Abstract
Epstein-Barr virus (EBV)—specific cytotoxic T lymphocytes are considered pivotal to prevent lymphoproliferative disease (LPD) in allogeneic stem cell transplantation (SCT) recipients. We evaluated the recovery of EBV-specific CD8+ T cells after partially T-cell—depleted SCT and studied the interaction between EBV-specific CD8+ T cells, EBV reactivation, and EBV-LPD. EBV-specific CD8+ T cells were enumerated using 12 class I HLA tetramers presenting peptides derived from 7 EBV proteins. Blood samples were taken at regular intervals after SCT in 61 patients, and EBV DNA levels were assessed by real-time polymerase chain reaction. Forty-five patients showed EBV reactivation, including 25 with high-level reactivation (ie, more than 1000 genome equivalents [geq] per milliliter). Nine of these 25 patients progressed to EBV-LPD. CD8+ T cells specific for latent or lytic EBV epitopes repopulated the peripheral blood at largely similar rates. In most patients, EBV-specific CD8+ T-cell counts had returned to normal levels within 6 months after SCT. Concurrently, the incidence of EBV reactivations clearly decreased. Patients with insufficient EBV-specific CD8+ T-cell recovery were at high risk for EBV reactivation in the first 6 months after SCT. Failure to detect EBV-specific CD8+ T cells in patients with high-level reactivation was associated with the subsequent development of EBV-LPD (P = .048). Consequently, the earlier defined positive predictive value of approximately 40%, based on high-level EBV reactivation only, increased to 100% in patients without detectable EBV-specific CD8+ T cells. Thus, impaired recovery of EBV-specific CD8+ T cells in patients with high-level EBV reactivation may identify a subgroup at very high risk for EBV-LPD and supports that EBV-specific CD8+ T cells protect SCT recipients from progressive EBV reactivation and EBV-LPD.
Introduction
Epstein-Barr virus (EBV) is a ubiquitous γ-herpesvirus that infects more than 90% of the world population. Following primary infection in the oropharynx, EBV remains latently present in B lymphocytes.1 Latent EBV infection is normally controlled by a cell-mediated immune response and CD8+ T lymphocytes directed against the immunodominant latent proteins EBNA3A, 3B, and 3C, and the lytic proteins BZLF1 and BMLF1 are detectable in the blood of most healthy EBV-seropositive individuals.2, 3, 4
Latently EBV-infected B cells may give rise to EBV+ lymphoproliferative disease (EBV-LPD) in immunosuppressed recipients of allogeneic solid organ and hematopoietic stem cell transplantation (SCT), due to inhibition of immunologic control of these cells. In particular, EBV-LPD is a serious complication following T-cell—depleted SCT, being associated with considerable mortality.5, 6, 7, 8 The quantification of EBV DNA in plasma or whole blood is a suitable assay to monitor EBV reactivation in the transplantation setting.9, 10, 11, 12 EBV reactivation occurs frequently in both partially T-cell—depleted as well as T-cell—replete SCT recipients.11 However, recipients of T-cell—depleted SCT are at significantly increased risk for EBV-LPD.11 In these patients, EBV-LPD can be quantitatively predicted by the frequent monitoring of viral load in plasma.11 A viral load of 1000 genome equivalents (geq) per milliliter proved to be a level of EBV reactivation associated with a positive predictive value for EBV-LPD of 39% and a negative predictive value of 100%.11
HLA-restricted, EBV-specific cytotoxic T lymphocytes (CTLs) are important for the control of EBV and, in particular, for the prevention of the outgrowth of latently EBV-infected B cells into EBV-LPD.1,3 The pivotal role of EBV-specific CTLs to control EBV+ B cells in SCT recipients has been emphasized by the clinical success of adoptive cellular immunotherapy of EBV-LPD using EBV-specific CTLs.13, 14, 15 While the overall recovery of CD8+ T cells after allogeneic SCT (allo-SCT) has been studied extensively,16, 17, 18, 19 little is known with respect to the recovery of EBV-specific CD8+ T cells after SCT.20 The development and use of fluorochrome-conjugated, peptide-loaded major histocompatibility complex (MHC) class I tetramer complexes now allows the direct identification of class I HLA-restricted, peptide-specific CD8+ T cells.21 Tetramer-based studies revealed much higher frequencies of circulating HLA-restricted, peptide-specific CD8+ T cells than estimated before by limiting dilution assays.2,21, 22, 23 The latter technique requires multiple divisions and differentiation of the responder cells to allow their detection, while tetramer-based assays do not. The aim of this study was to evaluate the recovery of EBV-specific CD8+ T cells after partially T-depleted SCT using tetramer technology. In view of the relatively high incidence of EBV-LPD in these patients,11 we were specifically interested in the interaction between EBV-specific CD8+ T cells, EBV reactivation, and EBV-LPD.
Patients, materials, and methods
Patients
Approval for this study was obtained from the Erasmus MC Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki. Between January 1998 and December 2000, 100 patients received a T-cell—depleted SCT at the Department of Hematology, Erasmus MC. Sixty-one of them expressed at least one of the following HLA alleles— A*0201, A*1101, B*0702, B*0801, and B*3501—and could be studied with the panel of EBV tetramers available to us. The characteristics of the patients, their donors, and the grafts are listed in Table 1. The donors related to 39 patients were fully matched for HLA-A, -B, -C, -DP, -DQ, and -DR. The donors unrelated to the remaining 22 patients had been typed by high-resolution techniques for HLA-A, -B, -C, -DRB1, and -DQB1 and were selected to have 9 or 10 matches for the 10 HLA alleles tested. The distribution of (combinations of) these HLA alleles among the patients is shown in Table 2. Thus, the HLA-A*0201 allele was present in 35 patients, HLA-A*1101 in 7 patients, HLA-B*0702 in 17 patients, HLA-B*0801 in 17 patients, and HLA-B*3501 in 12 patients.
Patient characteristics
Parameter . | No. of patients . |
|---|---|
| Sex | |
| Male | 32 |
| Female | 29 |
| Median age, y (range) | 38 (16-55) |
| Diagnostic indication for SCT | |
| Acute myelogenous leukemia | 17 |
| Acute lymphoblastic leukemia | 10 |
| Myelodysplastic syndrome | 1 |
| Chronic myeloid leukemia | 8 |
| Non-Hodgkin lymphoma | 16 |
| Multiple myeloma | 8 |
| Severe aplastic anemia | 1 |
| Stem cell donor type | |
| HLA-matched related donor | 39 |
| HLA-matched unrelated donor | 22 |
| Conditioning regimen | |
| Cy + TBI | 35 |
| Cy + TBI + ATG | 23 |
| Bu + Cy | 3 |
| Stem cell source | |
| Bone marrow | 51 |
| Mobilized peripheral blood | 10 |
| Graft characteristics, median (range) | |
| CD34+ hematopoietic stem cells, × 106/kg | 1.6 (0.5-8.8) |
| CD3+T lymphocytes, × 105/kg | 2.0 (1.0-7.5) |
| EBV serology prior to SCT | |
| Recipient positive, SCT donor positive | 55 |
| Recipient positive, SCT donor negative | 3 |
| Recipient negative, SCT donor positive | 3 |
| Recipient negative, SCT donor negative | 0 |
Parameter . | No. of patients . |
|---|---|
| Sex | |
| Male | 32 |
| Female | 29 |
| Median age, y (range) | 38 (16-55) |
| Diagnostic indication for SCT | |
| Acute myelogenous leukemia | 17 |
| Acute lymphoblastic leukemia | 10 |
| Myelodysplastic syndrome | 1 |
| Chronic myeloid leukemia | 8 |
| Non-Hodgkin lymphoma | 16 |
| Multiple myeloma | 8 |
| Severe aplastic anemia | 1 |
| Stem cell donor type | |
| HLA-matched related donor | 39 |
| HLA-matched unrelated donor | 22 |
| Conditioning regimen | |
| Cy + TBI | 35 |
| Cy + TBI + ATG | 23 |
| Bu + Cy | 3 |
| Stem cell source | |
| Bone marrow | 51 |
| Mobilized peripheral blood | 10 |
| Graft characteristics, median (range) | |
| CD34+ hematopoietic stem cells, × 106/kg | 1.6 (0.5-8.8) |
| CD3+T lymphocytes, × 105/kg | 2.0 (1.0-7.5) |
| EBV serology prior to SCT | |
| Recipient positive, SCT donor positive | 55 |
| Recipient positive, SCT donor negative | 3 |
| Recipient negative, SCT donor positive | 3 |
| Recipient negative, SCT donor negative | 0 |
SCT indicates stem cell transplantation; HLA, human leukocyte antigen; Cy, cyclophosphamide; TBI, total body irradiation; ATG, antithymocyte globulin; Bu, busulfan; and EBV, Epstein-Barr virus.
Distribution of HLA alleles
HLA allele . | No. of patients . |
|---|---|
| A*0201 | 17 |
| A*0201 A*1101 | 1 |
| A*0201 B*0702 | 4 |
| A*0201 B*0702 B*0801 | 2 |
| A*0201 B*0702 B*3501 | 2 |
| A*0201 B*0801 | 8 |
| A*0201 B*3501 | 1 |
| A*1101 | 5 |
| A*1101 B*3501 | 1 |
| B*0702 | 6 |
| B*0702 B*0801 | 1 |
| B*0702 B*3501 | 2 |
| B*0801 | 5 |
| B*0801 B*3501 | 1 |
| B*3501 | 5 |
| Total | 61 |
HLA allele . | No. of patients . |
|---|---|
| A*0201 | 17 |
| A*0201 A*1101 | 1 |
| A*0201 B*0702 | 4 |
| A*0201 B*0702 B*0801 | 2 |
| A*0201 B*0702 B*3501 | 2 |
| A*0201 B*0801 | 8 |
| A*0201 B*3501 | 1 |
| A*1101 | 5 |
| A*1101 B*3501 | 1 |
| B*0702 | 6 |
| B*0702 B*0801 | 1 |
| B*0702 B*3501 | 2 |
| B*0801 | 5 |
| B*0801 B*3501 | 1 |
| B*3501 | 5 |
| Total | 61 |
All patients were prepared for SCT with cyclophosphamide (120 mg/kg) and total body irradiation (12 Gy in 2 fractions with partial lung shielding). Patients who had been treated with locoregional irradiation before were prepared for SCT using busulfan (4 mg/kg on each of 4 successive days) and cyclophosphamide (120 mg/kg). Rabbit antithymocyte globulin (ATG) (Imtix Sangstat, Amstelveen, The Netherlands) was given at days –7 to –4 prior to SCT (total dose, 8 mg/kg) to recipients with an unrelated SCT donor to prevent graft rejection. Partial T-cell depletion was performed using sheep erythrocyte (E) rosetting (n = 31) or selection of CD34+ hematopoietic stem cells (n = 30) using the CliniMACS device (Miltenyi, Bergisch Gladbach, Germany). The stem cells were derived from bone marrow in 51 transplantations and from mobilized peripheral blood in 10. From either the E-rosette—forming or CD34– fractions, T cells were added back to the grafts so as to reach 2 × 105 T cells per kilogram.
Cyclosporin A (3 mg/kg) was given as graft-versus-host prophylaxis from day –3 until day +100 after allo-SCT. All patients received ciprofloxacin and fluconazole for prevention of infection during neutropenia, and cotrimoxazole was given after neutrophil recovery until day 180 after allo-SCT. As prophylaxis for herpes simplex virus reactivation, oral aciclovir (200 mg 4 times daily) was administered during neutropenia. Erythrocyte and platelet products for transfusion were filtered to remove all leukocytes and subsequently irradiated (25 Gy). Patients developing clinically significant (grades II to IV) acute graft versus host disease (GVHD) were treated with prednisone, 1 mg/kg twice daily for 7 to 10 days, which was then tapered according to clinical response. Grade I GVHD was treated with topical steroids. Chronic GVHD was treated with the combination of cyclosporine and prednisone according to clinical response.
Quantitative EBV-specific polymerase chain reaction (PCR) assay
EBV DNA levels were measured in plasma samples as described previously.12 We tested plasma rather than leukocytes in view of the deep B lymphopenia persisting in most patients for up to 3 months after SCT. Briefly, Taqman PCR primers had been derived from the part of the EBV genome encoding for the nonglycosylated membrane protein BNRF1-p143. This set of primers generated a DNA product of 74 base pairs. A preparation with a predefined EBV copy number (Advanced Biotechnologies, Columbia, MD) was used as internal standard for the quantitative PCR assay. Serial dilutions ranging from 101 to 107 EBV DNA genome equivalents (geq) per milliliter were made to characterize linearity, precision, specificity, and sensitivity. This Taqman assay detected viral DNA in plasma samples in a linear fashion over a range from 50 to 107 geq/mL. Test results below 50 geq/mL were considered negative. Plasma samples for quantitation of EBV load were obtained from hospitalized patients at weekly intervals in patients without EBV-LPD and daily in patients with established EBV-LPD until resolution of their disease. SCT recipients attending the outpatient clinic were monitored at larger time intervals. EBV was considered to be reactivated if plasma EBV DNA levels exceeded 50 geq/mL. Low-level EBV reactivation was defined as low-level if plasma EBV DNA levels did not exceed 1000 geq/mL; if these levels were 1000 geq/mL or higher, EBV reactivation was classified as high-level.11 From January 1999 onward, patients with high-level EBV reactivation were treated preemptively with the CD20 monoclonal antibody (mAb) rituximab (375 mg/m2) (Roche, Basle, Switzerland) as recently described.24
Diagnosis and treatment of EBV-LPD
EBV-LPD was diagnosed using histology and/or cytology and was classified according to the criteria of Knowles et al.25 Immunohistology included staining with monoclonal antibodies specific for CD19 (BD Biosciences, San Jose, CA), EBV-encoded latent membrane protein 1 (LMP1), and κ and λ light chains (all from DAKOCytomation, Glostrup, Denmark). In situ hybridization was performed to detect the expression of small EBV-encoded RNA copies (EBER) using the EBV-EBER probe (DAKOCytomation). PCR was performed for detection of EBV DNA for the BamHIW fragment. Furthermore, the staging of EBV-LPD included physical examination, whole-body computed tomography scanning, and flow cytometric detection of monoclonal B cells in mononuclear cell suspensions derived from peripheral blood and bone marrow specimens. Upon diagnosis of EBV-LPD, patients were treated with rituximab, and immunosuppressive drugs were suspended as guided by viral load.26 Patients were treated with donor leukocyte infusions if no response ensued following rituximab.26
Enumeration of CD8+ T lymphocytes specific for class I HLA-restricted, EBV-encoded epitopes
Heparinized blood samples were obtained from SCT recipients at 2, 3, 6, 9, 12, 18, and 24 months after allo-SCT as well as from healthy EBV carriers—that is, 37 laboratory workers and 39 SCT donors prior to bone marrow donation or stem cell mobilization. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Isopaque density grade centrifugation and cryopreserved in liquid N2. Absolute numbers of CD8+ T cells were enumerated in parallel using a 3-color, single-platform, whole blood immunostaining technique.27 The following mAbs were used: CD45 (clone 2D1 conjugated with fluorescein isothiocyanate), CD8 (clone SK1 conjugated with phycoerythrin [PE]), both from BD Biosciences; and T-cell receptor (TCR) pan-α/β (clone BMA031 conjugated with PE—cyanine 5 [PE-Cy5]; Immunotech, Marseille, France). Per stained sample, ungated list mode data containing at least 5000 TCRα/β+ T cells were acquired using a FACSCalibur flow cytometer (BD Biosciences). During data analysis, CD8+ T cells were defined as events with low to medium forward light scatter, low sideward light scatter, and being CD45+, TCRα/β+, and CD8+. The proportion of EBV-specific CD8+ T cells was assessed using tetramers on cryopreserved and thawed PBMCs.28 The characteristics of the 12 class I EBV tetramers used in this study are summarized in Table 3; their validation has been reported elsewhere.2,20,29, 30, 31 Thawed PBMC suspensions were incubated with EBV tetramers and CD8 (clone SK1 conjugated with allophycocyanin) for 30 minutes on melting ice. After one wash, cells were resuspended in PBS containing 1 μg/mL 7-aminoactinomycin D (7-AAD; Sigma, St Louis, MO). Following acquisition of 20 000 living CD8+ T cells (defined as having low to intermediate forward light scatter, low sideward light scatter, as well as being 7-AAD– and CD8+), the proportions of living CD8+ T cells binding tetramer were assessed. The absolute numbers of circulating tetramer-binding CD8+ T cells were calculated from the proportion of CD8+ T cells binding tetramer and the simultaneously obtained absolute CD8+ T-cell count.28 The lower limit of detection was 0.5 EBV-specific CD8+ T cells per cubic millimeter. For the stem cell grafts, the number of transplanted EBV-specific CD8+ T cells per kilogram of body weight of the recipient was calculated from the proportion of CD3+ T cells coexpressing CD8 and binding any of the 12 tetramers under study (assessed using the cryopreserved and thawed cell fractions used for T-cell add-back) and the simultaneously established number of CD3+ T cells per kilogram transplanted.
EBV tetramers used in this study
EBV antigen (coordinates) . | Presenting HLA allele . | Amino acid sequence . | Normal range, cells per mm3† . | Reference no. . |
|---|---|---|---|---|
| Latent EBV epitopes | ||||
| EBNA1 (407-417) | B*3501 | HPVGEADYFEY | 2 (<0.5-21) [18] | 30 |
| EBNA3A (379-387) | B*0702 | RPPIFIRRL | 2 (<0.5-5) [20] | 20 |
| EBNA3A (502-510) | B*0702 | VPAPAGPIV | 0.6 (<0.5-3) [20] | 20 |
| EBNA3A (325-333) | B*0801 | FLRGRAYGL | 1 (<0.5-28) [20] | 20,29 |
| EBNA3A (458-466) | B*3501 | YPLHEQHGM | 1 (<0.5-51) [16] | 30 |
| EBNA3B (399-408) | A*1101 | AVFDRKSDAK | 1 (<0.5-5) [9] | UR |
| EBNA3B (416-424) | A*1101 | IVTDSVIK | 0.6 (<0.5-3) [11] | UR |
| EBNA3C (284-293) | A*0201 | LLDFVRMGV | 1 (<0.5-8) [21] | 20 |
| LMP2 (426-434) | A*0201 | CLGLLTMV | 0.6 (<0.5-6) [22] | 20 |
| Lytic EBV epitopes | ||||
| BMLF1 (280-288) | A*0201 | GLCTLVAML | 1 (<0.5-18) [24] | 29 |
| BZLF1 (190-197) | B*0801 | RAKFKQLL | 3 (<0.5-79) [18] | 29 |
| BZLF1 (54-64) | B*3501 | EPLPQGQLTAY | 5 (<0.5-133) [17] | 31 |
EBV antigen (coordinates) . | Presenting HLA allele . | Amino acid sequence . | Normal range, cells per mm3† . | Reference no. . |
|---|---|---|---|---|
| Latent EBV epitopes | ||||
| EBNA1 (407-417) | B*3501 | HPVGEADYFEY | 2 (<0.5-21) [18] | 30 |
| EBNA3A (379-387) | B*0702 | RPPIFIRRL | 2 (<0.5-5) [20] | 20 |
| EBNA3A (502-510) | B*0702 | VPAPAGPIV | 0.6 (<0.5-3) [20] | 20 |
| EBNA3A (325-333) | B*0801 | FLRGRAYGL | 1 (<0.5-28) [20] | 20,29 |
| EBNA3A (458-466) | B*3501 | YPLHEQHGM | 1 (<0.5-51) [16] | 30 |
| EBNA3B (399-408) | A*1101 | AVFDRKSDAK | 1 (<0.5-5) [9] | UR |
| EBNA3B (416-424) | A*1101 | IVTDSVIK | 0.6 (<0.5-3) [11] | UR |
| EBNA3C (284-293) | A*0201 | LLDFVRMGV | 1 (<0.5-8) [21] | 20 |
| LMP2 (426-434) | A*0201 | CLGLLTMV | 0.6 (<0.5-6) [22] | 20 |
| Lytic EBV epitopes | ||||
| BMLF1 (280-288) | A*0201 | GLCTLVAML | 1 (<0.5-18) [24] | 29 |
| BZLF1 (190-197) | B*0801 | RAKFKQLL | 3 (<0.5-79) [18] | 29 |
| BZLF1 (54-64) | B*3501 | EPLPQGQLTAY | 5 (<0.5-133) [17] | 31 |
EBNA indicates EBV nuclear antigen; LMP, latent membrane protein; BMLF, BamHIM leftward reading frame; BZLF, BamHIZ leftward reading frame; and UR, D.V.B. and F.M., unpublished results.
Median (minimum-maximum) results obtained in healthy EBV carriers. The number of carriers tested for each EBV tetramer is shown between brackets.
Statistical analysis
The kinetics of regeneration of EBV-specific CD8+ T cells was first analyzed for the individual epitope-specific CD8+ T-cell subsets (Figure 2). Thereafter, CD8+ T-cell immunity against EBV lytic or latent epitopes was summarized by selecting, per patient and blood sample, the highest count of CD8+ T cells specific to any of the 3 lytic or 9 latent epitopes studied (Figure 3). For the subsequent analyses addressing the questions of whether or not delayed recovery of EBV-specific CD8+ T cells was associated with (high-level) EBV reactivation and development of EBV-LPD, blood samples were classified “positive” for EBV-specific CD8+ T cells if any EBV-specific CD8+ T-cell subset (either for lytic or latent epitopes) in that sample had reached the level of 0.5 cells per cubic millimeter. Conversely, if none of the EBV-specific CD8+ T-cell subsets studied had reached the threshold of 0.5 cells per cubic millimeter, the sample was classified “negative.” Thus, each patient was entered into these analyses, at each time point studied, with a single EBV-specific CD8+ T-cell count. Fisher exact test (1-sided) was performed for the analysis of 2 × 2 tables. P values less than .05 were considered significant.
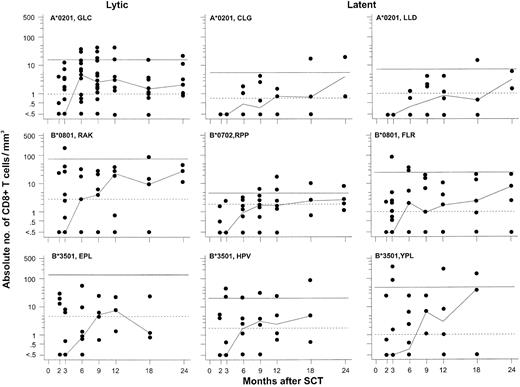
Repopulation of EBV-specific CD8+ T lymphocytes following partially T-cell—depleted SCT. Each panel shows the recovery of CD8+ T cells directed against a single EBV-specific epitope as measured by tetramer technology. Results of CD8+ T cells specific for 3 lytic epitopes are shown in the left panels and those of CD8+ T cells specific for 6 latent epitopes in the middle and right panels. The horizontal continuous lines indicate the upper limits of the normal ranges, which were defined as the maximum results obtained in 16 to 24 healthy EBV-seropositive individuals. The horizontal dotted lines indicate the median values of each T-cell subset in the healthy EBV-seropositive individuals. The lower limits of the normal ranges as defined by this criterion were less than 0.5 cells per cubic millimeter for all 9 T-cell subsets. See the legend to Figure 1 for further details.
Repopulation of EBV-specific CD8+ T lymphocytes following partially T-cell—depleted SCT. Each panel shows the recovery of CD8+ T cells directed against a single EBV-specific epitope as measured by tetramer technology. Results of CD8+ T cells specific for 3 lytic epitopes are shown in the left panels and those of CD8+ T cells specific for 6 latent epitopes in the middle and right panels. The horizontal continuous lines indicate the upper limits of the normal ranges, which were defined as the maximum results obtained in 16 to 24 healthy EBV-seropositive individuals. The horizontal dotted lines indicate the median values of each T-cell subset in the healthy EBV-seropositive individuals. The lower limits of the normal ranges as defined by this criterion were less than 0.5 cells per cubic millimeter for all 9 T-cell subsets. See the legend to Figure 1 for further details.
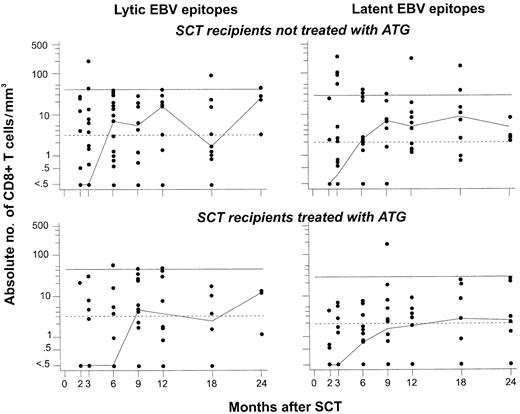
Reconstitution of CD8+ T cells specific for lytic or latent EBV epitopes is delayed in recipients of SCT from MUD pretreated with ATG, compared with recipients of SCT from an HLA-matched related donor (MRD) not treated with ATG. For this analysis, data on CD8+ T cells specific for any of the 3 lytic epitopes (left panels) or any of the 9 latent epitopes (right panels) studied were pooled (see “Patients, materials, and methods”). The normal ranges were defined as described in the legend to Figure 2 after the same procedure. Differences between recipients of MRD-SCT (top panels) and MUD-SCT (bottom panels) reached significance for CD8+ T cells specific for lytic EBV epitopes at 2 months after SCT (P = .008 using the Wilcoxon test). See the legend to Figure 1 for further details.
Reconstitution of CD8+ T cells specific for lytic or latent EBV epitopes is delayed in recipients of SCT from MUD pretreated with ATG, compared with recipients of SCT from an HLA-matched related donor (MRD) not treated with ATG. For this analysis, data on CD8+ T cells specific for any of the 3 lytic epitopes (left panels) or any of the 9 latent epitopes (right panels) studied were pooled (see “Patients, materials, and methods”). The normal ranges were defined as described in the legend to Figure 2 after the same procedure. Differences between recipients of MRD-SCT (top panels) and MUD-SCT (bottom panels) reached significance for CD8+ T cells specific for lytic EBV epitopes at 2 months after SCT (P = .008 using the Wilcoxon test). See the legend to Figure 1 for further details.
Results
Clinical outcome of EBV reactivation
Fifty-eight of the 61 SCT recipients were EBV-seropositive prior to SCT (ie, carried the virus); 55 received their SCT from EBV-seropositive donors and 3 from seronegative donors. The remaining 3 patients were EBV-seronegative prior to SCT; all received SCT from EBV-seropositive donors, and 2 of them became infected with EBV after SCT. In 45 of the 58 (78%) SCT recipients, at least 1 episode of EBV reactivation (ie, more than 50 geq/mL) was observed. The median time to first reactivation was 65 days after SCT (range, 4-447 days). High-level EBV reactivation (at least 1000 geq/mL) developed in 25 of the latter 45 patients. Ten of these 25 patients were preemptively treated with the anti—B-cell mAb rituximab.24 None of these patients progressed to EBV-LPD. Nine of the 15 patients who had not received preemptive treatment developed EBV-LPD. Seven of these 9 patients responded to therapy, while the 2 nonresponding patients died from progressive EBV-LPD. The median duration of follow-up for virologic and immunologic studies was 10 months after SCT (range, 2-38 months).
Repopulation kinetics of EBV-specific CD8+ T cells following SCT
We analyzed the repopulation of EBV-specific CD8+ T cells using a panel of 12 tetramers, 3 containing lytic epitopes and 9 containing latent epitopes (Table 3). Figure 1 shows the recovery pattern of total CD8+ T cells and Figure 2 the recovery patterns of the CD8+ T-cell subsets specific for the lytic epitopes GLC, RAK, and EPL (Figure 2 left panels) and the latent epitopes CLG, LLD, RPP, FLR, HPV, and YPL (Figure 2 middle and right panels). The data of the latent epitopes VPA, AVF, and IVT are not shown graphically. VPA-specific CD8+ T cells remained below the detection limit (ie, less than 0.5/mm3) in 40 of the 49 samples tested, and CD8+ T cells specific for AVF or IVT had been tested in only few (ie, less than 20) patient specimens. As reference, the normal ranges for each EBV-specific CD8+ T-cell subset were assessed in 9 to 24 healthy EBV-seropositive donors (Table 3).
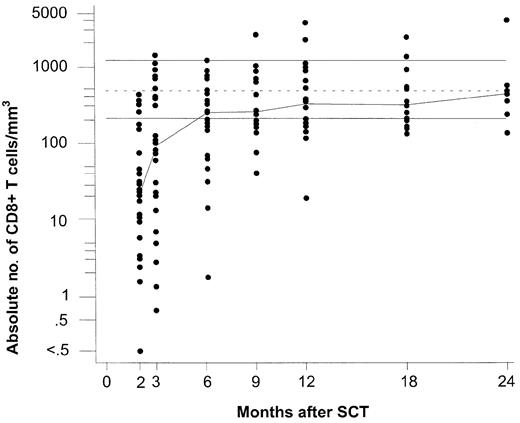
Repopulation of total CD8+ T lymphocytes following partially T-cell—depleted SCT. Logarithmic scales were used for the y-axis to compress the figure. The median values for each time point are connected with a line to indicate trend. The top and bottom horizontal lines indicate the normal range, which was defined as the range between the 5th and 95th percentiles of 60 healthy EBV-seropositive individuals, respectively; the dotted line in the middle is the median of this group.
Repopulation of total CD8+ T lymphocytes following partially T-cell—depleted SCT. Logarithmic scales were used for the y-axis to compress the figure. The median values for each time point are connected with a line to indicate trend. The top and bottom horizontal lines indicate the normal range, which was defined as the range between the 5th and 95th percentiles of 60 healthy EBV-seropositive individuals, respectively; the dotted line in the middle is the median of this group.
In the long run, EBV-specific CD8+ T cells (Figure 2) repopulated to higher levels in comparison with their normal ranges than the entire CD8+ T-cell population (Figure 1). During the first 3 months after SCT, total CD8+ T cells were still below the normal range, and EBV-specific CD8+ T cells remained undetectable in most patients. At 6 months, the group median of total CD8+ T cells had reached the lower limit of the normal range. At that time, EBV-specific CD8+ T cells had become detectable in most patients. The group median values of GLC- and FLR-specific CD8+ T cells exceeded the corresponding median values of healthy EBV carriers, while the group median values of the remaining subsets of EBV-specific CD8+ T cells were similar to (RAK and HPV) or still below (EPL, CLG, RPP, LLD, and YPL) those of the controls (Figure 2). However, at 12 months after SCT, when most patients had achieved normal CD8+ T-cell counts, the group median levels of all EBV lytic antigen-specific CD8+ T cells (ie, GLC, RAK, and EPL) and of 3 latent antigen-specific CD8+ T cells (ie, CLG, FLR, and YPL) exceeded those of healthy EBV carriers, while the group median levels of the remaining 6 subsets of EBV-latent antigen-specific T cells were similar to those of the control group.
The kinetics of recovery of EBV-specific CD8+ T cells during the first 6 months after SCT was delayed in recipients of SCT from matched unrelated donors compared with that of SCT recipients who had not been pretreated with ATG (Figure 3). This pattern was consistently observed for the first 6 months after SCT for both lytic and latent epitope-specific CD8+ T cells, although statistical comparisons stratified by individual time points generally did not reveal significant differences. From 9 months after SCT onward, both lytic and latent epitope-specific CD8+ T-cell counts were similar in both patient groups.
The numbers of EBV-specific CD8+ T cells in 44 stem cell grafts were not significantly correlated to the kinetics of EBV-specific CD8+ T-cell repopulation during the first 6 months after SCT, even after stratification for the administration of ATG to recipients of HLA-matched unrelated donor (MUD) transplantations (data not shown).
Grades II to IV acute GVHD developed in 39 of the 61 (64%) patients; the kinetics of EBV-specific CD8+ T-cell repopulation during the first 3 months after SCT was similar as in those with grades 0 to I acute GVHD. Extensive chronic GVHD developed in 13 of the 60 (22%) patients at risk; EBV-specific CD8+ T-cell counts between 6 and 18 months after SCT were clearly delayed in these patients compared with those without or with limited chronic GVHD (data not shown).
Recovery of EBV-specific CD8+ T cells and incidence of EBV reactivation
We analyzed the interaction between the recovery of EBV-specific CD8+ T cells and EBV reactivation by evaluating SCT recipients at risk by time interval, starting from each time point of enumeration of EBV-specific CD8+ T cells (ie, 2, 3, 6, 9, 12, and 18 months after SCT) until the next time point (ie, 3, 6, 9, 12, 18, and 24 months, respectively). At each time point, only those patients were included (1) whose EBV-specific CD8+ T cells had been enumerated at that time point and (2) whose EBV load had been monitored from that time point until the next time point. Table 4 shows that the proportion of patients having recovered their EBV-specific CD8+ T cells rapidly increased as a function of time after SCT. Specifically, only 7 of 35 (20%) and 13 of 37 (35%) patients had detectable EBV-specific CD8+ T cells at 2 and 3 months, respectively; this proportion increased to 73% (22 of 30 patients) at 6 months after SCT. Concurrently, the proportion of patients reactivating their EBV decreased from 31% at 2 months and 41% at 3 months to 20% of patients at 6 months after SCT. Thereafter, the proportion of patients having recovered their EBV-specific CD8+ T cells gradually increased to 100% at 24 months, while the proportion of patients with EBV reactivations gradually decreased to 0%. This analysis highlights that most EBV reactivations occurred during the period of insufficient EBV-specific CD8+ T-cell recovery. An additional analysis further illustrated the correlation between insufficient EBV-specific CD8+ T-cell recovery and EBV reactivation during the first 6 months after SCT. Only 3 of 16 (19%) patients who had recovered their EBV-specific CD8+ T cells at 2 and/or 3 months developed EBV reactivation versus 13 of 27 (48%) patients who failed to recover their EBV-specific CD8+ T cells during that time period (P = .053). These results indicate that patients having recovered their EBV-specific CD8+ T cells at 2 to 3 months after SCT are at lower risk for EBV reactivation than those who have not yet done so.
Interaction between EBV-specific CD8+ T-cell recovery and EBV reactivation after SCT
. | Time points in months after SCT* . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 2 . | 3 . | 6 . | 9 . | 12 . | 18 . | 24 . | ||||||
| No. of patients at risk† | 60 | 54 | 40 | 32 | 26 | 18 | 9 | ||||||
| No. of patients with complete virologic and immunologic data‡ | 35 | 37 | 30 | 22 | 17 | 14 | 3 | ||||||
| No. (%) of patients with detectable EBV-specific CD8+ T cells | 7 (20) | 13 (35) | 22 (73) | 16 (73) | 15 (88) | 12 (86) | 3 (100) | ||||||
| No. (%) of patients with EBV reactivation | 11 (31) | 15 (41) | 6 (20) | 4 (18) | 2 (12) | 1 (7) | 0 (0) | ||||||
. | Time points in months after SCT* . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 2 . | 3 . | 6 . | 9 . | 12 . | 18 . | 24 . | ||||||
| No. of patients at risk† | 60 | 54 | 40 | 32 | 26 | 18 | 9 | ||||||
| No. of patients with complete virologic and immunologic data‡ | 35 | 37 | 30 | 22 | 17 | 14 | 3 | ||||||
| No. (%) of patients with detectable EBV-specific CD8+ T cells | 7 (20) | 13 (35) | 22 (73) | 16 (73) | 15 (88) | 12 (86) | 3 (100) | ||||||
| No. (%) of patients with EBV reactivation | 11 (31) | 15 (41) | 6 (20) | 4 (18) | 2 (12) | 1 (7) | 0 (0) | ||||||
Patients were evaluated in time intervals, starting from each indicated time point until the next time point. The time interval from 24 months onward was 6 months.
Numbers of patients at risk per time interval: defined by the numbers of patients who survived during each time interval.
Numbers of patients with complete virologic and immunologic data per time interval: defined by (1) availability of data on EBV-specific CD8+ T cells at the initial time point and (2) follow-up for EBV reactivation from the initial time point until the next time point.
Recovery of EBV-specific CD8+ T cells and occurrence of high-level EBV reactivation
We have shown previously that high-level EBV reactivation (ie, with viral load at least 1000 geq/mL) predisposes for EBV-LPD.11 Therefore, we studied whether or not a delayed reconstitution of EBV-specific CD8+ T cells correlated with the development of high-level EBV reactivation. To this end, 24 patients in whom EBV-specific CD8+ T cells had been enumerated prior to the onset of EBV reactivation (high level, n = 15; low level, n = 9) were compared. Only 2 of 8 (25%) patients who had recovered their EBV-specific CD8+ T cells developed high-level EBV reactivation versus 13 of 16 (81%) patients without detectable EBV-specific CD8+ T cells (P = .01). This interaction between EBV-specific CD8+ T-cell recovery and high-level EBV reactivation did not extend to total CD8+ T-cell reconstitution. The repopulation kinetics of total CD8+ T cells preceding high- or low-level EBV reactivation was similar (data not shown).
Recovery of EBV-specific CD8+ T cells and occurrence of EBV-LPD
Next, we addressed the question of whether the presence or absence of EBV-specific CD8+ T-cells improved the positive predictive value as defined by quantitative viral load. The presence of EBV-specific CD8+ T cells was analyzed in 9 patients with high-level EBV reactivation (ie, at least 1000 geq/mL) who had not been treated preemptively with rituximab. EBV-LPD developed in all 5 patients without detectable EBV-specific CD8+ T cells. In contrast, only 1 of the 4 remaining patients with detectable EBV-specific CD8+ T cells developed EBV-LPD (P = .048). However, the EBV-specific CD8+ T cells in this patient had been detected 100 days prior to the development of LPD; meanwhile, extensive chronic GVHD had developed requiring immunosuppressive treatment, which may have abolished EBV-specific immune surveillance. Thus, the positive predictive value of viral load, when combined with EBV-specific CD8+ T-cell enumeration, was 100% in patients with high-level EBV reactivation and undetectable EBV-specific CD8+ T cells. Conversely, the positive predictive value was only 25% in patients with high-level reactivation but detectable EBV-specific CD8+ T cells. Because none of the patients with low-level EBV reactivation (ie, less than 1000 geq/mL) developed EBV-LPD, the negative predictive value of low-level reactivation for EBV-LPD remained 100% and therefore was not influenced by the assessment of EBV-specific CD8+ T cells.
In all 9 patients developing EBV-LPD, this complication was treated with a combination of rituximab and interruption of immunosuppression; 3 patients received lymphocytes from their SCT donors as well. In 7 patients, EBV-LPD responded to treatment: the EBV load gradually decreased to become undetectable after a median of 17 days (range, 5-59 days) following start of treatment. In all 7 responding patients, EBV-specific CD8+ T cells became detectable; in 4 of these patients, EBV-specific CD8+ T cells recovered rapidly (ie, within 4 weeks after start of therapy), while in the other 3 patients, EBV-specific CD8+ T cells did so at a more gradual rate. The remaining 2 patients died of rapidly progressive disease (ie, at 10 and 41 days following diagnosis of EBV-LPD, respectively). One of these 2 patients was studied at 5 days following diagnosis of EBV-LPD and had no detectable EBV-specific CD8+ T cells at that time.
The numbers of transplanted EBV-specific CD8+ T cells did not correlate significantly with the occurrence of (high-level) EBV reactivation or EBV-LPD (data not shown).
Discussion
Here, we evaluated the recovery of CD8+ T cells specific for several immunodominant EBV-encoded lytic or latent antigens in recipients of partially T-cell—depleted allogeneic hematopoietic stem cell grafts. We were particularly interested in the relation between the kinetics of EBV-specific CD8+ T-cell repopulation, the incidence of (high-level) EBV reactivation (ie, viral load at least 1000 geq/mL), and the development of EBV-LPD. Most EBV reactivations occurred during the first 3 months after SCT, when EBV-specific CD8+ T cells had not yet become detectable in most patients. In particular, EBV-specific CD8+ T cells were undetectable in most patients developing high-level EBV reactivation and EBV-LPD. The absence of EBV-specific CD8+ T cells in patients with high-level EBV reactivation was significantly associated with the progression to EBV-LPD, indicating that the timely recovery of EBV-specific CD8+ T cells after SCT may protect against uncontrolled EBV reactivation resulting in EBV-LPD. Thus, the absence of EBV-specific CD8+ T cells improved the positive predictive value for EBV-LPD of a viral load of at least 1000 geq/mL from approximately 40%11 to 100%. Our results are in agreement with those of a survey in recipients of liver allografts.32 In that study, EBV-specific CD8+ T cells were detected using induction of interferon-γ production by lymphocytes upon stimulation with autologous EBV-transformed lymphoblastoid cell lines. Similarly as in the SCT recipients, the combination of increased EBV viral load and absence of EBV-specific CD8+ T cells yielded a positive predictive value for EBV-LPD of 100% in the liver transplantation recipients.
Neither the in vitro functional characteristics of the tetramer-binding CD8+ T cells nor the recovery of EBV-specific CD4+ T-cells have been addressed in the current study. However, the association between detectable EBV-specific CD8+ T cells and protection against uncontrolled EBV reactivation indicates that the recovering EBV-specific CD8+ T cells do function in vivo. This contention is further supported by our earlier observations on recovery of cytomegalovirus (CMV)—specific CD8+ T cells after partially T-cell—depleted SCT.28 In that study, the detection of CMV-specific CD8+ T cells in patients reactivating their CMV was strongly associated with in vivo function—that is, protection against CMV disease. However, EBV-specific CD8+ T cells were detected in patients suffering from the acquired immunodeficiency syndrome (AIDS) complicated by the development of EBV+ B-cell non-Hodgkin lymphoma, but these T cells had lost their function.29 This loss of EBV-specific CD8+ T-cell function correlated with low CD4+ T-cell counts and high EBV load in the AIDS patients. Such a discrepancy between numbers and function of EBV-specific CD8+ T cells may explain the high incidence of EBV reactivation in recipients of unmanipulated SCT. These patients generally have higher numbers of circulating T cells than recipients of T-cell—depleted grafts but reactivate their EBV to the same levels as the latter.11 The need to administer higher amounts of immunosuppressive drugs to prevent and treat GVHD in recipients of unmanipulated SCT may result in stronger functional inhibition of their CD4+ T cells and thereby abrogation of the indispensable help of EBV-specific CD4+ T cells to CD8+ T cells, which may contribute to the occurrence of EBV reactivation.33
The first report of EBV-specific CD8+ T-cell reconstitution after allogeneic SCT using tetramer technology20 described a rapid return of EBV-specific CD8+ T cells in the blood of recipients of unmanipulated SCT, while EBV-specific CD8+ T cells remained undetectable in recipients of T-cell—depleted or unrelated cord blood grafts in spite of the occurrence of EBV reactivation. In our cohort of recipients of partially T-cell—depleted grafts, most patients had not yet regenerated EBV-specific CD8+ T cells by 3 months after SCT, although CD8+ T cells specific for some lytic (ie, RAK) and latent (ie, FLR, HPV, and YPR) epitopes already had reached supranormal levels by 3 months in some patients. In patients with infectious mononucleosis (ie, primary EBV infection), the CD8+ T-cell response to EBV is initially mainly directed against lytic epitopes to shift toward latent antigens upon resolution of the disease.34 We did not observe this discrepancy between lytic and latent epitope-specific CD8+ T-cell repopulation kinetics following SCT. This result may be explained by the fact that the early wave of T-cell reconstitution after SCT is dependent on the T cells present in the grafts. In the stem cell grafts as well as in the peripheral blood of healthy controls, we observed a slight numeric preponderance of lytic epitope-specific CD8+ T cells over latent epitope-specific CD8+ T cells. By and large, this balance between lytic and latent epitope-specific CD8+ T cells was reflected by the kinetics of EBV-specific CD8+ T-cell regeneration during the first 6 months after SCT.
Recovery of EBV-specific CD8+ T cells was delayed in the patients who received ATG as part of their conditioning regimen. ATG has been recognized as a particularly strong risk factor for the development of EBV-LPD: increased hazard ratios ranging from 5 to more than 10 have been reported.7,8,35 Furthermore, we found, in a larger group of patients, that ATG was associated with early EBV reactivation and increased the probability to develop EBV-LPD.11 Based on the combined results of these studies, we propose that the elimination of recipient and graft-derived EBV-specific T cells by ATG leads to delayed reconstitution of EBV-specific T-cell immunity during the first months after SCT. The half-life of our rabbit ATG (ie, 8 to 9 days) is consistent with this hypothesis. Thus, our observations further support the contention that early recovery of EBV-specific T-cell immunity is critical to prevent the development of EBV-LPD. Later after SCT, the development of extensive chronic GVHD requiring prolonged immunosuppressive therapy may interfere with the regenerating EBV-specific T-cell responses and put the patients at increased risk for high-level EBV reactivation and EBV-LPD.
We found an inverse correlation between EBV reactivation and EBV-specific CD8+ T-cell reconstitution. This result is in line with previous observations.20 Using real-time plasma PCR, we already observed EBV reactivation as early as within 1 month after SCT, while the median time to the first episode of EBV reactivation was 2 months after SCT. In a different patient cohort, oropharyngeal EBV production was already detectable during the peritransplantation period by a cord blood transformation assay.36 Thus, the immunologic stimulus for an early expansion of EBV-specific T cells may already be present from the day of transplantation onward. Importantly, the EBV virions produced in the oropharynx during the peritransplantation period may infect and transform residual recipient B lymphocytes and donor B lymphocytes infused with the graft.37 We suggest that the T-cell response directed to EBV antigens expressed during the lytic cycle may play a critical role in the early phase after SCT by limiting oropharyngeal EBV production and, subsequently, B-cell transformation.
Earlier, we observed that CMV-specific CD8+ T cells being below detection limit in the partially T-cell—depleted grafts predisposed CMV-seropositive recipients for progressive CMV infection after SCT.28 Here, we did not observe such a correlation for EBV-specific CD8+ T cells and high-level EBV reactivation or EBV-LPD. In our CMV study, 5 of the 16 (31%) patients received grafts from CMV-seronegative patients versus only 2 of 44 (5%) patients in our EBV study. Obviously, no virus-specific memory T cells are transferred with the grafts from seronegative donors. Therefore, we propose that the absence or presence of virus-specific T cells in the grafts per se, rather than the numbers of transferred virus-specific T cells as determined by available tetramers, may determine the outcome of CMV or EBV reactivation after partially T-cell—depleted SCT. In this context it is also relevant to recall that the CD8+ T-cell response in most HLA-A*0201+ CMV carriers is highly skewed toward the pp65-derived epitope NLVPMVATV.38 This epitope was the focus of our CMV study.28 In contrast, EBV-specific CD8+ T-cell immunity is less focused on a single protein.2,34 We have attempted to study EBV-specific CD8+ T-cell immunity as comprehensively as currently possible, given the limitations of tetramer availability (ie, 12 epitopes derived from 7 EBV proteins restricted by 5 class I HLA alleles). Nevertheless, any correlation between EBV-specific CD8+ T cells in the grafts and outcome of EBV reactivation after SCT may have been obscured in the current study by the complex interaction between T-cell antigen receptors on the one hand and HLA alleles presenting viral epitopes on the other. Indeed, this complexity poses a limitation on the applicability of tetramer technology as a clinical routine method to enumerate EBV-specific T cells.
In conclusion, the monitoring of the recovery of EBV-specific CD8+ T cells using tetramer technology significantly enhanced the predictive value of high-level EBV reactivation (ie, at least 1000 geq/mL) for development of EBV-LPD after SCT in our patients. Thus, a more accurate identification of patients at high risk for EBV-LPD appears to be possible by combining monitoring of EBV load and enumeration of EBV-specific CD8+ T cells. The combined use of these assays may enable us to further narrow preemptive treatment and avoid overtreatment of recipients who, in spite of high-level EBV reactivation, are able to mount an immune response that controls the proliferation of EBV-infected B cells.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-10-3001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Nicoline de Leeuw for technical assistance and coordinating the study at the laboratory. We also thank Corrien Groot-van Ruijven, Dicky van Duyvenbode-Maat, and Jaco Kraan for their technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal