Abstract
The prognostic value of histologic classification and single histomorphologic parameters in Hodgkin disease has been widely debated in the literature. Whereas several former studies identified single parameters to be of clinical relevance, some recent reports have doubted the prognostic value of histology using modern treatment. Grading of the largest histologic category of Hodgkin disease, nodular sclerosis (NS), has been controversially discussed concerning clinical relevance. In this study, 965 cases of NS were reviewed to assess 9 histomorphologic parameters. The histologic results were correlated with laboratory and clinical findings and with overall survival and disease-free survival. Based on these results, a new grading of the NS category was established. The new grading, based on the 3 criteria eosinophilia, lymphocyte depletion, and atypia of the Hodgkin/Reed-Sternberg cells, was a significant indicator of prognosis in intermediate and advanced stages. Patients investigated in this study represent an outstanding collection because all of them were enrolled in the prospective multicenter clinical trial of the German Hodgkin Lymphoma Study Group. All of them had been staged uniformly according to the Ann Arbor system and had received stage-adapted modern treatment according to multimodality protocols. A subtle analysis of histology could represent a possible way to identify patients with a significantly better or worse prognosis. This new grading should help to avoid overtreatment to reduce severe therapy-related side effects such as acute toxicity and chronic sequelae such as cardiopulmonary complications and secondary neoplasias.
Introduction
Various approaches in the histologic classification of Hodgkin disease (HD) have been developed since 1944 to predict prognosis on a histomorphologic basis. Because of the heterogeneity in clinical as well as histologic appearance, prognostic factors have always been a subject of interest in HD. Although HD is now generally considered to have a favorable outcome with modern therapeutic strategies, it remains important for pathologists and oncologists to establish reliable prognostic factors. In particular, distinguishing a low-risk group of patients might allow use of less aggressive treatment and thus reduce toxic side effects and late complications such as acute toxicity, chronic sequelae such as cardiopulmonary complications, and secondary neoplasias.
Among the histologic categories, nodular sclerosis (NS) was most commonly recognized in many early studies.1-6 This category was still very heterogeneous in its histologic appearance as well as patient survival.7,8 In a large study of the British National Lymphoma Investigation (BNLI), division of the cases into NS grade 1 (low-grade, NS1) and NS grade 2 (high-grade, NS2) showed statistically significant differences in overall survival, complete response rates, and disease-free survival.8 The former study included the degree of cytologic atypia as a criterion for distinguishing high- and low-grade NS in addition to the relative amounts of lymphocytes and Hodgkin/Reed-Sternberg (H/RS) cells.
The German Hodgkin Lymphoma Study Group (GHSG) also showed significant differences in clinical outcome between NS1 and NS2 treated according to modern, multimodality protocols9 10 and classified according to the British system. This significance was based on the differences in outcome in advanced stages of the disease only. No difference in clinical outcome could be observed between NS1 and NS2 in early and intermediate stages.
Grading of NS has been widely debated in the literature. Although some reports supported the British grading system,9-14significant differences in survival could not be confirmed by several other groups.15-18 Furthermore, tissue eosinophilia, which has recently been shown to be an important independent prognostic factor in NS, is not included in the British grading system.19
A useful grading of the NS category is desirable because it might be helpful in the development of risk-adapted therapies. Therefore, 9 single histomorphologic features and their clinical relevance were retrospectively evaluated in a large number of NS cases of the GHSG. The results were compared with the disputed BNLI grading system that was established with patients treated almost 30 years ago. The single components of the BNLI grading were thus re-evaluated in the context of optimal modern treatment. The aim was either to confirm the criteria or to propose a new grading of NS with risk factors adapted to new treatment and easier application for pathologists.
Patients and methods
All patients in this study (n = 965) belong to the second study generation (HD4-6) of the GHSG, which is a set of 3 parallel multicenter prospective clinical trials for early, intermediate, and advanced stages, respectively. They were recruited from throughout Germany between 1988 and 1993, staged uniformly according to the Ann Arbor criteria, and had received stage-adapted modern treatment according to standardized protocols. The patients of the study were aged 15 to 75 years at diagnosis.20,21 Detailed information about staging and treatment has been previously reported.19 Follow-up of up to nearly 10 years (range, 0-111 months; median, 70 months) with detailed clinical information was available for the evaluation of progression-free survival (PFS) and overall survival (OS).
From the study files in Hannover, Germany, all confirmed HD cases with the histologic subtype NS (n = 965) were included for review in the current study. Among the 965 NS cases reviewed in the current study, complete clinical data were available for 843 (87%). The retrospective histologic classification had been previously performed by the pathology review panel (A.G., R.F., K.H., and M.L.H.) without knowledge about patient outcome, always with a consensus decision.22 The diagnoses were supported by immunohistochemistry in the large majority of cases.23Glass slides of the involved lymph nodes were reviewed by 2 of the authors (S.v.W. and R.v.W.) to evaluate 9 single morphologic parameters without receiving any other clinical data. The biopsies of the diagnostic lymph nodes of all patients had always been collected prior to any therapy. The slides were each stained with hematoxylin and eosin and silver impregnation and examined by light microscopy. Nine different features that had been previously described in the literature were analyzed and semiquantitatively graded, similar to former studies.1,4,8,14,15,17 24-26 The following 9 parameters were evaluated in the present study: sclerotic thickening of the lymph node capsule, sclerotic bands within the lymph node, partial or total involvement of the lymph node by neoplastic and inflammatory cells, cohesive clusters or sheets of the H/RS cells, tissue infiltration by neutrophils, coagulative necrosis, morphologic atypia of the H/RS cells, the relative amount of lymphocytes, and tissue infiltration by eosinophils. Further details are shown in Table1.
Significance of the histologic parameters in NS by univariate analysis
| Histomorphologic parameter . | OS, P . | PFS, P . |
|---|---|---|
| Sclerotic thickening of the lymph node capsule* | ≥ .2 | ≥ .2 |
| Sclerotic bands within the lymph node† | .13 | ≥ .2 |
| Partial or total involvement of the lymph node by the neoplastic and inflammatory cells‡ | ≥ .2 | ≥ .2 |
| Cohesive clusters or sheets of the H/RS cells1-153 | .10 | ≥ .2 |
| Tissue infiltration by neutrophils1-155 | ≥ .2 | ≥ .2 |
| Presence of coagulative necrosis1-154 | .021-161 | ≥ .2 |
| Morphologic atypia of the H/RS cells# | .13 | .0081-161 |
| Relative amount of lymphocytes1-160 | .0031-161 | .071-161 |
| Tissue infiltration by eosinophils1-164 | ≤ .00011-161 | .0021-161 |
| Histomorphologic parameter . | OS, P . | PFS, P . |
|---|---|---|
| Sclerotic thickening of the lymph node capsule* | ≥ .2 | ≥ .2 |
| Sclerotic bands within the lymph node† | .13 | ≥ .2 |
| Partial or total involvement of the lymph node by the neoplastic and inflammatory cells‡ | ≥ .2 | ≥ .2 |
| Cohesive clusters or sheets of the H/RS cells1-153 | .10 | ≥ .2 |
| Tissue infiltration by neutrophils1-155 | ≥ .2 | ≥ .2 |
| Presence of coagulative necrosis1-154 | .021-161 | ≥ .2 |
| Morphologic atypia of the H/RS cells# | .13 | .0081-161 |
| Relative amount of lymphocytes1-160 | .0031-161 | .071-161 |
| Tissue infiltration by eosinophils1-164 | ≤ .00011-161 | .0021-161 |
Less than versus more than 1 mm in diameter on the microscopic glass slide.
Less than versus more than 50% of the lymph node tissue.
Total involvement versus focal residual secondary follicles.
No syncytia or small clusters versus syncytia (sheets of > 20 cohesive H/RS cells).
No or few neutrophils versus neutrophils approximately more than 15% of all cells.
No or fewer than 3 small foci of necrosis versus large areas of necrosis.
#Typical H/RS cells and variants versus numerous atypical cells (> 25% bizarre and highly anaplastic-appearing H/RS cells with pleomorphic nuclear features, hyperchromatism, and highly irregular nuclear outlines, according to MacLennan et al8).
Lymphocytes more versus less than 33% of all cells in the whole section.
Eosinophilia (> 5% of all cells or clusters in at least 5 high-power fields) versus no eosinophilia, according to von Wasielewski et al.19
Significant and borderline significant P values(P < .10).
The primary end point used in the present, retrospective investigation was PFS, counting early progression, failure to attain complete remission following first-line protocol therapy, relapse, and death due to HD as events. The secondary end point was OS, counting death from any cause as the event or, if no event was documented, until date of last information.
Data analysis began with univariate Kaplan-Meier analyses27 of the effect on PFS and OS of each histopathologic factor separately. By graphical inspection of the results, multicategory factors were converted into dichotomous factors by merging those categories showing similar PFS rates. Further analyses of the resulting dichotomous factors were stratified according to enrollment in the 3 parallel trials (HD4, HD5, HD6). The results of the univariate analyses were used to select histopathologic factors for multivariate analysis of PFS and OS using the Cox proportional hazards regression model.28 The Cox model was likewise stratified according to trial and also included the histopathologic factor CD15 positivity23 and the clinical factors stage, B symptoms, and the International Prognostic Factor Project (IPFP) score.29
First, clinical factors and CD15 positivity were selected by backward elimination (omitting morphologic factors), considering both end points PFS and OS together. Second, morphologic factors were investigated by adding them one at a time to the previously selected factors and recording their significance with respect to both PFS and OS. A subset of those morphologic factors that appeared to be prognostically relevant was chosen informally. Tentative prognostic scores were constructed using all plausible subsets of this initial subset. These tentative scores were then compared by estimating the hazard ratio (HR) for patients with at least one adverse factor compared with those having no adverse factors. The HR measures the degree of separation of prognostically poor from prognostically good cases.
Among the 9 histomorphologic parameters that were evaluated in the current study, syncytia and atypia were regarded as indicating a putative borderline between HD and non-Hodgkin lymphomas. Cases exhibiting these features were immunophenotyped by a large panel of antibodies to either ascertain the diagnosis of HD or exclude the case from the study (CD15, CD20, CD79a, CD30, CD3, CD43, leukocyte common antigen [LCA], epithelial membrane antigen [EMA], vimentin [VIM], ALK-1).
Results
Altogether, 965 NS cases were evaluated in this study. Among them, 256 cases showed unusual histologic features (ie, syncytia or atypical morphology of the H/RS cells and variants). In 135 of these cases, unstained slides or paraffin blocks were still available and they were stained with a broad panel of 10 immunohistochemical markers to either confirm the diagnosis of HD or exclude the case from the study (Table2). Of all cases examined, only one was excluded from the study because of an ALK-1 positivity that was weak and questionable, but still indicated a possible case of anaplastic large cell lymphoma. None of the remaining cases were excluded because all constellations of markers were fully compatible with HD. None of the cases showed positivity for CD45, CD43, or CD3, which could have indicated non-Hodgkin lymphoma. One case did not show CD 30 positivity, but was negative for all other markers except vimentin. Because none of the markers indicating non-Hodgkin lymphoma was positive, the case was not excluded from the study. Results of the immunohistochemical staining (positivity or negativity of the H/RS cells) are shown in Table 2. The exclusion of only one of a total of 965 cases of NS HD indicates that the panel diagnosis was reproducible. All further results are demonstrated for the remaining 964 cases.
Results of the immunohistochemical examination
| Marker . | Positivity of H/RS cells, % of cases . |
|---|---|
| CD15 | 82 |
| CD30 | 99 |
| CD20 | 10 |
| CD79a | 3 |
| CD3 | 0 |
| CD43 | 0 |
| CD45 | 0 |
| ALK-1 | 1 |
| EMA | 17 |
| Vim | 93 |
| Marker . | Positivity of H/RS cells, % of cases . |
|---|---|
| CD15 | 82 |
| CD30 | 99 |
| CD20 | 10 |
| CD79a | 3 |
| CD3 | 0 |
| CD43 | 0 |
| CD45 | 0 |
| ALK-1 | 1 |
| EMA | 17 |
| Vim | 93 |
Subdivision of NS according to the BNLI system showed 79.4% NS1 and 20.6% NS2 cases in this study. Comparison of NS1 versus NS2 showed a significant difference in OS (P = .003) and PFS (P = .005).
In the univariate analysis of the 9 histomorphologic criteria, the following parameters showed no significant association with PFS or OS: sclerotic capsule thickening (17.4% of cases; PFS, P≥ .2; OS, P ≥ .2), sclerotic bands within the lymph node (10.4% of cases; PFS, P ≥ .2; OS,P = .13), total lymph node involvement (79.4% of cases; PFS, P ≥ .2; OS, P ≥ .2), sheets of H/RS cell variants (14.4% of cases; PFS, P ≥ .2; OS,P = 0.1), and tissue neutrophilia (6.7% of cases; PFS,P ≥ .2; OS, P ≥ .2).
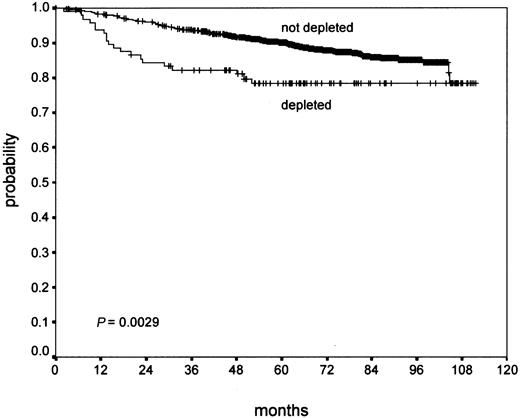
Confluent necrosis (19.3% of cases) showed no significant association with PFS (P ≥ .2), but a worse OS (P = .02) in univariate analysis. Atypia of the H/RS cells and variants (11.1% of cases) did not show a significant association with OS (P = .13). However, it showed a significant effect on PFS (P = .008). Lymphocyte depletion (9.3% of cases) showed a negative trend in PFS (P = .07) without statistical significance, but a significant negative effect on OS (P = .003; Figure 1). Tissue eosinophilia (43.0% of cases) showed a significant effect of PFS (P = .002) and a highly significant association with OS (P < .0001; Figure 2).
In brief, there was no evidence that the parameters capsule, sclerosis, degree of involvement, syncytia, and neutrophilia were associated with outcome. Necrosis was significantly associated with poor OS only. Atypia showed a negative trend for OS without statistical significance, but a significant effect on PFS. Lymphocyte depletion showed a significant effect on OS and a negative trend for PFS. Only the parameter eosinophilia showed highly significant results for PFS and OS (Table 1).
Therefore, the 4 morphologic factors eosinophilia, atypia, lymphocyte depletion, and necrosis were selected as the initial subset of apparently multivariately relevant parameters. Table3 displays the HRs with respect to both PFS and OS for Cox models based on all those subsets of this initial subset that contain (at least) the strongly prognostic parameter eosinophilia. These results suggest that the best prognostic score, that is, the score with the highest HR, should contain the following factors: eosinophilia (approximately > 5% of all cells or clusters in at least 5 high-power fields; atypia (> 25% bizarre and highly anaplastic-appearing H/RS cells with pleomorphic nuclear features, hyperchromatism, and highly irregular nuclear outlines); and lymphocyte depletion (< 33% of all cells in the whole section).
Stratified Cox model of the relevant morphologic factors together with clinical factors (stage, B symptoms, IPS)
| Factors in score . | PFS . | OS . | ||
|---|---|---|---|---|
| HR . | CI . | HR . | CI . | |
| EOS + LYM + MOR + NEC | 1.60 | 1.18-2.17 | 2.70 | 1.70-4.29 |
| EOS + MOR + NEC | 1.60 | 1.18-2.16 | 2.76 | 1.75-4.35 |
| EOS + LYM + NEC | 1.48 | 1.10-2.00 | 2.53 | 1.63-3.92 |
| EOS + LYM + MOR3-150 | 1.73 | 1.29-2.32 | 2.83 | 1.83-4.37 |
| EOS + MOR | 1.71 | 1.28-2.30 | 2.81 | 1.84-4.29 |
| EOS + LYM | 1.62 | 1.21-2.16 | 2.77 | 1.83-4.18 |
| EOS + NEC | 1.44 | 1.08-1.93 | 2.50 | 1.63-3.84 |
| EOS | 1.57 | 1.18-2.09 | 2.78 | 1.86-4.16 |
| Factors in score . | PFS . | OS . | ||
|---|---|---|---|---|
| HR . | CI . | HR . | CI . | |
| EOS + LYM + MOR + NEC | 1.60 | 1.18-2.17 | 2.70 | 1.70-4.29 |
| EOS + MOR + NEC | 1.60 | 1.18-2.16 | 2.76 | 1.75-4.35 |
| EOS + LYM + NEC | 1.48 | 1.10-2.00 | 2.53 | 1.63-3.92 |
| EOS + LYM + MOR3-150 | 1.73 | 1.29-2.32 | 2.83 | 1.83-4.37 |
| EOS + MOR | 1.71 | 1.28-2.30 | 2.81 | 1.84-4.29 |
| EOS + LYM | 1.62 | 1.21-2.16 | 2.77 | 1.83-4.18 |
| EOS + NEC | 1.44 | 1.08-1.93 | 2.50 | 1.63-3.84 |
| EOS | 1.57 | 1.18-2.09 | 2.78 | 1.86-4.16 |
Hazard ratios for the division of patients into 2 groups based on various prognostic scores. For each score, the patients are divided into those having at least one of the adverse factors included (listed in the first column) versus those having none of these factors. Hazard ratios are based on a Cox proportional hazards model including the score in question together with the clinical cofactors stage, B symptoms, and the IPFP prognostic score (IPS). Large hazard ratios imply greater prognostic separation between the 2 groups. EOS indicates eosinophilia; LYM, lymphocyte depletion; MOR, morphologic atypia; NEC, necrosis.
3-factor model separating best patients doing well and doing poorly.
Alternatively, a 2-factor model would include only eosinophilia and atypia, but omit the factor lymphocyte depletion, the differences between both models being small. These 2 models share the greatest HRs regarding both PFS and OS. They achieve markedly greater separation of prognostically poor and good patients than achieved with eosinophilia alone. We recommend the 3-factor score because the HR is slightly larger and the factor lymphocytopenia is easy to evaluate using our simplified approach (detailed results are shown in Table 3). Cases showing none of these 3 factors were graded as NS-low risk (NS-LR) and each case showing one or more of the 3 factors was graded as NS-high risk (NS-HR). Univariate Kaplan-Meier analyses for the 3-factor score are shown in Figures 3 and4.
Altogether, 480 of 964 NS cases showed at least one of the 3 risk factors (50%) and were recruited for the NS-HR category. Among the NS-HR cases, 78% showed only one risk factor, 19% showed 2 risk factors, and 3% showed all 3 risk factors. No differences in clinical course were detectable between cases with only one versus cases with 2 or 3 risk factors (data not shown). Therefore, they were grouped together for further analysis. Of NS-HR cases, 85% showed tissue eosinophilia that has recently been shown to be the strongest histologic prognostic factor in univariate and multivariate analysis,19 but 15% of cases contributed to the NS-HR category without presence of tissue eosinophilia.
Thus, the large NS category was divided almost exactly into halves, 50% belonging to the NS-LR and 50% belonging to the NS-HR group.
Among the 843 patients with complete clinical data, there were no significant differences in the age, sex, and stage distributions between NS-LR and NS-HR or the 2 grading systems. Among the other risk factors tested, there were no significant differences between NS-LR and NS-HR. A correlation of the new grading with clinicopathologic factors showed a significant linear association with the International Prognostic Score (IPS; P = .017) as the only factor. There was a trend that NS-HR is associated with an increased erythrocyte sedimentation rate (ESR; P = .061) and large mediastinal tumor (P = .089), but both were not significant. Unlike the BNLI grading, there was no difference in the frequency of low hemoglobin in the new grading (Table4).
Patient characteristics in the two NS grading systems
| Clinical feature . | BNLI grading . | New GHSG grading . | ||
|---|---|---|---|---|
| NS1 (n = 674) . | NS2 (n = 174) . | NS-LR (n = 420) . | NS-HR (n = 423) . | |
| % . | % . | % . | % . | |
| Age, y | ||||
| Younger than 20 | 12 | 9 | 12 | 11 |
| 20-29 | 40 | 39 | 38 | 43 |
| 30-39 | 25 | 24 | 28 | 22 |
| 40-49 | 11 | 17 | 12 | 12 |
| 50-59 | 8 | 7 | 7 | 8 |
| 60 and older | 3 | 5 | 3 | 4 |
| Sex | ||||
| Male | 47 | 49 | 45 | 49 |
| Female | 53 | 51 | 55 | 51 |
| Stage | ||||
| I | 8 | 11 | 11 | 7 |
| II | 49 | 47 | 47 | 50 |
| III | 28 | 27 | 29 | 28 |
| IV | 14 | 16 | 13 | 15 |
| B symptoms | 42 | 49 | 41 | 45 |
| Mediastinal4-150 | 23 | 30 | 22 | 27 |
| Extranodal4-151 | 23 | 29 | 23 | 26 |
| High ESR‡ | 53 | 58 | 51 | 58 |
| Low hemoglobin value4-153, g/dL | 13 | 27 | 15 | 15 |
| IPS29 | ||||
| 0 | 23 | 17 | 25 | 19 |
| 1 | 36 | 32 | 36 | 34 |
| 2 | 23 | 25 | 22 | 25 |
| 3 | 12 | 16 | 11 | 14 |
| 4 | 4 | 9 | 5 | 5 |
| 5 | 1 | 2 | 1 | 2 |
| 6 | 0 | 1 | 0 | 0 |
| CR | 90 | 80 | 92 | 84 |
| PR | 4 | 2 | 3 | 3 |
| PRO | 6 | 18 | 4 | 12 |
| Relapse | 21 | 29 | 17 | 28 |
| Clinical feature . | BNLI grading . | New GHSG grading . | ||
|---|---|---|---|---|
| NS1 (n = 674) . | NS2 (n = 174) . | NS-LR (n = 420) . | NS-HR (n = 423) . | |
| % . | % . | % . | % . | |
| Age, y | ||||
| Younger than 20 | 12 | 9 | 12 | 11 |
| 20-29 | 40 | 39 | 38 | 43 |
| 30-39 | 25 | 24 | 28 | 22 |
| 40-49 | 11 | 17 | 12 | 12 |
| 50-59 | 8 | 7 | 7 | 8 |
| 60 and older | 3 | 5 | 3 | 4 |
| Sex | ||||
| Male | 47 | 49 | 45 | 49 |
| Female | 53 | 51 | 55 | 51 |
| Stage | ||||
| I | 8 | 11 | 11 | 7 |
| II | 49 | 47 | 47 | 50 |
| III | 28 | 27 | 29 | 28 |
| IV | 14 | 16 | 13 | 15 |
| B symptoms | 42 | 49 | 41 | 45 |
| Mediastinal4-150 | 23 | 30 | 22 | 27 |
| Extranodal4-151 | 23 | 29 | 23 | 26 |
| High ESR‡ | 53 | 58 | 51 | 58 |
| Low hemoglobin value4-153, g/dL | 13 | 27 | 15 | 15 |
| IPS29 | ||||
| 0 | 23 | 17 | 25 | 19 |
| 1 | 36 | 32 | 36 | 34 |
| 2 | 23 | 25 | 22 | 25 |
| 3 | 12 | 16 | 11 | 14 |
| 4 | 4 | 9 | 5 | 5 |
| 5 | 1 | 2 | 1 | 2 |
| 6 | 0 | 1 | 0 | 0 |
| CR | 90 | 80 | 92 | 84 |
| PR | 4 | 2 | 3 | 3 |
| PRO | 6 | 18 | 4 | 12 |
| Relapse | 21 | 29 | 17 | 28 |
% indicates column percentage; CR, complete remission; PR, partial remission; PRO, progressive disease.
Large mediastinal tumor greater than or equal to one third of maximal transversal thoracic diameter (measured in a sagittal x-ray film).
Extranodal involvement.
Erythrocyte sedimentation rate 50 mm/h or higher without B symptoms or 30 mm/h or higher with B symptoms.
Hemoglobin 12 g/dL or less for men and 10.5 g/dL or less for women.
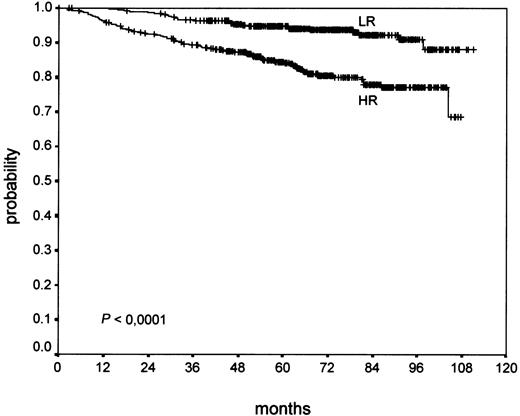
Univariate analysis of the new grading showed a highly significant correlation of NS-HR with a worse OS (P < .0001; Figure3) and PFS (P = .0003), which was clearer compared with the BNLI grading of the same patients.
An evaluation of OS and PFS separately in early (HD4), intermediate (HD5), and advanced (HD6) stages, respectively, was performed. It revealed no significant influence on outcome in early stages (PFS,P = .52; OS, P = .11), but a highly significant influence of the new grading in intermediate stages (PFS,P < .0001; OS, P < .0001) and a significant influence on outcome in advanced stages (PFS, P = .04; OS,P = .003).
In a multivariate analysis, the following factors were included in the Cox proportional hazards model: IPS,29 stage, B symptoms, CD15 negativity, BNLI grading, and new NS grading. Significant factors identified in a forward selection were the IPS (PFS,P = .024; OS, P = .016), CD15 negativity (PFS, P = .080; OS, P = .061), and the new NS grading (PFS, P = .005; OS, P < .001). The BNLI grading did not show significant results (PFS, OS,P > .2) in this multivariate model including the new NS grading.
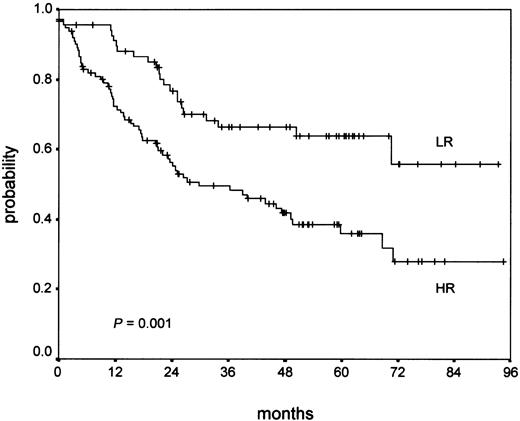
Additionally, an analysis of OS of NS-LR and NS-HR after therapy failure (relapse or early progression) was performed. From the 843 NS patients with clinical data, 192 patients showed therapy failure (23%). For 185 patients, follow-up after salvage therapy was available (95%). Salvage therapy depended on the first-line stage and treatment. For early-stage patients (HD4), conventional chemotherapy, chiefly COPP/ABVD (cyclophosphamide, vincristine [Oncovin], procarbazine, prednisone/doxorubicin, bleomycin, vinblastine, dacarbazine) or BEACOPP (bleomycin, etoposide, doxorubicin [Adriamycin], cyclophosphamide, vincristine [Oncovin], procarbazine, prednisone), was given at relapse in most cases. For intermediate and advanced stage patients (HD5 and HD6), therapy at relapse consisted of conventional salvage chemotherapy such as Dexa-BEAM (dexamethasone, cyclophosphamide/prednisone [BCNU], etoposide, cyctosine arabinoside [ara-C], melphalan; about 40%), radiotherapy (about 15%) and high-dose chemotherapy with stem cell transplantation (about 45%). Most patients suffering early progression of disease received Dexa-BEAM or similar salvage chemotherapy, followed by high-dose chemotherapy with stem cell transplantation in about one third of cases, although a minority received curative radiotherapy only. From the 185 patients with follow-up after salvage therapy, 38% belonged to the NS-LR group and 62% to the NS-HR group. Whereas in the NS-LR group 33% died (23 of 70), death was recorded for 55% (63 of 115) in the NS-HR group. This difference was statistically significant in the Kaplan-Meier analysis (P = .001) as is shown in Figure 4.
The numbers of NS1 and NS2 cases (British grading) contributing to the categories of the new grading are demonstrated in Table5. Of former NS2 cases, 75% contributed to the high-risk category, whereas 25% of former NS2 cases contributed to the low-risk category. Fifty-six percent of former NS1 cases became low-risk and 44% became high-risk cases. The new low-risk category (NS-LR) consists of 90% former NS1 and 10% former NS2 cases. The new high-risk category (NS-HR) consists of 69% former NS1 and 31% former NS2 cases.
Comparison of cases in the new NS grading with BNLI grading (n = 943)
| . | NS1 cases (BNLI) . | NS2 cases (BNLI) . | All cases . |
|---|---|---|---|
| NS-LR cases (new) | 417 (56%) | 48 (25%) | 465 |
| NS-HR cases (new) | 331 (44%) | 147 (75%) | 468 |
| All cases | 748 (100%) | 195 (100%) | 943 |
| . | NS1 cases (BNLI) . | NS2 cases (BNLI) . | All cases . |
|---|---|---|---|
| NS-LR cases (new) | 417 (56%) | 48 (25%) | 465 |
| NS-HR cases (new) | 331 (44%) | 147 (75%) | 468 |
| All cases | 748 (100%) | 195 (100%) | 943 |
Both the BNLI grading and the new grading are significantly associated with each other (χ2 test, 2-sided,P < .0001).
Discussion
NS is the category most commonly recognized in many early studies on HD in Western countries.1-6 Although NS is a well-defined category, it is still very heterogeneous in its histologic appearance and survival.8 The aim of the current study was to distinguish patients with a favorable prognosis who might be treated less aggressively thus developing less acute and late toxic complications such as second malignancies, chronic fatigue, or fertility problems30-33 from patients with a poor prognosis who may benefit from aggressive treatment.
Several inconclusive attempts have been made to correlate pathologic features with prognosis in NS. Parameters evaluated in those studies included the numbers of lymphocytes in relation to the neoplastic cells, the degree of fibrosis, and cytologic atypia of the malignant cells. Some studies grouped single parameters to establish a grading system, but most studies could not prove a statistically significant correlation. Overall, lymphocyte depletion at least tended toward a poor outcome in the majority of those studies.1,4,7,24-26 34-37
The most profound approach to distinguish risk groups has been suggested by the BNLI, which divides the cases into NS grade 1 (low-grade, NS1) and NS grade 2 (high-grade, NS2). Adapted from MacLennan et al,8 lymph nodes with nodular sclerosing HD were classified as grade 2, if: (1) more than 25% of the cellular nodules showed reticular or pleomorphic lymphocyte depletion, or (2) the majority of the cellular nodules (> 80%) showed the fibrohistiocytic variant of lymphocyte depletion, or (3) more than 25% of the nodules contained numerous bizarre and highly anaplastic-appearing Hodgkin cells without depletion of lymphocytes. These cells possessed large, hyperchromatic nuclei with prominent nucleoli and differed from the elongated, folded, and hyperlobated nuclei with small nucleoli.38
All cases that did not fulfill the criteria mentioned were classified as NS1. Patients with NS2 had a statistically significant worse clinical outcome, regardless of stage.8 When grading according to this scheme, the cases of the GHSG also showed significant differences in clinical outcome between NS1 and NS2, but a subanalysis revealed that this significance was based on the differences in outcome in advanced stages of the disease only, that is, 30% of the patients. No difference in clinical outcome was observed between NS1 and NS2 in early and intermediate stages.9
The BNLI grading is still not fully accepted, is controversial, and has been debated in the literature. Although some reports supported the British grading system,8,11-14 significant prognostic differences could not be confirmed by several other groups.15-18 However, the findings in these studies applying the BNLI grading system require comment.
A study by d'Amore et al15 did not find a significant prognostic value of grading NS with regard to OS. The latter study included only a limited number of patients (n = 123), excluded advanced stages, and treatment was not uniform. In another report by Hess et al,17 no significant difference of the grading could be found for OS and PFS. This study did not include patients with stage IV disease and contained only one case of NS2 with stage IIIB. Therefore, these studies supported the results of the GHSG concerning early and intermediate stages and did not contradict the results for advanced stages of NS2.9
In a study by Masih et al,16 no significant results of the BNLI grading were found, but this study included only 42 patients, all at advanced stages. More than 10% had received radiation prior to the investigation. The grading of this study showed a distribution of 31% NS1 and 69% NS2 cases. This selection of patients does therefore not seem comparable with a general distribution in developed countries and is furthermore too small to draw any substantial conclusions. The unusual distribution of NS1 and NS2 might be due to a differently interpreted use of the grading criteria or due to histologic alterations after previous treatment.
Van Spronsen et al18 claimed that significant differences between NS1 and NS2 in the period 1972-1980 disappeared in the period 1981-1992 due to more intensive treatment. This study included 109 patients in the latter period who received nonstandardized treatment. Moreover, NS2 patients were treated more intensively. Thus, this study presents a distorted picture regarding the comparison of clinical outcome.
Two studies by Wijlhuizen et al13 and Ferry et al14 showed a significant effect of the BNLI grading on OS. This corresponds to the results of the GHSG. However, these studies lack a subanalysis of the impact of NS grading in the different stages of disease, probably due to the limited numbers of patients.
In summary, a detailed analysis of published studies on NS grading reveals no convincing data contradicting the prognostic value of grading NS for advanced stages. On the other hand, the former studies did not reveal any prognostic impact of the BNLI grading in early and intermediate stages separately. Moreover, the criteria of the BNLI grading system appear to be complicated, somewhat imprecise, and often not easy to fulfill, especially in small sections. Nodules have to be counted and compared to fulfill the criteria: “more than 25% of the cellular nodules showed lymphocyte depletion; more than 25% of the nodules contained numerous bizarre and highly anaplastic-appearing cells.”8(p1689) Nothing was reported about how to assess different sizes of nodules or only small sections. Although some studies referred to MacLennan et al8 in their description of the evaluation method,13,17 others simplified the estimation of the questionable areas.15,16The BNLI grading was not fully introduced in the Revised European American Lymphoma (REAL) classification.39 This might have been due to the relatively difficult and equivocal applicability.
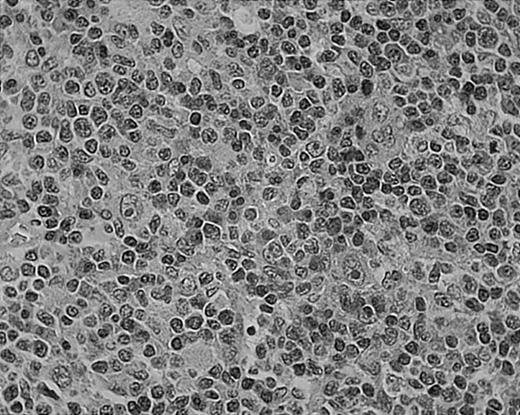
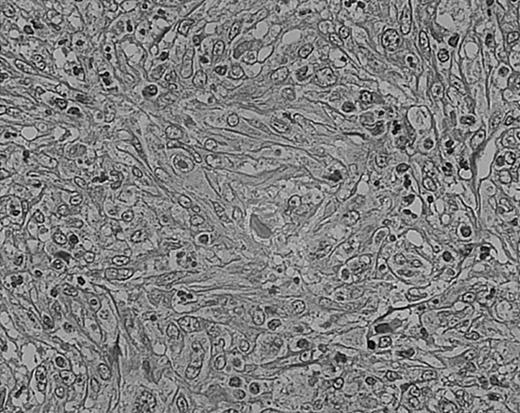
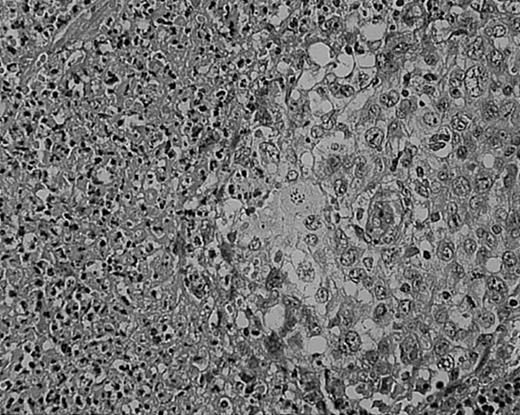
As we have previously reported, tissue eosinophilia is the strongest prognostic indicator in NS.19 Eosinophilia was not included in the BNLI criteria. We therefore propose a new grading system that includes this important risk factor and should be easier to use than the BNLI criteria. The 3 criteria of the new grading system are: eosinophilia (approximately > 5% of all cells or clusters in at least 5 high-power fields; new feature; Figure5); lymphocyte depletion (< 33% of all cells in the whole section; simplified feature of BNLI; Figure6); and atypia (> 25% of H/RS cells bizarre and highly anaplastic appearing, with pleomorphic nuclear features, hyperchromatism, and highly irregular nuclear outlines; simplified feature of BNLI; Figure 7). Cases showing none of these factors are called NS-low risk (NS-LR) and each case showing one or more of the factors is called NS-high risk (NS-HR).
Tissue eosinophilia in NS HD.
Note typical Hodgkin cells surrounded by clusters of eosinophils. Stained with hematoxylin and eosin (H&E). Original magnification, × 400.
Tissue eosinophilia in NS HD.
Note typical Hodgkin cells surrounded by clusters of eosinophils. Stained with hematoxylin and eosin (H&E). Original magnification, × 400.
Lymphocyte depletion in NS HD.
This example shows lymphocytes in less than 33% of all cells. Stained with H&E. Original magnification, × 400.
Lymphocyte depletion in NS HD.
This example shows lymphocytes in less than 33% of all cells. Stained with H&E. Original magnification, × 400.
Cellular atypia (left-side confluent necrosis) in NS HD.
This example shows more than 25% of H/RS cells presenting with bizarre and anaplastic appearances. Stained with H&E. Original magnification, × 400.
Cellular atypia (left-side confluent necrosis) in NS HD.
This example shows more than 25% of H/RS cells presenting with bizarre and anaplastic appearances. Stained with H&E. Original magnification, × 400.
The new grading showed highly significant results for PFS and OS. A separate analysis of the 3 clinical stage categories, that is, early, intermediate, and advanced stages (HD4, HD5, HD6) showed the new grading system to be prognostically relevant in intermediate and advanced stages. Therefore, the new grading carries important prognostic information for more than 80% of patients (51% intermediate and 30% advanced stages). Furthermore, the new grading showed a significant result for OS in cases after therapy failure.
Thus, we established a meaningful combination of histomorphologic parameters to find a low-risk and a high-risk group among the large number of NS patients. The new grading differentiates 50% of patients with a significantly better prognosis (NS-LR) compared with the 50% of worse prognostic outcome (NS-HR), as shown in Table 4 and Figure 3. The new grading is easy to apply and only after exclusion of all 3 risk factors (ie, eosinophilia, lymphocyte depletion, and atypia), can a case be graded NS-LR. The most frequent criterion (eosinophilia, 85% of high-risk cases) is highly reproducible.19
The new grading is a strong prognostic indicator not only for a small subset, but also for the large majority of patients with NS. These data imply that histology still plays an important role even under modern multimodality treatment.
How should these data be used for future clinical trials? Simplifying the grading of NS offers the chance for pathologists to grade every NS case reliably and we encourage other groups to confirm our grading score. A critical review of all relevant risk factors in HD seems to make sense only if based on a general acceptance of pathologists and the confirmatory results of other studies. Such revision should include both the long-established clinical factors and more recent findings of biologic and histopathologic risk factors. The purpose of this grading approach is obvious: helping to prevent patients being overtreated and thus being unnecessarily at risk of severe therapy-related side effects such as acute toxicity and chronic sequelae like cardiopulmonary complications and secondary neoplasias or fertility problems.30-33
Prepublished online as Blood First Edition Paper, January 23, 2003; DOI 10.1182/blood-2002-05-1548.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Reinhard von Wasielewski, Institut für Pathologie, Medizinische Hochschule Hannover Carl-Neuberg Str 1, D-30625 Hannover, Germany; e-mail:wasielewski.reinhard.von@mh-hannover.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal