Abstract
This phase 2 study evaluated the safety and efficacy of alemtuzumab in 22 patients with advanced mycosis fungoides/Sézary syndrome (MF/SS). Most patients had stage III or IV disease, reduced performance status, and severe itching. The overall response (OR) rate was 55%, with 32% of patients in complete remission (CR) and 23% in partial remission (PR). Sézary cells were cleared from the blood in 6 of 7 (86%) patients, and CR in lymph nodes was observed in 6 of 11 (55%) patients. The effect was better on erythroderma (OR, 69%) than on plaque or skin tumors (OR, 40%) and in patients who had received 1 to 2 previous regimens (OR, 80%) than in those who had received 3 or more prior regimens (OR, 33%). Itching, self-assessed on a 0 to 10 visual analog scale, was reduced from a median of 8 before treatment to 2 at end of therapy. Median time to treatment failure was 12 months (range, 5-32+ months). Cytomegalovirus (CMV) reactivation (causing fever without pneumonitis and responding to ganciclovir) occurred in 4 (18%) patients. Six additional patients had suspect or manifest infection (fever of unknown origin, 3; generalized herpes simplex, 1; fatal aspergillosis, 1). One patient had fatal Mycobacterium pneumonia at 10+ months. All serious infectious adverse events (except CMV) occurred in patients who had received 3 or more prior regimens. Progression of squamous cell skin carcinoma was noted in 1 patient. Alemtuzumab shows promising clinical activity and an acceptable safety profile in patients with advanced MF/SS, particularly in patients with erythroderma and severe itching and those who were not heavily pretreated.
Introduction
Mycosis fungoides and Sézary syndrome (MF/SS) are the most common cutaneous T-cell lymphomas (CTCLs).1 The clinical course of MF/SS is usually indolent, with pruritic erythematous areas slowly developing over long periods.2 Eventually, however, the erythematous patches become progressively infiltrated, developing into plaques and finally to ulcerating tumors. Some patients may acquire progressive, generalized erythema, frequently associated with severe itching. Other tissues and organs, such as peripheral blood, lymph nodes, or viscera, may also be involved. During disease progression, a defect in cell-mediated immunity becomes evident,3,4 and septicemia and other infections are common causes of death in patients with advanced MF/SS. Transformation to high-grade lymphoma may also occur during the course of the disease and is associated with a poor prognosis.5
The prognosis of MF/SS is based on the extent of disease at presentation.2,6 Patients with stage I disease have a median survival of 20 years or more, in comparison with a median survival of approximately 3 to 4 years for patients with stage III/IV disease.2,7
The traditional treatment of MF/SS includes topical and systemic therapies, alone or in combination.8 Psoralen and ultraviolet A radiation (PUVA) is effective in early-stage MF/SS, inducing complete remission (CR) in most patients.9, 10, 11 PUVA may also be combined with low doses of interferon-α to treat stage I and II disease.12, 13, 14 Early aggressive therapy with radiation and chemotherapy does not improve the prognosis.15 Local radiotherapy or total-skin electron-beam irradiation (TSEB) has been used with success to control advanced skin disease.16,17 Extracorporeal photopheresis may also be used successfully but is not generally available.18,19 Once the disease becomes refractory to topical therapy, interferon-α, bexarotene, single-agent chemotherapy, or combination chemotherapy may be given, but the duration of response is often less than 1 year, and ultimately all patients have relapses and the disease becomes refractory.8,20, 21, 22, 23, 24 Response rates following combined modality therapy with TSEB and chemotherapy/interferon-α appear similar to those of other therapies.25 Furthermore, the outcome for patients with MF/SS is similar regardless of age.26 There is, therefore, a great unmet need for novel treatment modalities for patients with advanced, symptomatic MF/SS.
Alemtuzumab (Campath-1H) is a humanized immunoglobulin G1 (IgG1) monoclonal antibody27 directed against CD52, a glycosylated peptide antigen expressed on most malignant B and T cells28 but not on hematopoietic stem cells.29 The effector mechanisms of alemtuzumab and other Campath antibodies are not fully understood but may include antibody-dependent cellular cytotoxicity,30,31 complement-mediated cell lysis,27,32 and apoptosis.33 Alemtuzumab has been developed primarily for the treatment of patients with B-cell chronic lymphocytic leukemia (B-CLL), in which response rates of 33% to 81%, depending on disease stage, have been reported.34, 35, 36
Malignant T cells express particularly high numbers of CD52 cell-surface markers (approximately 500 000 molecules/lymphocyte37 ), and the intensity of CD52 expression seems to correlate with the clinical effects.38 T-cell malignancies may be particularly responsive to therapy with alemtuzumab. A high CR rate has been reported in patients with T-cell prolymphocytic leukemia (T-PLL) treated with alemtuzumab.39 A phase 2 study40 of 50 patients with advanced, heavily pretreated, low-grade, non-Hodgkin lymphoma also included 8 pilot patients with MF/SS; 4 of 8 patients responded to alemtuzumab therapy, but otherwise no further details were reported. The aims of the present prospective phase 2 study in 22 additional patients with advanced symptomatic MF/SS were to examine in detail the response rate, response in relation to tumor site, impact on itching, and infusion toxicity and also to examine the antitumor effects versus infectious complications in relation to disease phase.
Patients, materials, and methods
Design
This was a phase 2, open-label study conducted in 8 European centers in patients with advanced MF/SS who did not respond adequately to treatment with at least PUVA, radiotherapy, or chemotherapy. Alemtuzumab was administered using a rapidly escalating initial dose regimen, followed by 30 mg 3 times a week for up to 12 weeks. The primary objective was to assess the overall response (OR) rate in these patients. Secondary objectives were to evaluate the safety profile of alemtuzumab and the clinical benefit (ie, relief of severe itching and time to progression) in this population. The ethics committee of each institution approved the study, and written informed consent was obtained from all patients.
Patients
The study was conducted in patients 18 years of age or older who had confirmed diagnoses of CD52+ MF/SS (stage II-IV1 ), who had received 5 or fewer previous systemic treatments, and who had a World Health Organization (WHO) performance status of 2 or less and a life expectancy of 15 weeks or longer. Eligible patients also had creatinine and bilirubin levels no higher than twice the upper limit of normal and had undergone previous therapy with PUVA and/or local radiotherapy, chemotherapy, or interferon-α, with documented failure to control local and generalized MF/SS. Failure was defined as lack of partial response (PR) or signs of progressive disease (PD) while undergoing PUVA/radiotherapy, chemotherapy, or interferon-α, or within 3 months of stopping previous treatment. All patients had clinical symptoms or signs (pruritus, skin ulcers, B-symptoms, symptomatic lymphadenopathy, anemia, or thrombocytopenia) requiring treatment.
Patients were excluded if they had stage I MF/SS, if they had previously untreated MF/SS with cutaneous involvement, if they needed only local/topical treatment, if their disease had undergone transformation to high-grade lymphoma, if they had CTCL other than MF/SS (the predominant form of CTCL), if they were HIV-positive, or if they had an active ongoing infection not controlled with antibiotics. Other exclusion criteria were history of anaphylaxis following exposure to rat- or mouse-derived monoclonal antibodies; less than 4 weeks since previous chemotherapy, PUVA, interferon-α, or radiotherapy; previous therapy with alemtuzumab; and oral corticosteroid therapy, apart from a maintenance dose of 10 mg or less prednisone. Also excluded were pregnant or lactating women and those of childbearing potential who were not using a reliable method of contraception.
Study treatment
Alemtuzumab (ILEX Pharmaceuticals, San Antonio, TX) was diluted in 100 mL 0.9% normal saline and administered over 2 hours through an intravenous infusion line containing a 0.22-μm filter. The first dose was 3 mg, which was increased to 10 mg and then to 30 mg as soon as infusion-related reactions were tolerated. The 30-mg dose was subsequently administered 3 times a week for up to 12 weeks. Treatment was stopped if the patient achieved CR or fulfilled the criteria for PD. Alemtuzumab therapy was also stopped in patients in whom there was no further tumor reduction or improvement in disease-related symptoms between the week 4 and week 8 assessments. Instead, these patients were followed up without therapy until disease progressed or further treatment was needed. Therapy was temporarily discontinued if grade 4 hematologic toxicity (platelets less than 25 × 109/L and absolute neutrophil count [ANC] less than 0.5 × 109/L) developed, and it was restarted on recovery of platelets to more than 50 × 109/L and of ANC to more than 1.0 × 109/L. If treatment was interrupted for more than 7 days, the dose was reinitiated at 3 or 10 mg.
Concomitant treatment
Patients received paracetamol (1 g orally) and antihistamine (2 mg clemastine intravenously) 30 minutes before the infusions. The use of corticosteroids (8 mg betamethasone or 100 mg hydrocortisone intravenously) as secondary prophylaxis during week 1 in case of flulike first-dose reactions was optional. Once all first-dose reactions disappeared, clemastine and then paracetamol were gradually withdrawn. Patients also received prophylaxis with cotrimoxazole, twice daily, 3 times weekly, and 500 mg valacyclovir, twice daily, for a minimum of 2 months following the discontinuation of alemtuzumab therapy. All patients received 300 mg allopurinol orally each day from days 1 to 28.
Disease evaluation
Disease was evaluated in all patients within 10 days before the start of treatment. Patients underwent routine physical and clinical examination, including measurement of lymph nodes, and standard laboratory tests, including blood counts, liver function tests, and serum protein and electrolyte measurements. In addition, they underwent chest x-ray examination, chest and abdominal computed tomography (CT), and bone marrow aspiration/trephine biopsy. Other investigations included assays for triiodothyronine (T3), free thyroxine (T4), thyroid-stimulating hormone (TSH), thyroid receptor antibody (TRAK), and thyroid peroxidase antibody (a-TPO). CD52 staining of tumor cells was performed by flow cytometry (FACScan; Becton Dickinson, Stockholm, Sweden) using fluorescein (FITC)–labeled anti-CD52 monoclonal antibodies (Serotec, Oxford, United Kingdom). Tumor cells analyzed were obtained from blood, bone marrow, or fine-needle aspiration biopsy of an involved lymph node or skin tumor nodule. The size and appearance of skin lesions were documented by physical examination and color photography, and patients self-assessed the severity of their itching on a visual analog scale (VAS), from 0 for no itching to 10 for worst possible itching. The tumor nodes and metastases classification system was used for clinical staging, as described previously.1,41 Fine-needle aspiration biopsy was performed on clinically enlarged lymph nodes to distinguish between stages II/III and IV. Clinical stage IV was determined mainly based on roentgenologic and clinical findings; biopsies from visceral organs were not routinely performed.
Total and differential white blood cell counts were repeated once weekly during treatment. Serum electrolyte, uric acid, and liver function tests were repeated every 4 weeks. Physical examination and assessment of skin lesions and lymph nodes were repeated after 4 and 8 weeks of therapy and again at the end of treatment, at 2 months after treatment, and then every third month until the development of PD or for a maximum of 2 years. The patient's self-assessment of itching was repeated after 4 and 8 weeks of therapy, at the end of treatment, and then every second month during follow-up until the development of PD or for a maximum of 2 years. T3, T4, TSH, TRAK, and a-TPO measurements were repeated at the end of alemtuzumab therapy and at 3, 6, 9, 12, and 18 months after completion. If they initially showed abnormal results, bone marrow aspiration, trephine biopsy, and chest and abdominal CT were repeated after 4 and 8 weeks, at the end of treatment, and thereafter every 6 months during follow-up until PD or for a maximum of 2 years. Patients achieving clinical CR underwent skin biopsy on the completion of alemtuzumab therapy.
Outcomes
The primary outcome measure was the OR rate (CR plus PR, according to the criteria detailed in “Response rate”). Secondary outcome measures were adverse events and clinical benefit (ie, the reduction of severe itching and time to treatment failure).
Response rate
Response was evaluated after 4, 8, and 12 weeks of treatment, mainly according to the criteria described previously.12,20,21 These were:
Complete remission. Complete remission was indicated by the disappearance of all previously detectable disease parameters and no new lesions; no palpable lymph nodes larger than 1 cm or nodes on CT scan larger than 1.5 cm; bone marrow negative if initially positive; absence of neoplastic, circulating lymphocytes; normal skin (no itching, no lesions); CR confirmed by skin biopsy; and duration of CR for 1 month or more.
Partial remission. Partial remission was indicated by a decrease of 50% or more from baseline in the sum of the products of the 2 largest perpendicular diameters in all measurable and evaluable lymph nodes and 5 predefined, representative skin lesions, respectively, and by no new development of lesions.
Stable disease. Stable disease was indicated by less than a 50% decrease from baseline in the sum of the products of the 2 largest perpendicular diameters in all measurable and evaluable lesions; less than a 50% increase from baseline/nadir in the sum of the products of the 2 largest perpendicular diameters in all measurable and evaluable lymph nodes and 5 predefined, representative skin lesions, respectively; and no new development of lesions.
Progressive disease. Progressive disease was indicated by more than a 50% increase from nadir in the sum of the products of the 2 largest perpendicular parameters of measurable lymph nodes or the 5 predefined, representative skin lesions, respectively, and the development of new lesions. Time to response was measured from the day of the initial treatment until the first objective documentation of OR. Time to treatment failure was measured in responding patients from the day of the initial treatment to objective documentation of PD; the requirement for additional therapy for MF/SS; death; or last follow-up.
Results
Patient population
Twenty-two patients, median age 61 years (range, 38-77 years), who had received a median of 3 prior therapies (range, 1-5 prior therapies) were enrolled. Patient characteristics are shown in Table 1. All patients had strong CD52 expression (arbitrary grading using flow cytometry) on the tumor cells. Most patients had advanced disease (stage II, 14%; stage III, 45%; stage IV, 41%).1 B-symptoms (fever, night sweats, weight loss) were present in 36% of the patients. Seven (32%) patients had Sézary cells in the blood. Seventeen patients had itching, with a median VAS score of 8 (range, 1-10). All patients were evaluable for safety and efficacy.
Patient characteristics
Age, y median (range) | 61 (38-77) |
| Clinical stage, % | |
| IA | 0 |
| IB | 0 |
| IIA | 5 |
| IIB | 9 |
| IIIA | 27 |
| IIIB | 18 |
| IVA | 32 |
| IVB | 9 |
| WHO performance status, % | |
| 0 | 22 |
| 1 | 55 |
| 2 | 23 |
| B-symptoms, % | |
| Yes | 36 |
| No | 64 |
| No. previous regimens, median (range) | 3 (1-5) |
| Itching according to VAS*, median (range) | 8 (1-10) |
Age, y median (range) | 61 (38-77) |
| Clinical stage, % | |
| IA | 0 |
| IB | 0 |
| IIA | 5 |
| IIB | 9 |
| IIIA | 27 |
| IIIB | 18 |
| IVA | 32 |
| IVB | 9 |
| WHO performance status, % | |
| 0 | 22 |
| 1 | 55 |
| 2 | 23 |
| B-symptoms, % | |
| Yes | 36 |
| No | 64 |
| No. previous regimens, median (range) | 3 (1-5) |
| Itching according to VAS*, median (range) | 8 (1-10) |
Total number of patients was 22.
n = 17 patients with itching.
Dosing
The alemtuzumab dose was rapidly escalated to the target dose of 30 mg per infusion after a median time of 5 days (range, 4-13 days). Median treatment time was 10 weeks. Eleven (50%) patients completed all 12 weeks of treatment. Reasons for withdrawal were nonresponse or PD (5 patients, at weeks 4, 7, 7, 8, and 8, respectively); infectious complications (5 patients, at weeks 4, 5, 5, 6, and 8, respectively); and fatigue grade 3 (1 patient, at week 6).
Efficacy
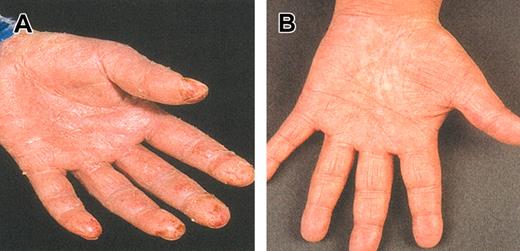
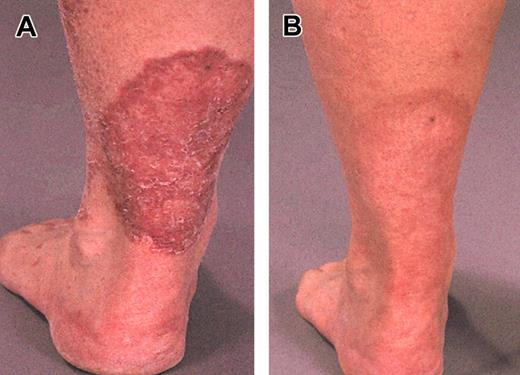
Examples of clinical responses in 2 patients with severe generalized erythroderma and plaque stage, respectively, are shown in Figures 1 and 2. The OR rate was 55%. Seven (32%) patients achieved CR (1 after 4 weeks, 3 after 8 weeks, and 3 after 12 weeks of therapy). PR was observed in 5 (23%) patients. Three (13%) patients had stable disease (SD), and 7 (32%) patients had PD. The OR rate in patients who had received 1 to 2 previous regimens was 80% (8 of 10) compared with 33% (4 of 12) in those who had received 3 or more prior regimens. Responses in relation to disease site are shown in Table 2. Tumor cells were cleared from blood (as assessed by morphologic examination and verified by negative flow cytometry analysis) in 6 of 7 (86%) patients, and CR with regard to lymphadenopathy was observed in 6 of 11 (55%) patients; all 6 patients had tumor-involved lymph nodes rather than dermatopathic lymphadenopathy. The OR rate in the skin was 55%, including 32% CR. Erythroderma responded in 69% of the patients, 38% of whom achieved CR. Corresponding number for plaque/tumors in the skin was 40% OR and 30% CR.
Skin appearance. Before (A) and after (B) 12 weeks of alemtuzumab treatment in patients with advanced erythrodermic MF/SS. Erythroderma, which was present all over the body and caused severe itching and repeated infections, responded gradually to alemtuzumab therapy, and the patient went into unmaintained CR that lasted for 18 months.
Skin appearance. Before (A) and after (B) 12 weeks of alemtuzumab treatment in patients with advanced erythrodermic MF/SS. Erythroderma, which was present all over the body and caused severe itching and repeated infections, responded gradually to alemtuzumab therapy, and the patient went into unmaintained CR that lasted for 18 months.
Plaque related to MF/SS. On the lower left leg before (A) and after (B) 12 weeks of alemtuzumab treatment.
Plaque related to MF/SS. On the lower left leg before (A) and after (B) 12 weeks of alemtuzumab treatment.
Response by disease site
. | N . | OR n (%) . | CR n (%) . | PR n (%) . | SD n (%) . | PD n (%) . |
|---|---|---|---|---|---|---|
| Blood | 7 | 6 (86) | 6 (86) | 0 (0) | 1 (14) | 0 (0) |
| Lymph nodes | 11 | 6 (55) | 6 (55) | 0 (0) | 3 (27) | 2 (18) |
| Skin | 22 | 12 (55) | 7 (31) | 5 (23) | 5 (23) | 5 (23) |
| Erythroderma | 16* | 11 (69) | 6 (38) | 5 (31) | 4 (25) | 1 (6) |
| Plaque/tumors | 10* | 4 (40) | 3 (30) | 1 (10) | 1 (10) | 5 (50) |
. | N . | OR n (%) . | CR n (%) . | PR n (%) . | SD n (%) . | PD n (%) . |
|---|---|---|---|---|---|---|
| Blood | 7 | 6 (86) | 6 (86) | 0 (0) | 1 (14) | 0 (0) |
| Lymph nodes | 11 | 6 (55) | 6 (55) | 0 (0) | 3 (27) | 2 (18) |
| Skin | 22 | 12 (55) | 7 (31) | 5 (23) | 5 (23) | 5 (23) |
| Erythroderma | 16* | 11 (69) | 6 (38) | 5 (31) | 4 (25) | 1 (6) |
| Plaque/tumors | 10* | 4 (40) | 3 (30) | 1 (10) | 1 (10) | 5 (50) |
N indicates total number of patients assessed (denominator for percentage).
Some patients had erythroderma and plaque/tumors simultaneously.
Self-assessment (VAS) of itching/pruritus before and after alemtuzumab treatment is shown in Table 3. Itching was reduced from a median of 8 (at baseline) to 2 (at the end of the treatment period). Itching was also improved by 10 mm or more (VAS) in 3 of 6 patients who did not meet the criteria for PR.
Self-assessment of itching/pruritus using VAS before and after alemtuzumab treatment
. | n . | Baseline VAS median (range) . | End-of-treatment VAS median (range) . |
|---|---|---|---|
| All patients | 17 | 8 (1-10) | 2 (0-9) |
| Responders | 11 | 8 (6-10) | 1 (0-6) |
| Nonresponders | 6 | 6 (1-9) | 5 (0-10) |
. | n . | Baseline VAS median (range) . | End-of-treatment VAS median (range) . |
|---|---|---|---|
| All patients | 17 | 8 (1-10) | 2 (0-9) |
| Responders | 11 | 8 (6-10) | 1 (0-6) |
| Nonresponders | 6 | 6 (1-9) | 5 (0-10) |
VAS severity: 0 indicates no itching; 10, worst possible itching.
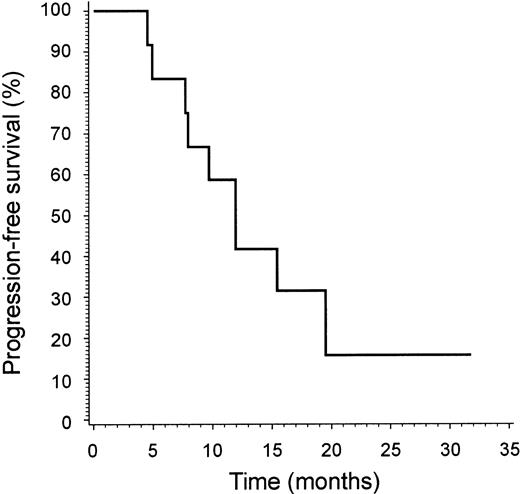
Median time to treatment failure in responding patients was 12 months (range, 5-32+ months; Figure 3). PD following a previous response to alemtuzumab was, in most patients, characterized by worsening of erythema, progression of plaque or tumors, or both.
Time to treatment failure. Time to treatment failure is shown for patients (n = 12) with advanced MF/SS who responded to alemtuzumab therapy.
Time to treatment failure. Time to treatment failure is shown for patients (n = 12) with advanced MF/SS who responded to alemtuzumab therapy.
Adverse events
Infusion-related adverse events. Infusion-related adverse events included fever, rigors, nausea, hypotension, rash/urticaria, bronchospasm, and fatigue. These were confined to the first infusion(s) and were largely mild to moderate in severity. Thirteen (59%) patients received intravenous corticosteroids as secondary prophylaxis during week 1. There were no National Cancer Institute (NCI) grade 4 reactions (Table 4), and no patient was withdrawn from the study because of first-dose reactions. After the first week, almost all side effects disappeared.
Infusion-related adverse events following intravenous administration of alemtuzumab in 22 patients with MF/SS
Event . | NCI grades 1-2 patients, % . | NCI grade 3 patients, % . |
|---|---|---|
| Fever | 68 | 5 |
| Rigors | 23 | 18 |
| Nausea | 27 | 0 |
| Hypotension | 23 | 0 |
| Rash/urticaria | 14 | 0 |
| Bronchospasm | 5 | 0 |
| Fatigue | 23 | 9 |
Event . | NCI grades 1-2 patients, % . | NCI grade 3 patients, % . |
|---|---|---|
| Fever | 68 | 5 |
| Rigors | 23 | 18 |
| Nausea | 27 | 0 |
| Hypotension | 23 | 0 |
| Rash/urticaria | 14 | 0 |
| Bronchospasm | 5 | 0 |
| Fatigue | 23 | 9 |
Most side effects disappeared after 1 week during continued alemtuzumab treatment. Thirteen (59%) patients received intravenous corticosteroids as secondary prophylaxis during week 1. No grade 4 reactions occurred.
Hematologic toxicity. Hematologic toxicity is shown in Table 5. Transient grade 4 neutropenia was recorded in 4 (18%) patients after a median time of 11 weeks (range, 8-12 weeks). One (5%) patient acquired transient grade 4 thrombocytopenia. Patients with grade 4 cytopenias recovered spontaneously (without granulocyte-colony stimulating factor usage) during continued alemtuzumab treatment or after the end of the treatment period (in patients with late-occurring neutropenia) after a median time of 2 weeks (range, 1-5 weeks).
Hematologic side effects
. | NCI grade . | . | . | ||
|---|---|---|---|---|---|
. | 0-1 n (%) . | 2-3 n (%) . | 4 n (%) . | ||
| Anemia | 21 (95) | 1 (5) | 0 (0) | ||
| Neutropenia | 17 (77) | 1 (5) | 4 (18)* | ||
| Thrombocytopenia | 18 (82) | 3 (13) | 1 (5) | ||
. | NCI grade . | . | . | ||
|---|---|---|---|---|---|
. | 0-1 n (%) . | 2-3 n (%) . | 4 n (%) . | ||
| Anemia | 21 (95) | 1 (5) | 0 (0) | ||
| Neutropenia | 17 (77) | 1 (5) | 4 (18)* | ||
| Thrombocytopenia | 18 (82) | 3 (13) | 1 (5) | ||
Total number of patients was 22.
After 8 to 12 weeks of treatment.
Infectious complications. Eleven (50%) of 22 evaluable patients had no infectious complications during or after alemtuzumab treatment. One patient had fatal pulmonary aspergillosis 2.5 months after the end of alemtuzumab treatment. This patient had chemotherapy-refractory disease, clinical stage IIIB, and rapid PD before and during treatment. One patient contracted fatal Mycobacterium pneumonia 10 months after the end of treatment. The patient had chemotherapy-refractory disease and PD at this time point and a history of recurrent bronchitis before alemtuzumab therapy. A causal relationship between study drug and fatality was regarded as probable. Four (18%) patients had reactivation of cytomegalovirus (CMV) infection, presenting as fever without pneumonitis; all 4 responded to intravenous ganciclovir. Two patients had fever of unknown origin (without neutropenia) that resolved with intravenously administered antibiotics. Another patient had febrile neutropenia 2 months after the last dose; fever resolved after intravenously administered antibiotics, and the neutrophil count normalized. One patient had a generalized herpes simplex virus (HSV) reactivation that resolved after foscarnet treatment. Another patient (with PD during alemtuzumab treatment) had long-lasting fever after the end of therapy that did not respond to antibiotics. No causative agent could be identified, and lymphoma-related fever was suspected.
All serious infectious adverse events occurred in the patients who had received 3 or more previous treatment regimens for MF/SS, except for CMV, which was equally distributed between less heavily pretreated patients (1-2 previous regimens) and those with advanced MF/SS.
Other side effects. Progression of a pre-existing squamous cell carcinoma was observed in one patient during alemtuzumab therapy.
Discussion
In this study, clinical responses after alemtuzumab treatment were recorded in more than 50% of patients with advanced MF/SS. Responses were obtained from all disease sites associated with MF/SS, with a preference for erythrodermic versus plaque/tumor stage of disease and for less heavily pretreated disease versus refractory disease. Furthermore, of 11 patients with lymphadenopathy at baseline, 6 (all of whom had tumor-involved lymph nodes) achieved CR in the nodes, a site considered less responsive to alemtuzumab therapy in patients with B-cell lymphomas or advanced B-CLL.34,40 Dermatopathic lymphadenopathy did not respond to alemtuzumab treatment in any of our patients. Itching, which was severe in many patients before therapy, improved markedly or disappeared during alemtuzumab treatment in most patients. Long-lasting (longer than 12 months) remissions were observed in half the responding patients. These results compare favorably with those of other targeted therapeutics that have been tested in patients with CTCL, such as chimeric anti-CD4 (OR, 7 of 8 patients; median response duration, 5 months)42 and denileukin diftitox (toxin-coupled anti-CD25), which attained an OR rate of 30% in 71 patients with CTCL for a median duration of 7 months.43 Clinical responses obtained in the present study were similar to those obtained with modern agents, such as 2-chlorodeoxyadenosine (2-CdA),22 pentostatin,23 and bexarotene.24 However, the median response duration with these agents is reportedly less than the median response duration achieved in the patients treated in the present study.
Alemtuzumab therapy resulted in moderate first-dose infusion-related side effects. In 13 (59%) patients, corticosteroids were administered intravenously as secondary prophylaxis during week 1; flulike symptoms, particularly rigors, were frequently reduced. Therefore, intravenous corticosteroid prophylaxis may be recommended in most patients during the first week of alemtuzumab treatment.
Treatment therapy was associated with acceptable hematologic toxicity; 18% of patients experienced transient grade 4 neutropenia, which occurred late (weeks 8-12) during therapy. This is in contrast to other recent reports in patients with B-CLL, who developed transient neutropenia after 2 to 4 weeks of therapy.34,36 This may be explained by the absence or the low degree of bone marrow infiltration in MF/SS compared with B-CLL. Growth factor support may be recommended in patients with prolonged or recurrent episodes of neutropenia while receiving alemtuzumab treatment.36
Given the T-cell–suppressive effects of alemtuzumab and the suppression of T-cell–mediated immunity that accompanies CTCL with advancing stage,3,4 an increased risk for opportunistic and other severe infections was expected during this trial mainly in the patients with advanced, heavily pretreated MF/SS. Even though half our patients completed therapy without any immediate or late-occurring major infectious complications, an increased risk was detected. This was considered to be attributable to alemtuzumab treatment in combination with the advanced disease stage of our patients. Notably, except for CMV, all serious infectious adverse events occurred in patients who had received 3 or more previous regimens for MF/SS. Reactivation of CMV and of other viruses must always be excluded in the event of fever of unknown origin in alemtuzumab-treated patients. The risk for opportunistic infections, such as Pneumocystis carinii pneumonia (not observed in this study), tuberculosis, and fungal infections, also must be taken into account. The introduction of antiviral and cotrimoxazole prophylaxis has reduced, but not eliminated, the risk for infectious complications during alemtuzumab treatment.34, 35, 36,40
In conclusion, this study indicates that alemtuzumab has clinically significant activity in patients with advanced MF/SS. The side effects reported in this study were acceptable in relation to the severity of the disease in our patients. The findings of this phase 2 multicenter study therefore support the role of alemtuzumab as treatment for patients with advanced MF/SS, provided that antibiotic and antiviral prophylaxis are used and that patients are kept under close observation during and after treatment. These results indicate that there is scope for the investigation of combination therapies with other modern drugs in the treatment of advanced CTCL. Our data indicate that early treatment with alemtuzumab may result in a higher efficacy, which also appears to be associated with a lower incidence of infections. Alemtuzumab should also be evaluated in patients with other types of T-cell lymphoma.
Prepublished online as Blood First Edition Paper, January 23, 2003; DOI 10.1182/blood-2002-09-2802.
Supported by the Cancer Society in Stockholm, the Swedish Cancer Society, the Karolinska Institutet Foundations, and ILEX Pharmaceuticals.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Gerd Ståhlberg and Cecilia Arnesson for their excellent secretarial help.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal