Abstract
We have generated transgenic mice overexpressing the human P2X1 ion channel in the megakaryocytic cell lineage. Platelets from transgenic mice exhibited a gain of P2X1ionotropic activity as determined by more prominent P2X1-mediated Ca2+ influx and platelet shape change. P2X1 overexpression enhanced platelet secretion and aggregation evoked by low doses of collagen, convulxin, or the thromboxane A2 mimetic U46619. In contrast, transgenic platelet responses to adenosine diphosphate (ADP) or thrombin were normal. Perfusing whole blood from transgenic mice over collagen fibers at a shear rate of 1000 seconds−1 resulted in increased P2X1-dependent aggregate formation and phosphatidylserine exposure. Platelet hyperreactivity to collagen was correlated with up-regulated extracellular signal-regulated kinase 2 (ERK2) phosphorylation. Accordingly, the MEK1/2 inhibitor U0126 potently inhibited the collagen-induced aggregation of transgenic platelets when stirred or when perfused over a collagen surface. In a viscometer, shear stress caused potent aggregation of transgenic platelets under conditions in which wild-type platelets did not aggregate. In an in vivo model of thromboembolism consisting of intravenous injection of a low dose of collagen plus epinephrine, transgenic mice died more readily than wild-type mice. Preinjection of U0126 not only fully protected transgenic mice against thrombosis, it also enhanced the survival of wild-type mice injected with a higher collagen dose. Hence, the platelet P2X1 ion channel plays a role in hemostasis and thrombosis through its participation in collagen-, thromboxane A2-, and shear stress–triggered platelet responses. Activation of the ERK2 pathway is instrumental in these processes.
Introduction
Adenosine triphosphate (ATP) is released as a cotransmitter from the sympathetic nerve endings, endothelium, and activated platelets. It is now established that ATP and other nucleotides act as extracellular signaling molecules.1 The receptors that mediate the action of the adenine nucleotides belong to 2 classes, the G-protein–coupled P2Y receptors and the P2X receptors, a family of ligand-gated ion channels.2 Seven distinct P2X purinergic receptors have been cloned from mammalian species (P2X1-7) and have been found to be widely expressed in excitable and nonexcitable cells.3 Subunits of these receptors can assemble to form homomeric and heteromeric functional channels. All P2X receptors are cation-selective channels with almost equal permeability to Na+ and K+ and significant permeability to Ca2+.3 The Ca2+ permeation through P2X receptors is considered to be an important component of the physiologic and pathophysiologic responses mediated by these receptors in vivo (reviewed by Burnstock4 and North5).
In platelets, ATP and adenosine diphosphate (ADP) are present at high concentrations in the dense granules and are coreleased during platelet activation.6 ADP has long been recognized as an important platelet activator, playing an essential role in enhancing secretion and in amplifying platelet aggregation induced by other agonists. Biologic effects of ADP are mediated by 2 distinct metabotropic receptors, the Gq-protein–coupled P2Y1receptor and the Gi-protein–coupled P2Y12receptor. The latter is the target for specific antithrombotic drugs (reviewed by Gachet7). Platelets express the P2X1 member of the P2X family of ligand-gated ion channels,8 which mediates a rapid ATP-induced Ca2+ influx.9,10 Because of the fast desensitizing property of the P2X1 ion channel11 and the lack of specific platelet P2X1 antagonists and because platelet studies have mainly been performed ex vivo at low extracellular Ca2+concentrations in citrated plasma, the function of P2X1 in platelet activation only recently started to be unraveled, and a physiologic role of ATP in this process is now being considered.
In human platelets, the selective P2X1 agonists αβ-methylene adenosine 5′-triphosphate (αβ-meATP) and βγ-methylene adenosine 5′-triphosphate (βγ-meATP) were shown to evoke a transient Ca2+ increase accompanied by reversible platelet shape change, provided that measures had been taken to avoid the activation and desensitization of P2X1 by ATP spontaneously released during platelet preparation.12,13Despite the fact that the sole P2X1 activation cannot cause platelet aggregation, the use of P2X1 desensitization strategies indicated that this ion channel contributes significantly to human platelet aggregation induced by collagen, a major platelet agonist able to trigger dense granule release.13 Recently, we have reported that this effect depends on P2X1-mediated activation of the extracellular signal-regulated kinase 2 (ERK2) mitogen-activated protein kinase (MAPK), which enhances platelet secretion initiated by collagen.14 According to our model, platelet stimulation with low doses of collagen rapidly causes minor dense granule release; ATP secreted during this early event activates a Ca2+- and a protein kinase C (PKC)–dependent P2X1-ERK2 signaling cascade needed to complete platelet aggregation by enhancing release from collagen-primed dense granules.14
To further investigate the physiologic role of the platelet P2X1 ion channel, we have generated transgenic mice overexpressing human P2X1 in the megakaryocytic cell lineage. Platelets from these mice displayed a gain of P2X1functionality accompanied by a mild prothrombotic phenotype. Combining ex vivo and in vivo analyses of platelet function, this mouse model enabled us to demonstrate the involvement of P2X1-mediated Ca2+ influx and the coupled ERK2 activation in platelet responses to collagen. We found that P2X1 overexpression promotes platelet secretion induced by the thromboxane A2mimetic U46619 and thereby enhances platelet aggregation. Moreover, P2X1 overexpression also increases platelet activation and aggregate formation under shear stress. Together, our findings suggest a regulatory role for P2X1 during in vivo hemostasis and thrombosis.
Materials and methods
DNA constructs
Polymerase chain reaction (PCR) cloning of the murine GPIIb promoter fragment extending from +23 to −508 relative to the initiation start site (TOPO TA cloning kit; Invitrogen, Carlsbad, CA) was performed using the following primers: sense, 5′-AGGAAGTGGGTAAATGTCCTACTC-3′; antisense, 5′-TCCCAAACGTCCTAAACAGGAATGG-3′. TheXhoI–HindIII promoter fragment was excised from the mGPIIb-PCR2.1–TOPO plasmid and inserted into the pGL3-basic luciferase reporter vector (Promega, Leiden, the Netherlands) digested with XhoI and HindIII. Megakaryocytic human erythroblastic leukemia (HEL) and nonmegakaryocytic HeLa cell lines were transiently transfected with the resultant reporter construct, and megakaryocytic-specific promoter activity was verified as described15; promoter-driven increases of luciferase expression were only measured in HEL cells, as expected. The promoter fragment was excised from the mGPIIb-pGL3 plasmid by digestion withKpnI and BamHI and inserted into theKpnI-BamHI–digested P2X1-pcDNA3 vector16 in front of the human P2X1(hP2X1) cDNA. DNA constructs were verified by sequencing on the automated A.L.F. sequencer (Pharmacia Biotech, Uppsala, Sweden). The 2.2-kilobase (kb)KpnI–DraIII fragment (GPIIb-hP2X1) was excised and purified for zygote injection. The GPIIb promoter has been successfully used to restrict transgene expression to the megakaryocytic cell lineage of mice.17
Generation of transgenic mice
Transgenic mice expressing human P2X1 were generated by zygote injection into the Friend leukemia virus, strain B (FVB) background according to previously published procedures.18 Transgenic offspring were identified by PCR screening using genomic DNA extracted from tail samples. The following primer pair was used: sense, mGPIIb promoter, 5′-AGGAAGTGGGTAAATGTCCTACTC-3′; antisense, hP2X1, 5′-TCAGGATGTCCTCATGTTCTCCTGCAGG-3′.
RNA isolation and RT-PCR
Total RNA was extracted from mouse washed platelets and leukocytes isolated from freshly drawn citrated blood using the High Pure RNA isolation kit (Roche Diagnostics, Brussels, Belgium). During reverse transcription–polymerase chain reaction (RT-PCR), specific amplification of the human P2X1 cDNA in transgenic mouse samples was accomplished with the following primers: sense, hP2X1, 5′-GTTCCAGGAGGAGCTGGCCGCCTTCC-3′; antisense, hP2X1, 5′-GGTCTTCATGTGGGCAGCATTCAC-3′. For the specific amplification of mP2X1 cDNA, the following primers were used: sense, mP2X1, 5′-CTGCAGGATGAGCTGTCAGCCTTCTTC-3′; antisense, mP2X1, 5′-GTAGAGGCATTTCTTCATGTAGGT-3′.
Materials
Adenosine 5′-diphosphate (ADP), αβ-meATP, βγ-meATP, apyrase (EC 3.6.1.5, grade 1: mixture of both high and low ATPase/ADPase ratio isoenzymes), and the thromboxane A2mimetic U46619 were from Sigma (St Louis, MO). ADP, αβ-meATP, and βγ-meATP were purified by high-performance liquid chromatography (HPLC) on an Adsorbosphere HS C18 7-μm, 250 × 4.6-mm column (Alltech, Bad Segeberg, Germany) as described.12Fibrillar collagen (Horm-type 1 collagen) was from Nycomed (Munich, Germany) and thrombin (Dade Thrombin Reagent) was from Dade Behring (Marburg, Germany). The MEK1/2 inhibitor U0126 was purchased from BioMol Research Laboratories (Plymouth Meeting, MA), and D-Phe-Pro-Arg chloromethyl ketone (PPACK) was from Calbiochem (San Diego, CA). OG 488–annexin V was from Nexins Research (Hoeven, the Netherlands). Fura-2 acetoxymethyl ester and Pluronic F-127 came from Molecular Probes (Leiden, the Netherlands). Recombinant saratin was produced in the yeast Hansenula polymorpha as described.19
Preparation of platelet-rich plasma and washed platelets
Eight- to 12-week-old mice were bled under sodium pentobarbital anesthesia (6 mg/kg) from the retro-orbital plexus. Mouse blood was collected in a saline solution containing either 4 U/mL heparin, 20 μM PPACK, and 0.1 U/mL apyrase or 20 μg/mL hirudin. Platelet-rich plasma (PRP) was obtained by centrifugation at 800g for 30 seconds followed by 5 minutes at 150g. PRP from 3 animals were pooled, and the platelet counts were adjusted to 2.5 × 105 platelets/μL with autologous platelet-poor plasma (PPP). Mouse washed platelets were prepared as previously described,14 using apyrase (1 U/mL) throughout the procedure. Platelets were resuspended in Ca2+-free Tyrode buffer containing 0.35% (wt/vol) human or bovine serum albumin and 1 U/mL apyrase, at a density of 2.5 × 105 platelets/μL.
Electron microscopy
Platelet-rich fractions were immediately fixed overnight at 4°C in 2.5% (wt/vol) glutaraldehyde and 0.1 M phosphate buffer, pH 7.2. After centrifugation at 800g for 10 minutes, a condensed pellet of platelets was formed. After fixation in 1% OsO4 (wt/vol), 0.1 M phosphate buffer, pH 7.2, and dehydration in a graded series of ethanol, the pellets were embedded in epoxy resin. Ultrathin sections were cut and stained with uranyl acetate and lead citrate before examination with a Zeiss EM 10 electron microscope (Oberkochen, Germany).
Platelet aggregation and ATP secretion analyses
Light transmission during mouse platelet aggregation was recorded using apyrase-treated washed platelets in the presence of 2 mM CaCl2 on an ELVI 840 aggregometer (Elvi Logos, Milan, Italy). Shear-induced platelet aggregations were performed in an annular ring-shaped viscometer generating laminar shear (Ravenfield viscometer; Heywood, Lancashire, United Kingdom) using mouse heparinized PRP. After 3 minutes, platelet samples were collected and fixed in 1% paraformaldehyde; the percentage of platelet aggregation was calculated by comparing single platelet counts before and after shearing. ATP secretion was monitored in hirudinized PRP in parallel with platelet aggregation by adding firefly luciferase and luciferin and comparing the luminescence generated by platelet ATP release or by an ATP standard (Chrono-Lume, Kordia, The Netherlands) as previously described.14
Immunoblotting
Western blot detection of the human P2X1 protein in transgenic mouse platelets (8 × 108 platelets) was performed by using a polyclonal rabbit anti-hP2X1antibody.16 Detection of ERK1/2 phosphorylation in human or mouse washed platelets (1 × 107 platelets) was accomplished with the PhosphoPlus p44/42 MAP Kinase Antibody kit (New England Biolabs, Hitchin, United Kingdom) according to the instructions of the manufacturer.
Ca2+ measurements
Apyrase (2 U/mL)–treated mouse washed platelets (2 × 105 platelets/μL) were loaded with 3.5 μM fura-2 acetoxymethyl ester in the presence of Pluronic F-127 for 15 minutes as described.20 The measurements were performed between 30 and 90 minutes after final platelet resuspension (0.7 × 105 platelets/μL). CaCl2 (2 mM) was added before the agonist. Fura-2 fluorescence was recorded from 0.2 mL aliquots of platelet suspension stirred at 37°C in an SLM-Aminco spectrofluorimeter (SLM Instruments, Rochester, NY) with excitation wavelengths of 340 and 380 nm and emission of 500 nm. Changes in intracellular Ca2+ concentration were monitored using the fura-2 340/380 fluorescence ratio and were calibrated according to the method of Grynkiewicz et al.21 The Ca2+signals evoked by αβ-meATP were assessed as the peak amplitude of the intracellular Ca2+ rise occurring within a few milliseconds after agonist application and returning to basal levels after 10 seconds.
Adhesion under flow conditions
Adhesion experiments under flow conditions were performed with anticoagulated mouse blood (4 U/mL heparin, 20 μM PPACK), basically as described.22 Whole blood was perfused for 4 minutes over a collagen-coated coverslip through a parallel-plate transparent flow chamber using a pulse-free pump, at a wall-shear rate of 1000 seconds−1. During the perfusion, high-resolution microscopic transmission or fluorescent images were recorded in real-time with a Visitech digital imaging system (Sunderland, United Kingdom). Exposure of phosphatidylserine (PS) was detected by postperfusion with the heparinized rinsing buffer containing OG488-labeled annexin V (1 μg/mL). Phase-contrast and fluorescent images were obtained from at least 10 different collagen-containing microscopic fields that were arbitrarily chosen. When indicated, apyrase (0.1 U/mL) was added during blood sampling; in some experiments, blood was incubated with saratin (10 μg/mL) blocking von Willebrand factor (VWF) binding to collagen19 1 minute before perfusion. Area coverage from phase-contrast images was analyzed off-line using ImagePro software (Media Cybernetics, Silver Spring, MD). Area coverage by platelets stained with OG488-annexin V was determined with Quanticell software (Visitech).
In vivo experiments
Thromboembolism was induced by injection of a mixture of collagen (0.125 or 0.06 mg/kg) and epinephrine (60 μg/kg) into the jugular veins of anesthetized mice. When indicated, mice received 200 μg/kg U0126 1 minute before the induction of thromboembolism. For bleeding time measurements, mice were anesthetized, and 3 mm of the tail tip was amputated with a scalpel. The tail was then blotted with filter paper every 15 seconds until the paper was no longer blood stained.
Statistical analyses
Statistical analyses of the data were made using the nonpaired Student t test and the 2-tailed Tukey-Kramer multiple comparisons test. Survival data were analyzed using 2 × 2 contingency tables.
Results
Generation of transgenic mice overexpressing the human P2X1 ion channel in the megakaryocytic cell lineage
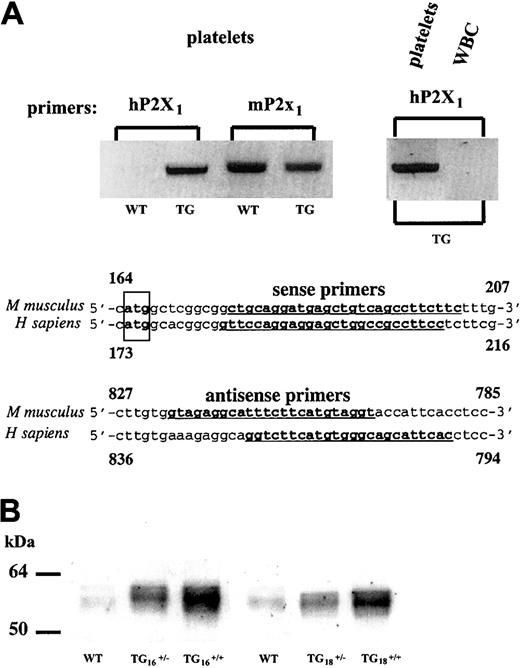
On zygote injection of a constructed GPIIb-hP2X1transgene, among 19 offspring mice obtained, 4 animals were found to be transgenic by PCR screening. All founders transmitted the transgene in a Mendelian fashion. RT-PCR analyses using primer pairs that selectively amplify hP2X1 versus mP2X1mRNAs revealed the presence of hP2X1transcripts in the platelets of the transgenic (TG) mice (Figure1A). The expression of the endogenous platelet mP2X1 remained comparable to that of wild-type (WT) platelets (Figure 1A). No transgene expression was found in the leukocytes (Figure 1A). Overexpression of the hP2X1 protein in platelets was demonstrated by immunoblotting of heterozygous (TG+/−) and homozygous (TG+/+) mouse platelet extracts (Figure 1B); similarly, immunohistochemistry of bone marrow sections revealed increased P2X1 staining in the TG megakaryocyte membranes (not shown). The homozygous mice of 2 founder lines (denominated TG16 and TG18) overexpressing similar amounts of platelet hP2X1 were characterized and showed identical phenotypes. These mice had no apparent physiologic abnormalities and displayed normal development, survival, and reproduction. Platelet count (Table1) and morphology (not shown), as well as other hematologic parameters, were identical to those of WT mice, with the exception of mild leukocytosis (Table 1). Values are represented as mean ± SD (P = .003).
hP2X1 overexpression in transgenic mouse platelets.
(A) RT-PCR performed on total RNA extracted from platelets or total leukocytes (WBCs) of wild-type (WT) or transgenic (TG) mice. Identical results were found for 2 independent transgenic founder lines. Partial sequences of the human and mouse P2X1 cDNAs are shown. Primers used to specifically amplify the hP2X1 and mP2X1 cDNAs are underlined and in boldface type. The translation initiation codon is boxed. (B) Western blotting detection of the hP2X1 protein in TG platelet whole-cell lysate with a polyclonal anti-hP2X1 antibody.12 The hP2X1 expression levels in identical numbers of platelets from heterozygous or homozygous transgenic mice originating from 2 independent transgenic founder lines (TG16 and TG18) are shown in parallel with those of the endogenous mP2X1 detected in WT mouse platelets.
hP2X1 overexpression in transgenic mouse platelets.
(A) RT-PCR performed on total RNA extracted from platelets or total leukocytes (WBCs) of wild-type (WT) or transgenic (TG) mice. Identical results were found for 2 independent transgenic founder lines. Partial sequences of the human and mouse P2X1 cDNAs are shown. Primers used to specifically amplify the hP2X1 and mP2X1 cDNAs are underlined and in boldface type. The translation initiation codon is boxed. (B) Western blotting detection of the hP2X1 protein in TG platelet whole-cell lysate with a polyclonal anti-hP2X1 antibody.12 The hP2X1 expression levels in identical numbers of platelets from heterozygous or homozygous transgenic mice originating from 2 independent transgenic founder lines (TG16 and TG18) are shown in parallel with those of the endogenous mP2X1 detected in WT mouse platelets.
Hematologic parameters of wild-type and transgenic mice
| Parameter . | Wild-type . | Transgenic . |
|---|---|---|
| Platelet count, ×104/μL | 69.5 ± 8.9 (n = 13) | 64.8 ± 2.8 (n = 9) |
| White blood cell count, ×103/μL | 1.7 ± 0.7 (n = 12) | 2.9 ± 0.5 (n = 10)* |
| Red blood cell count, ×106/μL | 7.07 ± 0.18 | 7.25 ± 0.15 |
| Hematocrit, % | 31.5 ± 0.9 | 32.9 ± 0.9 |
| Hemoglobin level, g/dL | 10.9 ± 0.3 | 11.7 ± 0.2 |
| Parameter . | Wild-type . | Transgenic . |
|---|---|---|
| Platelet count, ×104/μL | 69.5 ± 8.9 (n = 13) | 64.8 ± 2.8 (n = 9) |
| White blood cell count, ×103/μL | 1.7 ± 0.7 (n = 12) | 2.9 ± 0.5 (n = 10)* |
| Red blood cell count, ×106/μL | 7.07 ± 0.18 | 7.25 ± 0.15 |
| Hematocrit, % | 31.5 ± 0.9 | 32.9 ± 0.9 |
| Hemoglobin level, g/dL | 10.9 ± 0.3 | 11.7 ± 0.2 |
P = .03.
Overexpression of hP2X1 results in a gain of P2X1 functionality in the transgenic platelets
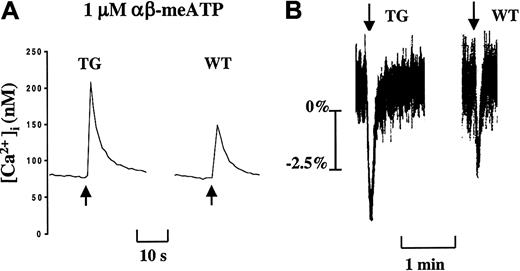
Apyrase, by degrading ATP spontaneously released during blood sample handling, is needed ex vivo to protect platelet P2X1channels from artificial desensitization.12 13 In the presence of this ectonucleotidase and physiologic Ca2+concentrations (2 mM CaCl2), the nonhydrolyzable P2X1 selective agonists αβ-meATP (1 μM, defined to be the optimal concentration) (Figure 2) and βγ-meATP (not shown) evoked a rapid Ca2+ influx in mouse platelets. The peak value of this intracellular Ca2+rise was increased by approximately 50% in TG platelets compared with WT platelets (TG, 146.5 ± 23.6 nM, n = 8; WT, 93.1 ± 21.4 nM, n = 8; P = .0003) (Figure 2). In the same experimental conditions, the WT and TG platelets displayed identical ADP-induced Ca2+ increases, reflecting P2Y1-mediated Ca2+ responses (WT, 399 ± 66 nM, n = 5; TG, 478 ± 74 nM, n = 5; P = .112).
Gain of P2X1 functionality in TG platelets.
(A) Increased P2X1-mediated Ca2+ influx in TG platelets. (B) Enhanced P2X1-mediated TG platelet shape change. Apyrase (2 U/mL)–treated washed platelets were stimulated with αβ-meATP (1 μM) in the presence of 2 mM CaCl2. Data are representative curves of at least 2 separate experiments performed in triplicate on platelet pools from 3 animals. Arrows indicate the addition of αβ-meATP (1 μM).
Gain of P2X1 functionality in TG platelets.
(A) Increased P2X1-mediated Ca2+ influx in TG platelets. (B) Enhanced P2X1-mediated TG platelet shape change. Apyrase (2 U/mL)–treated washed platelets were stimulated with αβ-meATP (1 μM) in the presence of 2 mM CaCl2. Data are representative curves of at least 2 separate experiments performed in triplicate on platelet pools from 3 animals. Arrows indicate the addition of αβ-meATP (1 μM).
In human platelets, P2X1 stimulation causes Ca2+ influx and subsequent platelet shape change.12 13 Similarly, αβ-meATP and βγ-meATP induced a quickly reversible shape change of WT mouse platelets proportional to Ca2+ influx. Platelets from TG mice exhibited a more prominent αβ-meATP–induced platelet shape change compared with WT platelet response (Figure 2B). Taken together, these data are compatible with a significant gain of P2X1functionality in the TG platelets.
Enhanced platelet aggregation induced by collagen in hP2X1 transgenic mice: role of the ERK2 signaling pathway
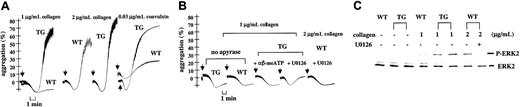
In an aggregometer, the P2X1 selective agonists αβ-meATP and βγ-meATP did not cause platelet aggregation or secretion either in WT or in TG mice. Functional studies of apyrase-treated platelets from TG mice, where P2X1desensitization is prevented, yet demonstrated strongly enhanced platelet aggregation evoked by low and intermediate doses of collagen (1 to 2 μg/mL) compared with WT platelets (Figure3A). A similar increase of TG platelet aggregation was observed after platelet stimulation with low concentrations of convulxin, a glycoprotein VI (GPVI)–selective agonist (0.025 to 0.04 μg/mL) (Figure 3A, right panel), indicating that P2X1 overexpression enhances platelet aggregation mediated by the collagen receptor GPVI. Platelet aggregations induced by higher collagen (4 μg/mL or greater) or convulxin (0.05 μg/mL or greater) concentrations were identical for WT and TG platelets (not shown).
Increased GPVI-mediated collagen-induced platelet aggregation and ERK2 activation with transgenic platelets.
(A) Light transmission (%) recordings in apyrase (1 U/mL)–treated washed WT or TG mouse platelets stimulated with collagen (1-2 μg/mL) or convulxin (0.03 μg/mL). (B) Platelet aggregations were induced with collagen (1 μg/mL) in the absence of apyrase (2 curves on the left). The other curves represent collagen-induced platelet aggregations performed in the presence of apyrase after 1-minute preincubation with αβ-meATP (1 μM) or with the MEK1/2 inhibitor U0126 (1 μM), as indicated. Arrows depict the time of collagen application. (C) Western blot analyses of ERK2 phosphorylation (P-ERK2) evoked by collagen in WT or TG apyrase-treated platelets. For TG, data from 2 independent platelet pools are shown. Samples were analyzed 3 minutes after collagen addition. Blockade of collagen-induced ERK2 phosphorylation with U0126 (1 μM) is shown for WT platelets in parallel with its effect on WT platelet aggregation (panel B). Data are representative of at least 3 independent experiments performed in duplicate on platelets from WT and TG mice.
Increased GPVI-mediated collagen-induced platelet aggregation and ERK2 activation with transgenic platelets.
(A) Light transmission (%) recordings in apyrase (1 U/mL)–treated washed WT or TG mouse platelets stimulated with collagen (1-2 μg/mL) or convulxin (0.03 μg/mL). (B) Platelet aggregations were induced with collagen (1 μg/mL) in the absence of apyrase (2 curves on the left). The other curves represent collagen-induced platelet aggregations performed in the presence of apyrase after 1-minute preincubation with αβ-meATP (1 μM) or with the MEK1/2 inhibitor U0126 (1 μM), as indicated. Arrows depict the time of collagen application. (C) Western blot analyses of ERK2 phosphorylation (P-ERK2) evoked by collagen in WT or TG apyrase-treated platelets. For TG, data from 2 independent platelet pools are shown. Samples were analyzed 3 minutes after collagen addition. Blockade of collagen-induced ERK2 phosphorylation with U0126 (1 μM) is shown for WT platelets in parallel with its effect on WT platelet aggregation (panel B). Data are representative of at least 3 independent experiments performed in duplicate on platelets from WT and TG mice.
Threshold concentrations of collagen, which only caused shape change of WT platelets, produced full aggregation of TG platelets (Figure 3A). Consistent with an event requiring functional P2X1channels, the enhanced reactivity to collagen was abrogated when the apyrase treatment was omitted (Figure 3B) or when these platelets were pretreated with αβ-meATP (1 μM) to selectively desensitize P2X1 (Figure 3B). Blockade of ERK2 activation with the selective MEK1/2 inhibitor, U0126 (1 μM) (Figure 3C, last 2 lanes) abolished collagen-induced platelet aggregation for TG (1 μg/mL) and WT (2 μg/mL) platelets (Figure 3B). Interestingly, full aggregation of TG platelets already evoked by 1 μg/mL collagen coincided with maximal ERK2 phosphorylation (Figure 3C). These data demonstrated that P2X1 and ERK2 are proximal and distal components of a common signaling cascade contributing to collagen-induced platelet aggregation and that this pathway has been up-regulated in TG platelets as a consequence of P2X1 overexpression.
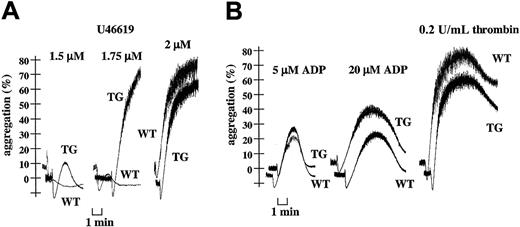
Further analyses of TG platelet aggregation revealed an increased response to low concentrations of the thromboxane A2mimetic U46619 (less than 2 μM) (Figure4A). In contrast, TG platelet aggregation provoked by any concentration of HPLC-purified ADP or of thrombin occurred normally (Figure 4B).
Increased U46619-induced platelet aggregation and normal responses to ADP and thrombin with transgenic platelets.
Light transmission (%) recordings in apyrase (1 U/mL)–treated washed WT or TG mouse platelets stimulated with thromboxane A2 mimetic U46619 (1.5-1.75-2 μM) (A) or HPLC-purified ADP (5-20 μM) and thrombin (0.2 U/mL) (B). Data are representative of at least 3 independent experiments performed in duplicate on platelets from WT and TG mice.
Increased U46619-induced platelet aggregation and normal responses to ADP and thrombin with transgenic platelets.
Light transmission (%) recordings in apyrase (1 U/mL)–treated washed WT or TG mouse platelets stimulated with thromboxane A2 mimetic U46619 (1.5-1.75-2 μM) (A) or HPLC-purified ADP (5-20 μM) and thrombin (0.2 U/mL) (B). Data are representative of at least 3 independent experiments performed in duplicate on platelets from WT and TG mice.
Enhanced platelet secretion induced by collagen and U46619 in hP2X1 transgenic mice
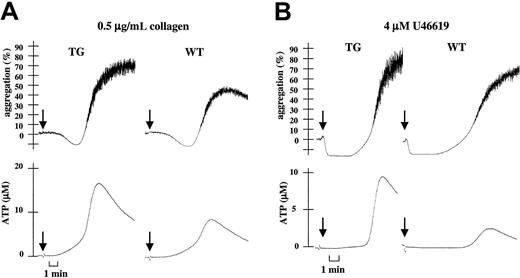
To investigate the mechanism responsible for the observed increased aggregation of TG platelets, we compared platelet secretion induced by low concentrations of collagen or of U46619 in WT and TG PRP. As in human platelets, the desensitization of P2X1with αβ-meATP strongly inhibited ATP secretion from WT platelets, indicating a P2X1 contribution to this platelet response (not shown). Figure 5 shows increased ATP secretion of TG platelets (1.9 ± 0.6- and 3.3 ± 0.2-fold increases in response to 0.5 μg/mL collagen and 4 μM U46619, respectively [P < .001; n = 3]) paralleled with enhanced platelet aggregation. Thus, P2X1 overexpression in TG platelets potentiates platelet-dense granule release initiated by low doses of collagen or of the thromboxane A2 mimetic U46619, leading to the amplification of the initial platelet response and the completion of aggregation. ATP secretion triggered by ADP or thrombin was found to be normal (not shown).
Increased collagen- or U46619-induced platelet ATP secretion with transgenic platelets.
(A) Parallel recordings of light transmission (%) and ATP secretion (μM) in WT or TG mouse hirudinized PRP during platelet stimulation with 0.5 μg/mL collagen. (B) Identical experiment after platelet stimulation with 4 μM U46619. Data are representative of at least 3 independent experiments performed in duplicate on platelets from WT and TG mice.
Increased collagen- or U46619-induced platelet ATP secretion with transgenic platelets.
(A) Parallel recordings of light transmission (%) and ATP secretion (μM) in WT or TG mouse hirudinized PRP during platelet stimulation with 0.5 μg/mL collagen. (B) Identical experiment after platelet stimulation with 4 μM U46619. Data are representative of at least 3 independent experiments performed in duplicate on platelets from WT and TG mice.
Increased whole blood aggregate formation and platelet phosphatidylserine exposure on a collagen-coated surface under flow
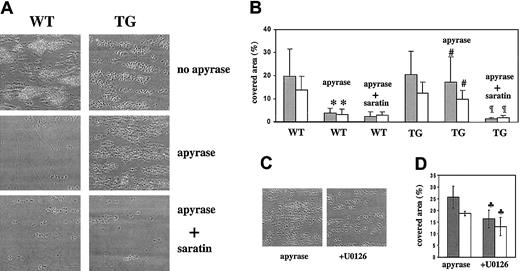
To further investigate the TG platelet hyperreactivity to collagen, aggregate formation and exposure of coagulation-active negatively charged phosphatidylserine (PS) on a collagen surface were analyzed in whole mouse blood under conditions of flow. Using videomicroscopy, aggregate formation was monitored from phase-contrast images, and PS exposure (procoagulant activity) was imaged after annexin V staining. As previously described,22 when WT mouse blood was perfused over collagen at a shear rate of 1000 seconds−1, platelets tethered, adhered, and assembled on the surface, with the adherent cells responding by a rapid increase in cytosolic Ca2+,22 and subsequent surface-exposure of PS (Figure 6). A similar picture was observed following perfusion of TG blood (Figure6). The use of ATP/ADP-degrading apyrase, needed to protect P2X1 from ATP-provoked desensitization, considerably reduced aggregate formation and platelet PS exposure after the perfusion of WT blood (Figure 6A-B). This is compatible with the reported role of the P2Y1 and P2Y12 receptors interacting with released ADP during aggregate formation on collagen.23 24 Perfusion of apyrase-treated TG blood yet resulted in prominent platelet aggregation coinciding with high PS exposure (Figure 6A-B), suggesting that P2X1 overexpression in TG platelets has compensated the inhibitory effects of apyrase by promoting ATP-dependent platelet adhesion, activation, and aggregation under these flow conditions.
Increased aggregate formation and PS exposure over a collagen surface under flow with transgenic platelets.
Inhibitory effect of U0126. Whole blood from WT or TG mice was perfused at a wall shear rate of 1000 seconds−1 for 4 minutes over a fibrillar collagen-coated surface. Blood from these 2 groups was assessed in the absence or presence of apyrase (0.1 U/mL) with or without saratin (10 μg/mL) and with or without U0126 (1 μM) for TG blood, as indicated. (A,C) Representative phase-contrast microscope images after perfusion. (B,D) Gray bars (░) represent the surface area coverage by platelets calculated from the phase-contrast images at the end of the perfusion period. White bars (■) represent the area coverage by platelets binding OG488-annexin V after perfusion (platelets exposing negatively charged PS) (n = 3-6) (*P < .001 vs WT;#P < .001 vs WT + apyrase;¶P < .001 vs TG + apyrase;♣P < 0.035 vs TG + apyrase).
Increased aggregate formation and PS exposure over a collagen surface under flow with transgenic platelets.
Inhibitory effect of U0126. Whole blood from WT or TG mice was perfused at a wall shear rate of 1000 seconds−1 for 4 minutes over a fibrillar collagen-coated surface. Blood from these 2 groups was assessed in the absence or presence of apyrase (0.1 U/mL) with or without saratin (10 μg/mL) and with or without U0126 (1 μM) for TG blood, as indicated. (A,C) Representative phase-contrast microscope images after perfusion. (B,D) Gray bars (░) represent the surface area coverage by platelets calculated from the phase-contrast images at the end of the perfusion period. White bars (■) represent the area coverage by platelets binding OG488-annexin V after perfusion (platelets exposing negatively charged PS) (n = 3-6) (*P < .001 vs WT;#P < .001 vs WT + apyrase;¶P < .001 vs TG + apyrase;♣P < 0.035 vs TG + apyrase).
Figure 6 also shows how aggregate formation and PS exposure by apyrase-treated TG platelets were abrogated by saratin (Figure 6A-B), which, by blocking VWF binding to collagen, prevents GPIbα-VWF–dependent platelet adhesion,19 suggestive of a role for P2X1 in the initial control of platelet activation and aggregation on surface-bound VWF at the shear rate investigated.
Interestingly, area coverage by platelet aggregates and PS exposure was reduced by 30% to 35% during the perfusion of apyrase-treated TG blood in the presence of U0126 (Figure 6C-D). These data indicate the involvement of the ERK2 pathway in platelet aggregate formation on collagen under flow. U0126 did not affect aggregate formation or PS exposure when WT blood was perfused in P2X1 nonprotective conditions (no apyrase) (not shown). These observations thus support the existence of a P2X1-ERK2 pathway contributing to platelet activation by collagen and surface-bound VWF.
Potent shear-induced aggregation of TG platelets
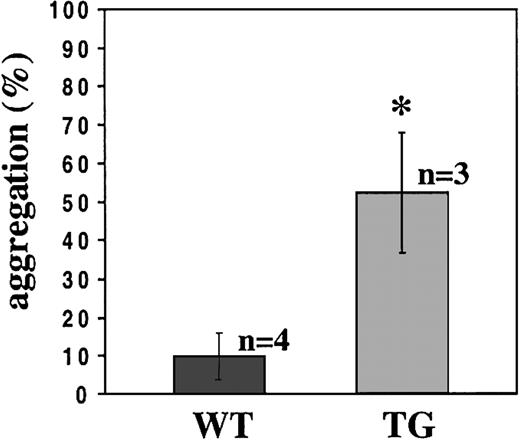
Platelet aggregation induced by shear stress can be measured in a viscometer producing a laminar flow. At high shear stress, VWF binding to GPIbα is essential to induce αIIbβ3-dependent platelet aggregation.25 We have investigated whether P2X1 would contribute to shear-dependent platelet aggregation. For this purpose, platelet aggregations were performed in apyrase-treated heparinized PRP from WT and TG mice, at a shear rate of 9000 seconds−1 corresponding to a shear stress of 124 dyne/cm2. At this shear rate, the transgenic platelets underwent potent aggregation (52.4% ± 15.5% of aggregation) compared with negligible aggregation of WT platelets (9.9% ± 6.3%) (Figure 7), showing that the TG platelets exhibited increased ability to respond to shear stress.
Potentiated shear-induced TG platelet aggregation.
Aggregation of platelets from WT or TG mice induced at a shear rate of 9000 seconds−1 in a viscometer for 3 minutes. WT and TG platelet aggregations were analyzed in apyrase (0.1 U/mL)–treated heparinized PRP (*P = .0039). At least 3 independent experiments were performed on PRP pools prepared from 2 mice.
Potentiated shear-induced TG platelet aggregation.
Aggregation of platelets from WT or TG mice induced at a shear rate of 9000 seconds−1 in a viscometer for 3 minutes. WT and TG platelet aggregations were analyzed in apyrase (0.1 U/mL)–treated heparinized PRP (*P = .0039). At least 3 independent experiments were performed on PRP pools prepared from 2 mice.
Increased thrombotic tendency in transgenic mice
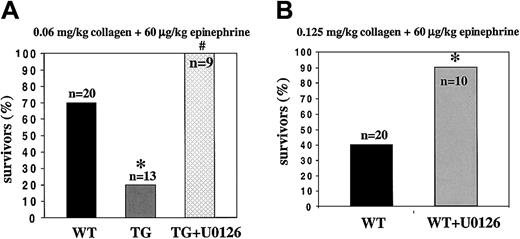
Bleeding times, considered to reflect primary hemostasis in vivo, were identical for WT and TG mice (WT, 5.07 ± 2.05 minutes, n = 22; TG, 4.98 ± 2.41 minutes, n = 12). Because in vitro collagen-induced transgenic platelet activation and aggregation were greatly enhanced, we used an in vivo model of pulmonary thromboembolism by intravenous injection of a mixture of a low dose of collagen (0.06 mg/kg body weight) and epinephrine (60 μg/kg). As shown in Figure 8A, 80% of the TG mice died after 4 minutes compared with only a 30% mortality rate for WT mice. These results indicate that P2X1 overexpression generates a prothrombotic phenotype.
Pulmonary thromboembolism.
(A) Increased lethal thrombosis in transgenic mice after the intravenous injection of collagen and epinephrine and protection against thrombosis by the inhibition of ERK2. Survival in WT and TG mice is shown as a percentage of animals treated (*P = .017 vs WT; #P = .017 vs TG). (B) Antithrombotic protection after the inhibition of ERK2 in WT mice by the preinjection of U0126 (200 μg/kg) versus control (*P = .0187).
Pulmonary thromboembolism.
(A) Increased lethal thrombosis in transgenic mice after the intravenous injection of collagen and epinephrine and protection against thrombosis by the inhibition of ERK2. Survival in WT and TG mice is shown as a percentage of animals treated (*P = .017 vs WT; #P = .017 vs TG). (B) Antithrombotic protection after the inhibition of ERK2 in WT mice by the preinjection of U0126 (200 μg/kg) versus control (*P = .0187).
Antithrombotic protection by blockade of the ERK2 pathway
To further examine the importance of the P2X1-coupled ERK2 signaling pathway for platelet function in vivo, we analyzed the effect of a 1-minute pretreatment with U0126 (200 μg/kg) on the mortality of TG mice. All mice pretreated with U0126 survived the injection of collagen (0.06 mg/kg) plus epinephrine (60 μg/kg) (Figure 8A). Inhibition of ERK2 also conferred antithrombotic protection to WT mice injected with a mixture of a higher dose of collagen (0.125 mg/kg) plus epinephrine (60 μg/kg); the number of survivors reached 90% compared with 40% in controls (Figure8B).
Discussion
We have generated transgenic mice overexpressing the human ATP-gated P2X1 ion channel in the megakaryocytic cell lineage. Platelets from these mice displayed a gain of P2X1ionotropic activity, as shown by more prominent Ca2+ influx and platelet shape change triggered by the 2 P2X1-selective agonists, αβ-meATP and βγ-meATP. Transgenic platelets underwent enhanced aggregation in response to low doses of collagen or of the thromboxane A2 mimetic U46619. We showed that the increased collagen- and U46619-induced platelet aggregations resulted from enhanced platelet-dense granule release. P2X1overexpression also increased platelet reactivity to shear stress. Transgenic mice manifested increased in vivo thrombosis after injections of a mixture of low-dose collagen and epinephrine. Thus, the overexpression of P2X1 in mouse platelets has generated a novel, mild, prothrombotic phenotype.
Enhanced secretion and aggregation of the transgenic platelets in response to collagen corroborate our previous findings in healthy human platelets.13,14 We have reported that low concentrations of collagen cause early minor ATP release that elicits a rapid P2X1-mediated Ca2+ influx, contributing to enhancement of the platelet release reaction, thus completing platelet aggregation. It is therefore likely that the enhanced TG platelet response to collagen results from an increased number of functional ATP-responsive P2X1 channels expressed at the surfaces of TG platelets. The higher level of P2X1 activation thereby leads to enhanced platelet secretion and aggregation. Similarly, the increased TG platelet aggregation induced by low concentrations of the thromboxane A2 mimetic U46619 was related to enhanced platelet secretion induced by this agonist. This is in agreement with earlier studies on the importance of secreted products, mainly ADP, during U46619-induced platelet aggregation.26 Collagen activates platelets by transducing signals through glycoprotein VI (GPVI). We investigated whether the observed enhanced platelet aggregation induced by collagen depended on this glycoprotein by using the GPVI-selective agonist convulxin. We found that TG platelet aggregation induced by low concentrations of convulxin was similarly enhanced, as with collagen, suggesting cooperation between secreted ATP- and GPVI-mediated signaling under mild stimulation of this receptor. In agreement with this finding and using ADP receptor antagonists, Quinton et al27 have reported a role for secreted ADP during platelet aggregation provoked by low concentrations of convulxin, whereas platelet aggregation at higher concentrations of convulxin was unaffected by these agents.
In human platelets, we have identified the ERK2 signaling pathway as an intracellular mechanism subserving the function of the ATP-gated P2X1 ion channel during platelet aggregation induced by low concentrations of collagen. We have shown that the P2X1-mediated ERK2 activation is needed to amplify dense-granule release initiated by this agonist. As in human platelets, the aggregation of wild-type mouse platelets evoked by low concentrations of collagen was abolished after blockade of the ERK2 pathway with the selective MEK1/2 inhibitor U0126. Accordingly, the increased collagen-induced aggregation of transgenic platelets coincided with the up-regulation of ERK2 and could equally be abolished by U0126. It appears that P2X1 and ERK2 are 2 components of a common signaling cascade in human and mouse platelets.
Thus, even though the P2X1-mediated Ca2+influx seems to be small, this necessarily local signal close to the plasma membrane, where ERK2 is also translocated, is likely to generate a significant local trigger for the promotion of Ca2+- and ERK2-dependent secretion.
To investigate the physiologic relevance of the P2X1-coupled ERK2 pathway in thrombosis, we used an in vivo model of pulmonary thromboembolism by injection of a mixture of collagen and epinephrine. We showed that inhibition of the ERK2 pathway by U0126 fully protected transgenic mice against lethal thrombosis induced by a low dose of collagen plus epinephrine. Wild-type mice were also partly protected after the administration of a higher dose of collagen. Together with the observation that P2X1-overexpressing transgenic platelets were hyperreactive to collagen in an ERK2-dependent fashion, our in vivo data support the existence of a P2X1-ERK2 signaling axis involved—among several other components—in the control of platelet function during hemostasis.
Shear-induced platelet aggregation requires initial VWF binding to platelet GPIbα and subsequent αIIbβ3activation.25 The fact that shear stress triggered potent aggregation of transgenic platelets whereas wild-type platelets hardly aggregated supports an additional role for the P2X1 ion channel in this process. Because the sustained elevation of cytosolic Ca2+ occurs in association with shear-induced platelet aggregation, possibly as the consequence of a rapid transmembrane ion flux,28 the contribution of P2X1-mediated Ca2+ influx to platelet activation can be hypothesized. This would be in analogy to the shear stress–activated Ca2+ influx into human endothelial cells through another member of the P2X family, P2X4.29 The authors proposed that P2X4 has a “shear-transducer” property through which shear stress is perceived directly or indirectly (shear-induced ATP release) and is transmitted to the cell through Ca2+ signaling. Further analyses of transgenic platelet responses to shear stress will enable us to determine whether P2X1 represents a platelet shear-transducer acting in conjunction with GPIb.
Where rapid blood flow creates high wall shear rates, such as in arterioles in the healthy circulation or in atherosclerotic arteries with restricted lumen, platelet thrombus formation depends on VWF immobilized on extracellular matrix components, in particular collagens.30 Compatible with the enhanced reactivity of transgenic platelets to collagen and shear stress, perfusion of whole blood from transgenic mice over a collagen surface at a shear rate of 1000 seconds−1 resulted in greatly enhanced aggregate formation and surface exposure of negatively charged phosphatidylserine, which is instrumental in coagulation activation.31 These platelet responses were inhibited by saratin, which blocks the binding of VWF to collagen,19 indicating enhancement of an event requiring collagen-bound VWF. Furthermore, consistent with the existence of the P2X1-ERK2 pathway, transgenic platelet aggregation on such a surface was significantly reduced after inhibition of the ERK2 pathway with U0126, possibly because of reduced secretion.
In vitro, because of the rapid desensitization of P2X1 by spontaneously released ATP, a high concentration of apyrase is often required to demonstrate this ion channel function.12-14 In addition, in the present experiments with isolated mouse platelets, apyrase was needed to detect αβ-meATP–induced Ca2+influx and platelet shape change. Using this ectonucleotidase leads to the simultaneous degradation of actively secreted ATP, the major agonist of P2X1, but also of ADP, acting at P2Y1 and P2Y12. Therefore, the use of apyrase for in vitro studies always constitutes a compromise between receptor protection and ligand breakdown. In vivo experiments in the transgenic mouse model, where endogenous ecto-ATPases prevent P2X1desensitization, essentially confirm the conclusions of our in vitro analyses of this ion channel function and thus demonstrate a physiologic role for P2X1 in platelets. This implies that, in vivo, mechanisms relying on fine-tuning ATPases32 must operate to control P2X1 activity (desensitization and resensitization). The importance of these mechanisms for platelet function has been highlighted in mice lacking the endothelial ecto-nucleoside triphosphate diphosphohydrolase CD39 (NTPDase 1). Indeed, these mice showed disordered hemostasis and thromboregulation.33
P2X1 knock-out mice display male infertility resulting from reduced neurogenic vas deferens contraction.34 In agreement with our study, preliminary data recently presented indicate impaired in vitro platelet aggregation induced by low doses of collagen.35 Furthermore, the perfusion of whole blood from these mice over a collagen surface revealed reduced aggregate formation under flow conditions.35
Finally, because P2X1 overexpression in platelets generates a prothrombotic tendency, we can speculate that pathologic deregulation of P2X1 expression may have a significant impact on platelet activation and may contribute to abnormal thrombosis. It is noteworthy that, in other tissues, the up-regulation of P2X1 mRNA levels has been described in pathophysiologic conditions (reviewed by Burnstock4). Thus, P2X1, as the predominant P2 receptor subtype in bladder smooth muscle, showed a considerably increased expression in the symptomatically obstructed bladder. The up-regulation of P2X1 mRNA in the hearts of rats with congestive heart failure has been reported.
Overall, the present study provides evidence that the P2X1ion channel plays a role in mediating the biologic effects of ATP during platelet activation. Platelet overexpression of P2X1resulted in increased platelet secretion and aggregation triggered by collagen and thromboxane A2 but also enhanced platelet responses under shear stress. A novel physiologic role of the P2X1-ERK2 signaling pathway in hemostasis and thrombosis is also proposed.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-10-3215.
Supported by the bilateral scientific and technological cooperation between Flanders and Hungary (BIL00/12) and from Fonds voor Wetenschappelijk Onderzoek (FWO) project G.0227.03. C.O. is holder of a postdoctoral research mandate of the FWO. E.T.Z. is recipient of a doctoral KULeuven scholarship. J.V. is holder of the Aventis Chair of Hemostasis Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marc Hoylaerts, Center for Molecular and Vascular Biology, University of Leuven, Herestraat 49, B-3000 Leuven, Belgium; e-mail:marc.hoylaerts@med.kuleuven.ac.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal