Abstract
Severe sepsis, defined as sepsis with acute organ dysfunction, is associated with high morbidity and mortality rates. The development of novel therapies for sepsis is critically dependent on an understanding of the basic mechanisms of the disease. The pathophysiology of severe sepsis involves a highly complex, integrated response that includes the activation of a number of cell types, inflammatory mediators, and the hemostatic system. Central to this process is an alteration of endothelial cell function. The goals of this article are to (1) provide an overview of sepsis and its complications, (2) discuss the role of the endothelium in orchestrating the host response in sepsis, and (3) emphasize the potential value of the endothelium as a target for sepsis therapy.
Introduction
Sepsis is the most common cause of death among hospitalized patients in noncoronary intensive care units. Thus, an important goal in critical care medicine is to develop novel therapeutic strategies that will impact favorably on patient outcome. Unfortunately, the pathophysiology of severe sepsis remains poorly defined. While it is generally accepted that sepsis-associated mortality is related to the host response and involves a multitude of cell types, inflammatory mediators, and coagulation factors, clinical studies have largely failed to identify an effective therapeutic target. Future advances in sepsis therapy will require a better understanding of how the individual components of the host response interact. The endothelium plays a critical role in mediating the sepsis phenotype. This article provides an overview of sepsis and its complications, discusses the role of the endothelium in orchestrating the host response in sepsis, and emphasizes the potential value of the endothelium as a target for sepsis therapy.
Overview of the sepsis continuum
Definition
Sepsis and its sequelae represent a continuum in clinical-pathologic severity. However, it is one with definable phases that characterize patients at risk for increased mortality.1 The American College of Chest Physicians and the Society of Critical Care Medicine established a set of definitions to facilitate early detection and treatment of sepsis and to standardize patient requirements for research protocols.2Infection is defined as an inflammatory response to microorganisms or the invasion of normally sterile host tissue by those organisms. Sepsis represents the systemic inflammatory response to infection and is manifested by 2 or more of the systemic inflammatory response syndrome (SIRS) criteria (eg, changes in body temperature, tachycardia, tachypnea and/or hyocapnia, and changes in the number and/or immaturity of white blood cells). Severe sepsis is sepsis complicated by organ dysfunction. Septic shock (hypotension despite adequate fluid resuscitation) is a subcategory of severe sepsis. At the end of the spectrum is multiple organ dysfunction syndrome (MODS), defined as the presence of altered organ function in an acutely ill patient such that homeostasis cannot be maintained without intervention.
Epidemiology
In 1990, the Centers for Disease Control and Prevention reported an estimate of 450 000 cases of septicemia per year in the United States, with over 100 000 deaths.3 Recently, Angus et al estimated that 750 000 cases of severe sepsis occur per year, with a mortality rate of 28.6%.4 Other studies suggest that 28-day mortality rates of severe sepsis may be 50% or greater.5 According to Angus et al, the mortality rate due to severe sepsis represented 9.3% of all deaths in the United States in 1995.4 The average cost per case was $22 100, with annual costs of $16.7 billion nationally. Importantly, the incidence of severe sepsis was projected to increase by 1.5% per year.4 Taken together, these data suggest that severe sepsis is a serious public health concern.
Clinical manifestations
The most common clinical findings in sepsis are related to SIRS (eg, fever, tachycardia, tachypnea, and leukocytosis) and organ dysfunction (eg, acute lung injury, acute respiratory distress syndrome, shock). Laboratory markers of inflammation include high circulating levels of interleukin 6 (IL-6), IL-8, and tumor necrosis factor alpha (TNF-α).6-8 Activation of the coagulation cascade is most often manifested by increased D-dimer levels (≈ 100% patients) and decreased levels of circulating protein C (≥ 90% patients).9-11 In contrast, less than half of patients with sepsis meet the definition of disseminated intravascular coagulation (DIC),1,12,13 a syndrome that is characterized by widespread activation of coagulation, fibrin deposition, and thrombotic occlusions and/or bleeding.14 15
Pathophysiology
There are several important themes in sepsis pathophysiology. First, it is the host response, rather than the nature of the pathogen, that primarily determines patient outcome. Second, monocytes and endothelial cells play a central role in initiating and perpetuating the host response. Third, sepsis is associated with the concomitant activation of the inflammatory and coagulation cascades. Finally, in a concerted effort to fend off and eliminate pathogens, the host response may inflict collateral damage on normal tissues, resulting in pathology that is not diffuse, but rather remarkably focal in its distribution. Each of these themes will be discussed in turn.
The importance of the host response.
Several findings point to the importance of host factors in determining outcomes in patients with severe sepsis. First, despite the prompt implementation of appropriate antibiotic therapy, sepsis mortality remains high, in the range of 28% to 50%. Second, patients with culture-positive and culture-negative sepsis or septic shock have comparable mortality rates.1,5 Third, administration of anti–endotoxin antibodies in large, clinical trials did not improve survival.16,17 Last, there is a direct correlation between the number of SIRS criteria and mortality rate, and there is a stepwise increase in mortality rates in the spectrum of SIRS, sepsis, severe sepsis, and septic shock.1 Clearly, the success of future therapies will rely on the ability to adequately target the host response.
Role of the monocyte and endothelial cell in mediating the host response.
Monocytes, tissue macrophages, other myeloid-derived cells, and to some extent endothelial cells, are the cornerstones of the innate immune response. As a first line of defense, these cells recognize invading pathogens through pattern recognition receptors that interact with conserved microbial structures.18-25 The interaction between pathogens and host cells results in the initiation of inflammatory and coagulation cascades (Figure1). These pathways yield soluble mediators that function in autocrine or paracrine loops to further activate the monocyte/tissue macrophage and/or endothelium.
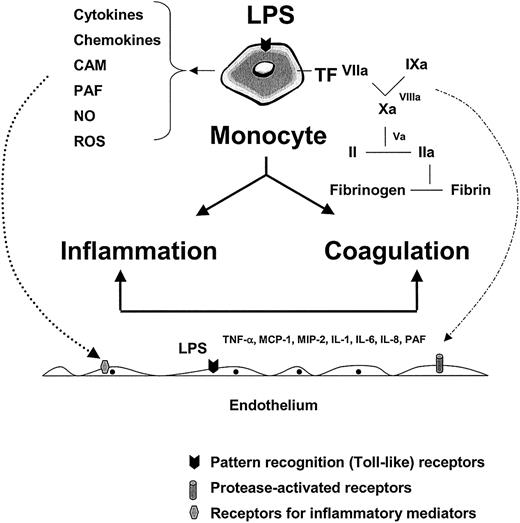
The role of the monocyte and endothelium in mediating the host response to infection.
LPS and/or other pathogen-associated properties activate pathogen recognition receptors (or toll-like receptors) on monocytes, tissue macrophages, and endothelial cells, leading to the release of inflammatory mediators and tissue factor (with subsequent activation of coagulation). Together with products of the contact system (eg, kinins) and complement cascade (eg, C5a) (not shown), inflammatory mediators function in autocrine and paracrine loops to further activate the monocyte and local endothelium (dotted line, left, shows paracrine pathway). The various components of the coagulation cascade serve not only to activate their downstream substrate (leading to fibrin formation) but also to trigger protease-activated receptors on the surface of a variety of cell types, including the endothelium (broken line, right). The combined effects of LPS, inflammatory mediators, and serine proteases on the endothelium may result in significant phenotypic modulation (not shown). CAM indicates cell adhesion molecules; PAF, platelet activating factor; NO, nitric oxide; ROS, reactive oxygen species; MIP-2, macrophage inflammatory protein 2.
The role of the monocyte and endothelium in mediating the host response to infection.
LPS and/or other pathogen-associated properties activate pathogen recognition receptors (or toll-like receptors) on monocytes, tissue macrophages, and endothelial cells, leading to the release of inflammatory mediators and tissue factor (with subsequent activation of coagulation). Together with products of the contact system (eg, kinins) and complement cascade (eg, C5a) (not shown), inflammatory mediators function in autocrine and paracrine loops to further activate the monocyte and local endothelium (dotted line, left, shows paracrine pathway). The various components of the coagulation cascade serve not only to activate their downstream substrate (leading to fibrin formation) but also to trigger protease-activated receptors on the surface of a variety of cell types, including the endothelium (broken line, right). The combined effects of LPS, inflammatory mediators, and serine proteases on the endothelium may result in significant phenotypic modulation (not shown). CAM indicates cell adhesion molecules; PAF, platelet activating factor; NO, nitric oxide; ROS, reactive oxygen species; MIP-2, macrophage inflammatory protein 2.
Activation of the inflammatory and coagulation pathways.
It is widely accepted that the inflammatory response plays an important role in mediating the sepsis phenotype. Pathogens promote the early activation of the contact system (factor XII, prekallikrein, and high-molecular-weight kininogen) and the complement cascade, and induce the rapid release of inflammatory mediators from a number of cell types (eg, monocytes and endothelial cells), changes that correspond to the clinical designation of SIRS. Simultaneously, endogenous antiinflammatory pathways are activated, which serve to dampen the inflammatory response.26-30 The latter process has been termed the compensatory anti-inflammatory response syndrome.27 Ideally, these 2 phases are coordinated to defend the host against invasion by pathogens. However, an excessive or sustained inflammatory response, an inadequate anti-inflammatory response, or perhaps an uncoupling of these 2 phases may contribute to tissue damage and death.
Besides activating the inflammatory system, pathogens also trigger the clotting cascade.31 During sepsis, tissue factor (TF) expression on the surface of circulating monocytes and tissue macrophages is up-regulated, resulting in activation of the extrinsic pathway, thrombin generation, and fibrin formation. Fibrin not only stabilizes platelet plugs, but may also play an important role in immobilizing pathogens on the surface of the leukocyte, facilitating their engulfment and disposal. Blood coagulation is initiated through the extrinsic pathway and is amplified through the intrinsic pathway by mechanisms that involve cross-talk and feedback.31-35 The clotting cascade is composed of a series of linked reactions in which a serine protease, once activated, is free to activate its downstream substrate. These reactions occur on activated phospholipid membranes and in some cases are accelerated by the presence of cofactors (factors VIIIa and Va). For every procoagulant response there is a natural anticoagulant reaction. Tissue factor pathway inhibitor (TFPI) controls the extrinsic pathway,36 antithrombin III (ATIII)–heparan neutralizes the serine proteases in the cascade,37 the thrombomodulin (TM)/protein C/protein S mechanism inactivates cofactors Va and VIIIa,38 and plasmin degrades preformed fibrin. Hemostasis represents a finely tuned balance between procoagulant and anticoagulant forces. Not only is there activation of the extrinsic pathway in sepsis, but there is also an attenuation of natural anticoagulant responses (eg, reduction in circulating levels of protein C and ATIII, decreased expression of TM on the surface of endothelial cells, impaired fibrinolysis).31 39-42 The resulting shift toward a procoagulant state results in excessive thrombin generation, fibrin formation, and consumption of clotting factors.
Once activated, the inflammatory and coagulation pathways interact with one another to further amplify the host response (Figure 1). For example, inflammatory mediators induce the expression of TF on the surface of circulating monocytes, tissue macrophages, neutrophils, and possibly some subsets of endothelial cells.43-49Conversely, serine proteases are capable of interacting with protease-activated receptors on the surfaces of monocytes and endothelial cells, leading to activation and additional inflammation.50,51 For example, thrombin signaling in endothelial cells results in changes in cell shape,52 cell permeability,53 proliferative response,54 and leukocyte adhesion.55-58 The latter changes are mediated in large part by the ability of thrombin to induce the expression of E-selectin,59 P-selectin,55,57 intercellular adhesion molecule 1 (ICAM-1),56,58 and vascular cell adhesion molecule 1 (VCAM-1).58,60 In addition, thrombin signaling in endothelial cells has been shown to induce the secretion of von Willebrand factor (VWF),61 increase the expression of protease-activated receptor 1 (PAR-1) mRNA,62 and stimulate the release of soluble mediators, including platelet-activating factor (PAF),63IL-8,59,64 monocyte chemoattractant protein 1 (MCP-1),65 growth factors, and matrix metalloproteinases.66 TF/VIIa complex and factor Xa may also bind to protease-activated receptors and trigger a proinflammatory response.67-70 Finally, fibrin(ogen) has been shown to interact with endothelial cells, leading to a number of phenotypic changes including increased expression of IL-8.71 72 The cross-talk between inflammatory and coagulation pathways contributes to the potentially explosive host response to sepsis.
Focal expression of sepsis phenotype.
A consistent feature of the pathologic lesions in severe sepsis and MODS is the focal nature of their distribution. Typically, patients only develop dysfunction in a limited number of organs. The endothelium is an important determinant of the focal response in sepsis. As discussed below, the endothelium displays remarkable heterogeneity in health and disease states, integrating systemic changes in inflammation and coagulation in ways that differ from one organ to the next.
Role of the endothelium in orchestrating the host response in sepsis
Endothelial cell activation and dysfunction
The endothelium is a truly pervasive organ; the human body contains approximately 1013 endothelial cells, weighing 1 kg and covering a surface area of 4000 m2 to 7000 m2.73 Among other functions, the endothelium mediates vasomotor tone, regulates cellular and nutrient trafficking, maintains blood fluidity, contributes to the local balance in proinflammatory and anti-inflammatory mediators, participates in generation of new blood vessels, and undergoes programmed cell death.74-76 Importantly, each of these activities is differentially regulated in space and time (a phenomenon that has been variably termed endothelial cell heterogeneity or vascular diversity).31,75 77-79
Under normal conditions, endothelial cells are highly active, constantly sensing and responding to alterations in the local extracellular environment, as might occur in the setting of transient bacteremia, minor trauma, and other common daily stresses. In other words, endothelial cell activation occurs as a normal adaptive response, the nature and duration of which depends not only on the type of stimulus, but also on the spatial and temporal dynamics of the system.80 For example, at any given time, the endothelial cells of a vein and artery may have distinct response patterns to a common systemic signal, while at any given site, the response will vary from one moment to the next, according to health and state of the whole organism. Therefore, endothelial cell activation is not an all-or-nothing response, nor is it necessarily linked to disease. Instead, endothelial cell activation represents a spectrum of response and occurs under both physiologic and pathophysiologic conditions.
Any response of the endothelium that benefits the host may be deemed functional, physiologic, or adaptive. For example, when pathogens invade a tissue, endothelial cells are induced locally to release inflammatory mediators, to recruit leukocytes, and to promote clotting as a means of walling off the infection. During this process, endothelial cells may undergo necrosis or apoptosis as tissue is reabsorbed and repaired. When viewed at the level of the single cell, necrosis and/or apoptosis are the ultimate expression of dysfunction. However, when considered in the larger context of host defense, the local loss of endothelium is part of a larger coordinated, adaptive response. Perhaps a fitting analogy is group altruism or group selection, an evolutionary mechanism of cooperation in animals, in which group-level positive effects outweigh the individual-level negative effects. The term endothelial cell dysfunction is better reserved for cases in which the endothelial cell response, whether local or systemic, represents a net liability to the host. For example, in severe sepsis, there is an excessive, sustained, and generalized activation of the endothelium. Without artificial organ support, virtually all patients with severe sepsis would die from their disease. In other words, most of these individuals have crossed the threshold from an adaptive to a maladaptive response. In so far as the endothelium contributes to the severe sepsis phenotype, its behavior may be characterized as dysfunctional.
Endothelial response in severe sepsis
Sepsis may induce phenotypic modulation of the endothelium by a number of different mechanisms. In some cases, pathogens directly infect intact endothelial cells.81 More commonly, components of the bacterial wall (eg, lipopolysaccharide [LPS]) activate pattern recognition receptors on the surface of the endothelium.22-25 Finally, a myriad of host-derived factors activate endothelial cells, including complement, cytokines, chemokines, serine proteases, fibrin, activated platelets and leukocytes, hyperglycemia, and/or changes in oxygenation or blood flow (see Table 1 and Figure 2 for an expanded list of host-derived mediators).
Treatment according to target(s)
| Therapies that target the host-pathogen interaction | Reference no. | ||
| Antibiotics* | 221 | ||
| Anti-endotoxin antibodies† | 16, 17, 222, 223 | ||
| Toll-like receptor or CD14 antagonists | 224 | ||
| Mediator-specific therapies that target inflammation | |||
| Anti–tumor necrosis factor antibodies† | 225, 226 | ||
| Platelet-activating factor acetylhydrolase | 227 | ||
| Bradykinin antagonists† | 228, 229 | ||
| Anti–factor XII antibodies‡ | 230 | ||
| Prostaglandin antagonists† | 228, 231, 232 | ||
| Anti–high mobility group 1 antibodies | 233-235 | ||
| Anti-C5a antibodies | 236, 237 | ||
| Granulocyte-macrophage colony-stimulating factor inhibition | 238, 239 | ||
| Macrophage inhibitory factor inhibition | 240, 241 | ||
| Anti-inflammatory agents (eg, interleukin 10) | 242-244 | ||
| Receptor antagonists | |||
| Interleukin-1r antagonist† | 12, 245 | ||
| Soluble tumor necrosis factor receptor† | 13 | ||
| Platelet-activating factor receptor antagonist† | 246, 247 | ||
| C5a receptor antagonists | 248 | ||
| Protease-activated receptor antagonists | 249, 250 | ||
| Mediator nonspecific therapies that target inflammation | |||
| CI-esterase inhibitor | 49, 251, 252 | ||
| Antioxidants | 253, 254 | ||
| Glucocorticoids1-153 | 148, 255-257 | ||
| Activated protein C1-153 | 10, 11 | ||
| Tissue factor pathway inhibitor† | 161, 258, 290 | ||
| Antithrombin III† | 145, 259 | ||
| Therapies that target cell-cell interactions | |||
| Antiplatelet agents | 260-262 | ||
| Antiadhesion strategies | 153, 263 | ||
| Therapies that target coagulation | |||
| Antithrombin agents | |||
| Heparin | 157 | ||
| Hirudin | 45 | ||
| Antithrombin III† | 145, 259 | ||
| Inhibition of extrinsic pathway | |||
| Anti–tissue factor antibodies | 264 | ||
| Factor VII inhibition | 162, 265 | ||
| Tissue factor pathway inhibitor† | 161, 258, 290 | ||
| Inhibition of cofactor activity | |||
| Activated protein C1-153 | 10, 11 | ||
| Enhanced fibrinolysis | |||
| Tissue plasminogen activator | 266 | ||
| Activated protein C1-153 | 10, 11 | ||
| Miscellaneous targets, amenable to inhibition | |||
| Apoptosis | 139 | ||
| Transcription factors (eg, NF-κB) | 188, 267 | ||
| Ubiquitin-proteasome pathway | 189 | ||
| Mechanical stretch, barotrauma1-153 | 147, 268 | ||
| Fever | 269 | ||
| Hyperglycemia1-153 | 149, 270 | ||
| Elastase | 271, 272 | ||
| Poly (ADP-ribose) synthetase (PARS) | 273 | ||
| Poly (ADP-ribose) polymerase 1 | 274, 275 | ||
| Nitric oxide | 209, 210, 213 | ||
| P38 MAPK | 196, 198 | ||
| PKCδ, ζ | 199, 276 | ||
| Miscellaneous targets, amenable to enhancement | |||
| Oxygenation | 173, 277-280 | ||
| Blood flow and hemodynamic forces1-153 | 150, 281-284 | ||
| Phospholipid oxidation products | 142 | ||
| Soluble lectin-like domain of TM | 285 | ||
| VEGF/Angiopoietin balance | 286 |
| Therapies that target the host-pathogen interaction | Reference no. | ||
| Antibiotics* | 221 | ||
| Anti-endotoxin antibodies† | 16, 17, 222, 223 | ||
| Toll-like receptor or CD14 antagonists | 224 | ||
| Mediator-specific therapies that target inflammation | |||
| Anti–tumor necrosis factor antibodies† | 225, 226 | ||
| Platelet-activating factor acetylhydrolase | 227 | ||
| Bradykinin antagonists† | 228, 229 | ||
| Anti–factor XII antibodies‡ | 230 | ||
| Prostaglandin antagonists† | 228, 231, 232 | ||
| Anti–high mobility group 1 antibodies | 233-235 | ||
| Anti-C5a antibodies | 236, 237 | ||
| Granulocyte-macrophage colony-stimulating factor inhibition | 238, 239 | ||
| Macrophage inhibitory factor inhibition | 240, 241 | ||
| Anti-inflammatory agents (eg, interleukin 10) | 242-244 | ||
| Receptor antagonists | |||
| Interleukin-1r antagonist† | 12, 245 | ||
| Soluble tumor necrosis factor receptor† | 13 | ||
| Platelet-activating factor receptor antagonist† | 246, 247 | ||
| C5a receptor antagonists | 248 | ||
| Protease-activated receptor antagonists | 249, 250 | ||
| Mediator nonspecific therapies that target inflammation | |||
| CI-esterase inhibitor | 49, 251, 252 | ||
| Antioxidants | 253, 254 | ||
| Glucocorticoids1-153 | 148, 255-257 | ||
| Activated protein C1-153 | 10, 11 | ||
| Tissue factor pathway inhibitor† | 161, 258, 290 | ||
| Antithrombin III† | 145, 259 | ||
| Therapies that target cell-cell interactions | |||
| Antiplatelet agents | 260-262 | ||
| Antiadhesion strategies | 153, 263 | ||
| Therapies that target coagulation | |||
| Antithrombin agents | |||
| Heparin | 157 | ||
| Hirudin | 45 | ||
| Antithrombin III† | 145, 259 | ||
| Inhibition of extrinsic pathway | |||
| Anti–tissue factor antibodies | 264 | ||
| Factor VII inhibition | 162, 265 | ||
| Tissue factor pathway inhibitor† | 161, 258, 290 | ||
| Inhibition of cofactor activity | |||
| Activated protein C1-153 | 10, 11 | ||
| Enhanced fibrinolysis | |||
| Tissue plasminogen activator | 266 | ||
| Activated protein C1-153 | 10, 11 | ||
| Miscellaneous targets, amenable to inhibition | |||
| Apoptosis | 139 | ||
| Transcription factors (eg, NF-κB) | 188, 267 | ||
| Ubiquitin-proteasome pathway | 189 | ||
| Mechanical stretch, barotrauma1-153 | 147, 268 | ||
| Fever | 269 | ||
| Hyperglycemia1-153 | 149, 270 | ||
| Elastase | 271, 272 | ||
| Poly (ADP-ribose) synthetase (PARS) | 273 | ||
| Poly (ADP-ribose) polymerase 1 | 274, 275 | ||
| Nitric oxide | 209, 210, 213 | ||
| P38 MAPK | 196, 198 | ||
| PKCδ, ζ | 199, 276 | ||
| Miscellaneous targets, amenable to enhancement | |||
| Oxygenation | 173, 277-280 | ||
| Blood flow and hemodynamic forces1-153 | 150, 281-284 | ||
| Phospholipid oxidation products | 142 | ||
| Soluble lectin-like domain of TM | 285 | ||
| VEGF/Angiopoietin balance | 286 |
References are not all-inclusive, but rather draw on a selection of basic, preclinical, early clinical and/or phase 3 clinical studies, as well as selected reviews.
Rapid institution of appropriate antibiotic therapy remains a mainstay in therapy of patients with severe sepsis.
Phase 3 clinical trials have been conducted and demonstrated no survival benefit.
The major physiologic role of Factor XII is not to mediate coagulation activation, but rather to increase the rate and extent of prekallikrein activation, resulting in generation of bradykinin, increased profibrinolytic activity and inhibition of thrombin-mediated platelet activation.287-289
Phase 3 clinical trials have been conducted and shown to improve survival. The degree to which treatment-related attenuation of endothelial cell activation contributed to overall benefit is unknown.
The endothelium as a therapeutic target.
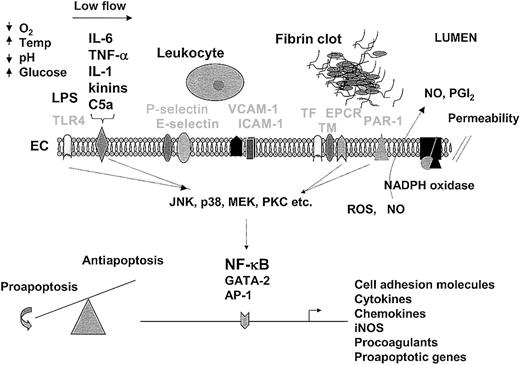
An understanding of the endothelial response to pathogens provides a foundation for therapeutic design. For purposes of illustration and discussion, the temporal sequence of events is depicted from left to right. In sepsis, the endothelium is activated by LPS-mediated engagement of the toll-like receptor (TLR4) or by the interaction of inflammatory mediators (IL-6, TNF-α, IL-1, kinins, and C5a are shown) with their respective receptors (drawn as a single representative receptor). At the same time (or later during the sepsis cascade), the endothelium may be conditioned by other environmental factors, such as hypoxia, low blood flow, changes in temperature, acid-base/electrolyte abnormalities, and/or hyperglycemia. The interaction of extracellular mediators with their receptors leads to activation of downstream signaling pathways (including MAPK and PKC), which in turn promote posttranscriptional changes in cell function or alter gene expression profiles through a number of transcription factors, including NF-κB, GATA-2, and AP-1. The up-regulation of cell adhesion molecules on the surface of the endothelium (P-selectin, E-selectin, VCAM-1, and ICAM-1 are shown) promotes increased adhesion, rolling, and transmigration of circulating leukocytes. Leukocyte-endothelial interactions further modulate the phenotype of these cells. The release of cytokines from the endothelium results in additional activation of monocytes and endothelial cells. Increased expression of procoagulants (eg, TF) and/or reduced expression of anticoagulants (eg, TM, EPCR) promote increased thrombin generation and fibrin formation. Various components of the coagulation pathway (including serine proteases, fibrin, and platelets) may signal directly in the endothelium through protease-activated receptors (PAR-1 is shown). Changes in the expression of proapoptotic and antiapoptotic genes (along with a multitude of posttranscriptional changes) may result in a shift in balance toward programmed cell death. During the process of activation, NADPH oxidase may induce the formation of reactive oxygen species (ROS), nitric oxide (NO) is released, and cell permeability is increased. In keeping with the theme of spatial and temporal dynamics, the relative activity of the various pathways will vary between different endothelial cells and from one moment to the next. Not shown are the critical interactions between the endothelium and underlying extracellular matrix and parenchymal cells. Temp indicates temperature; ICAM-1, intracellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule; EC, endothelial cell; TF, tissue factor; TM, thrombomodulin; EPCR, endothelial protein C receptor; NO, nitric oxide; PGI2, prostacyclin. Receptors are labeled in light font.
The endothelium as a therapeutic target.
An understanding of the endothelial response to pathogens provides a foundation for therapeutic design. For purposes of illustration and discussion, the temporal sequence of events is depicted from left to right. In sepsis, the endothelium is activated by LPS-mediated engagement of the toll-like receptor (TLR4) or by the interaction of inflammatory mediators (IL-6, TNF-α, IL-1, kinins, and C5a are shown) with their respective receptors (drawn as a single representative receptor). At the same time (or later during the sepsis cascade), the endothelium may be conditioned by other environmental factors, such as hypoxia, low blood flow, changes in temperature, acid-base/electrolyte abnormalities, and/or hyperglycemia. The interaction of extracellular mediators with their receptors leads to activation of downstream signaling pathways (including MAPK and PKC), which in turn promote posttranscriptional changes in cell function or alter gene expression profiles through a number of transcription factors, including NF-κB, GATA-2, and AP-1. The up-regulation of cell adhesion molecules on the surface of the endothelium (P-selectin, E-selectin, VCAM-1, and ICAM-1 are shown) promotes increased adhesion, rolling, and transmigration of circulating leukocytes. Leukocyte-endothelial interactions further modulate the phenotype of these cells. The release of cytokines from the endothelium results in additional activation of monocytes and endothelial cells. Increased expression of procoagulants (eg, TF) and/or reduced expression of anticoagulants (eg, TM, EPCR) promote increased thrombin generation and fibrin formation. Various components of the coagulation pathway (including serine proteases, fibrin, and platelets) may signal directly in the endothelium through protease-activated receptors (PAR-1 is shown). Changes in the expression of proapoptotic and antiapoptotic genes (along with a multitude of posttranscriptional changes) may result in a shift in balance toward programmed cell death. During the process of activation, NADPH oxidase may induce the formation of reactive oxygen species (ROS), nitric oxide (NO) is released, and cell permeability is increased. In keeping with the theme of spatial and temporal dynamics, the relative activity of the various pathways will vary between different endothelial cells and from one moment to the next. Not shown are the critical interactions between the endothelium and underlying extracellular matrix and parenchymal cells. Temp indicates temperature; ICAM-1, intracellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule; EC, endothelial cell; TF, tissue factor; TM, thrombomodulin; EPCR, endothelial protein C receptor; NO, nitric oxide; PGI2, prostacyclin. Receptors are labeled in light font.
The endothelium responds in ways that differ according to the nature of the pathogen, host genetics, underlying comorbidity, age, gender, and the location of the vascular bed.82-91 Endothelial cells may undergo structural changes, including nuclear vacuolization, cytoplasmic swelling, cytoplasmic fragmentation, denudation, and/or detachment.92 Functional changes are more common and include shifts in the hemostatic balance, increased cell adhesion and leukocyte trafficking, altered vasomotor tone, loss of barrier function, and programmed cell death.
Procoagulant properties.
Inflammatory mediators may interact with endothelial cells to induce a net procoagulant phenotype. Under in vitro conditions, the addition of LPS and/or cytokines to endothelial cells has been shown to decrease synthesis of TM, tissue-type plasminogen activator and heparan, to increase expression of TF and plasminogen activator inhibitor 1 (PAI-1), and to generate procoagulant microparticles.76,93-96 The extent to which these changes occur in the intact endothelium is not entirely clear. In a recent study of patients with meningococcemia, TM levels were reduced in dermal microvessels, an effect that would be predicted to yield decreased levels of activated protein C.42 In a mouse model of endotoxemia, the administration of LPS resulted in reduction in total tissue TM antigen in the lung and brain, but not in the kidney,41 suggesting that sepsis-associated changes in TM expression may vary between organs. While sepsis is associated with increased levels of PAI-1,84,97 an endothelial source of PAI-1 has not been established. With few exceptions,46 47sepsis studies have consistently failed to demonstrate TF in the intact endothelium.
When the endothelium is viewed in the context of its native environment, additional properties emerge that may contribute to a procoagulant state. For example, activated endothelial cells attract platelets, monocytes, and neutrophils—cells that are capable of initiating or amplifying coagulation. Endothelial activation may result in translocation of cell surface phospholipids that enhance binding of coagulation complexes. Endothelial cells undergoing apoptosis may express an increasingly procoagulant phenotype.98 The development of a low blood-flow state in sepsis, whether secondary to reduced cardiac output, vasoconstriction, or occlusive lesions, may reduce clearance of activated serine proteases, thus promoting additional clotting.
As with other properties of the endothelium, the hemostatic balance is differentially regulated between vascular beds.31,75,77,99In a mouse model of endotoxemia, the systemic administration of LPS resulted in organ-specific deposition of fibrin in the kidney and adrenal gland.100 In another study, LPS administration resulted in detectable levels of fibrin in the lung, but not the brain, of wild-type mice.41 Still others have shown that LPS injection in wild-type mice yields increased fibrin levels in the kidney, liver, and myocardium, but not the lung.101 In a baboon sepsis model, the administration of lethal doses of E coli resulted in increased fibrin deposition in the marginal zone and sinusoids of the spleen, the hepatic sinusoids, the glomeruli, and peritubular vessels of the kidney, but little or no fibrin in the portal vessels of the liver, cerebral cortex, skin, myocardium, or aorta.47 The discrepant patterns of fibrin deposition in the above studies may be related to differences in the species/strain being analyzed, the type of sepsis model, and/or the nature of the fibrin assays. Nevertheless, when taken together, the data are consistent in demonstrating an association between sepsis and organ-specific coagulation.
In genetic mouse models of hypercoagulability, sepsis results in an accentuated shift in the hemostatic balance. For example, in mice that carry a TM gene mutation that disrupts TM-dependent activation of protein C, LPS administration resulted in higher levels of fibrin deposition in the lung and kidney but not the brain, compared with wild-type mice.41 In heterozygous ATIII-deficient mice, LPS challenge resulted in increased deposition of fibrin in the kidney, liver, and heart.101 These studies demonstrate the importance of underlying genetics in modulating the sepsis phenotype.
Proadhesive properties.
The endothelium responds to inflammatory mediators by expressing adhesion molecules on the cell surface, including P-selectin, E-selectin, ICAM-1, and VCAM-1. Collectively, these alterations result in increased rolling, strong adherence, and transmigration of leukocytes into underlying tissue. These changes are not universal, but rather occur locally in certain organs and segments of the vascular loop.102-108 Activated endothelial cells also recruit increased numbers of platelets to the blood vessel wall.109-113 The importance of adhesion molecules in mediating the sepsis phenotype is supported by studies in knock-out mice.114-116
Vasomotor properties.
Vasomotor tone is regulated by a combination of endothelial-dependent and endothelial-independent mechanisms. Endothelial cells produce vasoactive molecules that regulate arteriolar tone and contribute to blood pressure control. These include the vasodilators (nitric oxide [NO] and prostacyclin) and the vasoconstrictors (endothelin, thromboxane A2, and platelet-activating factor).117 In sepsis, activated endothelium undergoes site-specific changes that impact the net balance of vasoconstrictor and vasodilatory properties.118
Increased permeability.
In the intact vasculature, the endothelium forms a continuous, semipermeable barrier that varies in integrity and control for different vascular beds.119 A central feature of the endothelium in sepsis is an increased permeability or loss of barrier function, resulting in a shift of circulating elements and tissue edema. TNF-α induces an increase in endothelial cell permeability both in vitro and in vivo.120-123 Under in vitro conditions, thrombin also increases endothelial cell permeability, while TNF-α and thrombin act synergistically to induce barrier dysfunction in vitro.124 Redistribution of fluid from the intravascular to the extravascular compartment may contribute to hypovolemia, hemoconcentration, and stasis of blood flow.
Endothelial cell apoptosis.
Endothelial cell apoptosis is a highly regulated process.125 Normally, only a small percentage (< 0.1%) of endothelial cells are apoptotic. Under in vitro conditions, certain pathogens are capable of inducing endothelial cell apoptosis.126 The incubation of cultured endothelial cells with LPS has been reported to induce apoptosis in some, but not all, studies.126-129 LPS has been shown to up-regulate the Bcl-2 homologue, A1, and the zinc finger protein, A20, in cultured endothelial cells.130 The sepsis cascade involves a large number of other mediators that may induce endothelial cell apoptosis, including TNF-α, IL-1, interferon, oxygen free radicals, and hypoxia.125,129,131 The interaction between circulating cells and the endothelium may further augment proapoptotic signaling. For example, LPS-activated monocytes promote programmed cell death in endothelial cells by a combination of TNF-α–dependent and –independent mechanisms.132
Endothelial cell apoptosis results in an accentuated proinflammatory response. For example, under in vitro conditions, apoptotic endothelial cells mediate IL-1–dependent paracrine induction of ICAM-1 and VCAM-1,133 increased production of reactive oxygen species (ROS), increased procoagulant activity,98 decreased production of prostacyclin,134 and activation of complement.135 In addition, endothelial cells undergoing apoptosis demonstrate increased binding to nonactivated platelets.136
In a mouse model of endotoxemia, intraperitoneally delivered LPS resulted in widespread apoptosis of the endothelium.137 In other studies, the intravenous administration of LPS in mice was shown to induce endothelial cell apoptosis in the lung, but not the liver, pointing to organ-specific differences in programmed cell death.138 139
Local versus systemic activation of the endothelium
The innate host response evolved as a locally operative mechanism to eradicate pathogens and necrotic tissue.140The endothelium orchestrates the local response by promoting the adhesion and transmigration of leukocytes, inducing thrombin generation and fibrin formation, altering local vasomotor tone, increasing permeability, and triggering programmed cell death.118 The activation of coagulation serves a number of potential roles, including the walling off of pathogens, the activation of protease-activated receptors, and the extravascular stimulation of macrophage chemokine expression.141 Normally, local and systemic negative feedback mechanisms are activated, dampening the response at distal sites.140 142 Compartmentalization of the innate immune response limits collateral damage to the host and preserves integrity and adaptability of uninvolved endothelium. Hence, the endothelium as a whole is not locked into a single response but remains poised to deal with other insults. When the host response generalizes, it escapes the well-developed local checks and balances and results in a dysregulated, undirected inflammatory response. Under these conditions, widespread involvement of endothelium and monocytes/tissue macrophages, together with the more generalized activation of inflammation and coagulation, may lead to SIRS and MODS.
Link between endothelial cell dysfunction and MODS
Despite an increasing appreciation that inflammatory and coagulation cascades are activated in severe sepsis, little is known about the mechanisms that ultimately lead to organ dysfunction and death. The inflammatory and coagulation pathways and the various cell types are so tightly coupled that they cannot and should not be viewed as discrete entities in severe sepsis. Activation of the inflammatory cascade impacts the coagulation pathway, and vice versa. Activated monocytes affect the endothelium, and the reverse is also true. Dysfunction of any one organ has a downstream effect on all other organs. Therefore, the host response to sepsis is highly integrated, and the whole is far greater than the sum of its constituent parts (Figure 3).
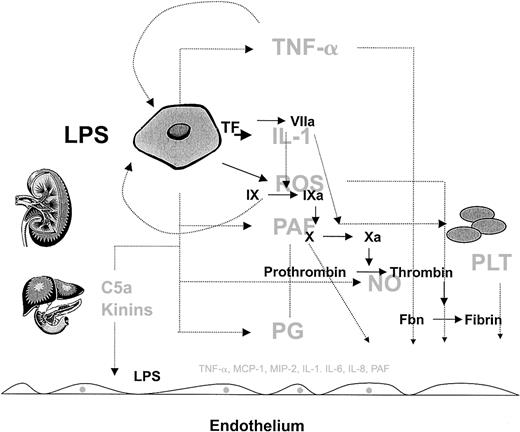
The complexity of the host response to infection.
The host response to infection involves a wide array of cells and soluble mediators, which include but are not limited to monocytes, endothelial cells, and platelets and components of the complement, inflammatory, and coagulation cascades. Rather than showing the detailed connections, this figure is intended to convey the interdependent, redundant, and pleiotropic nature of the host response. Several factors modulate the phenotype, including the type of pathogen, and host factors such as genetic make-up, age, gender, and the health of other organ systems (liver and kidney are shown as examples). Normally, the host mechanisms are highly coordinated in both space and time to defend the host against pathogens. However, when the response is disproportionate to the threat (eg, excessive, sustained, or poorly localized), then the balance of power shifts in favor of the pathogen, resulting in the sepsis phenotype. Given the highly integrated and nonlinear nature of this response, it will be difficult to identify a single component whose therapeutic modulation will short-circuit the sepsis cascade and improve outcome. As long as the complexity of the sepsis response remains outside our grasp the best hope for therapeutic advances will depend on broad base targeting, in which multiple components are targeted at the same time.
The complexity of the host response to infection.
The host response to infection involves a wide array of cells and soluble mediators, which include but are not limited to monocytes, endothelial cells, and platelets and components of the complement, inflammatory, and coagulation cascades. Rather than showing the detailed connections, this figure is intended to convey the interdependent, redundant, and pleiotropic nature of the host response. Several factors modulate the phenotype, including the type of pathogen, and host factors such as genetic make-up, age, gender, and the health of other organ systems (liver and kidney are shown as examples). Normally, the host mechanisms are highly coordinated in both space and time to defend the host against pathogens. However, when the response is disproportionate to the threat (eg, excessive, sustained, or poorly localized), then the balance of power shifts in favor of the pathogen, resulting in the sepsis phenotype. Given the highly integrated and nonlinear nature of this response, it will be difficult to identify a single component whose therapeutic modulation will short-circuit the sepsis cascade and improve outcome. As long as the complexity of the sepsis response remains outside our grasp the best hope for therapeutic advances will depend on broad base targeting, in which multiple components are targeted at the same time.
Based on these considerations, how can we fairly assess the endothelium's role in mediating the sepsis phenotype? Available evidence suggests that the function of the endothelium is altered in severe sepsis in ways that differ from one site of the vascular tree to another. These changes, while part of a larger, integrated host response, may help to initiate and perpetuate site-specific cycles of inflammation, coagulation, and cellular interactions that ultimately lead to microvascular occlusion, hypoxia, and organ dysfunction. To argue that the endothelium plays a more or less central role compared with the monocyte, or that inflammation is more or less important than the coagulation cascade in sepsis pathogenesis is misguided. Perhaps a more productive line of reasoning is as follows: the endothelium is a critical, but not the sole, component of the host response to sepsis; the endothelium is strategically located between blood and underlying tissue; the endothelium is a highly malleable and flexible cell layer; therefore, the endothelium is a potentially valuable target for sepsis therapy.
The endothelium as a therapeutic target
Therapeutic perspectives
Over the past decade, enormous resources have been expended on sepsis trials, with more than 10 000 patients enrolled in over 20 placebo-controlled, randomized phase 3 clinical trials.143,144 Most of these therapies have failed to reduce mortality in patients with severe sepsis, including antiendotoxin, anticytokine, antiprostaglandin, antibradykinin, and anti-PAF strategies, ATIII, and TFPI.143,145,146At the time of this writing, a total of 5 phase 3 clinical trials have demonstrated improved survival in critically ill patients or patients with severe sepsis. These include the use of low tidal volume ventilation,147 activated protein C,11low-dose glucocorticoids,148 intensive insulin therapy,149 and early goal-directed therapy.150
Strategies for targeting the endothelium
In principle, there are 2 strategies for attenuating the endothelial response in sepsis. One is to target nonendothelial components of the host response, including soluble mediators or other cell types (eg, leukocytes, platelets), which negatively modulate endothelial cell function. The other is to target endothelial components (eg, cell surface receptors, signaling pathways, transcriptional networks, or endothelial cell gene products) that are involved in mediating the sepsis phenotype (Figure 2; Table 1). The targets that are listed in Table 1 are derived from a combination of basic and clinical studies. While a number of these therapies have reached phase 3 clinical trials, others are in preclinical or early-phase clinical stages. The extent to which these latter targets will translate into clinical efficacy remains to be seen.
Antimediator therapy.
Several efforts have been made to target LPS or inflammatory mediators that directly activate endothelial cells either at the level of the extracellular factor or its receptor. In large phase 3 clinical trials, the use of specific antimediator therapy has consistently failed to improve survival in patients with severe sepsis.146,151 152
Antiadhesion therapy.
The interaction of circulating cells with the endothelium is likely to play an important role in the host response to infection. Several strategies have been used to inhibit leukocyte-endothelial cell interactions in animal models of sepsis, including the use of monoclonal antibodies.153 Moreover, nonactivated platelets roll on stimulated endothelium, in a process that involves P-selectin and E-selectin,154,155 suggesting that therapy aimed at these cell adhesion molecules may also attenuate platelet-endothelial interactions. Activated platelets have been shown to adhere to the endothelium through a GPIIbIIIa-dependent mechanism,156pointing to a potential role for GPIIbIIIa inhibitors in sepsis. At the present time, antiadhesion therapy in sepsis remains investigational.
Anticoagulant therapy.
Several natural anticoagulant molecules have been studied in nonhuman primate models of sepsis. Heparin and active-site-blocked factor Xa inhibited the activation of coagulation, but did not protect against organ dysfunction or mortality.157,158 These results suggest that activation of the coagulation cascade is not in and of itself sufficient to induce mortality in this syndrome. In contrast to agents that inhibit thrombin activity or thrombin generation, the administration of active-site-blocked factor VIIa, ATIII, activated protein C, or TFPI blocked activation of the coagulation and inflammatory pathways, reduced organ damage, and prevented lethality in a baboon model of sepsis.159-162 The anti-inflammatory effect of these agents is related, at least in part, to their ability to block protease-activated receptor–mediated signaling and/or to activate protective pathways within the endothelium.163-165 Together with the results of the failed anticytokine/antimediator trials, these data suggest, but by no means prove, that mortality in severe sepsis is linked to the combined activation of the coagulation and proinflammatory pathways.
The therapeutic potential of activated protein C was evidenced in the recent phase 3 Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial, in which the administration of human recombinant activated protein C (drotrecogin alfa [activated]) to patients with severe sepsis resulted in reduced mortality.11 A total of 1690 patients with a diagnosis of severe sepsis were randomized to receive either drotrecogin alfa (activated) or placebo. There was a statistically significant reduction in 28-day all-cause mortality (24.7% vs 30.8% in the treatment and placebo groups, respectively,P < .005).11 The PROWESS trial is the first published clinical trial to demonstrate a survival benefit in patients with severe sepsis.
In contrast to the promising results of preclinical and early phase 1/2 studies, phase 3 clinical trials with ATIII or TFPI failed to demonstrate improved survival in patients with severe sepsis.145,290 One possible explanation for these findings relates to study design. For example, patients in the phase 3 clinical trials may have received suboptimal doses of ATIII and/or TFPI. Moreover, in the ATIII study, a potential benefit of the drug may have been obscured by the concomitant administration of heparin.166,167 An alternative explanation is that activated protein C has unique biologic effects that set it apart from ATIII and TFPI in humans with severe sepsis (as distinct from the baboon model of sepsis). Indeed, while TFPI and ATIII are likely to indirectly exert their anti-inflammatory effect through protease-activated receptors (to date, there is no evidence of an ATIII receptor), activated protein C binds to and activates a unique receptor, the endothelial protein C receptor (EPCR), which is expressed on the surface of endothelial cells and possibly monocytes. The interaction between activated protein C and its receptor has been implicated in its profound anti-inflammatory and antiapoptotic function (see “Note added in proof”).164
Antiapoptosis therapy.
As discussed earlier, apoptosis may play a critical role in mediating the sepsis phenotype. Interestingly, inhibition of apoptosis represents a common thread in established sepsis therapies. For example, activated protein C has been shown to inhibit apoptosis in cultured endothelial cells by mechanisms that may include transcriptional down-regulation of the proapoptotic genes calreticulin and TRMP-2, and induction of the antiapoptotic genes A1 Bcl-2 homolog and inhibitor of apoptosis (IAP) homolog B.164 The maintenance of blood flow and hence shear stress may be an important inhibitor of endothelial cell apoptosis,168 and the benefits of early goal-directed therapy may reflect, at least in part, the protective effect of hemodynamics on endothelial cell function.150Hyperglycemia has been reported to promote endothelial cell apoptosis.169,170 Moreover, insulin promotes Akt-dependent endothelial cell survival.171 In light of these findings, it is interesting to speculate that intensive insulin therapy and tight blood glucose control in critically ill patients has a protective (prosurvival) effect on the endothelium.149
Transcription factors as targets.
Several transcription factors in the endothelium have been implicated in the host response to infection, including NF-κB,25,176-179 epithelium-specific Ets factor-1 (ESE-1),180 activator protein-1 (AP-1),181-183 GATA-2,60,184,185 and Egr-1.186 In addition, LPS administration in rodents has been shown to down-regulate DNA-binding activity of Sp1 and AP-2.187
Of the various transcription factors involved in mediating the sepsis phenotype, NF-κB has received the most attention as a potential therapeutic target. In a mouse model of endotoxemia, the intravenous somatic gene transfer with IκBα resulted in increased survival.177 In a rat model of sepsis, the systemic administration of pyrrolidine dithiocarbamate inhibited NF-κB–mediated gene expression of TNF-α, cyclooxygenase-2 (COX-2), and ICAM-1.188 More selective NF-κB inhibitors, such as the antibacterial peptide PR39, may hold greater promise.189
ATIII and activated protein C have each been shown to inhibit NF-κB activation of endothelial cells.163,164 A recent study demonstrated that low-dose glucocorticoids reduce mortality in patients with severe sepsis.148 The beneficial effects of steroids may be related, in part, to an attenuation of NF-κB activity.184 190
As an important caveat, NF-κB has been shown to attenuate TNF-α–mediated apoptosis of endothelial cells, perhaps through the induction of cytoprotective proteins such as IAPs, Bcl-2–like factors, and A20.191 Moreover, the selective blockade of NF-κB sensitized endothelial cells to the proapoptotic effects of TNF-α.192 These observations suggest that NF-κB may play a protective role during the sepsis continuum and underscore the need for caution in developing anti–NF-κB therapies.
Signaling pathways as targets.
The p38 mitogen-activated protein kinase (MAPK) signaling pathway is believed to play an important role in mediating proinflammatory responses and endothelial cell apoptosis.175,193,194 Mice that are null for MAPKAP kinase 2, a downstream p38 MAPK target, demonstrate increased resistance to LPS, an effect that is explained by reduced TNF-α production.195 Several studies have targeted p38 MAPK signaling in animal models, with mixed results.196,197 Interestingly, in a human model of endotoxemia, the oral administration of a new p38 MAPK inhibitor reduced cytokine production and leukocyte responses.198The extent to which the treatment impacted on endothelial cell dysfunction was not addressed.
Novel and atypical isoforms of protein kinase C (PKC) have also been implicated endothelial cell activation. Under in vitro conditions, thrombin-mediated induction of ICAM-1 in endothelial cells is dependent on a PKC-δ–NF-κB signaling pathway, whereas TNF-α–mediated stimulation of ICAM-1 involves PKC-ζ–NF-κB.56,199 Thrombin stimulation of VCAM-1 in endothelial cells is mediated by PKC-δ–NF-κB and PKC-ζ–GATA-2 signaling pathways.200 PKC-ζ has also been shown to mediate TNF-α stimulation of NADPH oxidase–derived ROS in endothelial cells.201 Compared with wild-type mice, LPS administration to PKC-ζ−/− mice resulted in significantly less NF-κB activation in the lung, but not the liver.202 These latter results suggest that the PKC-ζ isoform plays an important role in mediating the host response in select organs and may represent a valuable target for site-specific therapy in severe sepsis.
Nitric oxide synthase (NOS) inhibitors.
Sepsis is associated with increased inducible NOS (iNOS) activity and decreased endothelial NOS (eNOS) activity.203-205 However, the relative role of iNOS and eNOS in mediating the sepsis phenotype remains unclear. In genetic mouse models, the absence of iNOS or eNOS does not significantly alter the sepsis phenotype.206,207 Indeed, the chronic overexpression of eNOS in the endothelium of mice resulted in increased resistance to LPS-induced hypotension and death.208 In some studies, the use of NOS inhibitors yielded beneficial results,209-211 whereas other studies reported the opposite findings.212 In a rabbit model of sepsis, the administration of L-arginine, but not L-NAME (N(G)-nitro-L-arginine methyl ester), attenuated LPS-mediated endothelial cell injury.213 LPS-mediated induction of platelet-endothelial interactions in mice has been shown to be attenuated by NO donor and exacerbated by NOS inhibitor or eNOS deficiency, suggesting a beneficial effect of eNOS-derived NO.214 Further work is required before considering NOS inhibition therapy in sepsis.
Therapeutic challenges
Many reasons have been postulated to explain the long history of failed clinical trials in sepsis. These include inapplicability of results from animal models of sepsis, nonuniformity of supportive care, heterogeneity in patient populations, confounding effects of cointervention, inappropriate timing, and poor choice of outcome measures.143,146,151,152,166,215 216 An underemphasized explanation relates to the complexity of the host response. These themes are important to consider when approaching the endothelium as a therapeutic target.
Timing.
Sepsis represents a continuum in clinical and pathologic severity. In sepsis trials, the choice of inclusion and exclusion criteria may significantly influence the outcome. For example, at one end of the spectrum, the inclusion of low-risk patients may hide an otherwise beneficial response. In these individuals, the adverse effects of treatment (eg, anticoagulant-mediated bleeding) may outweigh any small benefit. Another important consideration is the adaptive nature of the host response. As long as the overall response is protective (eg, during the early stages of the sepsis continuum), targeted therapy may have no effect, or even a negative impact on survival.144 At the other end of the spectrum, patients who present with late-stage disease may be relatively resistant to therapy. Sepsis-induced cascades that were once amenable to therapeutic intervention may no longer be responsive. When designing therapies that target the endothelium, it will be important to define the optimal timing and spectrum of disease severity.
Complexity of the host response.
Traditionally, reductionist approaches have been applied to an understanding of sepsis pathophysiology. Indeed, the vast majority of basic studies in this field have focused on isolated and specific mechanisms of the host response. These data have given rise to linear models of pathophysiology, which in turn have guided the choice of therapeutic targets. The notion that the various components of the host response are aligned in series predicts that the attenuation of any one component (eg, TNF-α) will abort the sepsis cascade. This “domino-type model” is giving way to a more realistic paradigm of nonlinear complexity, in which the various cell types, inflammatory mediators, coagulation factors, cell surface receptors, intracellular signaling pathways, transcription factors, and genes interact as part of spatially and temporally coupled networks.80,217 218The nonlinear dynamics of the host response may help to explain, at least in part, the disappointing results of single-target therapy trials in severe sepsis.
One way to deal with the inherent complexity of the host response is to develop broad-base targeting schemes in which multiple components are attenuated at once, for example the inflammatory and coagulation cascades. It is perhaps by casting a wider “therapeutic net” that activated protein C succeeded, where so many other agents before it have failed. Although the precise mechanism(s) by which drotrecogin alfa (activated) improved survival in these patients is not known, several lines of evidence point to a multifaceted role of this agent in inhibiting the proinflammatory and procoagulant response, promoting fibrinolysis, and attenuating the activation of endothelial cells and white blood cells.38,219 220
An alternative approach is to target a nonredundant component of the host response that is central to the initiation and perpetuation of the sepsis phenotype. Examples might include a single function of the endothelium (eg, apoptosis), or a single transcription factor (eg, NF-κB). However, in designing such strategies, it is important that we acknowledge the unpredictable behavior of complex nonlinear systems and readjust our expectations accordingly. While in theory the host response to infection (for any one patient at a single time point) may be modeled by a highly complex series of nonlinear equations, these formulas are not only elusive, but are likely to be exquisitely sensitive to initial conditions. As a result, single-component targeting may not only fail to modulate the host response, but may have unintended, deleterious consequences. An important scientific challenge for the 21st century is to learn how to leverage nonlinear interactions for mechanistic and therapeutic gain. Future progress in understanding complex networks will rely both on improved readouts and more complete statistical and mathematical tools, including advanced clustering techniques, other data mining and pattern recognition strategies, Bayesian techniques, differential equations, and simulation tools. By studying and embracing more realistic biologic models that involve complex networks, we may improve our capacity to reconfigure the host response in our favor.
Implications for clinical trials.
In clinical trials, patients may be alike, but they are never identical. From a therapeutic standpoint, what may save one patient may actually harm another. Moreover, a therapeutic intervention that benefits a given patient at one moment in time may be deleterious at another point in time. Thus, the optimal therapy for sepsis is highly patient and time dependent. However, until we can better characterize the complex behavior of the host response, we are restricted to classic randomized control trial design, in which a single intervention is tested in a heterogeneous group of patients. An important goal, which can be achieved only through large clinical trials, is to identify subgroups of patients that appear to benefit from treatment. This information may then be used to design new preclinical/clinical studies. Such an approach should help to reduce patient heterogeneity (or noise) and to develop more tailored therapy, for exampl, against one or another component of the endothelial response or toward specific vascular bed(s).
Conclusions
Despite new information about the pathophysiology and treatment of severe sepsis, this disorder continues to be associated with an unacceptably high mortality rate. Future breakthroughs will require a conceptual shift that emphasizes relationships between the various mediators and cells involved in host response. The endothelium is key in initiating, perpetuating, and modulating the host response to infection. Additional studies promise to provide new insight into the endothelium, not as an isolated mechanism of sepsis pathophysiology, but rather as the coordinator of a far more expansive, spatially and temporally orchestrated response.
I thank Derek Angus, John Marshall, Wes Ely, and Ary Goldberger for their helpful input.
Prepublished online as Blood First Edition Paper, January 23, 2003; DOI 10.1182/blood-2002-06-1887.
Supported in part by National Institutes of Health grants HL 60585-03, HL 63609-02, and HL 65216-02.
References
Note added in proof
A recent study demonstrated that activated protein C signals through PAR-1 in cultured endothelial cells, by an EPCR-dependent mechanism.291 Consistent with these results, both EPCR and PAR-1 were shown to be required for mediating the cytoprotective function of activated protein C in hypoxic cultured human brain endothelial cells and in a stroke model of mice.292Collectively, these findings suggest that activated protein C signals through the PAR-1 receptor both in vitro and in vivo. Since PAR-1 is also a receptor for thrombin, these studies raise interesting questions as to how two distinct ligands, namely activated protein C and thrombin, mediate opposing PAR-1 responses (eg, protective and proinflammatory responses, respectively).
Author notes
William C. Aird, Molecular Medicine, Beth Israel Deaconess Medical Center, RW-663, 330 Brookline Ave, Boston, MA 02215; e-mail: waird@caregroup.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal