Abstract
Although hyperhomocysteinemia is an independent risk factor for cardiovascular disease, a direct role for homocysteine (Hcy) in this disease remains to be shown. Whereas diet-induced hyperhomocysteinemia promotes atherosclerosis in animal models, the effects of Hcy on atherogenesis in the absence of dietary perturbations is not known. We have generated double knock-out mice with targeted deletions of the genes for apolipoprotein E (apoE) and cystathionine β-synthase (CBS), which converts Hcy to cystathionine. ApoE−/−/CBS−/− mice developed aortic lesions even in the absence of dietary manipulation; lesion area and lesion cholesteryl ester (CE) and triglyceride (TG) contents increased with animal age and plasma Hcy levels. Plasma total cholesterol was significantly increased, whereas high density lipoprotein (HDL) cholesterol and TG concentrations of apoE−/−/CBS−/− mice were decreased. Cholesterol esterification and activities of enzymes catalyzing CE or TG formation in the vessel wall and in peritoneal macrophages were not changed by hyperhomocysteinemia. However, uptake of human acetyl-LDL, but not native low density lipoprotein (LDL), by mouse peritoneal macrophages was higher in the presence of hyperhomocysteinemia. These results suggest that isolated hyperhomocysteinemia is atherogenic and alters hepatic and macrophage lipoprotein metabolism, in part, by enhancing uptake of modified LDL.
Introduction
Despite advances in our understanding of the causes of cardiovascular disease (CVD), the established risk factors do not fully account for its occurrence. Numerous clinical studies have shown that homocysteine (Hcy) is a significant and independent CVD risk factor. It is not known whether Hcy is a causative agent or only a marker.
Several dietary animal models of hyperhomocysteinemia have been used to study Hcy-mediated vascular pathogenesis. Diet-induced hyperhomocysteinemia is associated with vascular dysfunction in the monkey, probably due to the inhibition of nitric oxide (NO) synthesis,1 linked with vascular structural damage in the minipig because of elastic lamina fragmentation,2correlated with increased postinjury intimal hyperplasia,3and impaired endothelial function and leukocyte-endothelium interaction in the rat.4
A genetic hyperhomocysteinemia model with the gene deletion of cystathionine β-synthase (CBS), which catalyzes Hcy to cystathionine, has been used in studies of Hcy pathology. Homozygous CBS-deficient mice (CBS−/−) have plasma Hcy levels that are 50 times higher than wild type and similar to human hyperhomocysteinemia. Heterozygous CBS-deficient mice (CBS−/+) have plasma Hcy levels approximately 2 times wild type.5CBS−/− mice have a short life span and develop hepatic steatosis. CBS−/+ mice have endothelial dysfunction, probably due to NO inactivation resulting from increased production of reactive oxygen species (ROS) following the inhibition of glutathione peroxidase, an antioxidant enzyme.6,7 This effect is associated with inhibition of S-adenosylmethionine (SAM)–dependent methylation.8 CBS−/+ mice fed a hyperhomocysteinemic diet have increased hepatic cholesterol and triglyceride (TG) levels through increased hepatic expression of genes involved in cholesterol and TG synthesis, uptake, and storage.9 However, atherosclerotic lesions have not been observed in CBS−/− mice. Therefore, it is necessary to test the hyperhomocysteinemia model of atherosclerosis in animals that develop spontaneous atherosclerosis to determine whether hyperhomocysteinemia is atherogenic.
Several genetic models of atherosclerosis have been established and characterized.10 Mice with a targeted disruption of the apolipoprotein E (apoE) gene, which mediates the removal of plasma lipoproteins via the low density lipoprotein (LDL) receptors and other receptors, are severely hypercholesterolemic and develop spontaneous aortic atherosclerotic lesions.11,12 These mice develop lesions at an early age if fed an atherogenic diet. Diet-induced hyperhomocysteinemia in apoE−/− has revealed accelerated atherosclerosis13 and enhanced vascular inflammation.14 However, dietary manipulations elicit broad physiologic changes that can confound data interpretation. Therefore, an animal model of hyperhomocysteinemia, which is isolated from broad dietary effects, would be useful in better defining the mechanistic relationship between hyperhomocysteinemia and atherogenesis.
In this study, we created double knock-out (KO) mice with targeted deletions of the apoE and CBS genes. This animal is a good model of human hypercholesterolemia and hyperhomocysteinemia and is susceptible to atherosclerosis. We used this model to determine the effect of Hcy on atherosclerosis and lipid metabolism in animals fed a regular diet, an atherogenic high-fat (HF) diet, or an HF plus hyperhomocysteinemic high-methionine (HF+HM) diet.
Materials and methods
Gene-targeted mice and diet
Mice deficient in both CBS and apoE were obtained by breeding CBS−/+ females with apoE−/− males. The mice were purchased from Jackson Laboratory (Bar Harbor, ME) and backcrossed 6 generations for CBS−/+ and 10 generations for apoE−/− to achieve approximately 98% purity in a C57BL/B6 genetic background. Mice were genotyped by polymerase chain reaction (PCR) as described.5 12 Age-matched littermates with evenly distributed sex were selected for each experimental group. Mice were fed a regular rodent diet (0% cholesterol, 5.23% fat, 0.37% methionine, 2.39 mg/g choline, 3.19 mg/kg folate, 54.6 μg/kg B12, 14.5 mg/kg B6; catalog no. 8640, Harlan Teklad, Madison, WI) after weaning. To induce dietary hyperhomocysteinemia, mice were switched to an HF diet (0.2% cholesterol, 21.2% fat, 0.75% methionine, 1.43 mg/g choline, 2.23 mg/kg folate, 32.5 μg/kg B12, 22.25 mg/kg B6; catalog no. TD88137, Harlan Teklad) or to an HF+HM diet (TD88137 with 2% methionine, catalog no. TD99338, Harlan Teklad) at 8 weeks of age and were maintained on this diet for 12 weeks.
Hcy, nonesterified fatty acid (NEFA), lipid, and lipoprotein distribution analysis
Mouse plasma was collected at the end of each experiment. Hcy concentrations were measured by liquid chromatography electrospray tandem mass spectrometry methods using 25 μL mouse plasma diluted to 100 μL with reverse osmosis water as previously described.15 Plasma levels of NEFA, total cholesterol (TC), and TG were measured with enzymatic kits according to the manufacturer's instructions (NEFA C, Total cholesterol CII, and Triglyceride kits, Wako Chemicals USA, Richmond, VA). The distribution of lipids within the plasma lipoproteins was assessed by size fractionation of individual mouse plasma using a Helena Laboratories Rapid Electrophoresis cholesterol profile system as described.16 Plasma lipoprotein standard electrophoresis was performed using 0.75% agarose gel.
Analyses of atherosclerotic lesions
Mouse aortas were removed 2 mm from the heart and excised from the aortic arch to just beyond the renal artery, cut longitudinally, pinned onto a silicon dish, and stained with saturated Sudan IV in propylene glycol.17 Total area, atherosclerotic lesion area of the aortic arch (2 mm from the heart extending to the left subclavian artery), and lesion area of the whole aorta were measured using the Image Plus program (Media Cybernetics, Sliver Spring, MD).
Vessel wall lipid analysis and thin-layer chromatography (TLC)
After lesion analysis, equal length of aorta, as measured 2 mm from the heart to the branching point of the renal artery, was sonicated in 0.3 mL of 0.1 M Tris (tris(hydroxymethyl)aminomethane)–HCl, pH 7.4. Lipids were extracted with 0.9 mL Folch reagent (chloroform-methanol [2:1, vol/vol]) 3 times, dried using nitrogen (N2) gas, and dissolved in chloroform. Lipid extracts were separated by TLC in hexane–diethyl ether–acetate (75:35:1, vol/vol/vol) and visualized by exposure to iodine vapor. Spots corresponding to cholesteryl ester (CE), TG, NEFA, free cholesterol (FC), and phospholipid were identified by comparing the positions with those of standards.
Mouse peritoneal macrophage culture and intracellular cholesterol esterification
Mouse peritoneal macrophages were isolated from mice at 25 to 30 weeks of age, plated on 24-well plates (about 4 × 105cells per well) in Dulbecco modified Eagle medium (DMEM) containing 10% calf serum (CS) to 90% confluence, cultured with 2 μCi/mL (0.074 MBq) [3H]FC in a [3H]FC–Mβcyclodextrin (MβCD, a cholesterol carrier) mixture (molar ratio 1:1) containing 1 μg/mL of inhibitor of acyl coenzyme A–cholesterol acyltransferase (ACAT) (S-58035, gift from Novartis) in dimethyl sulfoxide (DMSO) (0.1% DMSO final concentration), and incubated for 24 hours. [3H]FC–MβCD mixture was prepared as described.18 Cellular lipids were extracted with hexane/methanol (3:1, vol/vol) and transferred to a dry silica column. CE or FC was eluted with hexane–diethyl ether (6:1, vol/vol) or pure ether and analyzed by liquid scintillation counting. Cellular protein was extracted with 0.2 N NaOH/0.2% sodium dodecyl sulfate (SDS) and assayed for protein concentration. Cholesterol esterification was analyzed as [3H]CE counts versus [3H]CE plus [3H]FC counts per milligram of protein.
ACAT and triglyceride synthase (TGS) activities in mouse macrophages
Mouse peritoneal macrophages were cultured in DMEM with 10% lipoprotein-free serum (LPFS) on 24-well plates (about 4 × 105 cells per well) for 12 hours, changed to normal medium containing 10% CS, and 100 μL [3H]oleate–bovine serum albumin (BSA) per milliliter (final 5 μCi/mL [0.185 MBq], 1 mM oleic acid [OA]) at 37°C for 24 hours. Cellular lipids were extracted with hexane-isopropanol (3:2, vol/vol), counted for the incorporation of [3H]oleate, and analyzed by TLC as described above. The activity of ACAT or TGS was assayed by determining the incorporation of radiolabeled oleic acid into CE and TG. Spots of CE and TG were scraped off for radioactivity counting and normalized for protein. Cellular protein was extracted with 0.2 N NaOH/0.2% SDS and assayed for protein. Relative intracellular enzymatic activity is expressed as percentage of [3H]CE or [3H]TG counts versus total lipid counts per milligram of protein.
LDL isolation, acetylation, and radioiodination
LDL (density = 1.0919 to 1.063) was isolated by sequential ultracentrifugation of EDTA (ethylenediaminetetraacetic acid)–anticoagulated plasma obtained from healthy normolipidemic volunteers. LDL was dialyzed against saline containing 1 mM EDTA (pH 7.4). Acetylation of LDL was carried out by chemical modification of LDL with acetic anhydride.19 The extent of modification was confirmed by changes in relative electrophoretic mobility on 0.75% agarose gels. LDL was radioiodinated with [125I] according to the iodine monochloride method as described.20 Radioiodinated LDL was assayed for protein, stored at 4°C under N2 in the dark, and used within 3 days.
LDL uptake
Mouse peritoneal macrophages were cultured in DMEM with 10% LPFS on 24-well plates (about 4 × 105 cells per well) for 12 hours and changed to normal medium containing 10% CS with [125I]acetylated-LDL (Ac-LDL) or [125I]native-LDL (20 μg/mL) for 2 hours. Cellular protein was extracted with 0.2 N NaOH/0.2% SDS, counted for incorporation of [125I]LDL, and assayed for protein and protein-associated [125I] radioactivity.21LDL uptake activity was expressed as [125I] counts per milligram of protein. The uptake activity of [125I]Ac-LDL in macrophages from CBS−/+/ApoE−/− mice under HF diet was set as the control value.
Statistics
Statistical comparisons were performed with a Studentt test using SigmaStat 2.03 (Chicago, IL).
Results
CBS/apoE double KO mice
CBS/apoE double KO mice were produced by crossbreeding CBS−/+ females with apoE−/− males. The general health and body weight of CBS+/+/apoE−/− and CBS−/+/apoE−/− were not different compared with normal mice. CBS−/−/apoE−/− had a high incidence of death during the first 3 postnatal weeks. About 5% of CBS−/−/apoE−/− survived to 15 weeks of age, about 2% to 6 months.
CBS/apoE double KO mice are hyperhomocysteinemic and hypercholesterolemic
CBS gene deficiency, on an apoE KO background, resulted in an about 2-fold increase in plasma Hcy levels in CBS heterozygote mice (CBS−/+/apoE−/−) compared with CBS wild-type animals (CBS+/+/apoE−/−). Severe hyperhomocysteinemia was found in CBS homozygotes (CBS−/−/apoE−/−) (Table1). The ratio of plasma Hcy levels was 1:2.1:54 (CBS+/+/apoE−/− to CBS−/+/apoE−/− to CBS−/−/apoE−/−), which is greater than that in CBS single KO mice (1:2:40).5 The double KO mice had significantly increased plasma TC levels, which were similar to those in apoE single KO mice.12 Hyperhomocysteinemia caused by CBS gene deletion did not change the levels of plasma NEFA but was associated with significantly increased plasma TC levels and decreased plasma TG levels in the absence of dietary manipulation. Plasma very low density lipoprotein (VLDL) and LDL cholesterol, as well as high density lipoprotein (HDL) and LDL TG, were not markedly changed in the double KO mice (Table 2). However, plasma VLDL TG levels were significantly decreased. Although it did not reach statistical significance, the HDL cholesterol levels exhibited a trend to decreases in CBS homozygous mice.
Plasma levels of Hcy, NEFA, TC, and TG
| Genotype . | No. . | Hcy, μM . | NEFA, mEq/L . | TC, mg/dL . | TG, mg/dL . |
|---|---|---|---|---|---|
| Regular diet | |||||
| CBS+/+/ApoE−/− | 14 | 3.8 ± 0.9 | 0.74 ± 0.42 | 387 ± 130 | 128 ± 64 |
| CBS−/+/ApoE−/− | 15 | 7.4 ± 2.9 | 0.73 ± 0.12 | 442 ± 111 | 145 ± 56 |
| CBS−/−/ApoE−/− | 12 | 210.4 ± 80.1 | 0.83 ± 0.03 | 559 ± 84 | 72 ± 21 |
| P < .001*,† | P < .001* | P = .009* | |||
| P = .006† | P < .001† | ||||
| High-fat diet | |||||
| CBS+/+/ApoE−/− | 12 | 5.9 ± 2.0 | 1.37 ± 0.35 | 1271 ± 233 | 166 ± 40 |
| P = .039* | P = .099* | P < .001* | P = .095* | ||
| CBS−/+/ApoE−/− | 12 | 14.2 ± 7.8 | 1.61 ± 0.54 | 1366 ± 303 | 259 ± 166 |
| P = .011† | P = .029† | P < .001† | P = .02† | ||
| High-fat plus high-methionine diet | |||||
| CBS−/+/ApoE−/− | 14 | 154.9 ± 90.7 | 1.10 ± 0.27 | 1545 ± 264 | 162 ± 89 |
| P < .001‡ |
| Genotype . | No. . | Hcy, μM . | NEFA, mEq/L . | TC, mg/dL . | TG, mg/dL . |
|---|---|---|---|---|---|
| Regular diet | |||||
| CBS+/+/ApoE−/− | 14 | 3.8 ± 0.9 | 0.74 ± 0.42 | 387 ± 130 | 128 ± 64 |
| CBS−/+/ApoE−/− | 15 | 7.4 ± 2.9 | 0.73 ± 0.12 | 442 ± 111 | 145 ± 56 |
| CBS−/−/ApoE−/− | 12 | 210.4 ± 80.1 | 0.83 ± 0.03 | 559 ± 84 | 72 ± 21 |
| P < .001*,† | P < .001* | P = .009* | |||
| P = .006† | P < .001† | ||||
| High-fat diet | |||||
| CBS+/+/ApoE−/− | 12 | 5.9 ± 2.0 | 1.37 ± 0.35 | 1271 ± 233 | 166 ± 40 |
| P = .039* | P = .099* | P < .001* | P = .095* | ||
| CBS−/+/ApoE−/− | 12 | 14.2 ± 7.8 | 1.61 ± 0.54 | 1366 ± 303 | 259 ± 166 |
| P = .011† | P = .029† | P < .001† | P = .02† | ||
| High-fat plus high-methionine diet | |||||
| CBS−/+/ApoE−/− | 14 | 154.9 ± 90.7 | 1.10 ± 0.27 | 1545 ± 264 | 162 ± 89 |
| P < .001‡ |
Mice were grown under regular diet or fed an HF or an HF + HM diet at 8 weeks of age for 3 months. Values are mean ± SD; P values from t test. Only significant P values are shown.
Hcy indicates homocysteine; NEFA, nonesterified fatty acid; TC, total cholesterol; and TG, triglyceride.
Comparison versus CBS+/+/ApoE−/−.
Comparison versus CBS−/+/ApoE−/−.
Comparison versus CBS−/+/ApoE−/− on high-fat diet.
Lipoprotein cholesterol and TG profile in mice without dietary modification
| Genotype . | No. . | Cholesterol, mg/dL . | Triglyceride, mg/dL . | ||||
|---|---|---|---|---|---|---|---|
| HDL . | VLDL . | LDL . | HDL . | VLDL . | LDL . | ||
| CBS+/+/ApoE−/− | 14 | 24.4 ± 14.9 | 345 ± 142 | 10.1 ± 10.0 | 6.2 ± 3.0 | 75.6 ± 35.3 | 6.4 ± 4.5 |
| CBS−/+/ApoE−/− | 15 | 23.6 ± 14.4 | 334 ± 107 | 53.9 ± 50.6 | 4.1 ± 2.5 | 87.8 ± 51.6 | 12 ± 12 |
| CBS−/−/ApoE−/− | 12 | 19.9 ± 6.9 | 440 ± 87 | 48.0 ± 36.8 | 4.1 ± 6.3 | 38.4 ± 22.6 | 7.5 ± 4.8 |
| P = .036* | P = .006* | ||||||
| P = .079† | P = .007† | ||||||
| Genotype . | No. . | Cholesterol, mg/dL . | Triglyceride, mg/dL . | ||||
|---|---|---|---|---|---|---|---|
| HDL . | VLDL . | LDL . | HDL . | VLDL . | LDL . | ||
| CBS+/+/ApoE−/− | 14 | 24.4 ± 14.9 | 345 ± 142 | 10.1 ± 10.0 | 6.2 ± 3.0 | 75.6 ± 35.3 | 6.4 ± 4.5 |
| CBS−/+/ApoE−/− | 15 | 23.6 ± 14.4 | 334 ± 107 | 53.9 ± 50.6 | 4.1 ± 2.5 | 87.8 ± 51.6 | 12 ± 12 |
| CBS−/−/ApoE−/− | 12 | 19.9 ± 6.9 | 440 ± 87 | 48.0 ± 36.8 | 4.1 ± 6.3 | 38.4 ± 22.6 | 7.5 ± 4.8 |
| P = .036* | P = .006* | ||||||
| P = .079† | P = .007† | ||||||
Values are mean ± SD; P values fromt test.Only significant P values are shown.
HDL indicates high density lipoprotein; VLDL, very low density lipoprotein; and LDL, low density lipoprotein.
Comparison versus CBS+/+/ApoE−/−.
Comparison versus CBS−/+/ApoE−/−.
Dietary hyperhomocysteinemia increases the concentration of plasma TC but not fatty acid (FA) and TG
An HF diet doubled Hcy levels in both CBS+/+/apoE−/− and CBS−/+/apoE−/−. This HF diet also increased plasma NEFA by 2-fold and TC levels about 3-fold, with no significant differences between CBS+/+/apoE−/− and CBS−/+/apoE−/− mice (Table 1). An HF+HM diet resulted in severe hyperhomocysteinemia in CBS−/+/apoE−/− mice, which resembles that of CBS−/−/apoE−/−. This severe hyperhomocysteinemia moderately increased the concentration of plasma TC but not NEFA and TG.
Hyperhomocysteinemia accelerates aortic lesion in CBS/apoE double KO mice
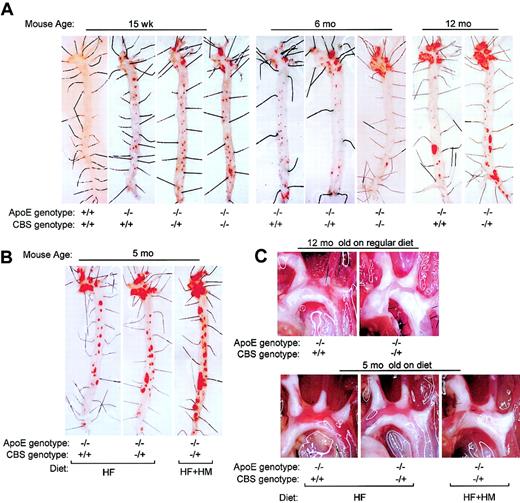
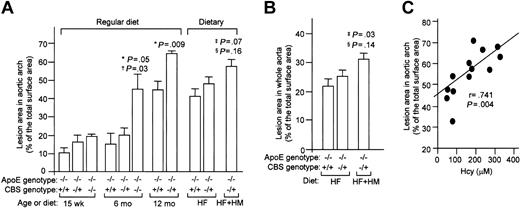
At 15 weeks, atherosclerotic lesions were apparent in apoE−/− mice at the branch points of the aortic arch and at all the ostia of the intercostal arteries (Figure1A). Hyperhomocysteinemic apoE−/− mice had a slightly larger lesion area in the aortic arch, but this was not statistically significant at this time point (Figures 1A and 2A). At 6 months, lesions were enhanced in the aortic arch and significantly increased with CBS gene deletion in a dose-dependent manner. At 1 year of age, advanced lesions were observed in all apoE−/− mice in the aortic arch; however, lesions were significantly increased by the coexistence of hyperhomocysteinemia. Advanced lesions in the aortic arch were also observed at 5 months of age in CBS−/+/apoE−/− mice that had been fed with HF or HF+HM diet for 3 months (Figure 1B). These lesions were comparable to those of CBS−/+/apoE−/− at 1 year of age on regular diet (Figure 1A), readily visible upon exposure of the aortic arch (Figure 1C). Similar to that seen in hyperhomocysteinemic mice on a regular diet, dietary hyperhomocysteinemic mice had a markedly increased lesion area in the aortic arch and in the whole aorta (Figure 2A-B). Interestingly, extensive lesions were found in the descending aorta in dietary mice, whereas lesions were found mostly in the aortic arch in regular diet–fed mice. In hyperhomocysteinemic mice, the increases of plasma Hcy levels were significantly correlated with increases in atherosclerotic lesion area in the aortic arch (Figure 2C). These results indicate that genetically induced mild hyperhomocysteinemia enhances atherogenesis in old mice, whereas both genetic and dietary severe hyperhomocysteinemia significantly increases lesion formation in young mice. Interestingly, there was a trend of increasing lesion size in CBC heterozygous male compared with female mice fed an HF or HF+HM diet. However, this did not achieve statistical significance.
Sudan IV staining and gross appearance of atherosclerotic lesions in mouse aortas.
Mice were killed at the ages indicated. Lesions were examined by Sudan IV staining (A,B) and by direct photography (C). Lesions showed up as red (A,B) and white (C) plaques. The extent of atherosclerotic lesions is shown in Figure 2. Original magnification, × 6.5.
Sudan IV staining and gross appearance of atherosclerotic lesions in mouse aortas.
Mice were killed at the ages indicated. Lesions were examined by Sudan IV staining (A,B) and by direct photography (C). Lesions showed up as red (A,B) and white (C) plaques. The extent of atherosclerotic lesions is shown in Figure 2. Original magnification, × 6.5.
Quantitation of atherosclerotic lesions in mouse aortas.
Atherosclerotic lesion area in aortic arch was measured in Sudan IV–stained aortas and expressed as percentage of aortic arch (A) or percentage of whole aorta (B). The mean lesion areas per each group were shown (n = 5 to 9). Values represent mean ± SD. *P value versus CBS+/+/ApoE−/−; †P value versus CBS−/+ /ApoE−/−; ‡P value versus CBS+/+/ApoE−/− on HF diet; §P value versus CBS−/+/ApoE−/−on HF diet. (C) Regression analysis shows the correlation of lesion area in aortic root versus plasma concentrations of Hcy in mice under HF+HM diet. Each dot represents one mouse. r indicates correlation coefficient.
Quantitation of atherosclerotic lesions in mouse aortas.
Atherosclerotic lesion area in aortic arch was measured in Sudan IV–stained aortas and expressed as percentage of aortic arch (A) or percentage of whole aorta (B). The mean lesion areas per each group were shown (n = 5 to 9). Values represent mean ± SD. *P value versus CBS+/+/ApoE−/−; †P value versus CBS−/+ /ApoE−/−; ‡P value versus CBS+/+/ApoE−/− on HF diet; §P value versus CBS−/+/ApoE−/−on HF diet. (C) Regression analysis shows the correlation of lesion area in aortic root versus plasma concentrations of Hcy in mice under HF+HM diet. Each dot represents one mouse. r indicates correlation coefficient.
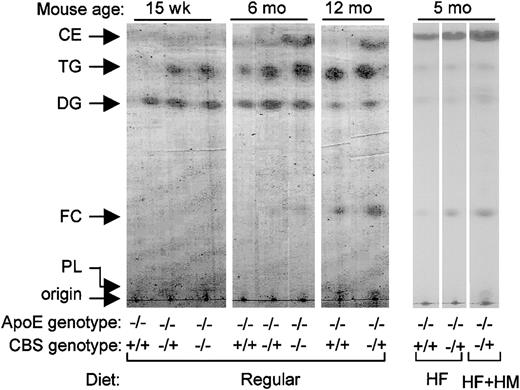
Hyperhomocysteinemia increases accumulation of CE and TG in the vessel wall in CBS/ApoE double KO mice
We next analyzed lipid composition in the vessel wall. Hyperhomocysteinemia increased vascular CE content in 6-month-old and 1-year-old double KO mice, and this correlated with the dose of CBS gene deletion (Figure 3). TG was found in early lesions at 15 weeks in CBS−/+/apoE−/−and CBS−/−/apoE−/− mice but not in CBS+/+/apoE−/− mice, and this was slightly increased with aging. As mice aged to 1 year, TG content in CBS+/+/apoE−/− matched those in CBS−/+/apoE−/− mice. FC content increased with aging but independent of hyperhomocysteinemia. Dietary hyperhomocysteinemia increased lesion CE and FC content without affecting lesion TG content. These results suggest that mild, moderate, and severe hyperhomocysteinemia increases CE and TG content in the vessel wall in an age- and dose-dependent fashion with or without dietary manipulation.
Effect of hyperhomocysteinemia on lipid profile of mouse aortas.
Shown is a representative of thin-layer chromatography analysis of lipids from aortas. Each lane represents lipids from one mouse. All mice are on apoE−/− background. CE indicates cholesteryl ester; TG, triglyceride; DG, diglyceride; FC, free cholesterol; and PL, phospholipid.
Effect of hyperhomocysteinemia on lipid profile of mouse aortas.
Shown is a representative of thin-layer chromatography analysis of lipids from aortas. Each lane represents lipids from one mouse. All mice are on apoE−/− background. CE indicates cholesteryl ester; TG, triglyceride; DG, diglyceride; FC, free cholesterol; and PL, phospholipid.
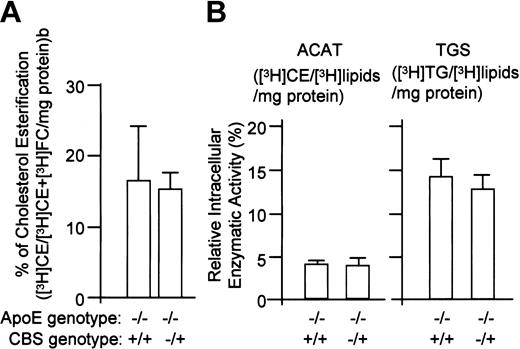
Hyperhomocysteinemia does not affect [3H]cholesterol esterification in mouse peritoneal macrophages
We speculated 2 potential mechanisms that might be responsible for the accumulation of CE and TG in the vessel wall of the double KO mice. One involves alteration in cellular lipid metabolism, while the other relates to increases in cellular LDL uptake. Because enhanced intracellular cholesterol esterification has been considered a major feature of atherosclerosis, we examined the cellular conversion of [3H]FC into [3H]CE in mouse peritoneal macrophages. We found that the relative size of cellular [3H]FC and [3H]CE pools were consistent between CBS+/+/apoE−/− and CBS−/+/apoE−/− in macrophages incubated with [3H]FC for 24 hours (Figure4A), indicating that hyperhomocysteinemia did not change macrophage intracellular cholesterol esterification.
Effects of hyperhomocysteinemia on intracellular cholesterol esterification and on activities of lipid catalytic enzymes (ACAT or TGS).
The values represent the means ± SD from 3 independent experiments (n = 6).
Effects of hyperhomocysteinemia on intracellular cholesterol esterification and on activities of lipid catalytic enzymes (ACAT or TGS).
The values represent the means ± SD from 3 independent experiments (n = 6).
Hyperhomocysteinemia does not affect TG or CE synthesis
To better understand the effect of hyperhomocysteinemia on the cellular metabolism of CE and TG, we examined enzymatic activities of ACAT and TGS, which are the major enzymes catalyzing CE and TG metabolism in mouse macrophages. Intracellular activities of ACAT and TGS were not different in the double KO mice (Figure 4B). Similar results were observed in enzymatic assays using microsomes isolated from mouse aorta or peritoneal microphages (data not shown). Taken together, these data suggest that hyperhomocysteinemia does not affect CE and TG intracellular metabolism in the vessels and in peritoneal macrophages from the double KO mice.
Hyperhomocysteinemia increases the uptake of Ac-LDL in peritoneal macrophages
The second potential mechanism by which CE accumulates in the lesions of the double KO mice is via enhanced LDL uptake. To test this possibility, we incubated mouse macrophages with [125I]Ac-LDL or [125I]native-LDL (20 μg/mL) for 2 hours and then measured protein-associated [125I] radioactivity. As shown in Figure5, native-LDL uptake was somewhat decreased in macrophages from hyperhomocysteinemic mice. In sharp contrast, hyperhomocysteinemia resulting from CBS gene deletion significantly increased Ac-LDL uptake. A similar pattern was observed in macrophages from dietary hyperhomocysteinemic mice. These data indicate that the uptake of Ac-LDL by macrophages from genetically severe hyperhomocysteinemic mice and by genetically moderate hyperhomocysteinemic mice is increased relative to those from control mice.
Effect of hyperhomocysteinemia on LDL uptake.
Mouse peritoneal macrophages were incubated with 20 μg/mL [125I]Ac-LDL or [125I]native-LDL for 2 hours. Protein-associated [125I]radioactivity was measured and normalized as [125I] counts per milligram of protein. The uptake activity of [125I]Ac-LDL in macrophages from CBS−/+/ApoE−/− mice under HF diet was set as the control. LDL uptake activity is expressed as relative cellular [125I]protein (% of control mean values). (Inset) The image of LDLs on 0.75% agarose gel shows the electrophoretic mobility change by acetylation. Values represent mean ± SD from 3 independent assays (n = 9). *P < .01 versus CBS+/+/ApoE−/−; †P < .01 versus CBS−/+/ApoE−/− in the same group.
Effect of hyperhomocysteinemia on LDL uptake.
Mouse peritoneal macrophages were incubated with 20 μg/mL [125I]Ac-LDL or [125I]native-LDL for 2 hours. Protein-associated [125I]radioactivity was measured and normalized as [125I] counts per milligram of protein. The uptake activity of [125I]Ac-LDL in macrophages from CBS−/+/ApoE−/− mice under HF diet was set as the control. LDL uptake activity is expressed as relative cellular [125I]protein (% of control mean values). (Inset) The image of LDLs on 0.75% agarose gel shows the electrophoretic mobility change by acetylation. Values represent mean ± SD from 3 independent assays (n = 9). *P < .01 versus CBS+/+/ApoE−/−; †P < .01 versus CBS−/+/ApoE−/− in the same group.
Discussion
In the present study, we found accelerated aortic atherosclerosis in CBS/apoE double KO mice, a genetic model of hyperhomocysteinemia. Increased lesion formation was observed in a genetically determined mild form of hyperhomocysteinemia in old mice (CBS−/+/apoE−/−, 12 months old, regular diet), in a diet-induced moderate hyperhomocysteinemia in young mice (CBS−/+/apoE−/−, 5 months old, HF diet), and in severe hyperhomocysteinemia produced by combined genetic and dietary means in young and old mice (CBS−/−/apoE−/−, 6 months old, regular diet; and CBS−/+/apoE−/−, 6 months old, HF+HM diet). Lesion formation was positively associated with Ac-LDL uptake by peritoneal macrophages from the affected mice, plasma TC, lesion CE and TG content, and was negatively associated with HDL cholesterol. To our knowledge, this study is the first to reveal the atherogenic property of Hcy in an animal model free of dietary perturbation.
Hyperhomocysteinemia is an independent CVD risk factor and does not correlate with most traditional risk factors, such as hyperlipidemia. Our identification of increased plasma TC and decreased HDL cholesterol in genetic hyperhomocysteinemic mice without dietary manipulation suggests a possible link between hyperhomocysteinemia and altered hepatic lipid metabolism. This finding is partially consistent with the report of Werstuck et al9 showing that Hcy increases cholesterol and TG content of HepG2 cells and that diet-induced hyperhomocysteinemia increases the accumulation and synthesis of hepatic cholesterol and TG in mice. The increased lesion formation in CBS−/−/apoE−/− animals is associated with elevated TC and lower HDL cholesterol, both of which are known to be strong risk factors for CVD, suggesting that plasma Hcy affects hepatic lipid metabolism, thereby further increasing CVD risk. Plasma TG and VLDL TG probably do not correlate with increased lesion formation because these analytes remain within the normal range of TG concentrations in the double knock-out mice. In addition, the role of plasma TG as an independent CVD risk factor is controversial and unresolved. An HF+HM diet consistently resulted in severe atherosclerosis and hyperhomocysteinemia in CBS−/+/apoE−/− mice, thereby confirming the atherogenicity of Hcy and validating the model for severe hyperhomocysteinemia.
Notably, an HF diet not only elevated NEFA and TC concentrations but also doubled Hcy levels in both CBS wild-type and heterozygous mice. However, this may be related, in part, to the higher content of methionine and lower content of choline in the HF diet. A prior study using a high-methionine plus low-folate diet in CBS−/+mice found a 15-fold increase (6.2 to 92.8 μM) in Hcy levels,22 whereas we observed a 21-fold increase (7.4 to 154.9 μM) in Hcy levels in CBS−/+/apoE−/−mice fed a high-methionine plus HF diet compared with mice on a control diet and an 11-fold increase (14.2 to 154.9 μM) compared with mice on only an HF diet (Table 1). These suggest that the high intake of dietary cholesterol and fat might contribute to the increase of Hcy levels as well. Thus, the combination of hyperhomocysteinemia and hyperlipidemia may increase the occurrence of atherosclerosis. The combination of HF plus high-methionine diet in CBS heterozygous animals is an easily produced model of severe hyperhomocysteinemia, which will permit large-scale in vivo functional assessments that were not possible with the CBS homozygous mice.
We observed that both genetic and dietary hyperhomocysteinemia increased aortic lesion formation and neutral lipid (CE and TG) content in the lesions of apoE−/− mice. The accumulation of CE in macrophages, which gives rise to macrophage foam cells, is a prominent feature of atherosclerotic lesions. Macrophage and plasma TG metabolism may be mechanistically linked. In rats, plasma TG levels increase after oral administration of Hcy, an effect that is mediated by the inhibition of fatty acid oxidation.23 However, the accumulation of TG in early lesions in hyperhomocysteinemia has not been previously reported. Our finding suggests that TG accumulation in the vessel wall is an early event of atherogenesis and could play a mediating role in Hcy vascular pathogenesis.
Several lines of evidence suggest that atherogenic lipids in lesions are derived from circulating lipoproteins, particularly LDL. Models for the mechanism of atherogenic lipid accumulation in vascular lesions emphasize increased LDL uptake by macrophages into the vessel wall or increased cholesterol esterification catalyzed by ACAT. TG synthesis involves several enzymes, which are collectively identified as TGS in this study. Because there were no significant changes in microsomal and cellular enzymatic activities of ACAT and TGS, we conclude that altered cellular lipid metabolism is not responsible for the increased lipid accumulation in the lesions of hyperhomocysteinemic apoE−/− mice.
In vitro studies have established that LDL can be modified by oxidation, acetylation, glycation, methylation, and other conditions.24,25 During Hcy autooxidation, liberated ROS could initiate lipid peroxidation and lead to impaired endothelial function and the formation of atherogenic LDL.26 Although Hcy and other thiols induce LDL peroxidation in vitro,27,28 no difference in the extent of oxidation of LDL has been found in patients with moderate and severe hyperhomocysteinemia in case-control studies.29,30 It has been proposed that hypomethylation is a specific biochemical mechanism by which Hcy induces vascular injury.31 Hcy can utilize adenosine to form S-adenosylhomocysteine (SAH), a potent inhibitor of cellular methylation. Elevated Hcy levels in patients are linked to increased SAH and impaired erythrocyte membrane protein methylation.32 CBS-deficient mice have increased SAH levels and decreased DNA methylation.8 Hcy arrests endothelial cell (EC) growth and increases cellular SAH in a cell type–specific way.33,34 It is relevant that methylation of LDL abolished its recognition by LDL receptors,35retarded the degradation of aggregated LDL by macrophages,36 and decreased CE formation in macrophages.37 It is possible that hyperhomocysteinemia may inhibit lipid or protein methylation in LDL, which may result in increased endocytosis of LDL-derived CE in the lesions.
In addition, we considered that enhanced uptake of modified LDL may account for the increase in lesion lipid content. Modified LDL stimulates the secretion of cytokines and growth factors from vascular cells and, in contrast to native LDL, is avidly taken up by macrophages in a process that is mediated by interaction with a family of scavenger receptors (SRs).38 Modified LDL binds to SRs (class A and B). SR-A and SR-B are detected in macrophage-rich areas within atherosclerotic lesions of apoE KO mice39 and in human atherosclerotic lesions.40 SR-A is proatherogenic under hyperlipidemic conditions, and both apoE and LDL receptor–deficient mice have reduced atherosclerosis in the absence of SR-A.
The interaction of SR-A with ligands induces cellular signaling leading to gene transcription and cytokine release. SR-B1 binds to HDL with high affinity.41 The expression of SR-B family members (SR-B1 and CD-36) is inducible. Unlike LDL receptors (LDLRs), macrophage SRs are not regulated by the cellular cholesterol content; hence, macrophage uptake of modified LDL can contribute to the cellular accumulation of CE and eventually to increased atherosclerosis. Whereas uptake of native LDL by macrophages from hyperhomocysteinemic mice was decreased, uptake of Ac-LDL was higher in the hyperhomocysteinemic mice than in control mice. Thus, the enhanced uptake of a modified LDL by macrophages from the hyperhomocysteinemic mice could account for the observed increase in lesion severity in these mice.
In summary, these data support the concept that Hcy causes atherosclerosis and is not merely associated with the disease. Our findings support a model in which hyperhomocysteinemia promotes atherosclerosis by altering hepatic lipid metabolism and increasing the uptake of modified LDL in macrophages, leading to the accumulation of CE and TG in the vessel wall. Results from lipid analyses and the LDL uptake assay suggest that hyperhomocysteinemia increases plasma cholesterol, decreases HDL cholesterol, and increases the number and/or activity of receptors for modified LDL. Additional studies are now underway to closely examine cholesterol and HDL metabolisms and SR regulation by hyperhomocysteinemia in the hyperhomocysteinemic mice. These studies should yield insights into the mechanistic link between hyperhomocysteinemia and atherosclerosis.
We thank Dr Larry Chan for important suggestions and critical review of this manuscript, Drs Mark Entman and David Via for helpful discussions, and Novartis for the ACAT inhibitor S-58035.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/ blood-2002-08-2606.
Supported by National Institutes of Health grants HL-67033 (H.W.), HL-62467 and HL-59976 (A.I.S.), HL-56865 and HL-30914 (H.J.P.), and HL-59976 (W.D.); American Heart Association Texas Affiliate grant 0160041y (H.W.); American Heart Association grant 0140067N (W.D.); and American Health Assistance Foundation grant H2001-010 (H.W.). W.D. is an Established Investigator of the American Heart Association. H.W. is an awardee of the Junior Faculty Scholar Award from the American Society of Hematology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hong Wang, VA Medical Center, Baylor College of Medicine, 2002 Holcombe Blvd 109-129, Houston, TX 77030; e-mail:hongw@bcm.tmc.edu.

![Fig. 5. Effect of hyperhomocysteinemia on LDL uptake. / Mouse peritoneal macrophages were incubated with 20 μg/mL [125I]Ac-LDL or [125I]native-LDL for 2 hours. Protein-associated [125I]radioactivity was measured and normalized as [125I] counts per milligram of protein. The uptake activity of [125I]Ac-LDL in macrophages from CBS−/+/ApoE−/− mice under HF diet was set as the control. LDL uptake activity is expressed as relative cellular [125I]protein (% of control mean values). (Inset) The image of LDLs on 0.75% agarose gel shows the electrophoretic mobility change by acetylation. Values represent mean ± SD from 3 independent assays (n = 9). *P < .01 versus CBS+/+/ApoE−/−; †P < .01 versus CBS−/+/ApoE−/− in the same group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/10/10.1182_blood-2002-08-2606/4/m_h81034332005.jpeg?Expires=1769240364&Signature=4w15u6pZiNmT8M5B1iWKInmW-2-eR1u909NFINGuiXgE5S~N98l4lq1ks5stLYzTifUzWalGSPykNtMMfiBsDLZjVo0QhpnlzLezRAEb0mtULfysaVvX3njqZIL2U-dtRzVdhntN5N0ARLqNDsZ3ZvxabQlW3L9l3byHoSocT7DmCGJwSdW643KdYJ2aaYLkqqs4mHH-PalEZt85qpUJPel2TAC-aK2HKL-V43TtP~21gxPqk39GS9IbSxfZse5k1XbJTKQyrPvxjX6plQdCAzsp7U-nowzmu4uTwvrhoo6NOkzsbhgifDDZUa1~jIj3qCMUVniGibV00kGrD4Wt3w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal