Abstract
In septic shock, tissue factor (TF) activates blood coagulation, and cytokines and chemokines orchestrate an inflammatory response. In this study, the role of Egr-1 in lipopolysaccharide (LPS) induction of TF and inflammatory mediators in vivo was evaluated using Egr-1+/+ and Egr-1−/− mice. Administration of LPS transiently increased the steady-state levels of Egr-1 mRNA in the kidneys and lungs of Egr-1+/+ mice with maximal induction at one hour. Egr-1 was expressed in epithelial cells in the kidneys and lungs in untreated and LPS-treated mice. LPS induction of monocyte chemoattractant protein mRNA in the kidneys and lungs of Egr-1−/− mice was not affected at 3 hours, but its expression was significantly reduced at 8 hours compared with the expression observed in Egr-1+/+ mice. Similarly, LPS induction of TF mRNA expression in the kidneys and lungs at 8 hours was reduced in Egr-1−/− mice. However, Egr-1 deficiency did not affect plasma levels of tumor necrosis factor α in endotoxemic mice. Moreover, Egr-1+/+ and Egr-1−/− mice exhibited similar survival times in a model of acute endotoxemia. These data indicate that Egr-1 does not contribute to the early inflammatory response in the kidneys and lungs or the early systemic inflammatory response in endotoxemic mice. However, Egr-1 does contribute to the sustained expression of inflammatory mediators and to the maximal expression of TF at 8 hours in the kidneys and lungs.
Introduction
Septic shock is induced in humans by the presence of pathogenic bacteria or fungi in the blood and is associated with a high mortality.1 In Gram-negative sepsis, lipopolysaccharide (LPS [endotoxin]) is released from Gram-negative bacteria and activates monocytes, endothelial cells, and epithelial cells. In sepsis, the procoagulant molecule tissue factor (TF) is expressed by circulating monocytes, vascular endothelial cells in the spleen, and epithelial cells in the lungs and kidneys.2-7TF-dependent activation of coagulation and the resulting intravascular fibrin deposition contributes to multiorgan failure.8Administration of anti-TF antibodies reduced mortality in baboon and mouse models of septic shock.9,10 In addition, administration of other coagulation inhibitors, such as antithrombin III, protein C, and tissue factor pathway inhibitor in various animal models of sepsis, reduced mortality.11-13 Plasminogen activator inhibitor type 1 (PAI-1) inhibits both types of plasminogen activators and reduces fibrinolysis.14 PAI-1 activity is increased in the plasma of patients with Gram-negative sepsis15 and is likely to contribute to intravascular fibrin deposition. Indeed, inhibition of PAI-1 prevents renal fibrin deposition in endotoxemic rabbits.16 In a mouse model of endotoxemia, maximal levels of TF mRNA are observed at 8 hours, and induction is observed in bronchial and tubular epithelial cells in the lungs and kidneys, respectively.2,5-7 In contrast, LPS treatment of mice induces maximal PAI-1 mRNA expression at 3 hours, and PAI-1 is expressed by endothelial cells.17 18
In Gram-negative sepsis, LPS triggers an inflammatory response that is orchestrated by monocytes.19 LPS-stimulated monocytes express a variety of inflammatory mediators, which include tumor necrosis factor alpha (TNFα), interleukin 1 (IL-1), and interleukin 6 (IL-6).1 Systemic exposure to LPS also initiates a rapid recruitment of neutrophils and monocytes into specific host tissues.20 The influx of inflammatory cells into tissues is coordinated by the expression of chemoattractants, such as monocyte chemoattractant protein (MCP-1), and adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1).21-23Importantly, ICAM-1−/− mice are resistant to the lethal effects of LPS.24
The early growth response (Egr) family of zinc finger transcription factors includes Egr-1, Egr-2, Egr-3, and Egr-4.25 Egr-1 is the prototypic member of this family and is induced in various cell types in response to a variety of stimuli.26,27 In addition, Egr-2 is induced in activated peripheral blood monocytes.28 Functional Egr-1 binding sites have been identified in many genes, including TF, TNFα, and ICAM-1.29-31 We found that LPS induction of TF and TNFα gene expression in human monocytic cells is mediated, in part, by Egr-1.29,30 Other studies have analyzed the expression of inflammatory mediators in Egr-1−/− and Egr-1+/+ mice and have identified additional target genes, such as IL-1β, IL-6, MCP-1, and PAI-1, that are directly or indirectly regulated by Egr-1.32 33
In this study, we used Egr-1−/− mice and Egr-1+/+ mice to evaluate the role of Egr-1 in the expression of TF and inflammatory mediators in a mouse model of endotoxemia.
Materials and methods
Mice
All studies were approved by The Scripps Research Institute Animal Care and Use Committee and comply with National Institutes of Health guidelines. Egr-1+/−(LacZ)mice34 on a C57BL/6 background were a generous gift from Drs P. Topilko and P. Charnay (Molecular Biology of Development Laboratory, Paris, France). Egr-1+/−(LacZ)mice were bred to generate Egr-1+/+ and Egr-1-(LacZ)/-(LacZ) (referred to as Egr-1−/−) mice. Mice were genotyped by a combination of LacZ staining and polymerase chain reaction (PCR).34
Model of endotoxemia
LPS (Escherichia coli serotype O111:B4; Sigma Chemical, St Louis, MO) was diluted to the appropriate concentration in 0.2 mL sterile saline (Sigma Chemical) and injected intraperitoneally into mice anesthetized by inhalation of methoxyflurane (Metofane; Pitman-Moore, Mundelein, IL). At the conclusion of experiments, mice were anesthetized with methoxyflurane inhalation and killed by cervical dislocation. Tissues were rapidly removed and either snap frozen in liquid nitrogen or fixed in a 10% formalin solution. Egr-1+/+ and Egr-1−/− mice were injected intraperitoneally with a low (2 mg/kg) or high (12 mg/kg) dose of LPS. For measurement of cytokine and chemokine levels, blood samples were collected by retro-orbital bleeding at different time points (0, 1, 2, 4, 6, and 8 hours) after LPS injection and plasma was prepared. For the survival-time experiments, Egr-1+/+ (n = 12) and Egr-1−/− (n = 10) mice were injected intraperitoneally with LPS (12 mg/kg) and monitored for 3 days.
Northern blot analysis
Total RNA from the kidneys and lungs of mice was extracted using Trizol reagent (Invitrogen, Carlsbad, CA). Total RNA (10 μg) was separated by electrophoresis, transferred to a nylon membrane, and hybridized with cDNA probes specific for mouse Egr-1 (303-1956 bp), TF (226-1044 bp), PAI-1 (1-1085 bp), ICAM-1 (140-887 bp), and glyceraldehyde-3-phosphate dehydrogenase (G3PDH; Clontech Laboratories, Palo Alto, CA). The cDNA fragments were labeled with [α32P]-deoxycytidine triphosphate (ICN, Costa Mesa, CA) using a Prime-It kit (Stratagene Cloning Systems, San Diego, CA). Bands were visualized by autoradiography.
Enzyme-linked immunosorbent assay (ELISA)
Levels of murine TNFα, IL-6, and MCP-1 were measured using commercial ELISA kits (R & D Systems, Minneapolis, MN).
RNAse protection assay (RPA)
Total RNA (10 μg) from the kidney and lung was hybridized with mouse cytokine (mCK-3b) and chemokine (mCK-5 and mCK-5b) RNA probes using a Riboquant Multiprobe RPA System (Pharmingen, San Diego, CA), following the manufacturer's instructions. The samples were separated by electrophoresis on 6% Tris borate-EDTA (TBE) urea-containing gels (Invitrogen), dried, and bands visualized by autoradiography. G3PDH and ribosomal protein L32 (L32) were used to monitor loading.
In situ hybridization
Expression of Egr-1 and TF mRNAs in kidneys and lungs from untreated and LPS-treated C57BL/6 mice were examined by in situ hybridization5 using 35S-labeled sense and antisense RNA riboprobes generated from a plasmid containing mouse TF cDNA (226-1044 bp)5 or from pSKEgr-1 (303-1959 bp), which was kindly provided by Dr V. Lindner (Maine Medical Center Research Institute, Portland).
Immunoprecipitation and Western blotting
Total protein from the kidneys and lungs was isolated and quantitated as described.35 Total protein (1 mg) from the kidney was immunoprecipitated overnight at 4°C using 300 μL radioimmunoprecipitation assay (RIPA) buffer (Santa Cruz Biotechnology, Santa Cruz, CA), 1:100 dilution of anti–Egr-1 (sc-110) antibody (Santa Cruz Biotechnology), and 40 μL protein A Sepharose beads (Amersham Pharmacia Biotech, Piscataway, NJ). Immunoprecipitates were collected by centrifugation and washed twice in RIPA buffer with detergent and twice with RIPA buffer without detergent. The final pellet was resuspended in 50 μL 4 × sample buffer, boiled for 5 minutes, and centrifuged at 14 000 rpm for 5 minutes at 4°C. Supernatant (20 μL) was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blot analysis using a rabbit anti–Egr-1 antibody (1:1000).
LacZ expression
LacZ staining of kidneys was performed using a β-Galactosidase Staining Kit (Stratagene Cloning Systems, La Jolla, CA). LPS or saline-treated Egr-1+/−(LacZ) mice were perfused intracardially with 6 mL phosphate-buffered saline (PBS) followed by 6 mL fixing solution (2% formaldehyde, 0.2% glutaraldehyde in PBS). Kidneys and lungs were cut into pieces approximately 2- to 3-mm thick and fixed for another 30 minutes. After washing with PBS, the tissue was placed in X-Gal staining solution and incubated at 37°C for 30 minutes. After staining, the tissue was washed 3 times with PBS and placed in 70% ethanol. For microscopic analysis, the tissue was embedded in paraffin and 7- to 8- μm sections were cut and counterstained with eosin. The cell-type distribution of LacZ expression was analyzed by microscopy. Images were captured using a digital Nikon camera (Melville, NY).
Histology
For the histologic studies, kidneys and lungs from control or LPS-treated mice were fixed with a 10% formaldehyde solution and embedded in paraffin. Sections (3 μm) were stained with hematoxylin and eosin. Leukocytes in the tissues were identified using the napthol AS-D chloroacetate esterase reaction (Sigma Chemical), which stains esterase-positive cells red.36 For quantification of the neutrophil infiltration in the lung, 10 random fields (grid 10 × 10 mm area, × 400 magnification) were counted in LPS-treated Egr-1+/+ (n = 2) and Egr-1−/− (n = 2) mice by an investigator blinded to the groups.
Data analysis
Band intensities were quantified by densitometric analyses using a Personal Densitometer and ImageQuant software (Molecular Dynamics, Sunnyvale, CA). Statistical analysis was performed using either the 2-tailed unpaired student t test or the log-rank test and differences were determined to be statistically significant at a P value less than .05.
Results
LPS induction of Egr-1, TF, and inflammatory mediators in the kidney
We measured the steady-state levels of Egr-1 mRNA in the kidney at various times after administration of LPS to mice. LPS induced a transient increase in Egr-1 mRNA expression that was maximal at one hour (Figure 1A). LPS also induced Egr-1 protein expression (Figure 1B). These data indicate that LPS induces Egr-1 expression in the kidneys of endotoxemic mice.
LPS induction of Egr-1, PAI-1, ICAM-1, and TF mRNAs in the kidney.
(A) Egr-1+/+ mice (2 per group) were either untreated (0 hours) or injected intraperitoneally with LPS (2 mg/kg) for various times (0.5 to 8 hours). Egr-1, PAI-1, ICAM-1, and TF mRNA levels were measured by Northern blotting. G3PDH was used as a loading control. (B) LPS induction (2 hours) of Egr-1 protein was detected by immunoprecipitation and Western blotting.
LPS induction of Egr-1, PAI-1, ICAM-1, and TF mRNAs in the kidney.
(A) Egr-1+/+ mice (2 per group) were either untreated (0 hours) or injected intraperitoneally with LPS (2 mg/kg) for various times (0.5 to 8 hours). Egr-1, PAI-1, ICAM-1, and TF mRNA levels were measured by Northern blotting. G3PDH was used as a loading control. (B) LPS induction (2 hours) of Egr-1 protein was detected by immunoprecipitation and Western blotting.
Local fibrin deposition in glomeruli and in the renal interstitium of the kidney results from increased TF and PAI-1 expression. In addition, inflammation is driven, in part, by the binding of leukocytes to ICAM-1 expressed by endothelial cells. Previous studies have reported that Egr-1 directly or indirectly regulates TF, PAI-1, and ICAM-1.29,31,32 37 We found that LPS induction of Egr-1 mRNA expression preceded the induction of PAI-1, ICAM-1, and TF mRNA expression in the kidneys of mice (Figure 1A). Maximal levels of PAI-1 and ICAM-1 mRNA were observed between 2 and 4 hours, whereas maximal levels of TF mRNA were observed at 8 hours (Figure 1A).
Role of Egr-1 in LPS induction of TF, PAI-1, and ICAM-1 expression in the kidney
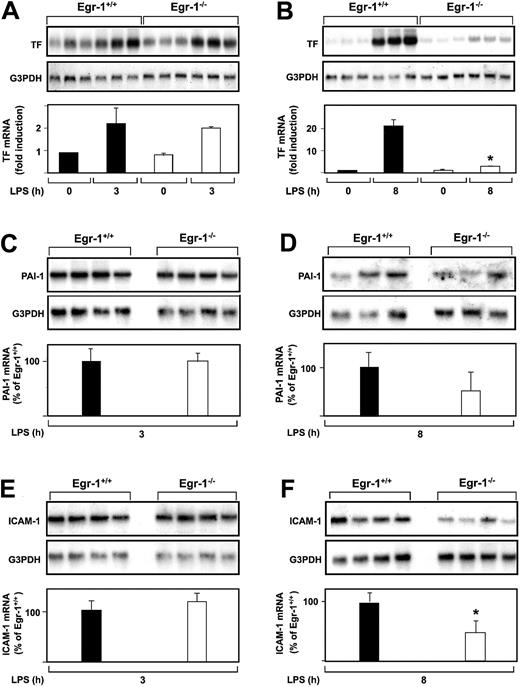
The role of Egr-1 in LPS induction of TF, PAI-1, and ICAM-1 mRNA expression in the kidneys of mice was determined by comparing the levels of expression of these genes in Egr-1−/− mice with those observed in Egr-1+/+ mice. At 3 hours, there was no difference in the level of LPS-induced TF mRNA expression in the kidneys of Egr-1+/+ and Egr-1−/− mice (Figure2A). In contrast, at 8 hours, TF mRNA levels were significantly reduced in Egr-1−/− mice compared with Egr-1+/+ mice (Figure 2B). LPS induced TF mRNA levels 23.9-fold in the kidneys of Egr-1+/+ mice and only 2.4-fold in the kidneys of Egr-1−/− mice, which represents a decrease of 86% (P = .008). Egr-1 deficiency did not affect LPS induction of PAI-1 and ICAM-1 mRNA expression at 3 hours (Figure 2C,E) but resulted in a 52% decrease in PAI-1 mRNA expression and a 52% (P = .047) decrease in ICAM-1 mRNA expression at 8 hours (Figure 2D,F). These results indicated that Egr-1 does not contribute to LPS induction of TF, PAI-1, and ICAM-1 mRNA expression at 3 hours but contributes to their expression at 8 hours in the kidneys of endotoxemic mice.
LPS induction of TF, PAI-1, and ICAM-1 mRNA expression in the kidneys of Egr-1+/+ and Egr-1−/− mice.
(A-F) Egr-1+/+ and Egr-1−/− mice (3 or 4 per group) were either untreated or injected with LPS for either 3 or 8 hours. TF, PAI-1, and ICAM-1 mRNA levels were measured by Northern blotting. Normalized levels of the various mRNAs are shown (mean ± SD). TF mRNA levels are expressed as fold induction, whereas PAI-1 and ICAM-1 mRNA levels are expressed as a percentage of the LPS-induced Egr-1+/+ levels because of the absence of detectable expression in untreated mice. The asterisks indicate decreases that were statistically significant (P < .05).
LPS induction of TF, PAI-1, and ICAM-1 mRNA expression in the kidneys of Egr-1+/+ and Egr-1−/− mice.
(A-F) Egr-1+/+ and Egr-1−/− mice (3 or 4 per group) were either untreated or injected with LPS for either 3 or 8 hours. TF, PAI-1, and ICAM-1 mRNA levels were measured by Northern blotting. Normalized levels of the various mRNAs are shown (mean ± SD). TF mRNA levels are expressed as fold induction, whereas PAI-1 and ICAM-1 mRNA levels are expressed as a percentage of the LPS-induced Egr-1+/+ levels because of the absence of detectable expression in untreated mice. The asterisks indicate decreases that were statistically significant (P < .05).
Role of Egr-1 in LPS induction of IL-6 and MCP-1 in the kidney
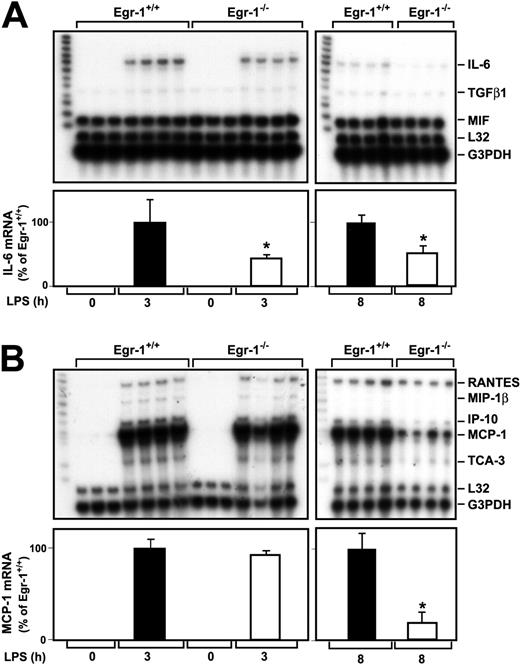
A previous study indicated that Egr-1 regulates the expression of various inflammatory mediators, such as MCP-1, in a murine model of lung ischemia-reperfusion injury.32 We determined whether Egr-1 regulates LPS induction of various cytokine and chemokine mRNAs in the kidneys of endotoxemic mice using RNAse protection assays. LPS induced IL-6 mRNA expression at 3 and 8 hours in the kidneys of Egr-1+/+ mice (Figure 3A). The level of IL-6 mRNA expression was decreased by 57% (P = .047) and 49% (P = .0013) at 3 and 8 hours, respectively, in the kidneys of Egr-1−/− mice injected with LPS compared with the kidneys of Egr-1+/+mice (Figure 3A). Low levels of transforming growth factor β1 (TGFβ1) mRNA were detected in the kidneys of endotoxemic mice, and these were not changed in Egr-1−/− mice at 3 hours but reduced at 8 hours (Figure 3A). At 3 hours, LPS induced only very low levels of TNFα mRNA, which was detected in longer exposures, and this expression was not reduced in Egr-1−/− mice (data not shown). We did not detect expression of TNFβ, lymphotoxin β (LTβ), interferon γ (IFNγ), IFNβ, TGF-β2, and TGF-β3 mRNAs.
LPS induction of IL-6 and MCP-1 mRNA expression in the kidneys of Egr-1+/+ and Egr-1−/− mice.
Egr-1+/+ and Egr-1−/− mice were either untreated (3 mice) or injected with LPS (4 mice per group) for either 3 or 8 hours. Total RNA was isolated from the kidney. (A) Various cytokine mRNAs were measured by RPA using the mouse cytokine multiprobe template set mCK-3b (Pharmingen). This probe set contains (left lane of each gel) TNFβ, LTβ, TNFα, IL-6, IFNγ, IFNβ, TGFβ1, TGFβ2, TGFβ3, and MIF. LPS induced IL-6 mRNA expression, whereas the constitutive expression of MIF was not affected by LPS. Levels of IL-6 mRNA normalized to G3PDH are shown below the gel (mean ± SD). (B) Various chemokine mRNAs were measured by RPA using the mouse chemokine multiprobe template set mCK-5 (Pharmingen). This probe set (left lane of each gel) contains Ltn, RANTES, Eotaxin, MIP-1β, MIP-1α, MIP-2, IP-10, MCP-1, and TCA-3. LPS induced RANTES, MIP-1β, MIP-2, MCP-1, and TCA-3 mRNA expression. Levels of MCP-1 mRNA normalized to G3PDH are shown below the gel (mean ± SD). The asterisks indicate decreases that were statistically significant (P < .05).
LPS induction of IL-6 and MCP-1 mRNA expression in the kidneys of Egr-1+/+ and Egr-1−/− mice.
Egr-1+/+ and Egr-1−/− mice were either untreated (3 mice) or injected with LPS (4 mice per group) for either 3 or 8 hours. Total RNA was isolated from the kidney. (A) Various cytokine mRNAs were measured by RPA using the mouse cytokine multiprobe template set mCK-3b (Pharmingen). This probe set contains (left lane of each gel) TNFβ, LTβ, TNFα, IL-6, IFNγ, IFNβ, TGFβ1, TGFβ2, TGFβ3, and MIF. LPS induced IL-6 mRNA expression, whereas the constitutive expression of MIF was not affected by LPS. Levels of IL-6 mRNA normalized to G3PDH are shown below the gel (mean ± SD). (B) Various chemokine mRNAs were measured by RPA using the mouse chemokine multiprobe template set mCK-5 (Pharmingen). This probe set (left lane of each gel) contains Ltn, RANTES, Eotaxin, MIP-1β, MIP-1α, MIP-2, IP-10, MCP-1, and TCA-3. LPS induced RANTES, MIP-1β, MIP-2, MCP-1, and TCA-3 mRNA expression. Levels of MCP-1 mRNA normalized to G3PDH are shown below the gel (mean ± SD). The asterisks indicate decreases that were statistically significant (P < .05).
MCP-1 mRNA expression was strongly induced in the kidneys of endotoxemic mice (Figure 3B). At 3 hours, LPS induction of MCP-1 mRNA expression in the kidney was similar in Egr-1+/+ and Egr-1−/− mice, but at 8 hours MCP-1 mRNA expression was reduced by 81% (P = .0005) in the Egr-1−/−mice (Figure 3B). Similarly, levels of IFNγ-inducible 10 kDa protein (IP-10) and T-cell activation 3 (TCA-3) were reduced in the kidneys of Egr-1−/− mice at 8 hours but not at 3 hours. LPS induction of regulated upon activation, normal T cells expressed and secreted (RANTES) mRNA expression was not different at 3 or 8 hours in the kidneys of Egr-1+/+ and Egr-1−/− mice (Figure 3B). We did not detect expression of lymphotactin (Ltn), Eotaxin, macrophage inflammatory protein (MIP) 2, and MIP-1α mRNAs. These data indicated that Egr-1 contributes to IL-6, MCP-1, IP-10, and TCA-3 mRNA expression but does not contribute to RANTES mRNA expression in the kidneys of endotoxemic mice.
Role of Egr-1 in LPS induction of TF and chemokine mRNAs in the lung
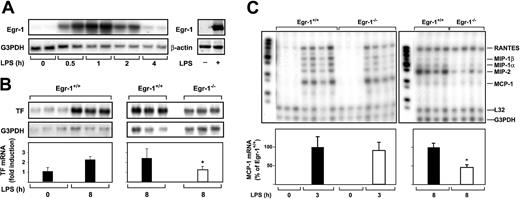
We next determined whether Egr-1 was required for LPS induction of TF and chemokine mRNAs in the lung by comparing the induction of these genes in Egr-1+/+ and Egr-1−/− mice. Previous studies have shown that maximal levels of TF mRNA are expressed at 8 hours in the lungs of endotoxemic mice.5 38 Administration of LPS increased the steady-state levels of Egr-1 mRNA in the lungs with maximal levels at one hour (Figure 4A). LPS also induced Egr-1 protein expression (Figure 4A). LPS did not induce a significant increase in the steady-state levels of TF mRNA at 3 hours (data not shown) but induced a 2.2-fold increase in TF mRNA levels at 8 hours in the lungs of Egr-1+/+ mice (Figure 4B). Egr-1 deficiency resulted in a 51% (P = .035) decrease in TF mRNA expression in the lungs at 8 hours (Figure 4B). In addition, Egr-1 deficiency did not affect the level of MCP-1 mRNA at 3 hours, but its expression was reduced by 54% (P = .012) at 8 hours (Figure 4C). The expression of MIP-2, MIP-1α, and MIP-1β mRNAs was also reduced at 8 hours. LPS induction of RANTES mRNA was not affected by loss of Egr-1 (Figure 4C). These results indicate that Egr-1 contributes to LPS induction of TF and MCP-1 mRNAs at 8 hours but does not contribute to the induction of RANTES mRNA in the lungs of endotoxemic mice.
LPS induction of Egr-1 and TF chemokine expression in the lungs of Egr-1+/+ and Egr-1−/− mice.
(A) (Left panel) Egr-1+/+ mice (2 per group) were either untreated (0 hours) or injected intraperitoneally with LPS (2 mg/kg) for various times (0.5 to 4 hours). (Right panel) LPS induction of Egr-1 mRNA and protein (2 hours) was detected by Northern and Western blotting, respectively. (B) Egr-1+/+ mice (3 per group) were either untreated or injected with LPS for 8 hours. In a second experiment, Egr-1+/+ and Egr-1−/− mice (3 per group) were injected with LPS for 8 hours. TF mRNA levels were determined by Northern blotting. (C) Various chemokine mRNAs were measured in the lungs of untreated and LPS-treated Egr-1+/+and Egr-1−/− mice by RPA using the mouse chemokine multiprobe template set mCK-5 (Pharmingen). This probe set (left lane of each gel) contains Ltn, RANTES, MIP-1β, MIP-1α, IL-10, MIP-2, MCP-1, and TCA-3. G3PDH was used as a loading control. Normalized levels of TF mRNA (mean ± SD) and MCP-1 mRNA (mean ± SE) are shown below. The asterisk indicates that the decrease was statistically significant (P < .05).
LPS induction of Egr-1 and TF chemokine expression in the lungs of Egr-1+/+ and Egr-1−/− mice.
(A) (Left panel) Egr-1+/+ mice (2 per group) were either untreated (0 hours) or injected intraperitoneally with LPS (2 mg/kg) for various times (0.5 to 4 hours). (Right panel) LPS induction of Egr-1 mRNA and protein (2 hours) was detected by Northern and Western blotting, respectively. (B) Egr-1+/+ mice (3 per group) were either untreated or injected with LPS for 8 hours. In a second experiment, Egr-1+/+ and Egr-1−/− mice (3 per group) were injected with LPS for 8 hours. TF mRNA levels were determined by Northern blotting. (C) Various chemokine mRNAs were measured in the lungs of untreated and LPS-treated Egr-1+/+and Egr-1−/− mice by RPA using the mouse chemokine multiprobe template set mCK-5 (Pharmingen). This probe set (left lane of each gel) contains Ltn, RANTES, MIP-1β, MIP-1α, IL-10, MIP-2, MCP-1, and TCA-3. G3PDH was used as a loading control. Normalized levels of TF mRNA (mean ± SD) and MCP-1 mRNA (mean ± SE) are shown below. The asterisk indicates that the decrease was statistically significant (P < .05).
Cell type–specific expression of Egr-1 and TF in the kidneys and lungs of untreated and LPS-treated mice
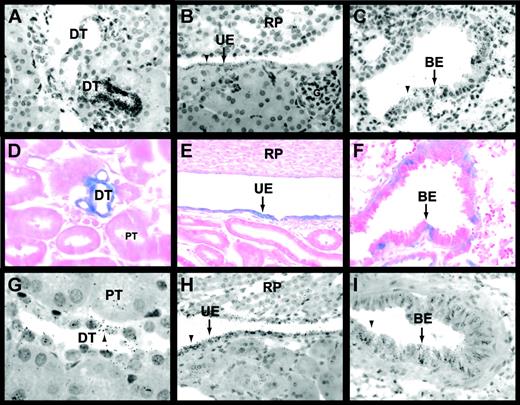
Cell type–specific expression of Egr-1 mRNA in the kidneys and lungs of untreated mice was analyzed by in situ hybridization. In the kidney, Egr-1 mRNA was expressed in the urinary epithelium and by epithelial cells of the distal tubules and Bowman capsule (Figure 5A-B; data not shown). Proximal tubules, glomeruli, and endothelial cells did not express detectable levels of Egr-1 mRNA (Figure 5A-B). In the lung, Egr-1 mRNA was expressed in the bronchial epithelium (Figure 5C). LacZ expression was analyzed in tissues from untreated and LPS-treated Egr-1+/−(LacZ)mice. LacZ was expressed in the urinary epithelium and in epithelial cells of distal tubules and the Bowman capsule in kidneys of untreated and LPS-treated Egr-1+/−(LacZ) mice (Figure 5D-E; data not shown) in the same pattern as Egr-1 mRNA. LacZ expression in epithelial cells in the kidneys and lungs of mice treated with LPS for 2 hours was increased compared with untreated mice and no additional cell types were found to express LacZ (data not shown). LacZ was also expressed in bronchial epithelium in the lung (Figure 5F). These results indicate that epithelial cells in the kidney and lung express Egr-1 in untreated and LPS-treated mice.
Egr-1 and TF expression in the kidneys and lungs of untreated and LPS-treated mice.
In situ hybridization experiments using an antisense Egr-1 riboprobe (A-C) detected Egr-1 mRNA expression in epithelial cells of distal tubules (A), urinary epithelium (B), and the bronchial epithelium (C) of untreated mice. Black grains indicate Egr-1–positive cells. LacZ expression (D-F) was analyzed in tissues from LPS-treated (2 hours) Egr-1+/−(LacZ) mice. Distal tubules (D), urinary epithelium (E), and bronchial epithelium (F) expressed LacZ (blue). In situ hybridization experiments (G-I) showed that TF mRNA was expressed by distal tubules (G), urinary epithelium (H), and bronchial epithelium (I) in LPS-treated (8 hours) mice. Original magnification was × 400 for all panels except panels D and G (× 1000). BE indicates bronchial epithelium; DT, distal tubule; G, glomerulus; PT, proximal tubule; RP, renal papilla; and UE, urinary epithelium. Arrowheads indicate Egr-1– and TF mRNA–positive cells.
Egr-1 and TF expression in the kidneys and lungs of untreated and LPS-treated mice.
In situ hybridization experiments using an antisense Egr-1 riboprobe (A-C) detected Egr-1 mRNA expression in epithelial cells of distal tubules (A), urinary epithelium (B), and the bronchial epithelium (C) of untreated mice. Black grains indicate Egr-1–positive cells. LacZ expression (D-F) was analyzed in tissues from LPS-treated (2 hours) Egr-1+/−(LacZ) mice. Distal tubules (D), urinary epithelium (E), and bronchial epithelium (F) expressed LacZ (blue). In situ hybridization experiments (G-I) showed that TF mRNA was expressed by distal tubules (G), urinary epithelium (H), and bronchial epithelium (I) in LPS-treated (8 hours) mice. Original magnification was × 400 for all panels except panels D and G (× 1000). BE indicates bronchial epithelium; DT, distal tubule; G, glomerulus; PT, proximal tubule; RP, renal papilla; and UE, urinary epithelium. Arrowheads indicate Egr-1– and TF mRNA–positive cells.
Previous studies have shown TF expression in glomeruli but not in tubules in kidneys of humans, baboons, and rabbits.4,7,39In contrast, TF is predominantly expressed in tubules and not glomeruli in mouse kidneys.2,5 In endotoxemic mice, TF mRNA expression is induced in tubular epithelium.2 In this study, we observed TF mRNA expression in distal tubules and urinary epithelium in kidneys and bronchial epithelium in lungs of untreated and LPS-treated mice (Figure 5G-I; data not shown). TF mRNA expression in epithelial cells was increased in kidneys and lungs from LPS-treated mice compared with untreated mice (data not shown). These data indicate that Egr-1 and TF are coexpressed in epithelial cells in the kidneys and lungs of untreated and LPS-treated mice.
Does Egr-1 play a role in the recruitment of leukocytes to the lungs and kidneys in endotoxemic mice?
Inflammation in the lungs and kidneys of endotoxemic Egr-1+/+ and Egr-1−/− mice was evaluated to determine whether the absence of Egr-1 resulted in a decrease in the recruitment of leukocytes. LPS induced a large increase in the number of leukocytes in the lungs at 8 hours, and similar numbers were observed in Egr-1+/+ and Egr-1−/− mice (3 per group) (Figure 6A-F). In the kidney, we observed fewer inflammatory cells compared with the lung, but, again, similar numbers of leukocytes were observed adhering to the endothelium in endotoxemic Egr-1+/+ and Egr-1−/− mice (Figure 6G-L). Leukocytes in the lung were identified by esterase staining, which permitted more accurate quantification of numbers (Figure 7A-C). Similar numbers of leukocytes were observed in the lungs from endotoxemic Egr-1+/+ and Egr-1−/− mice (Egr-1+/+ mice, 80 ± 12 leukocytes/10 fields compared with Egr-1−/− mice, 94 ± 7 leukocytes/10 fields). These data suggest that Egr-1 does not play a significant role in leukocyte recruitment into the lungs and kidneys at 8 hours.
Effect of Egr-1 deficiency on the expression of inflammatory mediators in the systemic circulation and on the survival of mice in a model of endotoxemia
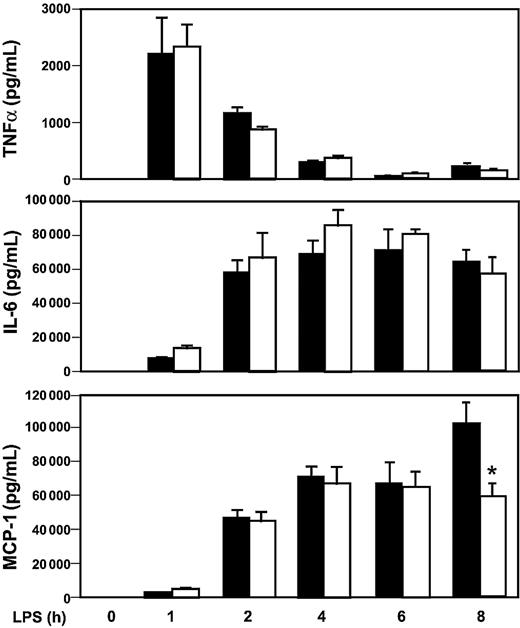
We have shown that Egr-1 regulates the expression of TF and inflammatory mediators in the kidneys and lungs of endotoxemic mice. However, it is not known whether an absence of Egr-1 will affect the levels of circulating inflammatory mediators during endotoxemia. The expression of cytokines and a chemokine in the systemic circulation was determined in Egr-1+/+ and Egr-1−/− mice injected intraperitoneally with a high dose of LPS. Maximal levels of TNFα in plasma were observed at one hour in endotoxemic mice, and levels were not different in Egr-1+/+ and Egr-1−/− mice (Figure 8). Similar levels of IL-6 and MCP-1 were observed in endotoxemic Egr-1+/+ and Egr -1−/− mice between 0 and 6 hours, but MCP-1 levels were reduced in Egr-1−/− mice at 8 hours by 42% (Figure 8). These data indicate that Egr-1 does not contribute to the early expression of these inflammatory mediators in the systemic circulation but contributes to MCP-1 expression at 8 hours in a model of endotoxemia.
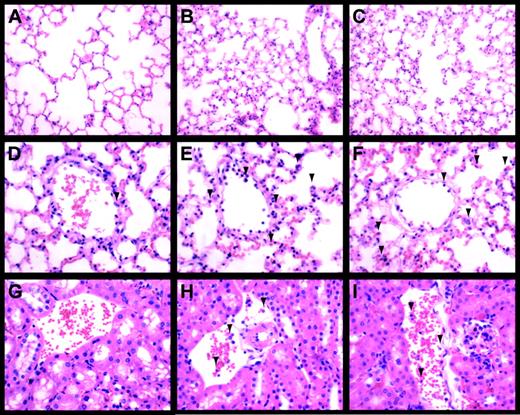
LPS-induced inflammation in the lung and kidney.
Lungs (A-F) and kidneys (G-I) from control (A,D,G), LPS-treated (8 hours) Egr-1+/+ (B,E,H), and LPS-treated (8 hours) Egr-1−/− (C,F,I) mice were stained with hematoxylin and eosin. Original magnification was × 250 (A-C) and × 400 (D-I). Leukocytes are indicated by the arrowheads.
LPS-induced inflammation in the lung and kidney.
Lungs (A-F) and kidneys (G-I) from control (A,D,G), LPS-treated (8 hours) Egr-1+/+ (B,E,H), and LPS-treated (8 hours) Egr-1−/− (C,F,I) mice were stained with hematoxylin and eosin. Original magnification was × 250 (A-C) and × 400 (D-I). Leukocytes are indicated by the arrowheads.
LPS-induced inflammation in the lung.
Leukocytes present in lungs from control (A), LPS-treated (8 hours) Egr-1+/+ (B), and LPS-treated (8 hours) Egr-1−/− (C) mice were stained with the napthol AS-D chloroacetate esterase reaction. Red-stained leukocytes are indicated by the arrowheads.
LPS-induced inflammation in the lung.
Leukocytes present in lungs from control (A), LPS-treated (8 hours) Egr-1+/+ (B), and LPS-treated (8 hours) Egr-1−/− (C) mice were stained with the napthol AS-D chloroacetate esterase reaction. Red-stained leukocytes are indicated by the arrowheads.
LPS induction of inflammatory mediators in Egr-1+/+ and Egr-1−/− mice.
Egr-1+/+ and Egr-1−/− mice (5 or 6 per group) were injected intraperitoneally with LPS (12 mg/kg) and serial blood samples were collected at various times. Levels of TNFα (top), IL-6 (middle), and MCP-1 (bottom) were determined by ELISA in Egr-1+/+ (▪) and Egr-1−/− (■) mice. Data are shown as mean ± SE. The asterisk indicates that the change in MCP-1 is statistically significant (P < .05).
LPS induction of inflammatory mediators in Egr-1+/+ and Egr-1−/− mice.
Egr-1+/+ and Egr-1−/− mice (5 or 6 per group) were injected intraperitoneally with LPS (12 mg/kg) and serial blood samples were collected at various times. Levels of TNFα (top), IL-6 (middle), and MCP-1 (bottom) were determined by ELISA in Egr-1+/+ (▪) and Egr-1−/− (■) mice. Data are shown as mean ± SE. The asterisk indicates that the change in MCP-1 is statistically significant (P < .05).
Finally, we determined the survival of Egr-1+/+ and Egr-1−/− mice injected with a high dose of LPS. Egr-1−/− mice were not protected from the lethal effects of LPS compared with Egr-1+/+ mice (Figure9). In fact, there was a trend toward increased lethality in Egr-1−/− mice, although this difference in survival was not statistically significant by a log-rank test. The absence of a role for Egr-1 in the expression of the majority of inflammatory mediators in the early inflammatory response is consistent with the observation that Egr-1 deficiency does not protect mice from the lethal effects of LPS.
Survival of Egr-1+/+ and Egr-1−/− mice in a model of endotoxemia.
Kaplan-Meier plots showing survival profiles of Egr-1+/+(n = 12, solid line) and Egr-1−/− (n = 10, dashed line) mice injected intraperitoneally with LPS (12 mg/kg). Survival was monitored for 3 days.
Survival of Egr-1+/+ and Egr-1−/− mice in a model of endotoxemia.
Kaplan-Meier plots showing survival profiles of Egr-1+/+(n = 12, solid line) and Egr-1−/− (n = 10, dashed line) mice injected intraperitoneally with LPS (12 mg/kg). Survival was monitored for 3 days.
Discussion
In this study, we showed that Egr-1 mRNA expression is rapidly and transiently induced in the kidneys and lungs of endotoxemic mice with maximal levels at one hour. LPS induction of Egr-1 mRNA in these organs preceded the induction of mRNAs for the inflammatory mediators IL-6, MCP-1, and ICAM-1, which are maximal at 3 hours, and TF mRNA, which is maximal at 8 hours. We observed several different patterns of Egr-1–dependent and Egr-1–independent gene expression. First, LPS induction of IL-6 mRNA expression in the kidney was reduced at both 3 and 8 hours in Egr-1−/− mice compared with Egr-1+/+ mice, indicating a role for Egr-1 in the induction of IL-6. Second, LPS induction of TF, ICAM-1, PAI-1, MCP-1, IP-10, and TCA-3 mRNA expression was not affected at 3 hours but was significantly reduced at 8 hours in the kidneys of Egr-1−/− mice compared with Egr-1+/+ mice. Similarly, TF mRNA expression in the lung was not reduced at 4 hours32 but was reduced by 51% at 8 hours. The expression of MCP-1, MIP-2, MIP-1α and MIP-1β was also reduced at 8 hours in the lungs of Egr-1−/− mice. Finally, Egr-1 did not contribute to the LPS induction of RANTES in the kidneys or lungs of endotoxemic mice. Analysis of the expression of inflammatory mediators in the systemic circulation of endotoxemic mice showed that Egr-1 did not contribute to the early expression of TNFα, IL-6, or MCP-1 but did contribute to MCP-1 expression at 8 hours. These results indicate that Egr-1 directly or indirectly regulates a subset of inflammatory genes via transcriptional and/or posttranscriptional mechanisms.
Our data indicate that Egr-1 does not contribute to the expression of the majority of inflammatory mediators in the early inflammatory response in endotoxemic mice. IL-6 expression at 3 hours is an exception because it is mediated, in part, by Egr-1. A recent study reported that LPS induction of IL-6 expression in epithelial cells was mediated by a nuclear factor–κB (NF-κB)–independent pathway, although the role of Egr-1 was not investigated.40 Previous studies have shown that the early inflammatory response in endotoxemic mice is primarily mediated by an NF-κB–dependent induction of inflammatory mediators.41-43 LPS induces the rapid activation of NF-κB in monocytes/macrophages, endothelial cells, and epithelial cells by activating intracellular signaling pathways that result in degradation of inhibitors of κB (IκBs).44,45NF-κB sites are present in the promoters of the majority of LPS-inducible inflammatory genes, such as TF, TNFα, IL-6, MCP-1, and ICAM-1.46 TNFα protein expression in the plasma of endotoxemic mice was not affected by loss of Egr-1. The number of inflammatory cells infiltrating into the lungs, kidneys, and livers of endotoxemic mice was similar in Egr-1+/+ and Egr-1−/− mice, which is consistent with the observation that Egr-1 does not significantly contribute to the early inflammatory response. Moreover, Egr-1−/− mice were not protected from the lethal effects of LPS and exhibited similar survival to Egr-1+/+ mice. We observed a trend toward a more rapid death in Egr-1−/− mice, which may indicate that Egr-1 regulates the expression of genes involved in protection from the toxic effects of LPS. Indeed, MCP-1 is reduced at 8 hours in Egr-1−/− mice, and this chemokine has been shown to be protective in a mouse model of lethal endotoxemia.47
This study shows that Egr-1 is required for the sustained expression of inflammatory mediators in endotoxemic mice. Unlike NF-κB, Egr-1 expression requires induction of the egr-1 gene and de novo synthesis of Egr-1 protein. This may explain why Egr-1 contributes to the expression of inflammatory mediators at a later time. The expression of IL-6, MCP-1, and ICAM-1 was reduced at 8 hours in the kidneys of endotoxemic Egr-1−/− mice compared with Egr-1+/+ mice. Similarly, MCP-1 protein expression in the plasma was reduced in Egr-1−/− mice at 8 hours. What is the role of the sustained expression of these inflammatory mediators? Clearly, a reduction in the expression of IL-6, MCP-1, and ICAM-1 at 8 hours in Egr-1−/− mice does not protect the mice from the toxic effects of LPS. However, the Egr-1–dependent sustained expression of these inflammatory mediators in endotoxemic mice may contribute to the activation of inflammatory cells in the tissue and possibly to the clearance of the pathogenic organisms. Future studies will examine this idea by challenging Egr-1−/− and Egr-1+/+ mice with live bacteria rather than LPS.
Analysis of the cell type–specific expression of Egr-1 demonstrated that Egr-1 was expressed by tubular epithelial cells in the kidneys and bronchial epithelial cells in the lungs of untreated and LPS-treated mice. Importantly, this study and previous studies showed that TF was also expressed in tubular epithelial cells and bronchial epithelial cells but not in endothelial cells in the kidneys and lungs, respectively, of untreated and LPS-treated mice.2,5 Other studies have shown TF expression in epithelial cells in the kidneys and lungs of endotoxemic baboons and rabbits.4 7 Attempts to show reduced TF mRNA expression in epithelial cells in the kidneys and lungs of LPS-treated Egr-1−/− mice by in situ hybridization were not successful. Nevertheless, the colocalization of Egr-1 and TF in epithelial cells in the kidneys and lungs and the reduced TF expression observed in Egr-1−/− mice strongly suggest that Egr-1 contributes to LPS induction of TF in epithelial cells in endotoxemic mice.
Epithelial cells express both CD14 and the LPS receptor toll-like receptor 4 (TLR4),48,49 which would permit the cells to respond directly to LPS. Indeed, one study using biotin-labeled LPS showed that LPS directly binds to epithelial cells.50 TLR4 expression by epithelial cells in the kidney is functionally important because C3H/HeJ mice, which express a dominant-negative form of TLR4, are more susceptible to infection byE coli than wild-type C3H/HeN mice.51 Our studies showing LPS induction of Egr-1 in epithelial cells suggest that Egr-1 may play a role in the innate immune response to microbial pathogens invading the body through epithelial surfaces.
We would like to acknowledge excellent technical assistance from M. Szeto, M. Tencati, and T. Holscher; preparation of the manuscript by C. Johnson; and critical reading of the manuscript by E. Adamson.
Prepublished online as Blood First Edition Paper, January 23, 2003; DOI 10.1182/blood- 2002-07-2303.
Supported by grants HL48872 and HL65226 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nigel Mackman, The Scripps Research Institute, Departments of Immunology and Cell Biology, 10550 N Torrey Pines Rd, C-204, La Jolla, CA 92037; e-mail:nmackman@scripps.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal