Abstract
Human platelets express 2 G protein–coupled nucleotide receptors: the platelet adenosine diphosphate (ADP) receptor coupled to stimulation of phospholipase C (P2Y1) via heterotrimeric guanosine 5-triphosphate (GTP)–binding protein Gq, and the platelet ADP receptor coupled to inhibition of adenylyl cyclase (P2Y12) via heterotrimeric GTP-binding protein Gi. Although these 2 receptors are encoded on the same chromosome and have similar pharmacologic profiles, they have different reactivities toward thiol reagents. The thiol agent p-chloromercuribenzene sulfonic acid (pCMBS) and the active metabolites from antiplatelet drugs, clopidogrel and CS-747, inactivate the P2Y12 receptor and are predicted to interact with the extracellular cysteine residues on the P2Y12 receptor. In this study we identified the reactive cysteine residues on the human P2Y12 receptor by site-directed mutagenesis using pCMBS as the thiol reagent. Cys97Ser and Cys175Ser mutants of the P2Y12 receptor did not express when transfected into Chinese hamster ovary (CHO-K1) cells, indicating the essential nature of a disulfide bridge between these residues. The Cys17Ser, Cys270Ser, and Cys17Ser/Cys270Ser double mutants had similar median effective concentration (EC50) values for ADP and 2-methylthio–ADP (2-MeSADP) when compared with the wild-type P2Y12. Similarly, the median inhibitory concentration (IC50) values for BzATP (2′,3′-O-(4- benzoylbenzoyl) adenosine 5′-triphosphate), an antagonist of the P2Y12 receptor, also did not differ dramatically among these mutants and the wild-type P2Y12receptor. pCMBS inactivated the wild-type P2Y12 receptor in a concentration-dependent manner, whereas it had no effect on the P2Y1 receptor. Finally, pCMBS partially affected the Gi coupling of Cys17Ser or Cys270Ser receptor mutants, but had no effect on Cys17Ser/Cys270Ser P2Y12receptor–mediated inhibition of adenylyl cyclase. These results indicate that, unlike the P2Y1 receptor, which has 2 essential disulfide bridges linking its extracellular domains, the P2Y12 receptor has 2 free cysteines in its extracellular domains (Cys17 and Cys270), both of which are targets of thiol reagents. We speculate that the active metabolites of clopidogrel and CS-747 form disulfide bridges with both Cys17 and Cys270 in the P2Y12 receptor, and thereby inactivate the receptor.
Introduction
Extracellular nucleotides act on cell-surface receptors, known as P2 receptors, leading to a number of physiologic responses including cardiac muscle contraction and platelet aggregation.1 Adenosine triphosphate (ATP) and adenosine diphosphate (ADP) are released from several sources in the body, including purinergic nerve endings, platelets, chromaffin cells, and endothelial cells.2 The P2 receptors are divided mainly into 2 classes: P2X ligand-gated channels and P2Y receptors coupled to heterotrimeric G proteins.3 Several members of the P2X family and the P2Y family have been cloned.3-8 Of the P2Y receptor subtypes characterized to date, most couple to heterotrimeric guanosine 5c-triphosphate (GTP)–binding protein that activates phospholipase C (Gq), but some couple to heterotrimeric GTP-binding protein that inhibits adenylyl cyclase (Gi). These Gi-coupled P2Y receptors are found in platelets,9 C6 rat glioma cells,10,11 and B10 microvascular endothelial cells.12
Of the G protein–coupled P2Y receptors, platelet ADP receptor coupled to inhibition of adenylyl cyclase (P2Y12) is expressed in platelets and in the brain,7,8 whereas the platelet ADP receptor coupled to stimulation of phospholipase C (P2Y1) receptor is expressed ubiquitously.13 The genes for P2Y12 and P2Y1 receptors are colocalized on chromosome 3q21-q2514 and share 22% sequence identity.7,8 The P2Y1 and the P2Y12 receptors play an important role in the activation of platelets, and coactivation of both of the receptors is essential for ADP-induced activation of platelet fibrinogen receptor.9,15,16 In platelets, these 2 receptors have identical pharmacologic profiles (ADP and 2-MeSADP agonists; ATP and BzATP [2′-,3′-O-(4-benzoylbenzoyl) adenosine 5′-triphosphate] antagonists).17-20 An antiplatelet drug, clopidogrel, is metabolized in the liver to an active metabolite containing a free thiol group, which inactivates the P2Y12 receptor through formation of a disulfide bridge with extracellular cysteine residues.21-23 Another antiplatelet drug, CS-747, also neutralizes the P2Y12 receptor by the same mechanism.24,25 In addition, the P2Y12receptor is irreversibly antagonized by the thiol reagent p-chloromercuribenzene sulfonic acid (pCMBS).22,26 27
Most G protein–coupled receptors (GPCRs), including the P2Y receptors, possess 2 conserved extracellular cysteine residues located on the first and second extracellular loops (Figure1). A disulfide bridge formed between these 2 cysteines is essential for the function of a number of GPCRs, including rhodopsin, the thyroid-stimulating hormone–releasing hormone receptor, the thromboxane receptor, the gonadotrophin-releasing hormone receptor, and the P2Y1receptor.28-30 In addition to this essential disulfide bridge between Cys124 in the first extracellular loop and Cys202 in the second extracellular loop of the P2Y1 receptor, Hoffmann et al31 found an additional essential disulfide bridge between 2 other extracellular cysteines (Cys42 in the amino terminal and Cys296 in the third extracellular loop) of the P2Y1receptor. Although the P2Y12 receptor also has these corresponding cysteine residues (Cys17 and Cys270) in its extracellular regions (Figure 1), the function of these cysteines has not been investigated.
Alignment of the human P2Y1 and P2Y12 receptors.
The alignment of the cloned human platelet P2Y receptors7-9 13 was performed using a GCG program (GCG Software, Madison, WI). The conserved cysteine residues in the extracellular domains are indicated by an arrow. Putative transmembrane domains are underlined.
Alignment of the human P2Y1 and P2Y12 receptors.
The alignment of the cloned human platelet P2Y receptors7-9 13 was performed using a GCG program (GCG Software, Madison, WI). The conserved cysteine residues in the extracellular domains are indicated by an arrow. Putative transmembrane domains are underlined.
In this study, we evaluated the function of extracellular cysteine residues of the P2Y12 receptor by site-directed mutagenesis and conclude that there is no essential disulfide bridge between Cys17 and Cys270. Furthermore, we conclude that thiol reagents such as pCMBS can inactivate the P2Y12 receptor by targeting either Cys17 or Cys270 or both. Thus, we postulate that Cys17 and Cys270 are possible targets of the antithrombotic drugs clopidogrel and CS-747 through the action of an active metabolite.
Materials and methods
Materials
pCMBS was purchased from Toronto Research Chemicals (North York, ON, Canada). Anti-FLAG M2 monoclonal antibody fluorescein isothiocyanate (FITC) conjugate, ADP, 2-MeSADP, and BzATP were from Sigma Chemical (St Louis, MO). Slow Fade Light Antifade Kit was purchased from Molecular Probes (Eugene, OR). Lab-Tek Chamber Slide Culture Chambers were purchased from Nunc (Naperville, IL). pcDNA3.1/Hygro(+) vector was purchased from Invitrogen (Carlsbad, CA). Ready-to-Go polymerase chain reaction (PCR) beads were purchased from Amersham Pharmacia Biotech (Piscataway, NJ). QuickChange Site-Directed Mutagenesis Kit was obtained from Stratagene (La Jolla, CA). Myo-2-[3H] inositol, [3H] adenine, and [14C] cAMP (3′,5′-cyclic adenosine monophosphate) were from NEN Life Science Products (Boston, MA).
Construction of the P2Y12 expression plasmid
Total RNA was isolated from human platelets by the RNAzol procedure (Tel-Test, Friendswood, TX), and cDNA was prepared from 5 μg total RNA using a first-strand synthesis kit (Gibco-BRL, Gaithersberg, MD). The PCR was carried out using forward and reverse primers specific for human P2Y12 receptor cDNA (GenBank accession no. AF313449).8 The nucleotide sequence encoding the FLAG epitope (DYKDDDDK) was inserted at the beginning of the translation initiation. The sense primer containing aHindIII restriction site and the FLAG epitope sequence is 5′-GCGCAAGCTTACCATGGACTACAAAGACGATGACGACAAGCAAGCCGTCGACAATCTC-3′. The antisense primer containing XbaI restriction site is 5′-GCTCTAGACATTGGAGTC-TCTTCATTTGG-3′. The restriction enzyme sites are underlined, and the coding sequence of FLAG epitope is given in bold letters. After an initial denaturation for 5 minutes at 94°C, the amplifications were carried out for 35 cycles using Ready-to-Go PCR beads as follows: denaturation at 94°C for 45 seconds, annealing at 50°C for 45 seconds, and extension at 72°C for one minute. The final cycle was followed by an additional extension for 7 minutes at 72°C. An expression plasmid (pcDNA3-HP2Y12) was constructed in pcDNA3.1-Hygro (+) vector by digesting the reverse transcription (RT)–PCR product with HindIII and XbaI and inserting it into the vector digested with the same set of restriction enzymes. The nucleotide sequence of the P2Y12 receptor cDNA in the expression plasmid was confirmed by DNA sequence analysis.
Site-directed mutagenesis
All mutations were introduced into pcDNA3-HP2Y12using the QuickChange site-directed mutagenesis kit from Stratagene. The resulting changes in oligonucleotides and the corresponding amino acids are shown in Table 1. Using Cys270Ser as a template and Cys17Ser mutation primers, a Cys17Ser/Cys270 double mutation was achieved by the same method. All mutations were confirmed by DNA sequencing.
Primers for site-directed mutagenesis of the P2Y12 receptor
| Amino acid and position . | Nucleotide change . | Amino acid change . | Sense . | Primers used . |
|---|---|---|---|---|
| Cys17 | TGC>TCC | Cys→Ser | + | 5′ -GGAACACCAGTCTGTCCACCAGAGACTAC-3′ |
| − | 5′ -GTAGTCTCTGGTGGACAGACTGGTGTTCC-3′ | |||
| Cys97 | TGT>TCT | Cys→Ser | + | 5′ -GGACCACTGAGAACTTTTGTGTCTCAAGTTACCTCCGTC-3′ |
| − | 5′ -GACGGAGGTAACTTGAGACACAAAAGTTCTCAGTGGTCC-3′ | |||
| Cys175 | TGC>TCC | Cys→Ser | + | 5′ -GCCGAGAGACAAGAATGTGAAGAAATCCTCTTTCCTTAAATC-3′ |
| − | 5′ -GATTTAAGGAAAGAGGATTTCTTCACATTCTTGTCTCTCGGC-3′ | |||
| Cys270 | TGC>TCC | Cys→Ser | + | 5′ -CCCGGGATGTCTTTGACTCCACTGCTGAAAATAC-3′ |
| − | 5′ -GTATTTTCAGCAGTGGAGTCAAAGACATCCCGGG-3′ |
| Amino acid and position . | Nucleotide change . | Amino acid change . | Sense . | Primers used . |
|---|---|---|---|---|
| Cys17 | TGC>TCC | Cys→Ser | + | 5′ -GGAACACCAGTCTGTCCACCAGAGACTAC-3′ |
| − | 5′ -GTAGTCTCTGGTGGACAGACTGGTGTTCC-3′ | |||
| Cys97 | TGT>TCT | Cys→Ser | + | 5′ -GGACCACTGAGAACTTTTGTGTCTCAAGTTACCTCCGTC-3′ |
| − | 5′ -GACGGAGGTAACTTGAGACACAAAAGTTCTCAGTGGTCC-3′ | |||
| Cys175 | TGC>TCC | Cys→Ser | + | 5′ -GCCGAGAGACAAGAATGTGAAGAAATCCTCTTTCCTTAAATC-3′ |
| − | 5′ -GATTTAAGGAAAGAGGATTTCTTCACATTCTTGTCTCTCGGC-3′ | |||
| Cys270 | TGC>TCC | Cys→Ser | + | 5′ -CCCGGGATGTCTTTGACTCCACTGCTGAAAATAC-3′ |
| − | 5′ -GTATTTTCAGCAGTGGAGTCAAAGACATCCCGGG-3′ |
Underlined nucleotide indicates the altered nucleotide for site-directed mutagenesis.
Cell culture
Chinese hamster ovary (CHO-K1) cells were grown in HAM F12 medium (Cellgro, Herndon, VA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin/amphotericin at 37°C with 5% CO2. CHO-K1 cells transfected with wild-type or mutant P2Y12 receptors were grown in the same medium supplemented with 400 μg/mL hygromycin. Astrocytoma cells (1321N1) stably expressing the human P2Y1 receptor32 were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin/amphotericin, and 500 μg/mL G418 (Cellgro) at 37°C with 5% CO2. Experiments were carried out in confluent cultures 2 days after plating in 6- or 12-well plates.
Stable expression of human P2Y12 receptor in CHO-K1 cells
The expression construct for the wild-type P2Y12receptor (pcDNA3-HP2Y12) or for each of the P2Y12 site-directed mutants (Cys17Ser, Cys97Ser, Cys175Ser, Cys270Ser, and Cys17Ser/Cys270Ser) (1 μg) was used to transfect CHO-K1 cells using lipofectamine as described previously.33 CHO-K1 cells were also transfected with pcDNA3.1/Hygro(+) to serve as a mock control. The growth medium was replaced after 6 hours with fresh medium containing 400 μg/mL hygromycin. Stable transfectants were selected on medium containing 400 μg/mL hygromycin and screened for the expression of wild-type or mutant P2Y12 receptor by second messenger (cAMP) inhibition.
FLAG-tag detection in CHO-K1 cells by immunofluorescence microscopy
Cells were cultured in Lab-Tek Chamber Slide Culture Chambers overnight and washed twice with Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS; 0.05 M Tris, 0.15 M NaCl, pH 7.4). Cells were fixed with freshly prepared methanol-acetone (1:1, using a glass vial) for one minute at room temperature, and then washed with TBS 4 times and incubated with anti-FLAG M2-FITC at 40 μg/mL in TBS at room temperature for 2 hours (gentle shaking in the dark). Cells were further washed twice with TBS, and 3 drops of component C of Slow Fade Light Antifade Kit were added and incubated for 10 minutes at room temperature. Finally, component C was washed away with TBS, and a small drop of component A was added, then covered with a cover slip, and examined using a Nikon Eclipse TE300 fluorescence microscope (Melville, NY).
Cyclic AMP assay
Cells were cultured in 6-well plates and labeled with 2 μL/mL [3H]-adenine (74 kBq/mL) for 4 hours or overnight at 37°C. The radiolabeling medium was replaced by fresh growth medium containing 1 mM IBMX (3-isobutyl-1-methylxanthine) and incubated for 10 minutes at 37°C. After stimulation with 20 μM forskolin for 1 minute, various concentrations of agonist and antagonist were added and incubated at 37°C for 10 minutes unless indicated. The reactions were terminated by addition of 1 M HCl containing 2000 cpm of [14C]-cAMP (2 GBq/mmol) as a recovery standard. Cyclic AMP levels were determined as described earlier32 34 and expressed as a percentage of total [3H]-adenine nucleotides. The median effective concentration (EC50)/median inhibitory concentration (IC50) values are calculated using Kaleidagraph 3.5 (Synergy Software, Reading, PA) fitting the curve to m1 + (m2 − m1)/[1 + (m0/m3)m4], where m0 is the difference between the maximal and minimal response, m1 is the minimal value of response, m2 is the maximal value of response, m3 is the estimated EC50 or IC50, and m4 is the slope. After fitting the curve, the calculated mean and SE of EC50 or IC50 is given automatically by the program.
Measurements of inositol phosphates
Inositol phosphates were measured essentially as described.35 36 Confluent cultures of cells in 12-well plates were labeled with 1 μCi/mL (0.037 MBq/mL) myo-2-[3H] inositol in inositol-free DMEM for 24 hours. Labeled cells were washed once, the medium was replaced with 890 μL fresh inositol-free 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered Eagle medium, pH 7.4, and the cells were incubated at 37°C for 30 minutes before proceeding; this step helped to reduce background levels of [3H] inositol phosphates, so that agonist-stimulated accumulation could be detected more easily. After incubation for 30 minutes at 37°C, 10 μL of 1 M LiCl was added to a final concentration of 10 mM, and the incubation continued for an additional 10 minutes. The cells were stimulated with 100 μL ADP at a final concentration of 10 μM for 15 minutes, and the reaction was terminated by aspiration of the medium, addition of 0.75 mL of 10 mM formic acid, and incubation at room temperature for 30 minutes. The solution containing the extracted inositol phosphates were neutralized by dilution with 3 mL of 10 mM NH4OH (yielding a final pH of 8-9) and then applied directly to a column containing 0.7 mL of the anion exchange resin, AG 1-X8, equilibrated with 40 mM ammonium formate. The column was washed with 4 mL of 40 mM ammonium formate, pH 5.0, to remove the free inositol and the glyceroinositol. Total inositol phosphates were eluted with 4 mL of 2 M ammonium formate, pH 5.0. The eluate (1 mL) was removed and counted with 9 mL of scintillation fluid.
Statistical analysis
All experiments were performed at least 3 times in duplicate. Data are expressed as means ± SE. Statistical significance was determined using the Student ttest and significance was designated to P values less than .05.
Results and discussion
Human platelets express 2 P2Y receptors, the P2Y1 and P2Y12 receptors,7,8,13 and each have 4 conserved extracellular cysteines capable of forming 2 disulfide bridges to stabilize the 3-dimensional structure of the receptor (Figure 1). In the case of the P2Y1 receptor, Cys124 in the first extracellular loop forms a disulfide bridge with Cys202 in the second extracellular loop, which is essential for the function of the receptor.31 In addition, the P2Y1 receptor has an additional disulfide bridge between Cys42 in the amino terminus and Cys296 in the third extracellular loop.31 Disruption of this bridge by site-directed mutagenesis of either Cys42 or Cys296 results in dramatic loss of the ability of the agonist to activate the receptor.31 As both the P2Y1 and the P2Y12 receptors have similar pharmacologic profiles in platelets,9,14,18 20 we first sought to determine whether the P2Y12 receptor also has 2 essential disulfide bridges between its 4 conserved extracellular cysteine residues. Secondly, we wanted to identify the cysteines in the P2Y12 receptor that interact with thiol reagents. To achieve these objectives, we constructed an expression plasmid for the P2Y12 receptor and mutated each of the 4 cysteines in the P2Y12 receptor (Cys17, Cys97, Cys175, and Cys270) to serines. In addition, we generated a double mutant of Cys17Ser and Cys270Ser by site-directed mutagenesis.
Stable expression of wild-type and mutant human P2Y12 receptors in CHO-K1 cells
CHO-K1 cells were transfected with the P2Y12 wild-type receptor, and clones resistant to 400 μg/mL hygromycin were selected. We screened our clones based on their response to 10 μM ADP in the adenylyl cyclase inhibition assay. From 16 cell clones, 1 wild-type clone was selected and designated P2Y12WT. The expression of P2Y12 wild type on the surface of CHO-K1 cells was also confirmed by immunofluorescence microscopy using a monoclonal antibody directed against the FLAG tag at the N-terminus of the receptor (Figure 2). Unfortunately, this monoclonal antibody was not useful in detecting the receptor expression by the enzyme-linked immunosorbent assay (ELISA) method.
Cell-surface expression of the FLAG-tagged human P2Y12 receptor on CHO-K1 cells.
The CHO-K1 cells transfected with (A) vector alone or (B) the FLAG-tagged human P2Y12 receptor cDNA construct were treated with FITC-conjugated anti-FLAG antibodies and visualized in a fluorescence microscope (original magnification, × 1000) after washing.
Cell-surface expression of the FLAG-tagged human P2Y12 receptor on CHO-K1 cells.
The CHO-K1 cells transfected with (A) vector alone or (B) the FLAG-tagged human P2Y12 receptor cDNA construct were treated with FITC-conjugated anti-FLAG antibodies and visualized in a fluorescence microscope (original magnification, × 1000) after washing.
Similarly, CHO-K1 cells transfected with expression constructs for P2Y12 mutants Cys17Ser and Cys270Ser were grown in the presence of hygromycin and screened by cAMP inhibition assay. There were 2 cell clones designated P2Y12Cys17Ser and P2Y12Cys270Ser selected from 15 and 12 cell clones, respectively. In contrast to the P2Y12 wild type and mutants Cys17Ser, Cys270Ser, and Cys17Ser/Cys270Ser, no positive clone was found after screening 61 cell clones stably transfected with Cys97Ser and 49 cell clones stably transfected with Cys175Ser. Immunofluorescence experiments with the antibody against the FLAG tag failed to detect any receptor expression on these cell clones. These results were not totally unexpected as similar results were obtained when Cys124 and Cys202 in the P2Y1 receptor were mutated to serines.31 These results indicate that a disulfide bridge between Cys97 and Cys175 might be essential for the receptor expression as is the case with the P2Y1 receptor.31However, it is also possible that a cysteine is required at both of these positions for the receptor expression. Finally, one cell clone, designated P2Y12Cys17Ser/Cys270Ser, was selected from 12 hygromycin-resistant clones by a similar method from CHO-K1 cells transfected with expression constructs for the P2Y12receptor double-mutant Cys17Ser/Cys270Ser.
Characterization of cloned human P2Y12 receptor and the mutants expressed in CHO-K1 cells
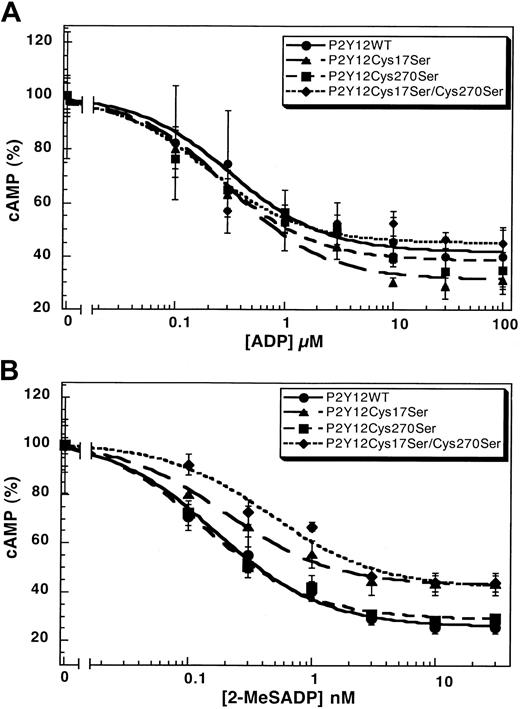
The mutation of Cys42 and Cys296 in the P2Y1 receptor leads to dramatically diminished responses to the agonist.31 We evaluated the function of the corresponding cysteines in the P2Y12 receptor with Cys17Ser mutant and Cys270Ser mutant and compared them with the wild-type P2Y12receptor. The wild-type P2Y12 receptor, the P2Y12Cys17Ser, or the P2Y12Cys270Ser mutant caused similar inhibition of forskolin-stimulated adenylyl cyclase upon stimulation by ADP (Figure 3A) or 2-MeSADP (Figure 3B) in a concentration-dependent manner. The EC50 values for these agonists are comparable in the wild-type and mutant P2Y12 receptors (Table2). We generated a cell line stably expressing the double-mutant P2Y12Cys17Ser/Cys270Ser and evaluated its ability to respond to the agonists. As shown in Figure 3, this double mutant also has similar EC50 values compared with the wild-type receptor (Table 2). These results indicate that mutations at Cys17 and/or Cys270 did not considerably alter the function or coupling of the P2Y12 receptor. Similar mutations in the P2Y1 receptor dramatically increased the EC50 values of the agonists.31 Thus, we conclude that, unlike in the P2Y1 receptor, a disulfide bridge between the cysteines in the amino terminus (Cys17) and the third extracellular loop (Cys270) is not essential for the function of the P2Y12 receptor.
Concentration-response curves.
Curves of (A) ADP- or (B) 2-MeSADP–induced inhibition of adenylyl cyclase stimulated with 20 μM forskolin in CHO-K1 cells stably expressing either P2Y12WT, P2Y12Cys17Ser, P2Y12Cys270Ser, or P2Y12Cys17Ser/Cys270Ser. Data were normalized to the maximal response obtained in the absence of ADP or 2-MeSADP (mean ± SE, n = 3-7).
Concentration-response curves.
Curves of (A) ADP- or (B) 2-MeSADP–induced inhibition of adenylyl cyclase stimulated with 20 μM forskolin in CHO-K1 cells stably expressing either P2Y12WT, P2Y12Cys17Ser, P2Y12Cys270Ser, or P2Y12Cys17Ser/Cys270Ser. Data were normalized to the maximal response obtained in the absence of ADP or 2-MeSADP (mean ± SE, n = 3-7).
Agonist-stimulated adenylyl cyclase inhibition by the wild-type P2Y12 and its mutants
| . | EC50 . | |
|---|---|---|
| ADP, μM . | 2-MeSADP, nM . | |
| P2Y12WT | 0.32 ± 0.08 | 0.17 ± 0.03 |
| P2Y12Cys17Ser | 0.32 ± 0.09 | 0.20 ± 0.03 |
| P2Y12Cys270Ser | 0.26 ± 0.10 | 0.13 ± 0.02 |
| P2Y12Cys17Ser/Cys270Ser | 0.26 ± 0.16 | 0.47 ± 0.16 |
| P2Y12Cys97Ser | NA* | NA* |
| P2Y12Cys175Ser | NA† | NA† |
| . | EC50 . | |
|---|---|---|
| ADP, μM . | 2-MeSADP, nM . | |
| P2Y12WT | 0.32 ± 0.08 | 0.17 ± 0.03 |
| P2Y12Cys17Ser | 0.32 ± 0.09 | 0.20 ± 0.03 |
| P2Y12Cys270Ser | 0.26 ± 0.10 | 0.13 ± 0.02 |
| P2Y12Cys17Ser/Cys270Ser | 0.26 ± 0.16 | 0.47 ± 0.16 |
| P2Y12Cys97Ser | NA* | NA* |
| P2Y12Cys175Ser | NA† | NA† |
The values are mean ± SE, n = 3-7.
NA indicates no activity or expression detectable among 61 clones tested.
NA indicates no activity or expression detectable among 49 clones tested.
Effect of antagonist on the wild-type and mutant P2Y12 receptors
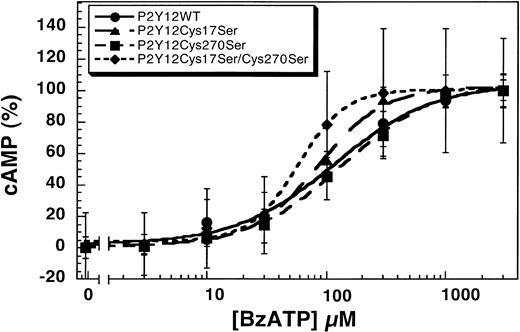
Human platelets express both the P2Y1 and P2Y12 receptors that contribute to various agonist-induced physiologic responses.9,15,16,37 BzATP inhibits both the Ca2+- and cAMP-dependent intracellular signaling pathways of ADP in endothelial cells and platelets through antagonism of the P2Y1 and P2Y12 receptors.17 We evaluated the effect of this nonselective antagonist on the wild-type and mutant P2Y12 receptors. As shown in Figure4, BzATP blocks 1 μM ADP-induced inhibition of adenylyl cyclase in CHO-K1 cells stably expressing P2Y12WT, P2Y12Cys17Ser, P2Y12Cys270Ser, or P2Y12Cys17Ser/Cys270Ser in a concentration-dependent manner. The IC50 values for the antagonism by BzATP are 116 ± 23 μM (wild type), 85 ± 6 μM (P2Y12Cys17Ser), 137 ± 15 μM (P2Y12Cys270Ser), and 58 ± 5 μM (P2Y12Cys17Ser/Cys270Ser). Compared with the wild type, the concentration-response curves of Cys17Ser/Cys270Ser mutant slightly shifted to the left, suggesting that double mutation renders the receptor more sensitive to the antagonist. It is possible that these 2 extracellular cysteines play a role in the binding of the BzATP and thereby alter the sensitivity of the receptor to the antagonist.
Effect of BzATP on the wild-type and mutant P2Y12 receptors.
BzATP antagonizes 1 μM ADP-induced inhibition of adenylyl cyclase in CHO-K1 cells stably expressing either P2Y12WT, P2Y12Cys17Ser, P2Y12Cys270Ser, or P2Y12Cys17Ser/Cys270Ser stimulated with 20 μM forskolin. Data were normalized to the maximal response obtained with antagonist (mean ± SE, n = 3).
Effect of BzATP on the wild-type and mutant P2Y12 receptors.
BzATP antagonizes 1 μM ADP-induced inhibition of adenylyl cyclase in CHO-K1 cells stably expressing either P2Y12WT, P2Y12Cys17Ser, P2Y12Cys270Ser, or P2Y12Cys17Ser/Cys270Ser stimulated with 20 μM forskolin. Data were normalized to the maximal response obtained with antagonist (mean ± SE, n = 3).
Effects of pCMBS on the function of the P2Y receptors
A critical role of cysteine residues in the function of the platelet ADP receptor has been suggested by the ability of pCMBS, a nonpenetrating thiol reagent, to abolish ADP responses in platelets.20,26 It was also shown that pCMBS affects the binding of the agonist to the ADP receptor mediating the adenylyl cyclase inhibition response.22,27 As both the P2Y1 and P2Y12 receptors have 4 conserved extracellular cysteine residues (Figure 1), we evaluated the effect of pCMBS on these 2 platelet P2Y receptors. As shown in Figure5, pCMBS blocked ADP-induced inhibition of forskolin-stimulated adenylyl cyclase in the cells expressing the human P2Y12 receptor in a concentration-dependent manner, whereas it did not inhibit agonist-induced inositol phosphate formation in the cells stably expressing the P2Y1 receptor. The effect of pCMBS on the human P2Y12 receptor is consistent with the data published by Savi et al.22 Thus, pCMBS inactivates the P2Y12 receptor but not the P2Y1receptor. As this thiol reagent is cell-impermeable and covalently binds to the free thiol groups, we predict that the Cys17 and Cys270 in the P2Y12 receptor exist as free cysteines and may be the target of pCMBS. On the other hand, the P2Y1 receptor does not have free extracellular cysteines, as the corresponding cysteines (Cys42 and Cys296) form an essential disulfide bridge, and hence is insensitive to pCMBS. Thus, we conclude that the P2Y12receptor does not have a disulfide bridge between Cys17 and Cys270 and that these residues are free cysteines in the native receptor and may be the target of the thiol reagents.
Effects of pCMBS on the P2Y1 and P2Y12 receptors.
(A) Cells stably expressing the P2Y12 receptor were pretreated with different concentrations of pCMBS for 10 minutes at 37°C before addition of 20 μM forskolin. Data were normalized to the value obtained with forskolin alone, taken as 100%, and expressed as mean ± SE (n = 4-6). (B) Cells stably expressing the P2Y1 receptor were pretreated with 20 μM pCMBS for 10 minutes at 37°C and stimulated with 10 μM ADP, and the total inositol phosphates were measured as described in “Materials and methods.” Data are expressed as percent of control as mean ± SE (n = 3).
Effects of pCMBS on the P2Y1 and P2Y12 receptors.
(A) Cells stably expressing the P2Y12 receptor were pretreated with different concentrations of pCMBS for 10 minutes at 37°C before addition of 20 μM forskolin. Data were normalized to the value obtained with forskolin alone, taken as 100%, and expressed as mean ± SE (n = 4-6). (B) Cells stably expressing the P2Y1 receptor were pretreated with 20 μM pCMBS for 10 minutes at 37°C and stimulated with 10 μM ADP, and the total inositol phosphates were measured as described in “Materials and methods.” Data are expressed as percent of control as mean ± SE (n = 3).
The sensitivity of the P2Y12 receptor to thiol reagents explains the mechanism of clopidogrel.22,23 Since its approval in 1988, clopidogrel has been broadly used as an antiplatelet drug.21 Although the P2Y12 receptor was thought to be the target of clopidogrel,38 its mechanism of action remained unclear until recently.22,23,39P2Y12 receptors play an essential role in ADP-induced platelet aggregation,40 and, hence, the P2Y12receptor–defective patients41-43 and mice deficient in the P2Y12 receptor have abnormal platelet aggregation.16 Savi et al23 proposed that the extracellular cysteine on P2Y12 was modified by formation of a disulfide bridge with the thiol group on the active metabolite of clopidogrel. Similarly, CS-747 was also predicted to target the extracellular cysteines in the P2Y12 receptor through conversion to an active metabolite in the liver.24 25
Effects of pCMBS on the wild-type and mutant P2Y12receptors
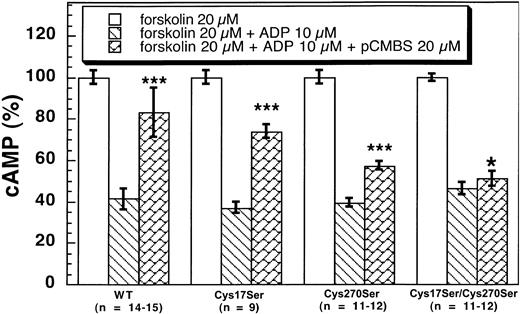
The active metabolite of clopidogrel has to be purified into an active enantiomer metabolite from the liver microsomes and is highly unstable.22 23 Hence, we used pCMBS as a tool to identify the reactive cysteine residue on the P2Y12 receptor using ADP-induced inhibition of adenylyl cyclase assay on CHO-K1 cells stably expressing the wild-type and mutant P2Y12 receptors. As shown in Figure 6, pCMBS (20 μM) significantly reversed the inhibitory effects of 10 μM ADP on adenylyl cyclase in CHO-K1 cells stably expressing wild type and Cys17Ser (41 ± 5 vs 83 ± 12 and 37.1 ± 2.7 vs 74 ± 3, respectively). Although pCMBS reversed the effect of ADP in the P2Y12Cys270Ser mutant, the effect was not as dramatic (39.4 ± 2.1 vs 57.3 ± 2.3). Finally, pCMBS did not reverse the ADP-induced inhibition of adenylyl cyclase in the cells expressing the double-mutant P2Y12Cys17Ser/Cys270Ser (46.2 ± 2.9 vs 51 ± 3, mean ± SE, P < .05). These results indicate that both Cys17 and Cys270 are the targets of pCMBS. The primary target appears to be Cys270, as the effect of pCMBS appears to be more dramatic on the P2Y12Cys17Ser mutant than on the P2Y12Cys270Ser mutant. However, a small effect on the single Cys270Ser mutant shows that even Cys17 is a target of the thiol reagent. Thus, we conclude that both Cys17 and Cys270 are the targets of the thiol reagents as they exist as the free cysteine residues in the native receptor in platelets.
Effects of pCMBS on 10 μM ADP-induced adenylyl cyclase inhibition in CHO-K1 cells stably expressing human platelet P2Y12 wild type and mutants.
Cells expressing the wild-type or mutant P2Y12 receptors were pretreated with pCMBS for 10 minutes at 37°C before the addition of 20 μM forskolin. Data were normalized to the value obtained with forskolin alone (mean, ± SE. *P > .05, ***P < .001 compared with forskolin 20 μM + ADP 10 μM by t test).
Effects of pCMBS on 10 μM ADP-induced adenylyl cyclase inhibition in CHO-K1 cells stably expressing human platelet P2Y12 wild type and mutants.
Cells expressing the wild-type or mutant P2Y12 receptors were pretreated with pCMBS for 10 minutes at 37°C before the addition of 20 μM forskolin. Data were normalized to the value obtained with forskolin alone (mean, ± SE. *P > .05, ***P < .001 compared with forskolin 20 μM + ADP 10 μM by t test).
As the active metabolites of clopidogrel and CS-747 form a disulfide bridge with the free thiol groups on the P2Y12 receptor, we postulate that Cys17 and Cys270 are the targets of the metabolites of these drugs. It should be noted that in order to achieve a complete effect, a thiol reagent should block both of the cysteine residues. Thus partial inhibition, such as that seen in humans when lower doses of clopidogrel are used,21 44 could be due to incomplete blockade of the Cys17 and Cys270 on the platelet P2Y12receptor.
Our results indicate that only one disulfide bridge is formed among the 4 extracellular cysteines and that 2 free extracellular cysteines are the targets of clopidogrel. Among the 4 extracellular cysteines on the platelet P2Y1 receptor, 2 disulfide bridges are formed, and both of them are essential to receptor function.31 Clopidogrel specifically targets the P2Y12 receptor.39 45 Our results successfully explain the specificity of clopidogrel on P2Y12 rather than P2Y1. As is the case with pCMBS, we believe that lack of free cysteines in the P2Y1 receptor makes it insensitive to clopidogrel. As the homology and positioning of the cysteine residues in the P2Y12 receptor relative to the P2Y1receptor suggest a disulfide bond should also exist in the P2Y12 receptor, it is possible that there may be a dynamic redox exchange operating between Cys17 and Cys270. Thus, a P2Y12 receptor–specific disulfide reductase operating in the external oxidative cellular environment could generate free cysteine residues from a disulfide bridge between Cys17 and Cys270 that could then be targeted by the thiol reagents.
There is considerable evidence to suggest that the metabolites of clopidogrel and CS-747 have specificity for the P2Y12receptor. Although both P2Y1 and P2Y12receptors have similar pharmacologic profiles, only the P2Y12 receptor is affected by the metabolites of clopidogrel and CS-747. This observation indicates that the specific ligand-binding pocket of the P2Y12 receptor might have only one of the cysteine residues (either Cys17 or Cys270) that would be accessible for modification by the metabolite. However, such modification of a single cysteine residue by the active metabolite of clopidogrel might be sufficient to block the binding of the agonist. It is possible that pCMBS did not achieve complete inhibition because of its small size in the single mutants, whereas clopidogrel metabolite might achieve this by interacting with only one of the cysteines because of its larger size. Hence, we would speculate that the active metabolites of clopidogrel and CS-747 might interact with only one of the cysteine residues (very likely Cys270) in the P2Y12receptor to cause blockade of agonist binding.
Unlike the thiol reagent pCMBS, the active metabolites of clopidogrel or CS-747, with a free thiol group, could also break disulfide bridges. Thus, these active metabolites could break the essential disulfide bridge between Cys97 and Cys175 of the P2Y12 receptor, thereby forming a separate disulfide bridge with each of these residues. Such action could result in an inactive receptor as the naturally formed disulfide bridge maintains the receptor in a conformation required for agonist binding. However, we believe that this is an unlikely mechanism. If the clopidogrel metabolite can break an existing disulfide bridge, then we expect that it also should inactivate a number of G protein–coupled receptors, including the P2Y1 receptor, by the same mechanism. Even if it is argued that the metabolite depends on an agonist binding pocket, we expect that the P2Y1 receptor should also be inactivated by this metabolite. However, despite having the same agonist profile, the P2Y1 receptor is insensitive to clopidogrel.
In conclusion, we have shown that unlike the P2Y1 receptor, which has 2 essential disulfide bridges in its extracellular domains, the P2Y12 receptor has 2 free cysteines in its extracellular domains (Cys17 and Cys270), both of which are the targets of thiol reagents. We predict that the P2Y1 receptor is insensitive to the thiol reagents and to the active metabolites of clopidogrel and CS-747 because of the lack of these free cysteines in the extracellular domains. Thus, we speculate that the active metabolites of clopidogrel and CS-747 form disulfide bridges with Cys17 and/or Cys270 in the P2Y12 receptor and thereby inactivate the receptor.
We thank Ms Mayosha Mendis for her technical assistance and Drs James L. Daniel, Barrie Ashby, A. Koneti Rao, Lee-Yuan Liu-Chen, Fujio Sekiya, and Todd M. Quinton, Temple University Medical School, for critically reading the manuscript.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-10-3027.
Supported by research grants HL60683 and HL64943 from the National Institutes of Health (S.P.K.). R.T.D. is supported by training grant T32 HL07777 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Satya P. Kunapuli, Department of Physiology, Temple University School of Medicine, Room 224 OMS, 3420 N Broad St, Philadelphia, PA 19140; e-mail: spk@temple.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal