Abstract
Heme oxygenase (HO) and carbon monoxide (CO) have been implicated in the modulation of various cardiovascular functions including blood pressure (BP) regulation. Up-regulating the HO/CO system lowers BP in young (8-week-old) but not in adult (20-week-old) spontaneously hypertensive rats (SHRs). The mechanisms for this selective effect are largely unknown. We investigated the effects of HO-1 inducer, hemin, on the HO/CO-soluble gyanylyl cyclase (sGC)/cGMP system in the aorta of prehypertensive (4-week-old) young and adult SHRs as well as age-matched Wistar-Kyoto rats (WKYs). Reduced expressions of HO-1, HO-2, and sGC proteins associated with depressed HO activity and cGMP levels were detected in young SHRs. These deficiencies were significantly reversed by hemin treatment. Macrophage infiltration of vascular tissues was more significant in adult SHRs than adult WKYs, but invisible in young SHRs and WKYs. Hemin treatment did not alter macrophage infiltration of vascular tissues in young SHRs. The same hemin administration resulted in a significant decrease in BP (from 148.6 ± 3.2 to 125.8 ± 2.6 mmHg, P < .01) in young SHRs, but not in prehypertensive or adult SHRs or WKYs of all ages. The HO inhibitor zinc protoporphyrin abrogated the hemin effect in young SHRs. Aortic tissues became desensitized to YC-1, an activator sGC, in adult SHRs. Thus, in young SHRs the expression and function of the HO/CO-sGC/cGMP system were suppressed, constituting a pathogenic mechanism for the development of hypertension. In adult SHRs, the HO/CO-sGC/cGMP system appeared normal, but desensitization of the sGC/cGMP pathway caused hypertension to prevail.
Introduction
One of the biggest health challenges in industrialized nations today is the high incidence and rapidly growing problem of hypertension, especially among the elderly (those older than 60 years) with approximately 50% morbidity.1Hypertension is a multifactorial pathology and considered the prevalent risk factor for many cardiovascular diseases including myocardial infarction, cardiac failure, and coronary and peripheral artery diseases. Among the known etiologic causes for hypertension is the disproportionate level of vasoconstrictors such as 20-hydroxyeicosatetraenoic acid (20-HETE) by cytochrome P450 monooxygenase2 compared with that of vasodilators such as epoxyeicosatrienoic acids3 and gasotransmitters.4 All known gasotransmitters, including nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S), relax vascular tissues through various mechanisms. CO induces relaxation of vascular smooth muscle cells (SMCs) by several pathways comprising the stimulation of soluble gyanylyl cyclase (sGC), opening of calcium-activated K+ channel, or inhibition of cytochrome P450–dependent mono-oxygenase.5 sGC with catalytic α1 (82 kDa) and β1 (70 kDa) subunits participates in a variety of physiologic processes responsible for converting guanosine triphosphatases to cGMP. Cyclic GMP regulates physiologic functions through gating of ion channels,6,7 activating cGMP-dependent protein kinases, or regulating phosphodiesterase hydrolysis. CO also is capable of blocking the production of constrictor substances such as endothelin8 and 20-HETE.3 Decreased 20-HETE would activate KCa channels in vascular SMCs.3This represents an alternative pathway for the potentiation of CO-induced vasodilatory response.
Among the key metabolic pathways that physiologically yield CO is the heme oxygenase (HO) catalyzed degradation of heme, which also produces bilirubin and iron as the coproducts of CO. Both vascular endothelium and SMCs express HO9 and produce CO that elicits vasorelaxation independent of NO.10 HO is a microsomal enzyme with 3 distinct isoforms.11 HO-1 is a 32-kDa protein that is not constitutively present in cells but can de induced by different stimuli,12,13 while HO-2, a 36-kDa protein, is normally expressed in many organs under physiologic conditions.14 HO-3 has a molecular weight of 33 kDa and shares considerable homology with HO-2 and is thought to regulate heme homeostasis.11 HO-2 expressed in endothelial and smooth muscle layers of blood vessels generates CO that intrinsically modulates vascular tone,6 while HO-1 up-regulation has been reported in atherogenesis,15 ischemia-reperfusion injury,16 and cardiac anaphylaxis.17Accordingly, transient HO-1 up-regulation that normally accompanies many pathophysiologic conditions represents the first line of defense mounted against tissue injury. Up-regulation of the HO/CO system and the sGC/cGMP pathway could subscribe a beneficial role not only in the pathogenesis of hypertension but also in many cardiovascular complications arising from elevated blood pressure (BP) or other etiologic sources. The deliverance of human HO-1 gene by retroviral vector intracardiac injection attenuated the development of hypertension in spontaneously hypertensive rats (SHRs).18The clinical relevance of the HO/CO system in hypertension can be appreciated even more from human studies showing that women with pregnancy-induced hypertension and pre-eclampsia have a significantly lower endogenous production of CO.19 Moreover, the incidence of hypertension as well as other clinical complications was reported in the first human HO-1–deficiency case.20
Although previous studies have shown that manipulation of the HO/CO system through HO-1 inducers lowers BP in young (8-week-old)21,22 but not in adult (20-week-old) SHRs,23,24 no study has correlated the actual expression levels of HO-1 and sGC proteins and cGMP production in the aorta to the extent of hypertension development. The integrity of the sGC/cGMP pathway is indispensable to the homeostatic control of contractile machinery of vascular SMCs.25 Alterations in the sGC/cGMP pathway resulted in the development of hypertension.26Accordingly, a defective HO/CO-sCG/cGMP system could constitute an important mechanism for the pathogenesis of hypertension. The present study was designed to examine the mechanisms underlying the changes in the development of hypertension in SHRs at different ages with the specific correlation with the HO/CO-sGC/cGMP system in vascular tissues. In this context, the basal expression and activity of the HO/CO-sGC/cGMP system in the aortas from SHRs and Wistar-Kyoto rats (WKYs) of different ages were evaluated. The functional status of the HO/CO-sGC/cGMP system, the vascular contractility, and the changes in blood pressure of SHRs at different ages were also compared before and after a 4-day administration of hemin to up-regulate HO expression. The same protocol has been used previously by other investigators.27,28 A shorter treatment period might not suffice to induce a stable change in BP caused by the altered expression of the targeted proteins. Prolonged treatment, on the other hand, might lead to certain secondary changes due to altered vascular remodeling and other age-dependent processes, which may complicate the interpretation of the functional changes of the acute HO up-regulation. Moreover, previous investigations using the 4-day treatment with hemin did not observe apparent toxicity to animals.27 28
Materials and methods
Animal preparation
Male SHR and WKY rats were purchased from Charles River Laboratories (Willington, MA). They were housed at 21°C with 12-hour light/dark cycles, fed with standard laboratory chow, and had access to drinking water ad libitum. The experimental protocol was approved by the University of Saskatchewan Standing Committee on Animal Care and Supply. Hemin (ferriprotoporphyrin IX), an endogenous chloroporphyrin (Sigma Chemical, St Louis, MO), was administered to the animals intraperitoneally at a concentration of 15 mg/kg/d for 4 consecutive days.27,28 Hemin and zinc protoporyphyrin IX (ZnPP) (Porphyrin Products, Logan, UT) were dissolved in 0.1 M NaOH, titrated to pH 7.4 with 0.1 M HCl, and diluted 1:10 with phosphate buffer.28 ZnPP was prepared in darkness and protected from light.
A total of 110 animals aged 4, 8, and 20 weeks were used, of which 55 were WKYs and 55 SHRs. The 4-week-old WKYs weighed 111 ± 1.4 g and the age-matched SHRs 95 ± 2.9 g, while the 8-week-old WKYs and age-matched SHRs weighed 197 ± 5.5 g and 180 ± 3.7 g, respectively. The 20-week-old WKYs and SHRs weighed 349 ± 7.3 g and 309 ± 4.3 g, respectively. Systolic BP was determined in conscious rats using a standard tail-cuff noninvasive BP measurement system (Model 29-SSP, Harvard Apparatus, St Laurent, QC, Canada) after acclimatization, before and 23 hours after the last intraperitoneal treatment with hemin or ZnPP (50 μmol/kg).17 27 At the end of the treatment period, the animals were anesthetized with pentobarbital sodium (50 mg/kg body weight) and killed. The aorta was isolated in ice-cold phosphate-buffered saline (PBS), cleaned, and snap-frozen in liquid nitrogen. PBS contained (mM) 140 NaCl, 3 KCl, 10 Na2HPO4, and 2 KH2PO4.
Western immunoblotting for HO-1, HO-2, and sGC proteins
Previously frozen aortic tissues were homogenized (1:10, wt/vol) in 10 mM Tris [tris(hydroxymethyl)aminomethane]-buffered saline (20 mM Tris-HCl, pH 7.4, 0.25 M sucrose, and 1 mM EDTA [ethylenediaminetetraacetic acid]) in the presence of freshly added cocktail of protease inhibitors as previously described29and centrifuged at 10 000g for 10 minutes at 4°C. The supernatant was decanted, and aliquots of 30 μg proteins were loaded on a 10% sodium dodecyl sulfate (SDS)–polyacrylamide gel for sGC, or 12.5% for HO-1 and HO-2. The fractionated proteins were electrophoretically transferred to nitrocellulose paper. Nonspecific bindings were blocked with 3% nonfat milk dissolved in PBS for 2 hours. Thereafter, the membranes were incubated overnight with polyclonal primary antibodies against α1 and β1 subunits of sGC (US Biologicals, Swampscott, MA) (1:1000 dilution), HO-2 (StressGen, Victoria, BC, Canada) (1:5000 dilution), or HO-1 (Affinity Bioreagents, Golden, CO) (1:1000 dilution) at 4°C. After several washes, the nitrocellulose blot was incubated with antirabbit IgG conjugated to horseradish peroxide in 1:5000 dilution (Bio-Rad, Hercules, CA) for 2 hours at room temperature. This was followed by another series of washes with PBS, and the immunoreactivity was visualized by use of enhanced horseradish peroxide/luminol chemiluminescence reagent (Perkin Elmer Life Sciences, Boston, MA). Densitometric scanning of respective bands of blot was quantified using UN-SCAN-IT software (Silk Scientific, Orem, UT). Equivalent loading was controlled by beta-actin detection. The protein concentration was determined by the Bradford method30 (Bio-Rad protein assay).
HO activity assay
HO activity was measured as bilirubin production.17In one experimental setting, aortic tissues isolated from animals treated in vivo with hemin or a combination of hemin and ZnPP17 were used. In other experiments aortas were incubated in vitro for 30 minutes at 37°C with one of the following substances: CO (1 μM), biliverdin (1 μM), ferrous ammonium sulfate (1 μM),31 or N2-aerated PBS.32 Some aortic tissues were preincubated in vitro with oxyhemoglobin (HbO2, 1 μM) for 30 minutes followed by hemin (1 μM) for 4 hours, ZnPP (1 μM) for 30 minutes, ZnPP plus hemin for 4 hours, or CO (1 μM) for 30 minutes at 37°C, respectively.33 The amount of bilirubin in each sample was determined spectrophotometrically at 560 nm, using Total Bilirubin Kit (Sigma Diagnostics, St Louis, MO) and expressed as nmol/mg protein/h. The protein concentration was determined by the Bradford method30 (Bio-Rad protein assay). HbO2 was prepared by reducing 1 mM solution of hemoglobin with a 10-fold molar sodium dithionite (Na2S2O4).34 A saturated solution of CO (1 mM) was prepared as previously described.35 N2-aerated PBS was made by bubbling PBS with N2 (99.99%, Praxair Products, Mississauga, ON, Canada) for 30 minutes at 37°C.32 36
Measurement of cGMP content
The concentration of cGMP was determined by a radioimmunoassay kit (125I-cGMP-RIA, Amersham International plc, Amersham, United Kingdom).17 Briefly, previously frozen tissues were homogenized in 6% trichloroacetic acid at 4°C in the presence of 3′-isobutyl-1-methylxanthine (IBMX, 50 μM)17 to inhibit phosphodiesterase activity37 and centrifuged at 2000g for 15 minutes. The supernatant was recovered and washed 3 times with water-saturated diethyl ether. The upper ether layer was aspired and discarded each time after wash, while the aqueous layer containing cGMP was recovered, frozen at −20°C, and subsequently lyophilized. The dry extract was dissolved in 1 mL cGMP-assay buffer, and the cGMP content was determined using the protocol of the manufacturer and expressed as picomol of cGMP per milligram of protein. The protein concentrations were determined by the Bradford method.30
Immunohistochemical determination of macrophage infiltration
The rats were perfused first with heparinized PBS and second with PBS containing 4% formaldehyde. Tissues including aorta and carotid artery were dissected out immediately and kept in the same fixative overnight. Samples were then incubated in a 30% sucrose solution for 3 days at 4°C for cryoprotection. After embedding in O.C.T. compound (Somagen Diagnostics, Edmonton, AB, Canada), 8-micrometer-thick cross sections were made using a freezing mocrotome and then collected on gelatin-chrome alum-coated slides. The sections were further incubated with serum to block nonspecific staining followed by incubation overnight with anti–ED-1 antibody (1:250 dilution; Serotec, Raleigh, NC), the marker for activated macrophages. Thereafter, the sections were incubated with Cy3-conjugated affiniPure goat antimouse IgG at the dilution of 1:200 for 30 minutes (Jackson ImmunoResearch Laboratories, Bar Harbor, ME) and examined under an Olympus fluorescence microscope (Markham, ON, Canada).
Vascular tissue contraction force development
The endothelium-free aortic rings were prepared as described previously.38 The aortic tissues were precontracted with a submaximal dose of phenylephrine (PHE, 0.3 μM) and, at the plateau phase of contraction, cumulative concentration-dependent responses to freshly prepared YC-1 [3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole] (Sigma Chemical) were determined.
Statistical analysis
All data were expressed as means ± SEM from at least 3 independent experiments performed in duplicate unless otherwise stated. Statistical analyses were done using unpaired Student ttest, analyses of variance in conjunction with Newman-Keuls test, and analyses of variance for repeated measures where appropriate. Group differences at the level of P < .05 were considered statistically significant.
Results
Selective modulation of blood pressure in young and adult SHRs by hemin treatment
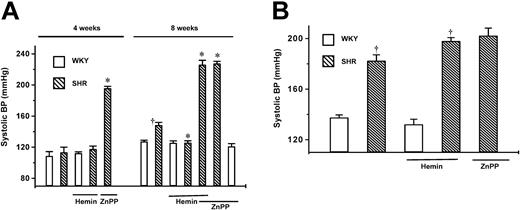
Prior to the implementation of a 4-day regimen of hemin treatment, tail-cuff measurements revealed a significantly higher BP in 8-week-old SHRs (148.6 ± 3.2 mmHg) than in the age-matched WKYs (P < .01) (Figure 1A), which was, however, significantly lower than that of 20-week-old adult SHRs (182.5 ± 4.6 mmHg) (P < .05) (Figure 1B). After completing the hemin treatment, there was a noticeable reduction in the BP (P < .01) of young SHRs (Figure 1A). In contrast, the BP of young WKYs (127.4 ± 1.5 mmHg) was not changed after hemin treatment (125.7 ± 2.5 mmHg) (Figure 1A). To ascertain the implication of HO-1 in the BP-lowering effect of hemin, the inhibitor of HO activity (ZnPP) was simultaneously administered with hemin or alone to the rats. ZnPP alone or with hemin significantly increased BP in prehypertensive (4 weeks) and young SHRs but not in young WKYs or adult SHRs (Figure 1). The BP-lowering effect of hemin in young SHRs was completely abolished (Figure 1A) in the presence of ZnPP. To investigate whether the hemin-elicited BP lowering was specific to young SHRs, a similar treatment was given to adult SHRs and prehypertensive 4-week-old SHRs as well as age-matched WKYs. BP remained elevated after hemin treatment in adult SHRs (182.5 ± 4.6 vs 198.1 ± 2.7 mmHg) (Figure 1B) and was unchanged in age-matched WKYs (137.5 ± 2.1 vs 138.2 ± 4 mmHg) (Figure1B). Similarly, the 4-day treatment with hemin did not alter the BP of the juvenile 4-week-old SHRs and WKYs (Figure 1A).
Effects of hemin treatment on systolic blood pressure of SHRs and age-matched WKYs.
The mean systolic pressure was calculated from 6 different measurements taken from each animal. (A) Changes in systolic BP in 4- and 8-week-old SHRs (▧) and WKYs (■). BP was higher in 8-week-old SHRs than in age-matched WKYs. (B) Changes in systolic BP in 20-week-old SHRs (▧) and WKYs (■). BP was higher in 20-week-old SHRs than in age-matched WKYs. Hemin treatment lowered BP in 8-week-old SHRs, but not in 4- week-old or 20-week-old SHRs or WKYs at all ages. ZnPP completely abolished the hemin-dependent BP-lowering effect in 8-week-old SHRs. ZnPP significantly increased BP in prehypertensive 4-week-old SHRs (A) but had no effect on BP of adult SHRs (B). Each group contained 10 animals. *P < .01 versus untreated age-matched SHRs. †P < .01 versus age-matched WKYs. Error bars indicate means ± SEM.
Effects of hemin treatment on systolic blood pressure of SHRs and age-matched WKYs.
The mean systolic pressure was calculated from 6 different measurements taken from each animal. (A) Changes in systolic BP in 4- and 8-week-old SHRs (▧) and WKYs (■). BP was higher in 8-week-old SHRs than in age-matched WKYs. (B) Changes in systolic BP in 20-week-old SHRs (▧) and WKYs (■). BP was higher in 20-week-old SHRs than in age-matched WKYs. Hemin treatment lowered BP in 8-week-old SHRs, but not in 4- week-old or 20-week-old SHRs or WKYs at all ages. ZnPP completely abolished the hemin-dependent BP-lowering effect in 8-week-old SHRs. ZnPP significantly increased BP in prehypertensive 4-week-old SHRs (A) but had no effect on BP of adult SHRs (B). Each group contained 10 animals. *P < .01 versus untreated age-matched SHRs. †P < .01 versus age-matched WKYs. Error bars indicate means ± SEM.
Effect of hemin on the expression of HO-1 in aortic tissues
To correlate the HO-1 expression levels to BP changes in young and adult SHRs, Western immunoblot assay was performed on aortic tissues from different groups of rats. The basal expression level of HO-1 increased in aortic tissues from WKYs in an age-dependent manner (P < .05 between 8- and 20-week-old WKYs) (Figure2). More importantly, the basal level of HO-1 was much lower in young SHRs than in adult SHRs or WKYs of all ages. The relative abundant level of HO-1 in 8-week-old SHRs was 49% ± 3% (n = 4) of that in the age-matched WKYs (n = 4). A 78% ± 1% increase in HO-1 expression level was observed in young SHRs treated with hemin (n = 4) in comparison to age-matched SHRs without hemin treatment. Similarly, the expression of HO-1 in young hemin-treated WKYs increased by 51% ± 3% (n = 4) in comparison to age-matched WKYs without hemin treatment. The basal expression level of HO-1 in adult SHRs was 101% ± 4% (n = 4) higher than that in age-matched WKYs (n = 4). Hemin treatment failed to up-regulate HO-1 expression in adult SHRs (n = 4). The same treatment, however, significantly enhanced the expression of HO-1 in adult WKYs by 31% ± 5% (n = 4) in comparison to adult WKYs that did not receive hemin treatment.
HO-1 expression in the aortas of adult (20-week-old) and young (8-week-old) SHRs and WKYs.
Representative Western immunoblotting (A) and the mean relative abundant levels of expressed proteins (B) revealed a significantly increased expression of HO-1 protein in the aortas from young SHRs that received hemin treatment. The basal HO-1 expression level from adult SHRs was significantly greater than in the age-matched WKYs, which were not affected by hemin. †P < .01 versus age-matched WKYs. *P < .05 versus all other groups. ΔP < .05 versus age-matched animals of same species without hemin treatment. The ages of the animals in weeks are indicated as “20” and “8” in (A) and (B). n = 4 for each group. Error bars indicate means ± SEM.
HO-1 expression in the aortas of adult (20-week-old) and young (8-week-old) SHRs and WKYs.
Representative Western immunoblotting (A) and the mean relative abundant levels of expressed proteins (B) revealed a significantly increased expression of HO-1 protein in the aortas from young SHRs that received hemin treatment. The basal HO-1 expression level from adult SHRs was significantly greater than in the age-matched WKYs, which were not affected by hemin. †P < .01 versus age-matched WKYs. *P < .05 versus all other groups. ΔP < .05 versus age-matched animals of same species without hemin treatment. The ages of the animals in weeks are indicated as “20” and “8” in (A) and (B). n = 4 for each group. Error bars indicate means ± SEM.
Effect of hemin on the expression of HO-2 in aortic tissues
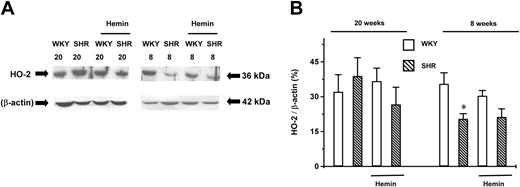
The expression level of constitutive HO-2 in young SHRs was 58% ± 4% (n = 4, P < .05) of that in the age-matched WKYs (n = 4). This lower expression level was not altered by hemin treatment (n = 4). There was no significant difference in the expression levels of HO-2 among young and adult WKYs and adult SHRs (n = 4 for each group). Hemin treatment also did not change the HO-2 expression in these groups (n = 4 for each group) (Figure3).
Expression of HO-2 in the aortas of adult (20-week-old) and young (8-week-old) SHRs and age-matched WKYs.
Representative Western immunoblotting (A) and the mean relative abundant levels of expressed proteins (B) revealed a significantly lower HO-2 expression level in 8-week-old SHRs, which were unaffected by hemin treatment. *P < .05 versus age-matched WKYs. The ages of the animals in weeks are indicated by the numbers “20” and “8” in (A). n = 4 for each group. Error bars indicate means ± SEM.
Expression of HO-2 in the aortas of adult (20-week-old) and young (8-week-old) SHRs and age-matched WKYs.
Representative Western immunoblotting (A) and the mean relative abundant levels of expressed proteins (B) revealed a significantly lower HO-2 expression level in 8-week-old SHRs, which were unaffected by hemin treatment. *P < .05 versus age-matched WKYs. The ages of the animals in weeks are indicated by the numbers “20” and “8” in (A). n = 4 for each group. Error bars indicate means ± SEM.
HO activity and its modulation by hemin treatment
The functionality of HO is the product of the quantities of expressed HO proteins and the enzymatic activity of individual HO proteins. The basal HO activity in young SHRs was significantly lower than in age-matched WKYs (Figure 4). The application of hemin increased HO activity by 3-fold in young WKYs (P < .01). However, an even more significant increase in HO activity (6-fold) was observed in young SHRs (P < .01, hemin-treated SHRs vs hemin-treated WKYs). ZnPP abated the hemin-induced increase of HO activity in young SHRs and WKYs. Incubation of aortic tissues for 30 minutes with iron (1 μM), biliverdin (1 μM), or N2-aerated PBS for 30 minutes had no effect on the activity of HO. Interestingly, CO (1 μM) incubation for 30 minutes induced a significant increase in HO activity (Figure4). Pretreatment of aortic tissues for 30 minutes with the CO scavenger HbO2 abrogated the CO- and hemin-induced increase in HO activity in a manner similar to ZnPP (Figure 4).
HO activities in the aortas of young (8-week-old) SHRs and age-matched WKYs.
A significantly lower HO-1 activity in SHRs than WKYs (†P < .05) was registered. Hemin (Hem) and CO greatly increased HO activity in SHRs and age-matched WKYs. ZnPP and HbO2 nullified the hemin-dependent increase in HO activity. **P < .01 versus control (Ctrl). *P < .05 versus hemin-treated animals. Nitrogen (N2), biliverdin (Bil), and iron (Fe) had no effect on HO activity. n = 4 for each group. Error bars indicate means ± SEM.
HO activities in the aortas of young (8-week-old) SHRs and age-matched WKYs.
A significantly lower HO-1 activity in SHRs than WKYs (†P < .05) was registered. Hemin (Hem) and CO greatly increased HO activity in SHRs and age-matched WKYs. ZnPP and HbO2 nullified the hemin-dependent increase in HO activity. **P < .01 versus control (Ctrl). *P < .05 versus hemin-treated animals. Nitrogen (N2), biliverdin (Bil), and iron (Fe) had no effect on HO activity. n = 4 for each group. Error bars indicate means ± SEM.
Effect of hemin on the expression of sGC protein in aortic tissues
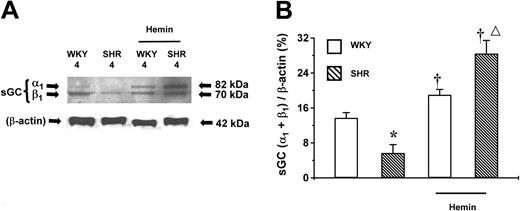
sGC is the downstream target of CO generated by HO. The role of sGC in the hemin-induced BP lowering in young SHRs was determined. The basal expression level of sGC protein was highest in adult (20-week-old), lower in young (8-week-old) (Figure5), and lowest in prehypertensive 4-week-old SHRs (Figure 6). The same age-dependent trend in sGC expression level was observed in WKY rats. Hemin enhanced the expression of sGC, which was positively correlated to the hemin-induced HO-1 expression. Notably, hemin administration up-regulated the expression levels of sGC proteins by 4- and 3-fold in 4- and 8-week-old SHRs, respectively, compared with the untreated age-matched SHRs (P < .05). The expression level of sGC in adult SHRs remained unaltered after hemin treatment (Figure 5). In addition, hemin injection enhanced sGC expression in WKYs of all ages, albeit to a lesser extent as occurred in the age-matched SHRs (Figures 5-6).
Expression of sGC proteins in the aortas of adult (20-week-old) and young (8-week-old) SHRs and WKYs.
Representative Western immunoblotting (A) and the mean relative abundant levels of expressed proteins (B) indicated a depressed basal sGC expression in 8-week-old SHRs. Hemin treatment significantly increased sGC expression in 8-week-old SHRs and in WKYs of all ages, although a higher magnitude of increase was registered in 8-week-old SHRs (†P < .05). ††P < .01 versus age-matched WKYs. *P < .05 versus all other groups. The ages of the animals in weeks were indicated as “20” and “8” in (A) and (B). n = 4 for each group. Error bars indicate means ± SEM.
Expression of sGC proteins in the aortas of adult (20-week-old) and young (8-week-old) SHRs and WKYs.
Representative Western immunoblotting (A) and the mean relative abundant levels of expressed proteins (B) indicated a depressed basal sGC expression in 8-week-old SHRs. Hemin treatment significantly increased sGC expression in 8-week-old SHRs and in WKYs of all ages, although a higher magnitude of increase was registered in 8-week-old SHRs (†P < .05). ††P < .01 versus age-matched WKYs. *P < .05 versus all other groups. The ages of the animals in weeks were indicated as “20” and “8” in (A) and (B). n = 4 for each group. Error bars indicate means ± SEM.
Expression of sGC proteins in the aortas of 4-week-old SHRs and WKYs.
Representative Western immunoblotting (A) and the mean relative abundant levels of expressed proteins (B) indicated a lower basal level of sGC expression in SHRs, which were enhanced by hemin treatment. *P < .05 versus age-matched WKYs. †P < .05 versus age-matched animals of the same species but without hemin treatment. ΔP < .05 versus age-matched and hemin-treated WKYs. n = 4 for each group. Unlabeled bars indicate untreated animals. Error bars indicate means ± SEM.
Expression of sGC proteins in the aortas of 4-week-old SHRs and WKYs.
Representative Western immunoblotting (A) and the mean relative abundant levels of expressed proteins (B) indicated a lower basal level of sGC expression in SHRs, which were enhanced by hemin treatment. *P < .05 versus age-matched WKYs. †P < .05 versus age-matched animals of the same species but without hemin treatment. ΔP < .05 versus age-matched and hemin-treated WKYs. n = 4 for each group. Unlabeled bars indicate untreated animals. Error bars indicate means ± SEM.
Effect of hemin treatment on cGMP content in aortic tissues
To examine whether hemin-enhanced sGC expression was paralleled by an increase in cGMP production, we determined the tissue content of cGMP. The basal cGMP content in aortas from adult SHRs was much greater than from any other group of animals and was not further enhanced by hemin treatment (Figure 7). The basal cGMP content in aortas from 8-week-old SHRs was significantly lower than in age-matched WKYs but greatly increased after hemin treatment (Figure 7A). In prehypertensive 4-week-old SHRs the content of cGMP was lower than that of age-matched WKYs but was enhanced by 5-fold following the administration of hemin, similar to the observation in young SHRs (Figure 7B). The basal cGMP content was highest in 20-week-old, lower in 8-week-old, and lowest in 4-week-old WKYs. Although notable increases in cGMP content were observed in WKYs of all ages following the administration of hemin, a significantly higher percentage increase (P < .05) was registered in 4- and 8-week-old SHRs compared with age-matched WKYs.
Effects of hemin on cGMP levels in the aortas from SHRs and WKYs.
(A) Changes in cGMP contents in 20- and 8-week-old SHRs and WKYs. (B) Changes in cGMP contents in 4-week-old SHRs and WKYs. The administration of hemin significantly enhanced the cGMP level in aortas from 4- and 8-week-old SHRs and the respective age-matched WKYs, but not 20-week-old SHRs or WKYs. *P < .05 versus all other groups. †P < .05 versus age-matched and hemin-treated WKYs. ††P < .05 versus any other groups without hemin treatment. ΔP < .05 versus age-matched animals of the same species but without hemin treatment. The ages of the animals in weeks were indicated as “20” and “8” in (A). n = 4 for each group. Error bars indicate means ± SEM.
Effects of hemin on cGMP levels in the aortas from SHRs and WKYs.
(A) Changes in cGMP contents in 20- and 8-week-old SHRs and WKYs. (B) Changes in cGMP contents in 4-week-old SHRs and WKYs. The administration of hemin significantly enhanced the cGMP level in aortas from 4- and 8-week-old SHRs and the respective age-matched WKYs, but not 20-week-old SHRs or WKYs. *P < .05 versus all other groups. †P < .05 versus age-matched and hemin-treated WKYs. ††P < .05 versus any other groups without hemin treatment. ΔP < .05 versus age-matched animals of the same species but without hemin treatment. The ages of the animals in weeks were indicated as “20” and “8” in (A). n = 4 for each group. Error bars indicate means ± SEM.
Impaired sGC/cGMP system in aortic tissues from adult SHRs
To assess the functions of the sGC/cGMP pathway in SHRs, a CO- and NO-independent activator of sGC YC-139 was used to relax the precontracted aortic tissues. YC-1 induced a concentration-dependent vasorelaxation in aortas from adult SHRs and age-matched WKYs. The EC50 of YC-1 in adult SHRs (7.8 ± 1.6 μM) was significantly higher than that of age-matched WKYs (5.5 ± 0.1 μM) (P < .05) or that of young SHRs (4.8 ± 0.1 μM) (n = 8, P < .05). This effect could be explained by a desensitized sCG/cGMP pathway in adult SHRs. The YC-1–induced relaxations of aortic tissues from young (5.5 ± 0.1 μM) and adult WKYs (5.5 ± 0.1 μM) were not different from each other.
Macrophage infiltration of vascular tissues
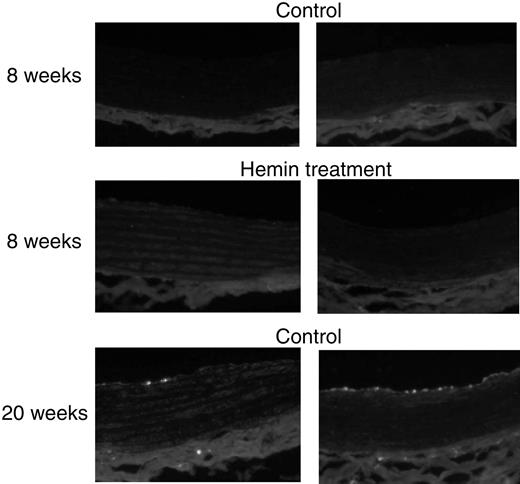
Immunocytochemistry staining (Figure8) revealed that ED-1–positive cells were not detectable in the aorta from either 8-week-old SHRs or age-matched WKYs with or without hemin treatment (n = 3 in each group). There was also no macrophage infiltration observed in carotid arteries from 8-week-old SHRs and WKYs treated with or without hemin (data not shown). Interestingly, ED-1–positive cells were present in aorta (Figure 8) and carotid arteries (data not shown) from both adult SHRs (20 weeks old, n = 3) and age-matched WKYs (n = 3) with fewer macrophages detected in the vascular tissues from WKYs.
ED-1 immunofluorescence staining in aorta from SHRs and WKYs at different ages and with different treatments.
Aortic tissues from adult SHRs (right panels; 20-week-old) and age-matched WKYs (left panels) exhibited macrophage infiltration, shown as bright stains, but more significantly in the former. Original magnification, × 200.
ED-1 immunofluorescence staining in aorta from SHRs and WKYs at different ages and with different treatments.
Aortic tissues from adult SHRs (right panels; 20-week-old) and age-matched WKYs (left panels) exhibited macrophage infiltration, shown as bright stains, but more significantly in the former. Original magnification, × 200.
Discussion
The objectives of this study were to investigate the alterations of the HO/CO-sGC/cGMP system in hypertension and the mechanisms underlying the hemin-induced selective BP lowering in young SHRs. We demonstrate that the basal expression levels of HO-1, HO-2, and sGC proteins as well as cGMP contents in aortic tissues from 8-week-old SHRs were significantly lower than that in age-matched WKYs. These deficiencies in young SHRs are indicative of genetic impairments of the HO/CO system, which possibly lead to blood pressure increase. After a 4-day regimen of hemin treatment, the elevated BP in young SHRs was regressed to normotensive level, which was accompanied by increased expressions of HO-1 and sGC and increased cGMP content. Although hemin treatment also increased the levels of HO-1 and sGC expression in all ages of WKYs, the net increment in 8-week-old SHRs was significantly higher. The hemin-enhanced HO/CO-sGC/cGMP system in WKYs may fall below the threshold necessary to trigger a BP-lowering response. The integrated functioning of other physiologic reactions also may be involved to maintain BP in WKYs at normotensive settings. In contrast, a greater increase in HO activity observed in young SHRs after hemin treatment might reach a threshold level high enough to exert a detectable BP-lowering effect. In 4-week-old SHRs with BP still in the normotensive range, sGC and cGMP levels were lower than in age-matched WKYs and significantly increased by hemin treatment. This observation further indicates a defective sGC/cGMP pathway in SHRs at birth whose contribution in counteracting hypertension is latent at 4 weeks of age. The defective sGC/cGMP pathway emerges and becomes determinant to the evolution of the disease at a later stage of life when the relative input of the sGC/cGMP pathway becomes indispensable for the regulation of the contractile machinery in vascular SMCs. A lower HO-2 protein expression level was found in young SHRs. Consequently, the level of endogenous CO generated via an HO-2–dependent pathway for intrinsic maintenance of vascular tone would be lower in young SHRs. This defect, together with the lower expression level of HO-1, would contribute to the aggravation of hypertension of young SHRs. Any attempt to counteract the deficit CO production would depend entirely on the manipulation of HO-1, as HO-2 is a constitutive noninducible enzyme. This was demonstrated by our results that hemin treatment did not affect HO-2 expression levels in the animals.
The levels of HO-1 and sGC expression and cGMP content in adult SHRs were significantly higher than those of age-matched WKYs, yet the former had hypertension and the latter was normotensive. Moreover, hemin treatment did not increase the expression levels of HO-1 and sGC proteins or the cGMP level in adult SHRs, nor did it lower the BP in these rats. One of the possible reasons for these controversial observations is that the stunning stress factors evoked by elevated BP induce HO-1 expression to compensate the increased oxidative stress and increased blood pressure. However, the compensatory increase in HO-1 expression may not suffice to lower blood pressure to the normotensive level since other elements of the HO-CO/sGC-cGMP systems become abnormal, too, in adult SHRs. The fully developed hypertension in adult SHRs may cause desensitization of the downstream targets of cGMP. Therefore, even the compensatory increase in HO-1 expression and CO production would still fail to bring BP to the normotensive level. Hemin treatment could not further increase the already fully expressed HO-1 or sGC proteins. To support this hypothesis, our contractility studies revealed a desensitized vasorelaxant response to YC-1 of aortic tissue in adult SHRs. As such, a decreased vasorelaxation and a consequentially increased vascular tone would be encountered, although CO production and the cGMP level were significantly higher than normal. To this end, mounting evidence has pinpointed a desensitized sGC/cGMP metabolic pathway in vascular SMCs from adult SHRs40 and in cultured rat vascular SMCs after the exposure to isobutyl-1-methylxanthine (IBMX) or folskolin.41 Tissue-specific down-regulation of the sGC/cGMP system was suggested as the underlying mechanism for the impairment of endothelium-independent but cGMP-mediated vasorelaxation in adult SHRs.26 Genetic hypertension and aging also were shown to be associated with blunted relaxation of smooth muscle at the level of or downstream of sGC.42
It is not a surprise that hemin increased the activity of sGC since the up-regulated HO-1 by hemin leads to increased CO production, and the latter is well known to stimulate sGC activity. The increased sGC expression by hemin treatment, on the other hand, is a novel observation that has not been previously reported. Either the hemin-derived CO via HO-1 up-regulation or hemin per se might directly increase the transcription and/or translation of sGC. At any rate, this up-regulation of sGC by CO or hemin may represent a self-reinforcement mechanism through which CO can influence the expression of its target, a mechanism that becomes especially important in pathophysiologic conditions where deficient sGC expression is characterized, such as the lower sGC expression encountered in 4- and 8-week-old SHRs in our study.
Metalloporphyrins, including ZnPP, have been reported to inhibit NO synthase (NOS) and sGC activities in addition to the inhibition of HO.43,44 With appropriate concentrations, however, the specific effect of ZnPP on HO activity can be investigated.45 Our previous study showed that intraperitoneal application of ZnPP (50 μmol/kg) did not alter sGC activity.17 In line with our observation is the report that ZnPP at the concentration of 50 μmol/kg specifically inhibited aortic HO activity without affecting sGC activity.46 In the present study, the coadministration of hemin and ZnPP abolished the hemin-elicited BP-lowering effect, indicating the direct implication of the HO/CO systems in the effect of hemin treatment to lower BP. To confirm the specificity of action of ZnPP for HO activity, we also have compared the effects of ZnPP with that of chromium mesoporphyrin (CrMP) (4 μmol/kg body weight intraperitoneally). Appleton et al had shown that CrMP selectively inhibits HO activity without inhibiting NOS or sGC activity.47 We found that the effects of CrMP on BP regulation and HO activities were similar to that of ZnPP (data not shown). We also observed that administration of ZnPP alone to 8-week-old SHRs evoked elevation in BP but not in age-matched WKYs. This suggests that the basal activity of the HO/CO system in 8-week-old SHRs, although lower than age-matched WKYs, plays an essential role in the regulation of BP in these rats, which is one of the major reasons that BP in these young rats is not too high. It is expected that without the basal BP-lowering input from the HO/CO system, the elevating BP in 8-week-old SHRs would be much higher. This was demonstrated by the blockage of the basal activity of the HO/CO system with ZnPP. That the up-regulation of the lower basal HO/CO system in 8-week-old SHRs through hemin administration normalizes the BP is also evidence of the importance of this system.
Since bilirubin, CO, and iron are the end products of the HO enzymatic activity, the interaction between these substances with HO activity may constitute an important feedback mechanism in modulating the activity of the HO/CO system. Our present study demonstrated that biliverdin and iron were ineffective in affecting HO activity, while exogenously administered CO significantly up-regulated HO activity by several fold, agreeing with previous reports.31,48 The increased HO-1 expression under different conditions in response to hypoxia induced by CO or other substances49 has been reported. However, under our experimental conditions, it is believed that CO increased HO activity instead of HO-1 expression, an effect unlikely mediated by hypoxia. Several lines of evidence support this notion. Our experiments were carried out by incubating vascular tissues with 1 μM CO for 30 minutes at 37°C. Within this short period, the possibility that increased HO-1 expression leads to new HO-1 protein synthesis is remote. The mechanism for the CO-induced hypoxia in vivo is related mainly to the competitive combination of CO to hemoglobin with oxygen. Our in vitro incubation study does not involve this hypoxia mechanism. N2 has been used widely as an internal control for the specificity of action of CO and to exclude the effects of hypoxic stunning.50 51 While CO alone significantly increased HO activity, N2 treatment does not affect HO activity. When the same samples pretreated with N2 were consecutively treated with CO, the enhanced HO activity was observed again (data not shown).
The expression and activity of HO-1 in macrophages have been reported.52 Different extents of macrophage infiltration of vascular tissues might be responsible for the different expression levels of HO-1 in young and adult SHRs with or without hemin treatment. Our results demonstrate that (1) the lower HO-1 level in young SHRs is not related to macrophage infiltration, (2) macrophage infiltration of vascular tissues does not occur after the hemin treatment of 8-week-old SHRs, and (3) macrophage infiltration of vascular tissues occurs in an age-dependent fashion, more severe in SHRs than in WKYs. Thus, it remains a possibility that elevated HO-1 expression in adult SHR vascular tissues resulted from, at least in part, the infiltrated macrophages during the remodeling of the vascular wall.
Collectively, our results indicate that the function of the HO/CO-sGC/cGMP system is closely related to the developmental stages of genetic hypertension, and deficiency of this system might contribute to the genesis of hypertension. In adult SHRs, the overexpressed HO/CO-sGC/cGMP system was consequential to the desensitization of the downstream targets of cGMP, thus canceling the antihypertensive effects of this system. By the same token, the activation of a functional HO/CO-sGC/cGMP system serves to lower BP if the cGMP targets retain their sensitivity to cGMP. The scenario is different in young SHRs, where the downstream targets of sGC/cGMP were not desensitized but the upstream of the sGC/cGMP pathway, that is, the HO/CO system, was impaired. HO-1 expression in WKY rats at all ages and young SHRs respond well to hemin challenge. The same hemin treatment failed to induce HO-1 expression in 20-week-old SHRs. It is speculated that the sensitivity of HO-1 in adult SHRs is somehow reduced to hemin treatment. This desensitization mechanism, albeit difficult to fully understand, could be related to the increased blood pressure as well as to the increased oxidative stress in these adult SHRs. The conclusions of our present study are derived from experimental observations on aortic tissues of the hemin-treated SHRs at different ages. With the acknowledgment of the important contribution of the increased stiffness of conduit arteries to the increased systolic blood pressure, especially in the elderly,53 elucidation of the structural and functional abnormalities of resistant arteries under the same experimental conditions may provide more revealing evidence for the role of the HO/CO-sGC/cGMP system in the regulation of blood pressure in SHRs at different developmental stages, which merits future investigation. Moreover, further intensive investigation on the efficacy and potency of different treatment regimens with hemin or other HO-1 inducers, including prolonged treatment periods and various administration routes and dosages, will better establish the HO/CO-sGC/cGMP system as an important therapeutic target for the management of genetic hypertension.
The authors are grateful for technical assistance from Ms Ginger Beal, Ms Koleen Safinuik, Mr Deryk Meszaros, and Mr Zoran Jakic.
L.W. is a New Investigator of Canadian Institutes of Health Research (CIHR). R.W. is an Investigator of the CIHR/Regional Partnership Program.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/ blood-2002-08-2608.
Supported by the Heart and Stroke Foundation of Saskatchewan, Canada. J.F.N. is supported by a postdoctoral fellowship from Health Services Utilization and Research Commission (HSURC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rui Wang, Department of Physiology, College of Medicine, University of Saskatchewan, 107 Wiggins Rd, Saskatoon, SK, Canada S7N 5E5; e-mail: wangrui@duke.usask.ca.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal