Abstract
Lymphocyte-predominant Hodgkin disease (LPHD) is a unique clinical entity characterized by indolent nodal disease that tends to relapse after standard radiotherapy or chemotherapy. The malignant cells of LPHD are CD20+ and therefore rituximab may have activity with fewer late effects than standard therapy. In this phase 2 trial, 22 patients with CD20+ LPHD received 4 weekly doses of rituximab at 375 mg/m2. Ten patients had previously been treated for Hodgkin disease, while 12 patients had untreated disease. All 22 patients responded to rituximab (overall response rate, 100%) with complete response (CR) in 9 (41%), unconfirmed complete response in 1 (5%), and partial response in 12 (54%). Acute treatment-related adverse events were minimal. With a median follow-up of 13 months, 9 patients had relapsed, and estimated median freedom from progression was 10.2 months. Progressive disease was biopsied in 5 patients: 3 had recurrent LPHD, while 2 patients had transformation to large-cell non-Hodgkin lymphoma (LCL). All 3 patients with recurrent LPHD were retreated with rituximab, with a second CR seen in 1 patient and stable disease in 2. Rituximab induced prompt tumor reduction in each of 22 LPHD patients with minimal acute toxicity; however, based on the relatively short response duration seen in our trial and the concerns about transformation, rituximab should be considered investigational treatment for LPHD. Further clinical trials are warranted to determine the optimal dosing schedule of rituximab, the potential for combination treatment, and the possible relationship of rituximab treatment to the development of LCL.
Introduction
Lymphocyte-predominant Hodgkin disease (LPHD) has long been recognized as the subtype of Hodgkin disease (HD) with the most favorable prognosis. Typical patients are males in the third or fourth decade of life who present with asymptomatic lymphadenopathy, usually limited to cervical, axillary, or inguinal regions. The disease is generally indolent, with slowly progressive lymphadenopathy that is often present for years prior to diagnosis.1 Pathologically, LPHD is characterized by the presence of atypical large cells known as lymphocytic and/or histiocytic (L&H) cells, or “popcorn” cells because of the unique appearance of their nuclei.2 L&H cells reside within a characteristic cellular background composed mostly of small B lymphocytes with fewer numbers of T cells. Recent advances in immunophenotyping and molecular genetics have confirmed that LPHD represents a B-cell neoplasm likely derived from a germinal center precursor cell.3 L&H cells express the B-cell marker CD20 and lack expression of CD15 and CD30, the markers characteristic of classical HD.1 This unique immunophenotype constitutes an integral component of the definition of LPHD in the current World Health Organization (WHO) classification.4
On average, fewer than 400 patients are diagnosed with LPHD each year in the United States.5 Therefore, our understanding of the clinical features of this subtype has generally evolved from retrospective reviews of cases seen at single institutions.6 Unfortunately, these studies differ in patient populations, pathological criteria for the diagnosis of LPHD, and approaches to treatment. The European Task Force on Lymphoma (ETFL) project on LPHD sought to address this problem by retrospectively evaluating a large group of patients previously diagnosed with LPHD using the pathological requirements of the WHO classification. The results of this study form the basis of our current knowledge of the pathologic and clinical features of LPHD and its response to standard Hodgkin disease treatment.7,8 The ETFL found that treatment of LPHD with radiotherapy and/or chemotherapy using standard Hodgkin disease protocols can lead to a complete response (CR) in more than 95% of patients.8
However, 2 observations suggest that this approach may not represent the optimal treatment for patients with LPHD. First, both radiotherapy and chemotherapy are associated with significant late toxic effects, including secondary malignancies.9 In the ETFL study at least as many patients died of secondary malignancies as did from LPHD itself.8 Second, despite the high initial CR rate, these patients tend to relapse continuously over time, with relapses often occurring more than 10 years after initial therapy. A plateau in the failure-free survival (FFS) curve at 10 to 20 years has only been observed in retrospective single institution studies that have remarkably long follow-up.10,11 Fortunately, most relapses are not life threatening, and overall survival is excellent, exceeding 90% at 10 years in most published studies.6
Therefore, current LPHD research aims to identify alternative treatment approaches that possess significant activity but less toxicity. Because L&H cells express CD20, the anti-CD20 antibody rituximab has naturally emerged as a potential treatment option for patients with LPHD. Two individual case reports have documented complete remission of advanced, recurrent, and chemotherapy-refractory LPHD after 4 weekly doses of rituximab.12,13 To formally define the overall response rate and response duration of this approach, we performed a phase 2 trial of rituximab (Rituxan, IDEC Pharmaceuticals) in patients with either newly diagnosed or recurrent LPHD. Here we describe the results of rituximab treatment in 22 patients.
Patients and methods
Patients
In 1999 we opened a phase 2 trial for LPHD patients at Stanford University and Washington University, St Louis, MO. The diagnosis was confirmed by institutional pathologists according to morphologic features and immunohistochemistry with the CD20+, CD15–, CD30– profile. We included patients with either untreated or previously treated LPHD. Patients were eligible if they were between the ages of 3 and 70 years and had an ECOG performance status of 0 to 2. Patients were required to have measurable disease, with at least one lymph node mass measuring 1.0 cm or more in largest dimension, or quantifiable extranodal disease. Adequate end-organ function was required as follows: absolute neutrophil count of more than 1500; platelet count higher than 50 000; serum creatinine level of less than 1.5 times the upper limit of normal; and alkaline phophatase, bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels all less than 2 times the upper limit of normal. All patients gave written informed consent. The protocol was approved by the human subjects review board at each of the participating institutions.
Patients were excluded for the following reasons: radiotherapy or chemotherapy within the previous 4 weeks, prior monoclonal antibody treatment, major surgery within the previous 4 weeks, current use of systemic glucocorticoids, or any other previous malignancy other than cured carcinoma in situ of the cervix or basal cell carcinoma of the skin. Women had to have no childbearing potential or be using adequate contraception with a negative pregnancy test at study entry. We also excluded patients with serious nonmalignant disease, such as congestive heart failure or active, uncontrolled bacterial, viral, or fungal infections.
All patients were evaluated within 4 weeks prior to initiating treatment, with routine medical history, physical examination, baseline laboratory tests, and baseline computed tomography (CT) scans of the neck, chest, abdomen, and pelvis to identify all sites of measurable disease.
Treatment and evaluation
Treatment consisted of rituximab given as an intravenous infusion of 375 mg/m2 weekly for 4 consecutive weeks. Patients were premedicated 30 minutes prior to treatment with oral doses of acetaminophen (650 mg) and diphenhydramine (25 mg). Premedication with glucocorticoids was prohibited. Patients were seen in follow-up at 1 month after the fourth dose of antibody and then again at 3, 6, 12, 15, 18, and 24 months. At each of these visits history, physical exam, and determination of complete blood cell count and serum chemistries were obtained. Repeat CT scans of the neck, chest, abdomen, and pelvis were required at 3, 6, 12, 18, and 24 months after the fourth dose of rituximab. Patients with bone marrow involvement prior to rituximab were required to have a bone marrow biopsy to confirm a complete response.
Data management and statistics
The primary outcome measure was overall response rate. Response to treatment was determined on posttreatment CT scans using the criteria of the National Cancer Institute (NCI) Workshop.14 Freedom from progression (FFP) was calculated starting with the date of the first rituximab treatment. FFP estimations were performed with the Kaplan-Meier method, using Statview 5.0 (SAS Institute, Cary, NC). Toxicity was measured using the NCI Common Toxicity Criteria.
A Simon 2-stage optimal trial design was employed.15 It was assumed that rituximab would be of no further interest if the true overall response rate was 20% or lower and would be of interest if the true response rate was 40% or higher. For rejection of the null hypothesis, 12 responses were required among a total of 43 patients targeted for enrollment. We terminated the study after 22 patients because the response rate was 100%, indicating that rituximab was of sufficient interest for further study.
Results
Patient characteristics
From March 1999 to April 2002, 22 patients with either recurrent or untreated LPHD met the enrollment criteria and agreed to participate. Nineteen patients were treated at Stanford University and 3 were treated at Washington University. The characteristics of the cohort are summarized in Table 1. Generally, involved regions were characterized by the presence of several distinctly abnormal lymph nodes that individually had little bulk. The largest lymph node in each patient ranged from 1.5 to 5.5 cm. Only 2 patients had nodes greater than 5 cm in maximum diameter.
Characteristics of 22 patients with LPHD
. | Relapsed, n = 10 . | Untreated, n = 12 . |
|---|---|---|
| Male-female ratio | 8:2 | 8:4 |
| Age, median (range), y | 44 (29-61) | 45 (18-63) |
| Stage/relapse stage | ||
| I | 3 | 4 |
| II | 4 | 3 |
| III | 3 | 5 |
| B symptoms | 0 | 2 |
| No. of lymph node regions involved | ||
| 1-2 | 5 | 7 |
| 3-4 | 3 | 4 |
| More than 4 | 2 | 1 |
| Relapse history | ||
| 1st relapse | 6 | NA |
| 2nd relapse | 3 | NA |
| 4th relapse | 1 | NA |
| Prior treatment | ||
| Radiotherapy | NA | |
| IF | 2 | NA |
| Regional* | 3 | NA |
| STLI | 2 | NA |
| Chemotherapy | ||
| MOPP | 3 | NA |
| ABVD | 2 | NA |
| MOPP/ABVD | 2 | NA |
| CHOP | 1 | NA |
. | Relapsed, n = 10 . | Untreated, n = 12 . |
|---|---|---|
| Male-female ratio | 8:2 | 8:4 |
| Age, median (range), y | 44 (29-61) | 45 (18-63) |
| Stage/relapse stage | ||
| I | 3 | 4 |
| II | 4 | 3 |
| III | 3 | 5 |
| B symptoms | 0 | 2 |
| No. of lymph node regions involved | ||
| 1-2 | 5 | 7 |
| 3-4 | 3 | 4 |
| More than 4 | 2 | 1 |
| Relapse history | ||
| 1st relapse | 6 | NA |
| 2nd relapse | 3 | NA |
| 4th relapse | 1 | NA |
| Prior treatment | ||
| Radiotherapy | NA | |
| IF | 2 | NA |
| Regional* | 3 | NA |
| STLI | 2 | NA |
| Chemotherapy | ||
| MOPP | 3 | NA |
| ABVD | 2 | NA |
| MOPP/ABVD | 2 | NA |
| CHOP | 1 | NA |
IF indicates involved field; STLI, subtotal lymphoid irradiation; MOPP, mechlorethamine, vincristine, procarbazine, prednisone; ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; and NA, not applicable.
Mantle (1 pt), Mini-mantle (1 pt), Inverted-Y (1 pt).
Ten patients had previously been treated for Hodgkin disease, as outlined in Table 1. The median time from original diagnosis of Hodgkin disease in the relapsed patients was 11.9 years (range, 1-33 years), and the median length of the most recent remission was 9 years (range, 0.4-27 years).
Twelve patients had untreated disease, with staging shown in Table 1. The median time from diagnosis to the start of treatment in these patients was 3 months (range, 1.8-7.5 months).
Toxicity
All 22 patients received the planned treatment on schedule and without dose modification. Rituximab treatment was well tolerated, and toxicity was limited to mild infusion-related reactions that were expected. No grade III or IV toxicity was seen, and no patient required hospitalization. Hematologic toxicity was limited to one instance each of leukopenia, thrombocytopenia, and anemia, all grade 1.
Initial response to treatment
All 22 patients were evaluable for response. As demonstrated in Table 2, the overall response rate to rituximab was 100%. Nine patients (41%) achieved a CR, 1 patient (5%) achieved an unconfirmed complete response (CRu), and 12 patients (54%) achieved a partial response (PR). Each patient's best response was seen within 3 months after treatment. Patients with relapsed disease had responses similar to those of untreated patients. We noted that patients with larger lymph nodes, transdiaphragmatic disease, and more than 2 nodal regions involved were generally less likely to achieve a CR (Table 2). However, these associations were not statistically significant (all P > .1 by χ2 test), likely owing to the limited number of patients in our study.
Results of treatment
. | CR . | CRu . | PR . |
|---|---|---|---|
| All patients | 9 | 1 | 12 |
| Untreated patients | 4 | 0 | 8 |
| Relapsed patients | 5 | 1 | 4 |
| Stage/restage | |||
| I | 5 | 0 | 2 |
| II | 2 | 1 | 4 |
| III | 2 | 0 | 6 |
| No. of lymph node regions involved | |||
| 1-2 | 6 | 1 | 5 |
| 3-4 | 3 | 0 | 4 |
| More than 4 | 0 | 0 | 3 |
| Largest lymph node size | |||
| 1.5-2.0 cm | 3 | 0 | 0 |
| 2.1-4.0 cm | 6 | 0 | 7 |
| 4.1-5.5 cm | 0 | 1 | 5 |
. | CR . | CRu . | PR . |
|---|---|---|---|
| All patients | 9 | 1 | 12 |
| Untreated patients | 4 | 0 | 8 |
| Relapsed patients | 5 | 1 | 4 |
| Stage/restage | |||
| I | 5 | 0 | 2 |
| II | 2 | 1 | 4 |
| III | 2 | 0 | 6 |
| No. of lymph node regions involved | |||
| 1-2 | 6 | 1 | 5 |
| 3-4 | 3 | 0 | 4 |
| More than 4 | 0 | 0 | 3 |
| Largest lymph node size | |||
| 1.5-2.0 cm | 3 | 0 | 0 |
| 2.1-4.0 cm | 6 | 0 | 7 |
| 4.1-5.5 cm | 0 | 1 | 5 |
CR indicates complete response; CRu, unconfirmed complete response; PR, partial response.
Response duration
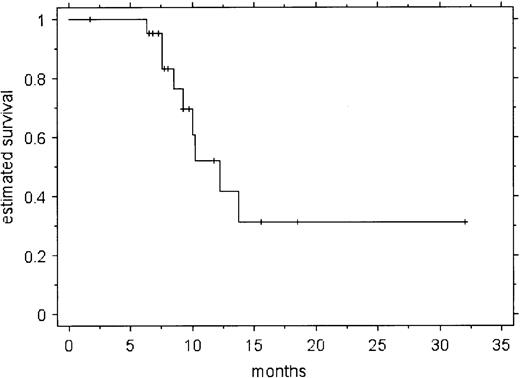
The responses we observed were in general short-lived. With a median follow-up of 13 months (range, 3-32 months), 9 patients have relapsed a median of 9 months after start of treatment (range, 6-14 months). The characteristics of these patients are shown in Table 3. Eight patients relapsed exclusively at sites previously involved with LPHD. We found that the quality of the response to rituximab correlated with relapse. At the time of the analysis, only 2 of 10 patients who attained a CR or CRu had relapsed, compared with 7 of 12 patients who had a PR (P = .0686 by χ2 test).
Summary of the 9 patients who relapsed after rituximab treatment
Patient no. . | Age . | Sex . | Stage/restage . | Initial response to rituximab . | FFP, mo . | Histology at progression . | Treatment . | Response . |
|---|---|---|---|---|---|---|---|---|
| Not previously treated | ||||||||
| 1 | 60 | M | IIIA | PR | 10 | TCR-BCL | CHOP | Responding |
| 2 | 23 | M | IIIA | PR | 9 | LPHD | Rituximab | SD (4.5 mo) |
| 3 | 38 | F | IA | PR | 6 | ND | Observation | NA |
| 4 | 45 | M | IA | PR | 10 | ND | Observation | NA |
| 5 | 32 | M | IIIA | PR | 7.5 | LPHD | Rituximab | SD (16.5 mo)* |
| 6 | 46 | F | IIIA | CR | 14 | ND | Observation | NA |
| Previously treated | ||||||||
| 7 | 51 | M | IIIA | CR | 12 | ND | Observation | NA |
| 8 | 25 | M | IIA | PR | 7.5 | LPHD | Rituximab | CR (11 mo) |
| 9 | 29 | M | IIA | PR | 8.5 | DLBCL | Rituximab followed by CHOP | CR (12 mo) |
Patient no. . | Age . | Sex . | Stage/restage . | Initial response to rituximab . | FFP, mo . | Histology at progression . | Treatment . | Response . |
|---|---|---|---|---|---|---|---|---|
| Not previously treated | ||||||||
| 1 | 60 | M | IIIA | PR | 10 | TCR-BCL | CHOP | Responding |
| 2 | 23 | M | IIIA | PR | 9 | LPHD | Rituximab | SD (4.5 mo) |
| 3 | 38 | F | IA | PR | 6 | ND | Observation | NA |
| 4 | 45 | M | IA | PR | 10 | ND | Observation | NA |
| 5 | 32 | M | IIIA | PR | 7.5 | LPHD | Rituximab | SD (16.5 mo)* |
| 6 | 46 | F | IIIA | CR | 14 | ND | Observation | NA |
| Previously treated | ||||||||
| 7 | 51 | M | IIIA | CR | 12 | ND | Observation | NA |
| 8 | 25 | M | IIA | PR | 7.5 | LPHD | Rituximab | CR (11 mo) |
| 9 | 29 | M | IIA | PR | 8.5 | DLBCL | Rituximab followed by CHOP | CR (12 mo) |
FFP indicates freedom from progression; M, male; PR, partial response; TCR-BCL, T-cell—rich B-cell lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; LPHD, lymphocyte-predominant Hodgkin disease; SD, stable disease; F, female; ND, not done; NA, not applicable; CR, complete response; and DLBCL, diffuse large B-cell lymphoma.
Progressive disease at 16.5 months.
Figure 1 shows the estimated freedom from progression (FFP) in all 22 patients. No relapses were seen before 6 months. The estimated probability of progressive disease at 10.2 months was 52% (± SE of 13%). While the number of patients enrolled makes subgroup analysis hazardous, patient age, sex, disease extent, and previous treatment did not appear to correlate with the probability of relapse. However, the quality of response to rituximab (CR/CRu vs PR) was a significant predictor of relapse (P = .0027 by log-rank test). There have been no deaths, yielding an overall survival of 100%.
Pathological analysis of relapsed disease
Of the 9 patients who relapsed, 5 underwent a repeat biopsy to assess the status of their disease. The characteristics of relapsed patients and the results of these biopsies are summarized in Table 3. Three patients were found to have recurrent LPHD. However, specimens from 2 other patients demonstrated evidence of transformation to large-cell non-Hodgkin lymphomas (LCLs). The subtypes were found to be T-cell—rich B-cell lymphoma (TCR-BCL) in patient 1 and diffuse large B-cell lymphoma (DLBCL) in patient 9.
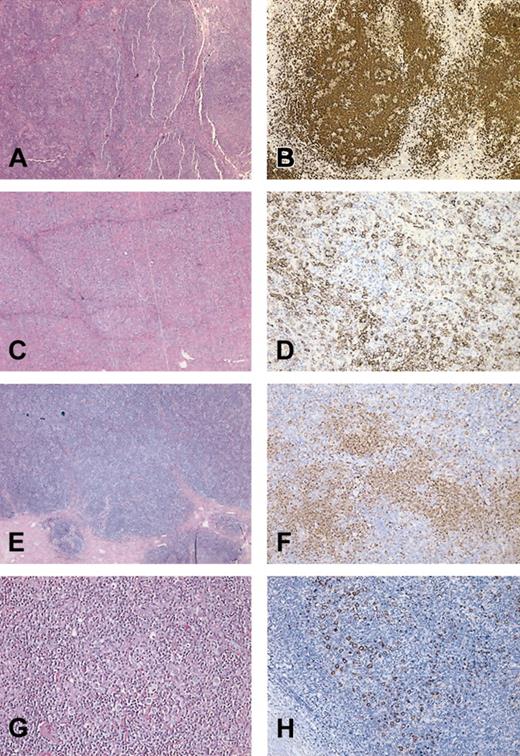
To better understand the potential effect of rituximab treatment on the transformation process, we performed a dedicated pathological review of the initial biopsy and subsequent LCL in these 2 cases. The initial biopsies of both patient 1 and patient 9 showed features typical of LPHD characterized by a nodular lymphocytic proliferation containing scattered large atypical CD20+ cells against a small lymphocytic background (Figure 2A,B,E,F). The biopsy of progressive disease in patient 9 demonstrated sheets of CD20+ large atypical cells without significant nodularity, a typical finding in DLBCL (Figure 2C). Areas rich in T cells (Figure 2D) were rare and did not warrant the diagnosis of TCR-BCL. The LCL that developed in patient 1 contained numerous small T cells in the background of scattered large neoplastic B cells, a pattern typical of TCR-BCL (Figure 2G-H).
Photomicrographs of LPHD and subsequent transformation. Shown are representative H&E stains (A,C,E,G) and CD20 immunohistochemistry (B,D,F,H) from patient 9 (A-D) and patient 1 (E-H). (A) H&E of initial biopsy of patient 9 showing nodular proliferation of lymphocytes. (B) CD20 stain of initial biopsy highlights large nodules of B cells containing scattered large B cells, characteristic of NLPHD. (C) H&E stain of postrituximab biopsy at low power showing sheets of large atypical lymphocytes, diagnostic of DLBCL. (D) CD20 stain from panel C highlights a focal area in which the large CD20+ B cells are surrounded by negatively stained T cells. (E) H&E stain of initial biopsy from patient 1 showing LPHD with vague nodularity. (F) Anti-CD20 antibody stain from highlights loose nodular aggregates of B lymphocytes. Scattered large CD20+ cells (L&H cells) are present within and outside the B-cell nodules. (G) H&E section of postrituximab transformation to TCR-BCL showing diffuse proliferation of lymphocytes. (H) CD20 stain of DLBCL demonstrates scattered CD20+ large cells with loss of nodularity. The large neoplastic cells are set against a background of T cells. Original magnification, × 150 (A,B,C,E,F) and × 600 (D,G,H).
Photomicrographs of LPHD and subsequent transformation. Shown are representative H&E stains (A,C,E,G) and CD20 immunohistochemistry (B,D,F,H) from patient 9 (A-D) and patient 1 (E-H). (A) H&E of initial biopsy of patient 9 showing nodular proliferation of lymphocytes. (B) CD20 stain of initial biopsy highlights large nodules of B cells containing scattered large B cells, characteristic of NLPHD. (C) H&E stain of postrituximab biopsy at low power showing sheets of large atypical lymphocytes, diagnostic of DLBCL. (D) CD20 stain from panel C highlights a focal area in which the large CD20+ B cells are surrounded by negatively stained T cells. (E) H&E stain of initial biopsy from patient 1 showing LPHD with vague nodularity. (F) Anti-CD20 antibody stain from highlights loose nodular aggregates of B lymphocytes. Scattered large CD20+ cells (L&H cells) are present within and outside the B-cell nodules. (G) H&E section of postrituximab transformation to TCR-BCL showing diffuse proliferation of lymphocytes. (H) CD20 stain of DLBCL demonstrates scattered CD20+ large cells with loss of nodularity. The large neoplastic cells are set against a background of T cells. Original magnification, × 150 (A,B,C,E,F) and × 600 (D,G,H).
Management of progressive disease
The management of disease progression depended largely on the results of repeat biopsy, if performed (Table 3). The 3 patients with recurrent LPHD were retreated with rituximab. One patient had a CR one month after retreatment. A second patient had stable disease 4.5 months after treatment. The third patient had stable disease for 16.5 months but then progressed. A total of 4 patients have been observed for periods ranging from 2 to 19 months. These patients remain asymptomatic and are doing well.
The patient with diffuse large B-cell lymphoma (patient 9) received 4 weekly infusions of rituximab followed by cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). Interestingly, a positron emission tomography scan that was positive at relapse became negative after rituximab was given but prior to initiation of CHOP. The patient achieved a complete remission and is currently free of both LPHD and large-cell lymphoma. The patient with TCR-BCL (patient 1) had advanced disease involving the bone marrow and was started on CHOP chemotherapy. At the time of this analysis he had received 4 cycles and was in complete remission, including having a negative bone marrow biopsy.
Discussion
We report here the results of a phase 2 trial of rituximab in patients with either untreated or recurrent LPHD. We have found that rituximab has high initial activity, with an overall response rate of 100%. Patients who had relapsed after standard chemotherapy or radiotherapy had responses similar to those who had not been previously treated. Rituximab appeared to be less effective in patients with greater disease bulk or more widespread disease at baseline. However, despite the high initial response rate, we found the duration of response to be relatively short. With a median follow-up of 13 months, a total of 9 patients had relapsed. The estimated probability of relapse reached 50% by 10 months after the start of treatment. Not surprisingly, we found that patients who achieved a PR were at significantly higher risk for relapse than those who attained a CR. Treatment with rituximab was well tolerated, with toxicity limited to transient infusion reactions.
The initial response rate we observed is essentially identical to that seen in a phase 2 trial performed by the German Hodgkin Lymphoma Study Group (GHSG).16 These investigators studied a heterogeneous group of patients with recurrent CD20+ disease, consisting of 10 patients with LPHD, 2 patients with lymphocyte-rich classical HD, and 2 patients with HD that had transformed to TCR-BCL. At the time of their most recent analysis, the OR was 100%, with 7 CR and 5 PR in 12 assessable patients; 9 of 12 patients were in remission 10 to 26 months after treatment.16 Differences in eligibility criteria and other patient characteristics may underlie the variable response durations seen in the 2 trials. The GHSG study was limited to patients with relapsed disease and included 4 patients who were not classified as LPHD. The patients in our study, particularly untreated patients, tended to have more advanced disease than typical LPHD. Nearly half of the untreated patients were stage III, and overall 10 patients had 3 or more nodal sites of disease. Of note, in the ETFL only 20% of patients were stage III or IV.8
In our study, 2 of the patients with recurrent disease after rituximab treatment were found on rebiopsy to have transformed to a large-cell lymphoma. The occurrence of these 2 cases within a cohort of 22 patients, when compared with the 2.9% incidence of non-Hodgkin lymphoma (NHL) in the ETFL study,1 raises concern that rituximab might facilitate transformation. Our cases showed morphologic and immunophenotypic patterns similar to those of transformed LCL in the absence of rituximab therapy.17 We found that in both cases the initial LPHD lesions showed an increased number of extranodular L&H cells, the presence of which has been observed to correlate with recurrence as well as progression to LCL (Z.F. et al, manuscript submitted). This observation suggested a high risk for transformation in these patients before rituximab was given. It is also possible that rituximab created a pathological artifact by depleting the background B-cell population, leaving a predominance of T cells and histiocytes. In support of this view, rituximab treatment has been shown to increase the ratio of T cells to B cells in lymph nodes affected by low-grade NHL.18
The optimal treatment for patients with LPHD remains uncertain. Radiotherapy for early-stage patients and chemotherapy or combined modality treatment for advanced-stage patients continue to represent the standard of care. Based on the relatively short response duration seen in our trial and the concerns about transformation, rituximab should be considered an investigational agent in LPHD. The high initial activity seen in our trial, along with the observed benefit of re-treatment for relapsed patients, suggests that perhaps alternative dosing schedules or combination therapy with other treatment modalities might increase response duration. With a disease as rare as LPHD, the optimal treatment approach can only be defined by cooperative efforts between community and academic physicians in prospective clinical trials. We therefore gratefully acknowledge referrals to our current study.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-08-2644.
Supported by a grant from Genentech and by National Institutes of Health grant R01-CA56060.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank the following physicians for referral of patients for this study: James Heckman, Alan Yuen, Peter Sayre, Joseph Szumawski, Tony Reid, Carol Portlock, Daniel Wong, Brian Lewis, Beverly McLeod, Lawrence Strieff, James Wong, Barbara Beach, Peter Wong, Thierry Jahan, Charlotte Kim, George Selby, Sidney Crain, Richard Robinson, Alex Denes, and Shabir Safdar.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal