart Association cardiac disease grade 3-4 excluded); no prior imatinib mesylate therapy; and no more than 1 month of IFN-α therapy. Women of childbearing potential were required to have a negative pregnancy test before starting imatinib mesylate; all patients at risk were required to use barrier contraception while on therapy. Chronic-phase CML was defined as less than 15% blasts, less than 20% basophils, and less than 30% blasts plus promyelocytes in the peripheral blood and bone marrow, and platelet counts of 100 × 109/L or higher.
Therapy and monitoring
Patients received imatinib mesylate 400 mg orally daily. Patient monitoring and dose reductions of imatinib mesylate for nonhematologic or hematologic toxicities were detailed previously.18 When possible, patients were not reduced below a minimal daily dose of 300 mg daily. If therapy at this dose resulted in severe myelosuppression (not manageable with growth factors, eg, epoetin alfa [Procrit], granulocyte colony-stimulating factor [G-CSF]), the treatment was interrupted and resumed, rather than the dose reduced to less than 300 mg daily. Severe extramedullary toxicities with doses of 300 mg daily resulted in discontinuation of therapy.
Marrow studies including morphology and cytogenetics or interphase fluorescence in situ hybridization (iFISH) were performed every 3 months; iFISH used the Vysis BCR-ABL ES probe which, in normal controls has a positive mean value of 0.4% ± 1.2% (2 SDs); the upper normal limit is 1.5%. The iFISH analysis is conducted on 200 interphases. Competitive quantitative polymerase chain reaction (QPCR) studies were conducted on marrow samples as previously described22-25 (Guo et al, manuscript submitted). The QPCR values were expressed as a ratio-percentage (BCR-ABL/ABL transcripts × 100). Side effects were graded according to National Cancer Institute (NCI) Common Toxicity Criteria, version 2.0.
Response criteria and statistical considerations
Response criteria were as described.6 8 A complete hematologic response (CHR) was defined as white blood cell (WBC) count less than 10 × 109/L, platelets less than 450 × 109/L, no immature peripheral cells (blasts, promyelocytes, myelocytes), and disappearance of all signs and symptoms related to leukemia, including palpable splenomegaly, lasting for at least 4 weeks. This was further categorized by the best cytogenetic response: complete, Ph+ 0%; partial, Ph+ 1% to 34%; and minor, Ph+ 35% to less than or equal to 90%. A major cytogenetic response included complete plus partial cytogenetic responses (Ph+ less than 35%). At least 20 metaphases were analyzed for a cytogenetic response to be evaluable. If cytogenetic analysis was not successful, the ratio of iFISH on therapy to pretreatment iFISH was considered for cytogenetic response evaluation.
Historical control groups
These included patients with Ph+ early chronic-phase CML (diagnosis to therapy < 12 months) treated with IFN-α alone or with hydroxyurea (Hydrea) or IFN-γ (n = 274), IFN-α and low-dose cytarabine (n = 257), or IFN-α with low-dose cytarabine and homoharringtonine (n = 90). These patients had similar follow-up studies as the patients on the current study.
Results and discussion
Study groups
The characteristics of the 50 patients in the study are detailed in Table 1. Their median age was 48 years (range, 15-79 years). The median time from diagnosis to therapy was 1.5 months (range, 0-11 months). Fifteen patients had no prior therapy; 35 patients had received short courses of hydroxyurea (33 patients) or IFN-α (less than 2 weeks; 2 patients).
Characteristics of the study groups
| . | Imatinib (N = 50) (%) . | IFN-α (N = 274) (%) . | IFN-α + ara-C (N = 257) (%) . | IFN-α + ara-C + HHT (N = 90) (%) . | P . | P for imatinib versus all IFN-α regimens . |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Age older than 60 y | 13 (26) | 34 (12) | 28 (11) | 4 (4) | .002 | .004 |
| Male | 26 (52) | 166 (61) | 146 (57) | 46 (51) | NS | NS |
| Splenomegaly | 11 (22) | 116 (42) | 72 (28) | 26 (29) | .001 | NS |
| Hemoglobin less than 12g/dL | 19 (38) | 107 (39) | 95 (37) | 28 (31) | NS | NS |
| WBC count more than 50 × 109/L | 11 (22) | 143 (52) | 86 (33) | 20 (22) | <.001 | .02 |
| Platelets more than 450 × 109/L | 17 (34) | 122 (44) | 81 (32) | 29 (32) | .01 | NS |
| Peripheral blasts (any) | 14 (28) | 94 (34) | 66 (26) | 17 (19) | .02 | NS |
| Marrow blasts more than 5% | 0 (0) | 11 (4) | 11 (4) | 2 (2) | NS | NS |
| Peripheral basophils 7% or higher | 10 (20) | 46 (17) | 42 (16) | 16 (18) | NS | NS |
| Marrow basophils 4% or higher | 12 (24) | 63 (23) | 28 (11) | 19 (21) | .02 | NS |
| Cytogenetic clonal evolution | 0 (0) | 5 (2) | 20 (8) | 4 (4) | NS | NS |
| Risk group (Sokal model) | ||||||
| Good | 25 (51) | 124 (52) | 111 (56) | 34 (53) | NS | NS |
| Intermediate | 13 (26) | 58 (24) | 52 (26) | 24 (37) | ||
| Poor | 11 (22) | 54 (23) | 34 (17) | 6 (9) | ||
| Unknown | 9 | 38 | 60 | 26 |
| . | Imatinib (N = 50) (%) . | IFN-α (N = 274) (%) . | IFN-α + ara-C (N = 257) (%) . | IFN-α + ara-C + HHT (N = 90) (%) . | P . | P for imatinib versus all IFN-α regimens . |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Age older than 60 y | 13 (26) | 34 (12) | 28 (11) | 4 (4) | .002 | .004 |
| Male | 26 (52) | 166 (61) | 146 (57) | 46 (51) | NS | NS |
| Splenomegaly | 11 (22) | 116 (42) | 72 (28) | 26 (29) | .001 | NS |
| Hemoglobin less than 12g/dL | 19 (38) | 107 (39) | 95 (37) | 28 (31) | NS | NS |
| WBC count more than 50 × 109/L | 11 (22) | 143 (52) | 86 (33) | 20 (22) | <.001 | .02 |
| Platelets more than 450 × 109/L | 17 (34) | 122 (44) | 81 (32) | 29 (32) | .01 | NS |
| Peripheral blasts (any) | 14 (28) | 94 (34) | 66 (26) | 17 (19) | .02 | NS |
| Marrow blasts more than 5% | 0 (0) | 11 (4) | 11 (4) | 2 (2) | NS | NS |
| Peripheral basophils 7% or higher | 10 (20) | 46 (17) | 42 (16) | 16 (18) | NS | NS |
| Marrow basophils 4% or higher | 12 (24) | 63 (23) | 28 (11) | 19 (21) | .02 | NS |
| Cytogenetic clonal evolution | 0 (0) | 5 (2) | 20 (8) | 4 (4) | NS | NS |
| Risk group (Sokal model) | ||||||
| Good | 25 (51) | 124 (52) | 111 (56) | 34 (53) | NS | NS |
| Intermediate | 13 (26) | 58 (24) | 52 (26) | 24 (37) | ||
| Poor | 11 (22) | 54 (23) | 34 (17) | 6 (9) | ||
| Unknown | 9 | 38 | 60 | 26 |
HHT indicates homoharringtonine; and NS, not significant.
Response
The median follow-up time is 9 months. One patient discontinued imatinib mesylate after 4 weeks because of recurrent severe hepatotoxicity; 49 patients (98%) achieved CHR (median time to CHR 2 weeks), and all 49 patients (98%) had a cytogenetic response: complete in 36 (72%), partial in 9 (18%), and minor in 4 (8%). The major cytogenetic response rate was thus 90% (45 of 50 patients). When only cytogenetic studies (without complementary iFISH studies) were considered, the cytogenetic response rates were complete in 34 (68%), partial in 9 (18%), and minor in 4 (8%). The complete cytogenetic response rate was 35% after 3 months and 53% after 6 months of therapy (Table 2). For interest, we also included the results of patients treated at our institution with imatinib mesylate for chronic-phase CML after IFN-α failure.26 Response rates were higher (all values ofP < .001) than those achieved with IFN-α regimens (Table2). Currently, 44 patients continue on imatinib mesylate therapy; 6 patients are off study because of development of myeloid blastic phase (n = 2; at 6 and 6 months), hematologic relapse (n = 1; after 6 months of therapy), severe transient liver toxicities (n = 2; after 1 month and 9 months); and noncompliance (n = 1). The 2 patients who developed blastic phase had low risk by the Sokal model. The patient with hematologic relapse had high risk. All patients are alive.
Major and complete cytogenetic responses at 3, 6, and 9 months on different regimens
| Regimen . | No. of patients . | % cytogenetic response, complete/major . | ||
|---|---|---|---|---|
| 3 mo . | 6 mo . | 9 mo . | ||
| Imatinib mesylate for early chronic-phase CML | 50 | 34/74 | 52/80 | 60/77 |
| IFN-α | 274 | 4/2 | 3/11 | 5/14 |
| IFN-α + cytarabine | 257 | 1/9 | 7/23 | 8/23 |
| IFN-α + cytarabine + HHT | 90 | 4/24 | 7/28 | 11/30 |
| Imatinib mesylate for chronic phase after IFN-α failure (M. D. Anderson study group)26 | 261 | 26/44 | 31/49 | 35/53 |
| Regimen . | No. of patients . | % cytogenetic response, complete/major . | ||
|---|---|---|---|---|
| 3 mo . | 6 mo . | 9 mo . | ||
| Imatinib mesylate for early chronic-phase CML | 50 | 34/74 | 52/80 | 60/77 |
| IFN-α | 274 | 4/2 | 3/11 | 5/14 |
| IFN-α + cytarabine | 257 | 1/9 | 7/23 | 8/23 |
| IFN-α + cytarabine + HHT | 90 | 4/24 | 7/28 | 11/30 |
| Imatinib mesylate for chronic phase after IFN-α failure (M. D. Anderson study group)26 | 261 | 26/44 | 31/49 | 35/53 |
QPCR studies
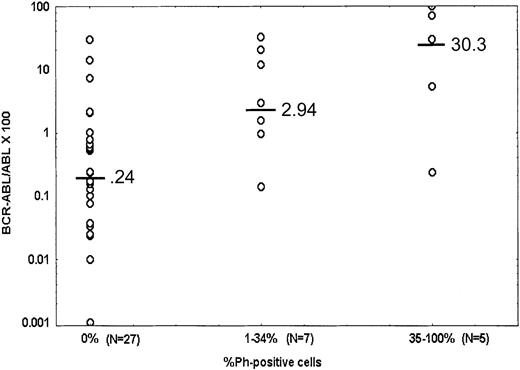
The QPCR values among patients treated with imatinib mesylate at 9 months on therapy are shown in Figure 1. The median overall QPCR value was 0.59%. Among 27 patients tested in complete cytogenetic response the median QPCR value was 0.24% (range, 0.001%-29.6%); 7 of them (26%) had a QPCR value less than 0.04%, and 21 (78%; 54% of 39 patients tested) had QPCR values less than 1%. Two patients in complete cytogenetic response had high QPCR values above 10%; the iFISH studies showed 1% and 3.5% positivity. The significance of this finding is unknown; it may be related to high BCR-ABL/ABL expression in resting nondividing cells and may be potentially predictive of relapse.
Competitive quantitative PCR values at 9 months on imatinib mesylate therapy by cytogenetic response.
Horizontal markings on graphs are median values.
Competitive quantitative PCR values at 9 months on imatinib mesylate therapy by cytogenetic response.
Horizontal markings on graphs are median values.
Side effects
Side effects of imatinib mesylate were similar to those reported in previous trials.18 Grade 3 to 4 toxicities were skin rashes in 6%, muscle cramps or aches in 2%, fatigue in 2%, and liver function abnormalities in 4%. Myelosuppression-associated complications were granulocytopenia less than 0.5 × 109/L in 20%, thrombocytopenia less than 50 × 109/L in 8%, and anemia less than 9 g/dL in 8%. These required dose interruptions (n = 1) or reductions to 300 mg daily (n = 2), but no patient had to discontinue therapy permanently because of myelosuppression.
This study demonstrates the superior efficacy of imatinib mesylate compared with IFN-α regimens in relation to previously defined early surrogate end points for long-term prognosis with IFN-α therapy3-11 but not yet convincingly with imatinib mesylate therapy18,19 (Table 2). The incidence of complete cytogenetic response with imatinib mesylate was 72% overall and 60% at 9 months of therapy (versus 4% to 11% with IFN-α). A randomized study of imatinib mesylate versus IFN-α plus cytosine arabinoside (ara-C) in early chronic phase showed better cytogenetic response rates (major 63% versus 10%, complete 40% versus 2%, respectively, at 6 months; P < .001), lower failure rates, and fewer side effects with imatinib mesylate therapy.27
Achievement of a complete cytogenetic response has been associated with 5- to 10-year survival rates of 70% to 90%,5,6,9-11 and has been a consistent reliable early surrogate marker of survival prolongation in CML with IFN-α therapy.3-6 9-11 The observation of a high incidence of complete cytogenetic response early with imatinib mesylate therapy suggests its potential benefit in improving long-term prognosis in CML.
Qualitative and quantitative PCR studies have been relevant in assessing minimal residual disease and prognosis. In the setting of allogeneic SCT, persistent reverse transcriptase (RT)–PCR positivity after 12 months from transplantation was associated with CML relapse in 30% to 40% of patients versus less than 5% for RT-PCR–negative patients.28 The QPCR values for patients “cured” after allogeneic SCT range from 0% to less than 0.03%.25 With IFN-α therapy, QPCR values of less than 0.05% have been associated with long-term event-free survival and low relapse rates.25This study demonstrates rapid achievement of very low QPCR values (median 0.59% in the total population; 54% with QPCR values < 1%) after a short period of 9 months of imatinib mesylate therapy. This compares favorably with the QPCR levels achieved at 9 months with imatinib mesylate therapy after IFN-α failure in chronic phase (median QPCR values 0.24% versus 0.89% [19 patients tested];P = .04). Among 190 samples in complete cytogenetic response after IFN-α therapy tested by QPCR, the median QPCR value was 0.087% (H.M.K., unpublished data, 2000). However, these complete cytogenetic responses had all persisted for more than 12 months, were tested after long-term IFN-α therapy (not at 9 months into therapy), and occurred in a minority of patients (20% to 25% in our studies). In the study of Hochhaus et al, the median QPCR values after 6 and 12 months in complete cytogenetic response with IFN-α were 0.46% and 0.135%, respectively. Again, the complete cytogenetic responses were achieved in a minority of patients (5%-10%).25 Because imatinib mesylate resulted in a median overall QPCR value of 0.59% in the total study group, it appears to induce significantly lower levels of molecularly detectable residual disease in CML compared with IFN-α therapy. However, the median follow-up time is short in this study, and longer follow-up is needed before definite conclusions can be drawn.
Prepublished online as Blood First Edition Paper, August 29, 2002; DOI 10.1182/blood-2002-02-0545.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hagop M. Kantarjian, Department of Leukemia, Box 428, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail:hkantarj@mdanderson.org.