Abstract
Rodent bone marrow cells can contribute to liver. If these findings are applicable to humans, marrow stem cells could theoretically be harvested from a patient and used to repair his/her damaged liver. To explore this potential, CD34+ or highly purified CD34+CD38−CD7− human hematopoietic stem cells from umbilical cord blood and bone marrow were transplanted into immunodeficient mice. One month after transplantation, carbon tetrachloride (CCl4) was administered into the mice to induce liver damage and hepatocyte proliferation. Mice were analyzed in comparison with CCl4-injured mice that did not receive transplants and noninjured controls that received transplants with the same stem cell populations, one month after liver damage. Human-specific albumin mRNA and protein were expressed in the mouse liver and human albumin was detected in the serum of mice that had received CCl4 injury. Human alpha-fetoprotein was never expressed, but in some mice, human cytokeratin 19 was expressed, which may indicate bile duct development in addition to the albumin-secreting hepatocyte-like cells. Human albumin was not expressed in the starting stem cell populations in injured mice that did not receive transplants nor in noninjured mice that had received transplants of human stem cells. Human albumin expression was detected only in CCl4-treated mice that received transplants of human stem cells, and recovery was increased by administration of human hepatocyte growth factor 48 hours after the CCl4-mediated liver injury. Our studies provide evidence that human “hematopoietic” stem/progenitor cell populations have the capacity to respond to the injured liver microenvironment by inducing albumin expression.
Introduction
There is an intriguing literature describing murine and rat stem cells that display plasticity. In one of the most definitive studies in the field to date, Lagasse et al reported that c-kit+ Thy-1.1 (lo) Lin− sca-1+(KTLS) stem cells, isolated from the bone marrow (BM) of adult mice, rescued the liver defect in the fumaryl acetoacetate hydrolase (FAH)−/− mouse, an animal model of tyrosinemia type I, by restoring the biochemical function of its liver. The same population of cells was also capable of radioprotection of the lethally irradiated mice.1 The c-kit+ Thy-1.1 (lo) Lin− sca-1+ (KTLS) population of murine stem cells had been shown to be radioprotective,2-4 but the elegant demonstration by Lagasse et al that the same population could also generate functional hepatocytes was highly novel.
Theise et al also demonstrated that in humans hepatocytes and cholangiocytes could be derived from bone marrow.5 They analyzed archival liver specimens from female recipients of bone marrow transplants (BMTs) from male donors, and found Y chromosome–positive hepatocytes and cholangiocytes in the female BMT recipients.5 This observation suggests that marrow-derived stem cells can generate liver in humans. There is little other data indicating that human counterparts to the rodent stem cells that have displayed plasticity exist because of a lack, so far, of appropriate in vivo models for studying human stem cells that may have the capacity to generate multiple tissues.
In the current studies, we sought to develop a model to extend the seminal reports on murine stem cell plasticity, to allow examination of the potential for highly purified human “hematopoietic” stem cells to contribute to liver regeneration in immunodeficient mice. We used both nonobese diabetic–severe combined immunodeficiency (NOD/SCID) and NOD/SCID/β2microglobulin (β2M)–null mice as the recipients for human CD34+ or CD34+/CD38−/CD7− cells purified by flow cytometry from human bone marrow and umbilical cord blood (UCB). The CD34+ stem/progenitor cell pool has been used in many human transplants over the past decade, and is known to have the capacity to regenerate all blood cell lineages. The human CD34+/CD38−/CD7− cell population is a more stringently isolated stem cell phenotype, which we have previously shown to be quiescent and to have the capacity to generate both lymphoid and myeloerythroid progeny.6
After exploring less successful approaches, we determined that human albumin and cytokeratin 19 (CK19)–expressing cells were best generated in NOD/SCID/β2M knock-out mice that had human stem cells transplanted one month prior to liver injury by 0.4 mL/kg carbon tetrachloride (CCl4). One month after the liver injury, livers were harvested from injured and noninjured mice that received stem cell transplants, and the expression of mRNA for human albumin, alpha-fetoprotein, and CK19, a marker of bile duct cells, was analyzed. Human albumin was also identified by enzyme-linked immunosorbent assay (ELISA) in the serum of the mice, and by immunohistochemistry on liver sections with documentation of human cells by in situ hybridization (ISH) for human DNA Alu sequences. Administration of human hepatocyte growth factor (HGF) after the liver injury increased the survival rate of the mice and increased the levels of human albumin mRNA that were expressed. This system provides the first model for generation of human hepatocyte-like cells from purified hematopoietic stem cells from human bone marrow and umbilical cord blood.
Materials and methods
Isolation of human hematopoietic progenitors
Normal human bone marrow (BM) cells were obtained from screens used to filter marrow during harvest of allogeneic donors. Umbilical cord blood (UCB) samples were collected at Kaiser Permanente, Los Angeles, CA. Use of these samples was approved by the Committee on Clinical Investigations at Childrens Hospital of Los Angeles (CHLA). CD34+ progenitors were isolated from mononuclear cell fractions from both BM and UCB by incubation with the monoclonal antibody HPCA-1 (Becton Dickinson, San Jose, CA), followed by goat antimouse–conjugated immunomagnetic beads (Dynal, Oslo, Norway) or by sequential passes through 2 MiniMACS columns as directed by the manufacturer (Miltenyi, Auburn, CA). CD34+CD38−CD7− cells were isolated from human marrow by pre-enrichment of CD34+ cells using MiniMACS columns, followed by fluorescence-activated cell-sorter (FACS) acquisition using a stringent gate as described,6 to obtain a highly purified population.
Mice
Studies used 8-week-old NOD/SCID and NOD/SCID/β2M-null mice initially obtained from Len Shultz at Jackson Laboratories (Bar Harbor, ME) and bred at CHLA. Sublethal conditioning was done by administering 300 cGy 2 to 4 hours prior to injection of human cells. Either 2000 CD34+/CD38−/CD7− or 1 × 105 CD34+ cells were transplanted in each experiment. At 3 weeks after transplantation, mice were screened for human cell engraftment by detection of human CD45+cells in the peripheral blood using FACS analysis. One month after transplantation, mice that had been shown by the screening to have more than 0.5% circulating human CD45+ cells were subjected to liver damage by intraperitoneal administration of 0.4 mL/kg carbon tetrachloride (CCl4) diluted to 100 microliters with corn oil (Sigma, St Louis, MO). In some sets of the animals, 4 injections of 1.5 μg recombinant human HGF (rhHGF) was administered at 12-hour intervals, 48 to 72 hours after CCl4 treatment, to attempt to increase the human hepatocyte differentiation. In other sets of the CCl4-treated mice, 1 × 106 enhanced green fluorescent protein (EGFP)–positive human mesenchymal stem cells (MSCs) transduced with a retroviral vector carrying the HGF cDNA (MND-HGF-IRES-EGFP)7,8 were transplanted intravenously 24 hours after CCl4 treatment to provide a sustained growth factor release, as we have previously described.9-11 The vector was produced in Donald Kohn's vector core at Childrens Hospital Los Angeles, and the HGF cDNA was a kind gift from Giovanni Gaudino, Novara, Italy. Mice were killed 5 (n = 3) or 30 (n = 27) days after CCl4 treatment. Liver was analyzed by first perfusing the entire mouse with saline under anesthesia and then preparing blocks for paraffin sections and isolating individual cells from sections of liver by mincing and digestion in 0.15% collagenase in phosphate-buffered saline for 10 minutes at 37°C, followed by washing. Cells from the digested liver sections were gently spun down and floating material was discarded; then the cell pellet was immediately taken for the cytospin preparation.
Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis.
Total cellular RNA was extracted from the human-mouse chimeric hepatocytes, murine tissues, and starting populations of human cells. Samples were quantitated using a spectrophotometer, and equal amounts of RNA from all samples were subjected to cDNA synthesis using Oligo (dT) (Invitrogen, Carlsbad, CA) to prime first-strand synthesis. Human-specific albumin12 and cytokeratin 19 (CK19) primers13 were used to detect human albumin and human CK19 expression in the mouse liver. Primers were selected from 2 different exons with at least one intervening intron to rule out false signals at the expected band size from contaminating DNA strands. The albumin genomic DNA fragment would have been 2019 bp, and the ck19 genomic fragment would have been 1197 bp. PCR conditions for albumin were as follows: 94°C for 1 minute; 55°C, 1.5 minutes; and 72°C, 1.5 minutes; and for CK19: 94°C, 1 minute; 64°C, 1 minute; and 72°C, 2 minutes. PCR products were run on an agarose gel and visualized using an Eagle Eye Gel reader (Stratagene, La Jolla, CA). Product sizes were 422 bp for human albumin and 461 bp for human CK19.
In situ hybridization (ISH) and immunohistochemistry staining.
Dissociated liver cells were examined on cytospin slides by in situ hybridization (ISH) to detect human Alu sequences, with simultaneous antibody staining for human albumin protein. Human Alu ISH was carried out using the ISH diaminobenzidine (DAB) kit (Innogenex, San Romon CA), according to the manufacturer's instructions. Colorfrost/plus glass slides (Fisher Scientific, Pittsburgh, PA) were used in all studies. Hepatocyte cytospin slides were fixed in acetone-methanol (1:1) for 30 seconds. To permeabilize, slides were covered by cold ethanol for 10 minutes at −20°C. Then slides were incubated with probe at 80°C for 5 minutes, then at 37°C for 90 minutes. Alu probes end-labeled with multiple fluorescein-linker molecules were obtained from Innogenex. The Alu probe was diluted to 1:1 in a buffer provided by the company, and 20 uL of this mixture per slide was used. An antifluorescein antibody (LINK-1) was used to detect the labeled probe. To amplify the signal, LINK-2, which carries biotin and recognizes and binds to the link 1 antibody, was added. To visualize the antibody/probe complex, horseradish peroxidase–conjugated streptavidin (label) was added next. Each biotin molecule on LINK-2 binds to a streptavidin moiety of the label. Upon addition of the chromogen DAB/substrate, an intense dark brown to black color appeared at the specific site of the hybridized probe. Next, fluorescent labeling or immunohistochemical detection of human albumin was performed. Slides were rinsed in Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS, pH 7.5) 2 times for 5 minutes each. Slides were then blocked with Power Block (Innogenex) supplemented with murine immunoglobulin G (Coulter, Hialeah, FL) for 15 minutes. Rabbit antihuman albumin–fluorescein isothiocyanate (FITC; DAKO, Carpenteria, CA) was used at a 1:100 dilution to counterstain the slides for 15 minutes.
In 0.6-μM paraffinized sections, immunohistochemistry was done as described in the previous paragraph, with Vectablue chromogen (Vector Laboratories, Burlingame, CA) deposited on the slides as the final chromogen in an immunohistochemical reaction using the antialbumin antibody, in place of visualization with direct fluorescence. The reaction was done exactly as recommended by the manufacturer of the SuperStreptavidin kit (Biogenix, San Ramon, CA). Slides were finally washed 3 times with TBS and mounted with Vectashield medium (Vector Laboratories) and coverslips.
Microscopy.
Images were viewed with a Leica DMRA microscope (Wetzlar, Germany) using a Plan Apo 40 ×/1.25 NA phase 3 DIC or Plan Apo 63 ×/1.32 oil immersion objective lens. The microscope was equipped with a Sutter LS175W ozone-free xenon arc lamp (Novato, CA). Images were acquired with an Applied Spectral Imaging SkyVision-2/VDS camera from EasyFISH software (Applied Spectral Imaging, Migdal Ha'Emek, Israel) and printed using Microsoft PowerPoint (Redmond, WA).
Determination of human serum albumin in the mouse plasma.
Plasma was collected for the human serum albumin assays, and ELISA was performed according to the manufacturer's instructions (MD Biosciences, Gewerbesteuer, Zurich, Switzerland). Normal human and nontransplanted murine sera were tested in comparison with the samples in each experiment in addition to the standards included in the kit.
Statistical analyses.
Analyses were done using Correlation Analysis in the GraphPad Prism software program (San Diego, CA), the Mann-Whitney test, and descriptive statistics analysis in the Minitab Computer Program (State College, PA). Means ± SEM are listed.
Results
Induction of liver damage in immune-deficient mice
The initial challenge to studying human hematopoietic stem cell to hepatocyte differentiation was to establish a model of acute liver damage in immunodeficient mice, in which the control mice that did not undergo transplantation would recover from the injury. Hepatic injury in NOD/SCID mice by several different doses of radiation and by intraperitoneal administration of varying doses of allyl alcohol was studied first. There was good survival of the mice in all arms of the allyl alcohol studies, and NOD/SCID and NOD/SCID/β2M-null mice tolerated up to 300 RADS without significant mortality. However, no evidence of human hematopoietic stem cell to hepatocyte (or albumin-expressing cell) differentiation was observed in those studies using RT-PCR and the assays detailed in the following sections (data not shown).
Next, hepatic injury using carbon tetrachloride (CCl4) was evaluated because it is known to induce periportal lipid peroxidation and protein denaturation after administration, yet will allow endogenous liver regeneration if not given in an excess dose.14 Mice were screened for engraftment prior to induction of liver damage. When as few as 0.5% human CD45+cells were found in the peripheral blood, the mouse was considered engrafted and the CCl4 treatment proceeded. Occasionally, human cells were not detected in the circulation of the mouse, although the marrow was later found to be engrafted. These animals were not used in the current studies. Successfully engrafted NOD/SCID and NOD/SCID/β2M-null mice were given 0.4 mL/kg CCl4 by intraperitoneal administration one month after human stem cell transplantation, and survival was studied. Half of the mice were given 4 injections of 1.5 μg recombinant human HGF (rhHGF) at 12-hour intervals, 48 to 72 hours after CCl4 treatment. The effects of these treatments on survival of the mice and on organ damage were assessed.
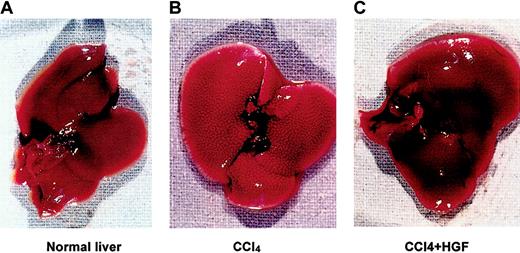
Compared with normal age-matched mouse liver (Figure1A), 0.4 mL/kg CCl4 induced massive liver damage in the form of numerous foci of periportal necrosis (Figure 1B), and 75% of the mice died within 4 weeks (n = 12). Injection of recombinant human HGF enhanced recovery of the necrotic areas in the liver (Figure 1C). None of the mice died in the initial CCl4-treated group that was given HGF treatment (n = 5). In spite of the toxicity to the non-HGF–treated mice, we chose to use this model for all subsequent studies because the first evidence of human stem cell to hepatocyte differentiation was obtained, as will be described in more detail.
Immunodeficient (NOD/SCID) mouse liver damaged by CCl4, with and without rescue by hepatocyte growth factor (HGF).
Human stem cell–engrafted mice were injected intraperitoneally with 0.4 mL/kg carbon tetrachloride (CCl4), and then treated with or without 4 intraperitoneal injections of 1.5 μg recombinant human HGF (rhHGF) at 12-hour intervals, 48-72 hours after CCl4 treatment. (A) Age-matched normal liver. (B) CCl4-treated liver. (C) Mouse liver treated with CCl4 and HGF.
Immunodeficient (NOD/SCID) mouse liver damaged by CCl4, with and without rescue by hepatocyte growth factor (HGF).
Human stem cell–engrafted mice were injected intraperitoneally with 0.4 mL/kg carbon tetrachloride (CCl4), and then treated with or without 4 intraperitoneal injections of 1.5 μg recombinant human HGF (rhHGF) at 12-hour intervals, 48-72 hours after CCl4 treatment. (A) Age-matched normal liver. (B) CCl4-treated liver. (C) Mouse liver treated with CCl4 and HGF.
Differentiation of human albumin–expressing hepatocyte-like cells in the immune-deficient mouse livers was augmented by injection of recombinant human HGF (rhHGF)
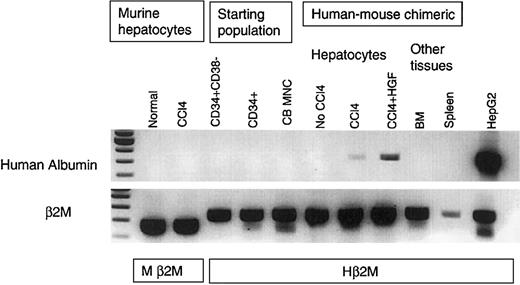
Mice were killed 2 months after transplantation with human cells and one month after liver injury using carbon tetrachloride (CCl4), with or without HGF injection. RNA samples were obtained from the livers of the mice in each group; controls that did not receive transplants versus mice that had received stem cell transplants alone, stem cell transplants with CCl4 damage, or stem cell transplants with CCl4 damage plus HGF administration. RT-PCR for human albumin was done in comparison with β2M in Oligo (dT) primed cDNA amplified from equivalent amounts of RNA isolated from each sample. In order to demonstrate the specificity of the primers to detect human albumin mRNA, we examined the mRNA in nontransplanted mouse liver and in injured (CCl4 treated) nontransplanted mouse liver. These samples were negative in every assay, and an example is shown in Figure2. Because of the phenomenon known as “stem cell priming,” in which primitive hematopoietic cells have been shown to express low levels of various mRNA before committing to a particular blood cell lineage,15 16 we also tested the human hematopoietic stem/progenitor cell starting populations for albumin expression. As shown in Figure 2, the starting stem cell populations did not express albumin when they were freshly isolated from umbilical cord blood. Equivalent levels of RNA from total cord blood mononuclear cell preparations, purified CD34+ cells, and CD34+38− cell samples were all negative for albumin expression (Figure 2). The same result was observed for stem cells isolated from human bone marrow (data not shown).
Human albumin mRNA was specifically expressed after injury in the human stem cell–engrafted NOD/SCID mouse liver.
Total cellular RNA was extracted from the human-mouse chimeric hepatocytes plus injured and noninjured controls, and Oligo (dT) was used to prime first-strand synthesis from equivalent levels of RNA. Human-specific albumin primers were used for PCR in comparison with human and murine-specific β2M, and amplifications were halted in log phase. Nontransplanted NOD/SCID mouse liver, including injured (CCl4-treated) mouse liver, did not express human albumin. There was no albumin expression in human cord blood mononuclear cells, or in the purified CD34+38−or CD34+ starting populations. There was no albumin expression in the human-mouse chimeric liver, marrow, or spleen in the absence of injury. However, in the human-mouse chimeric liver, CCl4 treatment induced human albumin expression. There was increased albumin expression after rhHGF was injected into mice that underwent stem cell transplantation that had received liver damage. HepG2 was used as positive control. Human β2M (Hβ2M) and murine β2M (Mβ2M) were internal controls for densitometry. RNA analyses were repeated 4 times, using different samples, with the same results. The first lane shows molecular weight markers.
Human albumin mRNA was specifically expressed after injury in the human stem cell–engrafted NOD/SCID mouse liver.
Total cellular RNA was extracted from the human-mouse chimeric hepatocytes plus injured and noninjured controls, and Oligo (dT) was used to prime first-strand synthesis from equivalent levels of RNA. Human-specific albumin primers were used for PCR in comparison with human and murine-specific β2M, and amplifications were halted in log phase. Nontransplanted NOD/SCID mouse liver, including injured (CCl4-treated) mouse liver, did not express human albumin. There was no albumin expression in human cord blood mononuclear cells, or in the purified CD34+38−or CD34+ starting populations. There was no albumin expression in the human-mouse chimeric liver, marrow, or spleen in the absence of injury. However, in the human-mouse chimeric liver, CCl4 treatment induced human albumin expression. There was increased albumin expression after rhHGF was injected into mice that underwent stem cell transplantation that had received liver damage. HepG2 was used as positive control. Human β2M (Hβ2M) and murine β2M (Mβ2M) were internal controls for densitometry. RNA analyses were repeated 4 times, using different samples, with the same results. The first lane shows molecular weight markers.
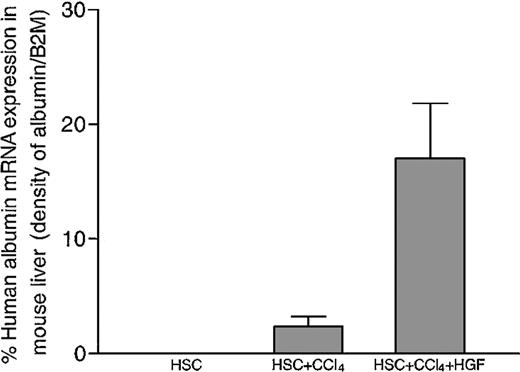
In the mice that received human stem cell transplants that were not subjected to liver injury by CCl4 injection, there was no human albumin expression. However, in mice that received human stem cell transplants that had received CCl4-mediated liver injury, a portion of the transplanted human stem cells had localized to the liver and had begun to express human albumin when analyzed one month after the liver damage (Figure 2). The human albumin mRNA levels in the livers of the mice in this group were positively correlated with the percentage of human cells in the BM (r2 = 0.8). Human alpha-fetoprotein mRNA was not detected in any sample (data not shown). In an attempt to increase the efficiency of HSC to hepatocyte differentiation, recombinant human HGF was injected into some of the mice that underwent stem cell transplantation, following CCl4 treatment. As shown in an example in Figure 2, there was an increase in human albumin expression in the HGF-treated mice. Human albumin RNA was detected specifically in the livers of the mice, but not in other tissues such as spleen and BM (Figure 2). When densitometry was performed to compare the levels of human albumin mRNA with the β2M message in each sample, an increase in albumin expression was present in the HGF-treated mice, as opposed to the CCl4-treated mice that had received the same stem cell transplant (Figure 3).
Human albumin expression was significantly increased in the livers of chimeric NOD/SCID mice treated with rhHGF after CCl4-mediated liver injury.
In human umbilical cord blood–derived stem cell–engrafted mouse liver, the densities of the bands representing human albumin expression in RT-PCR analyses were normalized against human β2M expression in the same sample. rhHGF injection enhanced the level of human albumin expression compared with CCl4 treatment alone. Error bars indicate SEM.
Human albumin expression was significantly increased in the livers of chimeric NOD/SCID mice treated with rhHGF after CCl4-mediated liver injury.
In human umbilical cord blood–derived stem cell–engrafted mouse liver, the densities of the bands representing human albumin expression in RT-PCR analyses were normalized against human β2M expression in the same sample. rhHGF injection enhanced the level of human albumin expression compared with CCl4 treatment alone. Error bars indicate SEM.
Morphology of human albumin–expressing cells in the immune-deficient mouse liver
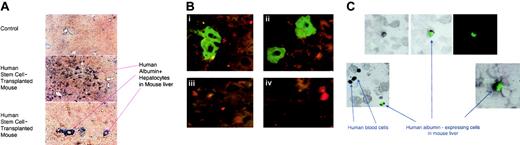
Livers from the mice in each experiment were sectioned and used for detection of human DNA by in situ hybridization and human albumin protein by immunohistochemistry. Figure4A shows detection of human albumin–expressing cells in a paraffinized liver section from one of the CCl4 + HGF–treated mice, as assessed by immunohistochemistry. The human albumin–expressing cells, stained blue, were found to be dispersed throughout some areas of the liver. There was little background staining in the control slides from mice that were injured but that had not undergone transplantation (Figure4A), and in those samples the blue chromogen did not localize to discrete cells but to the inner linings of vessels or to debris that was on the slide. The photos in Figure 4A (inset) show the human albumin–expressing cells at higher magnification, visualized under a fluorescent scope with the antihuman albumin antibody conjugated to FITC (panels A-B). Panels C and D of Figure 4A inset show the level of background autofluorescence obtained from the liver tissue damaged by CCL4 and HGF, in mice that did not undergo transplantation. It is important to include the injured liver controls shown in Figure 4A because the damaged tissue binds antibodies and probes with much higher background than intact tissue.
Immunohistochemistry and in situ hybridization to detect human albumin expression in human cells.
(A-B) Human albumin expression in the livers of chimeric NOD/SCID/β2M-null mice that had received liver injury with or without rhHGF. (A) Slides were made from paraffinized liver sections prepared from the chimeric and control mice, and immunohistochemistry was performed using an antihuman albumin antibody that did not cross-react with the murine hepatocytes (A). The antibody was visualized with a blue chromogen. Panel A shows a low magnification image of a control slide prepared using the same reagents. Panels B-C show low and high magnifications of the albumin-expressing human cells that were detected distributed throughout certain regions of the chimeric liver from one of the mice that had received recombinant human HGF after CCl4-mediated liver injury. Specific areas of the chimeric livers appeared heavily repopulated, as shown, although other areas were nearly devoid of human albumin–expressing cells. Panel B: (i-ii) High magnification of human albumin–expressing cells identified with an antihuman albumin-FITC antibody in liver sections. Nonalbumin-expressing human or mouse cells are seen surrounding the albumin–expressing cells. The background level of autofluorescence in injured tissues can be seen in the control sections (iii-iv) taken from mice that had received both CCl4 and HGF but no human stem cell transplantation. (C) Human cord blood stem cell–derived albumin-expressing cells recovered from the livers of injured, chimeric NOD/SCID/β2M-null mice. To reduce the chance of artifacts appearing from overlaid cells, cytospin slides of dissociated hepatocytes were prepared. Slides were stained with antihuman albumin-FITC Ab, and then in situ hybridization was performed using a probe specific for human DNA Alu sequences. The upper panel shows a brightfield image (left) and the fluorescent image (right) with an overlay image in the center. Human hepatocyte–like cells appear green in the cytoplasm, detected by the antihuman albumin-FITC antibody, and black in the nuclei, as detected by DAB reaction for human Alu sequences. Human blood cells showed black nuclei only (Alu+) without cytoplasmic albumin expression. Original magnifications: × 10 (A); × 100 (B); and × 20 (C).
Immunohistochemistry and in situ hybridization to detect human albumin expression in human cells.
(A-B) Human albumin expression in the livers of chimeric NOD/SCID/β2M-null mice that had received liver injury with or without rhHGF. (A) Slides were made from paraffinized liver sections prepared from the chimeric and control mice, and immunohistochemistry was performed using an antihuman albumin antibody that did not cross-react with the murine hepatocytes (A). The antibody was visualized with a blue chromogen. Panel A shows a low magnification image of a control slide prepared using the same reagents. Panels B-C show low and high magnifications of the albumin-expressing human cells that were detected distributed throughout certain regions of the chimeric liver from one of the mice that had received recombinant human HGF after CCl4-mediated liver injury. Specific areas of the chimeric livers appeared heavily repopulated, as shown, although other areas were nearly devoid of human albumin–expressing cells. Panel B: (i-ii) High magnification of human albumin–expressing cells identified with an antihuman albumin-FITC antibody in liver sections. Nonalbumin-expressing human or mouse cells are seen surrounding the albumin–expressing cells. The background level of autofluorescence in injured tissues can be seen in the control sections (iii-iv) taken from mice that had received both CCl4 and HGF but no human stem cell transplantation. (C) Human cord blood stem cell–derived albumin-expressing cells recovered from the livers of injured, chimeric NOD/SCID/β2M-null mice. To reduce the chance of artifacts appearing from overlaid cells, cytospin slides of dissociated hepatocytes were prepared. Slides were stained with antihuman albumin-FITC Ab, and then in situ hybridization was performed using a probe specific for human DNA Alu sequences. The upper panel shows a brightfield image (left) and the fluorescent image (right) with an overlay image in the center. Human hepatocyte–like cells appear green in the cytoplasm, detected by the antihuman albumin-FITC antibody, and black in the nuclei, as detected by DAB reaction for human Alu sequences. Human blood cells showed black nuclei only (Alu+) without cytoplasmic albumin expression. Original magnifications: × 10 (A); × 100 (B); and × 20 (C).
The dispersed colony shown in Figure 4A was one of the best areas located, and the overall detection of albumin-expressing human cells in the livers of the mice was low. The percentage of human cells in the livers of the mice was consistently between 1% and 10% when analyzed by FACS or imunohistochemistry using an antibody that detects all human major histocompatibility complex class 1 (MHC1) subtypes (obtained from Sigma). However, the total percentage of human hepatocyte-like cells in the liver is difficult to quantitate precisely because of interference from hematopoietic cells that have not been induced to express albumin. We would estimate, from counting cells on the double-stained slides, that 1 in 20 of the human DAB+cells was expressing albumin. This is only an estimate but gives some indication of the frequency, or lack thereof, of this event. Also, the level of human MHC+ CD45+ cells was consistently close to the number of total human MHC+ cells (data not shown). Therefore, the numbers of cells that had become CD45− albumin-expressing hepatocyte-like cells was low, and could be estimated to be less than 1% of the total liver in each mouse. This low level of induction of hematopoietic stem cells to a nonhematopoietic phenotype is consistent with observations from Lagasse et al,1 and future research will be required to better understand the signals that control this type of differentiation in response to injury.
Next, slides were used for both ISH and albumin staining. Cytospin preparations of dissociated liver cells from the treated mice were used to eliminate potential artifacts that might occur from performing double staining on the tissue sections that could be several cells in thickness. Following performance of ISH for human Alu sequences, followed by fluorescein-linked antihuman albumin antibody, slides were evaluated under a fluorescent microscope. Scanning the cytospin slides under bright field, numerous cells with dark DAB-stained nuclei were observed, indicating that they were human cells that contained Alu sequences. No cells with black nuclei were seen in the control mouse slides. When the bright field and fluorescein filters were combined, a portion of the human cells were observed to have human albumin in their cytoplasm, identified by the FITC-conjugated antibody (Figure 4B). The human cells that did not express albumin were likely hematopoietic cells or undifferentiated stem cells. The human albumin–positive cells in the tissues were not round like their human CD45+hematopoietic counterparts but were often oval or polygonal (Figure 4), and 2 nuclei were sometimes seen in the same cell (Figure 4B), as has been reported for both human and rat binucleate hepatocytes.17-19
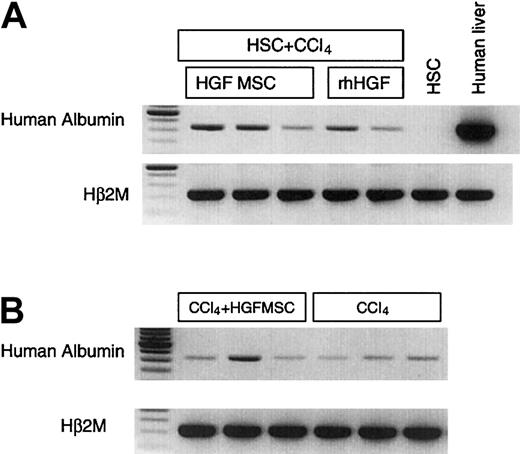
To sustain the systemic levels of human HGF in the immune-deficient mice that received stem cell transplants and were recovering from liver injury, we implanted human mesenchymal stem cells/stromal cells that had been engineered to secrete HGF using retroviral vectors into 6 NOD/SCID/β2M-null mice. We have previously reported this technique to be an efficient means to deliver supraphysiologic systemic levels of hematopoietic cytokines for several months.9-11 20 Mice that had received stem cell transplants one month before CCL4 injury were implanted with 1 000 000 HGF-secreting MSC 2 days after CCL4 administration. Human albumin could be detected in the livers of 3 of the mice 5 days after MSC implantation. When harvested one month after HGF-secreting MSC implantation, in some cases, the levels of human albumin mRNA were higher than in the mice that had received the same stem cell transplant and injury but had been treated with HGF injections rather than systemic production (Figure5A). The ratio of human albumin message to β2M message was 31.7 ± 7.9% and 22 ± 6.0% for HGF MSC implantation and HGF injections, respectively (P < .05).
Human albumin expression in mice that received transplants of HGF-secreting MSC.
(A) Systemic HGF production induced human albumin expression in CB HSC–transplanted NOD/SCID/β2M-null mouse liver. Normal human MSCs were transduced with a retroviral vector expressing HGF and transplanted into chimeric, CCl4-treated mice (n = 6). Human albumin expression appeared as early as 5 days after CCl4 and HGF injection. The comparison of human albumin expression in HGF MSCs and rhHGF-injected mouse liver is shown. (B) Human albumin expression in human bone marrow–derived stem cell transplanted mouse liver. Human marrow-derived CD34+ cells were transplanted into NOD/SCID/β2M-null mice. CCl4 with or without human HGF-secreting MSCs was administered into the engrafted mice (n = 6). Human albumin expression by RT-PCR in the mouse liver is shown. The first lane in both panels show molecular weights.
Human albumin expression in mice that received transplants of HGF-secreting MSC.
(A) Systemic HGF production induced human albumin expression in CB HSC–transplanted NOD/SCID/β2M-null mouse liver. Normal human MSCs were transduced with a retroviral vector expressing HGF and transplanted into chimeric, CCl4-treated mice (n = 6). Human albumin expression appeared as early as 5 days after CCl4 and HGF injection. The comparison of human albumin expression in HGF MSCs and rhHGF-injected mouse liver is shown. (B) Human albumin expression in human bone marrow–derived stem cell transplanted mouse liver. Human marrow-derived CD34+ cells were transplanted into NOD/SCID/β2M-null mice. CCl4 with or without human HGF-secreting MSCs was administered into the engrafted mice (n = 6). Human albumin expression by RT-PCR in the mouse liver is shown. The first lane in both panels show molecular weights.
In addition to the mice that had received transplants of human umbilical cord blood–derived stem cells, 6 NOD/SCID/β2M-null mice that had received transplants of human bone marrow–derived stem cells also had cells expressing human albumin mRNA in their livers one month after liver injury by CCl4 and implantation of HGF-secreting MSCs (Figure 5B). These data show that either source of human stem cells, cord blood or bone marrow, could be a potential source for human albumin–expressing cell differentiation in the liver.
Human serum albumin in the plasma of mice that received human stem cell transplants after liver injury
The levels of human serum albumin in the plasma of the CCl4-treated mice that underwent transplantation was next evaluated by ELISA. Normal mouse serum was tested and showed no cross-reaction with the ELISA reagents. Human serum albumin was detected in the plasma of mice that received human stem cell transplants, indicating that human hepatocyte-like cells produced in the mouse liver were capable of albumin secretion (Table1). Mice that had received transplants of stem cells derived from human bone marrow had significantly higher levels of human albumin in their plasma than mice that received transplants of human umbilical cord blood–derived stem cells (Table 1,P = .03), although the engraftment levels with human hematopoietic cells in their livers and bone marrow were comparable between the groups that had or had not been treated with HGF. Although more albumin mRNA had been detected in the mice that had been treated with HGF, there was not a significantly higher level in the serum in those groups. A positive correlation was found between the levels of engraftment of human cells in the bone marrow with the levels of human albumin in the liver, as tested by mRNA and immunohistochemistry, but not in the serum of the mice, as tested by ELISA. There may be additional, rate-limiting factors that control secretion of the albumin produced by the human cells into the bloodstream of the mice. It is possible that there could be different developmental stages of the human hepatocyte-like cells, with the earliest stages able to make albumin but not able to secrete it into the bloodstream, whereas the later stages could do both. A lack of correlation between systemic albumin levels and albumin mRNA detection from the livers could also be due to nonuniform distribution of the human cells in the livers. From the current studies it appears that a certain level of albumin-secreting cells was attained and that this level was not increased by HGF administration. Prevention of the host hepatocytes from outcompeting the human stem cells in the liver may increase the levels of development of the most mature forms of human hepatocytes. Novel methods for suppressing host hepatocyte regeneration have recently been suggested by Wang et al21 and Battaile et al.22
Human serum albumin in the transplanted mouse plasma
| Transplanted stem cells . | HGF . | Human serum albumin . |
|---|---|---|
| CB CD34+ | − | 8.9 ng (8.3-9.5, N = 2) |
| CB CD34+ | + | 17.6 ng (8.4-44.1, N = 4) |
| BM CD34+ | − | 26.2 ng (21.0-33.4, N = 3) |
| BM CD34+ | + | 29.4 ng (25.4-37.0, N = 3) |
| Normal human plasma | NA | 29.9 mg (20.2-39.5, N = 2) |
| Normal mouse plasma | NA | −2.5 ng (−3.2 to −1.8, N = 2) |
| Transplanted stem cells . | HGF . | Human serum albumin . |
|---|---|---|
| CB CD34+ | − | 8.9 ng (8.3-9.5, N = 2) |
| CB CD34+ | + | 17.6 ng (8.4-44.1, N = 4) |
| BM CD34+ | − | 26.2 ng (21.0-33.4, N = 3) |
| BM CD34+ | + | 29.4 ng (25.4-37.0, N = 3) |
| Normal human plasma | NA | 29.9 mg (20.2-39.5, N = 2) |
| Normal mouse plasma | NA | −2.5 ng (−3.2 to −1.8, N = 2) |
NOD/SCID mice received transplants of human cord blood– or marrow-derived CD34+ cells; then, one month later they were treated with CC14 with or without HGF administration. One month after CC14 damage, mouse plasma was collected and human serum albumin was assayed with ELISA. Means and their ranges are given. Human serum albumin in the normal mice tested was below the zero standard. − indicates without HGF; +, with HGF; NA, not applicable.
Development of human cytokeratin 19–expressing cells in the mouse liver
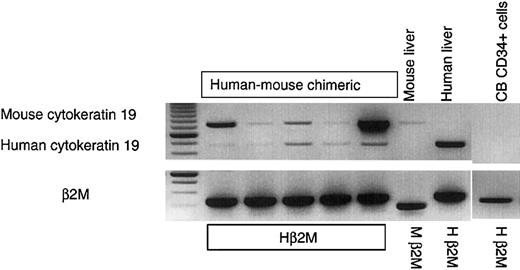
Finally, expression of the human cholangiocyte marker cytokeratin 19 (CK19) in the livers of the mice that received stem cell transplants was studied. No human CK19 was detected in freshly isolated CD34+ hematopoietic progenitors. No human CK19 was ever detected in NOD/SCID mice that received transplants of human hematopoietic stem/progenitor cells, either with or without liver injury and with or without human HGF supplementation by intravenous injection or MSC-mediated delivery (6 mice per arm tested; data not shown). However, in a different model of immune-deficient mice, the NOD/SCID/β2M-null strain, hepatic development of human CK19+ cells from the transplanted human stem cells was observed (Figure 6). In 6 NOD/SCID/β2M-null mice that underwent transplantation, 2 bands were observed. The first was murine CK19, which comigrated with the band from the control mice that did not undergo transplantation (Figure 6). The second band was a human-specific band of the expected size, which comigrated with the CK19 band from a human liver control (Figure 6). The ability of the CK19+ cells to develop in the livers of the NOD/SCID/β2M-null mice, but not the NOD/SCID parental strain, indicates that different xenograft models may be required to examine development of different tissues from transplanted stem cells in “stem cell plasticity” studies. The current studies provide a model in which the differentiation of human albumin–expressing cells and CK19-expressing cells from either bone marrow– or umbilical cord blood–derived hematopoietic stem/progenitor cells can be examined and provide the first animal model to study differentiation of human HSC into hepatocyte-like cells.
Human cytokeratin 19 expression in the livers of injured mice that underwent human stem cell transplantation and were treated with HGF.
When NOD/SCID/β2M-null mice received transplants of human stem cells (CB- or BM-derived CD34+ cells) and were treated with CCl4 and HGF MSCs, cells expressing mRNA for the human cholangiocyte marker cytokeratin 19 (CK19) were found in the mouse liver. In the injured, HGF-treated chimeric mice, 2 bands were observed. The first was murine CK19, which comigrated with the band from the control mice that did not undergo transplantation. The second band was a human-specific band of the expected size, which comigrated with the CK19 band from a human liver control. No human CK19 was detected in the murine control liver, or in freshly isolated CD34+ hematopoietic progenitors. The first lane shows molecular weights.
Human cytokeratin 19 expression in the livers of injured mice that underwent human stem cell transplantation and were treated with HGF.
When NOD/SCID/β2M-null mice received transplants of human stem cells (CB- or BM-derived CD34+ cells) and were treated with CCl4 and HGF MSCs, cells expressing mRNA for the human cholangiocyte marker cytokeratin 19 (CK19) were found in the mouse liver. In the injured, HGF-treated chimeric mice, 2 bands were observed. The first was murine CK19, which comigrated with the band from the control mice that did not undergo transplantation. The second band was a human-specific band of the expected size, which comigrated with the CK19 band from a human liver control. No human CK19 was detected in the murine control liver, or in freshly isolated CD34+ hematopoietic progenitors. The first lane shows molecular weights.
Discussion
The concept of stem cell plasticity is a very exciting and intriguing one. We can envision that a simple bone marrow aspirate could be used in the future to repair a patient's damaged liver, heart or skeletal muscle, nervous tissue, or perhaps any somatic tissue. The plasticity studies reported so far in the literature, demonstrating that murine stem cells can differentiate to tissues other than blood, are extremely exciting and provide us with groundbreaking ideas that are changing the way stem cell biologists perceive their field. In the current studies we demonstrate that purified human stem cells from marrow and umbilical cord blood can generate hepatocyte-like cells in the livers of immune-deficient mice. If human cord blood– or bone marrow–derived stem cells, which are relatively easily obtained and tissue matched, can contribute to liver repair in humans, this capability would impact health care in a major way.
It has been difficult to achieve sustained engraftment of mature human hepatocytes into the livers of immune-deficient mice. The mature hepatocytes initially engraft but die out within weeks after transplantation. Mature human hepatocytes are also difficult to culture and sustain in vitro, and cloning can be better accomplished from regenerating cells in the liver after injury. These data suggest that the matrix and soluble factors controlling survival and self-renewal of mature human hepatocytes are not yet fully understood and may be absent (nonspecies cross-reactive) in mice. The hepatocytes survive better under the kidney capsule than when injected via the portal vein. Many groups are working on promoting human hepatocyte survival in vitro for extended use in bioartificial livers.23-25 Braun et al recently reported that stimulation of c-Met, the receptor for hepatocyte growth factor, extended the duration of survival of mature human hepatocytes in immune-deficient mice to 5 months.26 Part of the challenge in this field may be that there could be inhibitory factors in adult liver to maintain the appropriate organ mass, and therefore human prehepatocytes might engraft there only after chemical- or radiation-induced injury or partial hepatectomy. The primitive, marrow-derived hepatocyte precursors/stem cells may have a better advantage for survival in the liver than mature hepatocytes. We have previously demonstrated that intravenously injected human hematopoietic stem cells home initially to the liver, as well as lung, spleen, and bone marrow.9,10,27 The human cells and their progeny persist for at least 6 months in immune-deficient mice, and human T lymphocytes are generated from CD34+/CD3− cells (selected to be devoid of mature T cells) in the liver.28 Although these data demonstrate only human hematopoietic cell differentiation, they demonstrate that the primitive human stem cells seed into the liver in xenograft models and persist there for months. In the current studies we sought to define the appropriate system to allow the primitive human stem/progenitor cells to induce differentiation to hepatocytes, rather than T cells. This model would be useful not only for experiments involving stem cell plasticity, but also for drug metabolism and virus (such as the different forms of hepatitis) treatment studies. The latter 2 models would require that the transdifferentiation of human stem cells to human hepatocytes become robust, which has not yet been accomplished. But, in the current manuscript we provide a model that is one of the first steps in that direction for human cells. Our data build upon the excellent work of other groups that studied rodent stem cell differentiation to hepatocytes,1,29,30 and on one intriguing recent study that obtained albumin-expressing cells from human complement receptor C1q (C1qRp)–positive cells.31 In the latter study by Danet et al,31 human cells were transplanted into NOD/SCID mice that had received 375 cGy radiation as the only liver injury. In our current study radiation did not induce albumin expression from transplanted human CD34+/CD38−/CD7− cells, and CCl4 treatment was required. These data may indicate differential trafficking or differentiative capacities between the 2 populations of human stem cells transplanted into immune-deficient mice in the current studies, versus the studies done by Danet et al.31
To promote differentiation of the human “hematopoietic” stem cells to hepatocyte-like cells, we developed an immune-deficient mouse liver injury model. We first tried liver injury with radiation, and then allyl alcohol, and were not successful at obtaining significant differentiation of the transplanted human hematopoietic stem cells into hepatocyte-like cells. We then learned that CCl4 is less damaging to small blood vessels in the liver than allyl alcohol. Because it is possible that this may be a critical route for the human hematopoietic cells to influx into the damaged liver, we tried CCl4 to reduce the damage to the liver microenvironment, because it may be key in directing differentiation of stem cells to hepatocytes. Administration of 0.4 mL CCl4 per kilogram body weight was toxic to the immunodeficient mice, resulting in 75% death if left untreated. However, in the groups of mice that were given administration of human hepatocyte growth factor, 100% survival was observed.
We used human CD34+ cells or CD34+/CD38−/CD7− cells from human bone marrow and umbilical cord blood as the starting human hematopoietic stem/progenitor cell sources in the current studies. CD34+/CD38− cells have been previously defined to be highly quiescent.32-35 The stem cell population used in the current studies, human CD34+/CD38−/CD7− cells highly purified by FACS isolation, was a subset of this progenitor pool. This population of quiescent human stem/progenitor cells has been previously demonstrated to generate both lymphoid and myeloerythroid progeny.6 There was no albumin expression in this population (or in CD34+ cells or total marrow mononuclear cells) prior to transplantation into the mice. At 2 months after transplantation into noninjured mice, there were human cells present in the murine livers, but they were all hematopoietic and none expressed human albumin. However, when CCl4 was administered one month after human stem cell transplantation, the human stem cells were induced to begin expressing human albumin. In some mice, human CK19 was expressed in addition to the human albumin, which might indicate differentiation toward a cholangiocyte/bile duct pathway. Of interest, although the human albumin–expressing cells could develop from stem cells in both NOD/SCID and NOD/SCID/β2 microglobulin (β2M)–null mice, the human CK19-expressing cells could develop only in the latter, as discussed further in the paragraphs below.
The NOD/SCID/β2M-null mouse is an excellent recipient for human hematopoietic stem cells, T cells, and committed progenitor populations.36-39 In spite of the short life span due to the development of a lethal thymoma in mice derived from the NOD/SCID strain by the age of 4 to 6 months,40 the NOD/SCID/β2M-null strain is the best currently available xenograft model because of its more severely immunodeficient phenotype. NOD/SCID/β2M knock-out mice have been shown to engraft more human hematopoietic progenitor populations than those that are able to engraft in NOD/SCID mice.38 41 In the current studies it was very interesting to find that no human CK19-expressing cells could be detected in the livers of NOD/SCID mice after stem cell transplantation and liver injury, but, in contrast, CK19 expression was detected in NOD/SCID/β2M knock-out mice that underwent transplantation in the same manner. It is feasible that the different strains of xenograft recipients may allow human cells with different properties, such as phenotype, adhesion molecule density, or cell-cycle status, to engraft with altered efficiencies. Or, the microenvironment within the injured livers of the mice might vary, with the endogenous cells in the liver of the NOD/SCID/β2M knock-out mice producing growth factors or other proteins that promote differentiation of human stem cells toward both bile duct and hepatocyte lineages, although the NOD/SCID microenvironment may be capable only of allowing or promoting the latter.
The phenomenon of different immune-deficient mouse strains promoting or allowing differential lineage development has been seen in the hematopoietic system. For instance, the proportion of each hematopoietic lineage that develops from the same populations of transplanted human hematopoietic stem and progenitor cells in the NOD/SCID and beige/nude/xid xenograft systems differs dramatically. NOD/SCID mice generate primarily human B lymphocytes from transplanted human CD34+ cells. Human T-lymphocyte development has seldom been observed in NOD/SCID mice in our hands or in other laboratories unless methods are undertaken to reduce residual murine natural killer activity.42-44 In contrast, human cells of all myeloid lineages and T lymphocytes develop in the bone marrow of bnx mice that received transplants of human CD34+ cells. Development of human B cells does occur inbnx mice, but to a much lesser extent than in the NOD/SCID system.20,27 45 It is likely that similar mechanisms may exist for induction of human stem cells toward different tissue-specific lineages in the various immune-deficient mouse strains. Future studies will determine which models are best to study human stem cell plasticity in each tissue.
An added advantage to the xenograft experiments described here is that this system will allow relatively straightforward fluorescence ISH–based murine versus human chromosomal analyses to determine whether cell fusion is contributing to the apparent “transdifferentiation” phenomena that are being observed in vivo. Because cells in vitro can fuse when put under strong selective pressure,46 47 it will be important to determine whether fusion can also occur in vivo. We have not ruled out cell fusion yet in the current studies, but have shown only that the damaged, but not normal, liver microenvironment can promote induction of human albumin expression from human DNA. In summary, the current studies define a model to study differentiation of purified human cord blood– and bone marrow–derived CD34+ and CD34+/CD38−/CD7− stem cells to albumin-expressing cells in the damaged livers of immune-deficient mice.
We very much appreciate the donation of umbilical cord blood samples from Kaiser Permanente, Los Angeles. We thank Sally Worttman, who heads our animal facility; Phillip Herrbrich, who runs our immunodeficient mouse colony; and Dr Janet Baer, for her extremely valuable veterinary assistance and advice. Light microscopy was performed at the CHLA Congressman Dixon Cellular Imaging Core. Giovanni Gaudino (University of Piemonte Orientale, Novara, Italy) kindly supplied the HGF cDNA. We thank Kathy Parker-Ponder (Washington University, St Louis, MO), Bryon Peterson (Gainesville, FL), and Neil Theise (New York University Medical Center, NY), who assisted us greatly with advice on liver damage and regeneration.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-05-1338.
Supported by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 1 R01DK61848-01 (J.A.N.), NIDDK RO1 DK53041 (J.A.N.), NIDDK RO1DK54567 (G.M.C.), P01CA59318 (G.M.C.), and NIH NHLBI SCOR no. 1-P50-HL54850 (J.A.N., G.M.C.). G.M.C. is a Scholar of the Leukemia, Lymphoma Society and is supported by the Seaver Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jan A. Nolta, Washington University School of Medicine, Department of Internal Medicine, Division of Oncology, Section of Stem Cell Biology, 660 S Euclid, Box 8007, St Louis, MO 63110; e-mail: jnolta@im.wustl.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal