Abstract
The mechanisms by which S-nitrosohemoglobin (SNOHb) stimulates vasodilation are unclear and underlie the controversies surrounding the proposal that this S-nitrosothiol modulates blood flow in vivo. Among the mechanistic complexities are the nature of vasoactive species released from SNOHb and the role heme and oxygen play in this process. This is important to address since hemoglobin inhibits NO-dependent vasodilation. We compared the vasodilatory properties of distinct oxidation and ligation states of SNOHb at different oxygen tensions. The results show that SNOHb in the oxygenated state (SNOoxyHb) is significantly less efficient than SNOHb in the ferric or met oxidation state (SNOmetHb) at stimulating relaxation of isolated rat aortic rings. Using pharmacologic approaches to modulate nitrogen monoxide radical (·NO)–dependent relaxation, our data suggest that SNOoxyHb promotes vasodilation in a ·NO-independent manner. In contrast, both SNOmetHb and S-nitrosoglutathione (GSNO), a putative intermediate in SNOHb reactivity, elicit vasodilation in a ·NO-dependent process. Consistent with previous observations, an increase in sensitivity of SNOHb vasodilation at low oxygen tensions also was observed. However, this was not exclusive for this protein but applied to a range of nitrosovasodilators (including a ·NO donor [DeaNonoate], an S-nitrosothiol [GSNO], and the nitroxyl anion donor, Angelis salt). This suggests that oxygen-dependent modulation of SNOHb vasoactivity does not occur by controlling the allosteric state of Hb but is a property of vessel responsiveness to nitrosovasodilators at low oxygen tensions.
Introduction
The physiologic functions of hemoglobin (Hb) include transporting the respiratory gases oxygen (O2) and carbon dioxide (CO2) and buffering of blood pH.1,2 Allosteric regulation of O2 affinity is a central facet of these processes that allow coordinated delivery of O2 to respiring tissues and CO2 to the lungs. More recently, via formation of S-nitrosohemoglobin (SNOHb), these concepts have been extended to include regulation of nitric oxide activity (NO) in the vasculature and have led to the suggestion that Hb can modulate blood flow and other NO-dependent signaling pathways.3, 4, 5, 6, 7
Classically, Hb is associated with an inhibition of NO reactivity arising from the rapid rate of reaction between ferrous heme and the nitrogen monoxide radical ·NO. (In this text, where discussion refers specifically to the nitrogen monoxide radical, the abbreviation ·NO will be used. Where discussion refers to a nitric oxide–dependent process that may be mediated by ·NO or other redox congeners derived thereof, the abbreviation NO will be used). The reactions that occur between ·NO and Hb in the ferrous deoxygenated (deoxyHb or Fe[II]) and ferrous oxygenated (oxyHb or Fe[II]O2) states are shown in Equations 1 and 2 and form nitrosylhemoglobin (Fe[II]NO), nitrate, and met or ferric hemoglobin (metHb or Fe[III]), respectively.8
It is clear that these reactions are important when Hb is outside the confines of the red blood cell and may have a role in mediating vascular dysfunction in sickle cell disease.9, 10, 11, 12, 13 Within the red blood cell, however, diffusional constraints provided by the cell membrane and the red blood cell–free zone created by flow significantly retard entry of NO into the erythrocyte and hence reaction with Hb.11,12,14,15 These physical constraints appear to be important in preventing the red blood cell from causing excessive hypertension, although interaction of NO with erythrocytic Hb may still occur, as indicated by formation of metHb during NO inhalation or increased production of NO during inflammation.16
Alternatively, NO reactions with Hb in vivo have been discussed in the context of mechanisms that preserve and stimulate NO function in the vasculature.4,7 Central in this proposal is the bioactivity of S-nitrosohemoglobin (SNOHb), a derivative in which NO is bound to a specific cysteine residue in the β chain of the protein (β93Cys). This interaction formally involves the nitrosonium cation (NO+) bound to the thiolate anion and as such is insensitive to reactions with ferrous heme. It has been suggested that SNOHb elicits NO-dependent vasodilation in environments of low oxygen tension and thereby stimulates blood flow to these regions.4 Specifically, deoxygenation of SNOHb is followed by transfer of the NO group via a transnitrosation reaction to either erythrocytic glutathione (GSH) or thiols on the anion exchange protein (AE-1, also referred to as band 3 protein).4,7,17,18 In the case of GSH, the resultant low molecular weight S-nitrosothiol (RSNO), S-nitrosoglutathione (GSNO), is thought to then leave the red blood cell and stimulate NO signaling in the vasculature. With the anion exchange protein, the corresponding high molecular weight RSNO then transduces the NO signal out of the red blood cell by undefined mechanisms.19
However, this model is controversial, with many aspects of the proposed mechanism disputed. These include the oxygen dependence and kinetics of SNOHb reactivity with thiols,20, 21, 22, 23, 24, 25 the mechanisms of SNOHb formation, and the physiologic concentrations of these species in the human arterial and venous circulation.10,23,26, 27, 28, 29 Also, it is unclear how SNOHb in the oxy ferrous state (SNOoxyHb) can elicit ·NO-dependent signaling in the presence of ferrous heme. To circumvent this problem, it has been suggested that the vasorelaxant stimulus is not ·NO per se, but an S-nitrosothiol (eg, GSNO) that is insensitive to reaction with oxyheme.4,7 However, it has also been argued by the same group that to avoid fatal hypotension, a significant portion of the bioactive NO released from SNOHb is autocaptured by ferrous heme.17 This would, however, necessitate the formation of ·NO.
These mechanistic complexities have contributed to the debate over what role SNOHb plays in physiology.30,31 It is important to note that despite the controversies surrounding the role of SNOHb in regulating blood flow, S-nitrosation of Hb may play an important role in inflammatory diseases and the therapeutic development of Hb-based blood substitutes in which converting Hb to a species that can stimulate NO-dependent vasodilation may circumvent the marked hypertensive responses observed upon infusion of these compounds.4,32,33
In order to gain further insights into the vasoactive mechanisms of SNOHb, we investigated the nature of the vasorelaxant stimulus released by SNOHb in the presence of low molecular weight thiols and the effects that heme oxidation state and oxygen have on this process. Our data suggest that SNOHb in the oxygenated state promotes vessel relaxation by mechanisms that are both GSNO and ·NO-independent. In contrast, the mechanisms of SNOHb in the ferric oxidation (SNOmetHb) state relax vessels in a process that is ·NO-dependent. Furthermore, our data clearly show that the oxygen dependence of SNOHb-mediated relaxation can be explained by the increased sensitivity of vessels to nitrosovasodilators at lower oxygen tensions. These data are discussed from the perspective of the possible biologic role of SNOHb.
Materials and methods
All reagents were purchased from Sigma Chemical (St Louis, MO). S-nitrosocysteine (SNOC) and S-nitroso-N-acetylpenicillamine (SNAP) were synthesized as previously reported.22 DeaNonoate and GSNO were purchased from Alexis Biochemicals (Carlsbad, CA). Human HbA was purified as described below.
Preparation of hemoglobin and S-nitrosohemoglobin
Human hemoglobin was prepared as previously described and stored as the carbonmonoxy derivative.22 Briefly, erythrocytes were washed, lysed, and Hb purified by ammonium sulfate precipitation at 4°C, dialyzed versus 3 mM sodium phosphate buffer, pH 7.4, and stored in liquid N2. Carbon monoxide was purged routinely through solutions to convert Hb into the carbon monoxide–bound state (to prevent oxidation to metHb). OxyHb was prepared by photolysis under oxygen at 4°C with no detectable formation of metHb and had similar oxygen affinities to high-performance liquid chromatography–purified Hb (not shown), suggesting organic phosphates are not present in the Hb preparations used in this study. MetHb was prepared by addition of a 1.2 to 1.4 molar excess of K3Fe(CN)6 to oxyHb and then purified by gel filtration chromatography using sephadex G-25 (10 × 1 mL, pre-equilibrated with phosphate-buffered saline (PBS), pH 7.4). SNOoxyHb was synthesized by incubating oxyHb (0.2-2 mM; all stated Hb concentrations are in terms of heme) with SNAP (2-20 mM) for 15 to 30 minutes at 20°C in 2% borate + 1 mM diethylenetriaminepentaacetic acid (DTPA). For some experiments S-nitrosocysteine (SNOC, 2-20 mM) was used instead, and incubations with Hb carried out at 20°C for 5 minutes in 2% borate + 1 mM DTPA. For SNOmetHb preparation, metHb was S-nitrosated as described above with oxyHb. All solutions were protected from light. Hb was separated from SNOC or SNAP by gel filtration using sephadex G-25 (10 × 1 mL, pre-equilibrated with PBS + 1 mM DTPA, pH 7.4). Using SNAP no significant metHb (measured by visible spectroscopy) was formed in SNOoxyHb preparations. Any preparations of SNOoxyHb that contained metHb (> 2% to 3% determined using the Winterbourn equations34 ) were discarded. OxyHb concentrations were determined by UV-Vis spectroscopy using the extinction coefficient per heme group ϵ577nm = 14.6 mM–1 cm–1.22 MetHb concentrations were determined by reduction to deoxyHb using sodium dithionite and S-nitrosation of Hb quantified by the Saville reaction with all mercury-dependent nitrite formation being protein precipitable. SNOHb preparations varying between 0.4 and 2 SNO per Hb tetramer were used in this study and are indicated in the figure legends.
Vessel reactivity studies
Isometric tension in isolated rat thoracic aortic ring segments was measured as described previously.35 The rat was humanely killed, and the aorta was excised and cleansed of fat and adhering tissue. Vessels were then cut into individual ring segments (2-3 mm wide) and suspended from a force-displacement transducer in a tissue bath. Ring segments were bathed in a bicarbonate-buffered Krebs-Henseleit (KH) solution of the following composition (mM): NaCl 118; KCl 4.6; NaHCO3 27.2; KH2PO4 1.2; MgSO4 1.2; CaCl2 1.75; Na2 EDTA (ethylenediaminetetraacetic acid) 0.03, and glucose 11.1. A passive load of 2 grams was applied to all ring segments and maintained at this level throughout the experiments. At the beginning of each experiment, indomethacin-treated ring segments were depolarized with KCl (70 mM) to determine the maximal contractile capacity of the vessel. Rings were then washed extensively and allowed to equilibrate. For subsequent experiments, vessels were submaximally contracted (50% of KCl response) with phenylephrine (PE) (∼3 × 10–8-10–7 M). In some experiments L-N-nitroarginine methyl ester (L-NAME) (1 mM) also was added to inhibit eNOS and endogenous NO production. This prevents any confounding effects on vascular tone that may occur due to reactions between added Hb and endogenous NO. After tension development reached a plateau, GSH (100 μM), superoxide dismutase (SOD) (100 U/mL), and then NO donors or Hb derivatives were added cumulatively to the vessel bath (as described in figure legends) and effects on tension monitored. At the end of each experiment, vessel viability was monitored by determining relaxation in response to sodium nitroprusside. No changes in vessel viability were observed in any of the experimental conditions. Real-time data were downloaded to an IBM PC and analyzed using commercially available software. Due to inherent differences in sensitivity of different vessel preparations, relaxation responses to different stimuli (eg, SNOoxyHb vs SNOmetHb or high vs low oxygen) were compared using vessels obtained from the same preparation and fold-difference in the EC50s (concentration at which 50% relaxation was observed) calculated. “n” values correspond to individual experiments conducted using different vessel and SNOHb preparations. Within a given experiment, each condition was repeated 2 to 4 times.
Low oxygen tension studies
For experiments at low oxygen tensions, a similar protocol described by McMahon et al17 was followed. Vessel baths were perfused with 5% CO2 95% N2 for 15 minutes prior to addition of relaxation stimuli. These studies were performed under “high oxygen” (perfusion with 95% O2, 5% CO2) first, followed by “low oxygen” (perfusion with 95% N2, 5% CO2), or vice versa. No differences in results were observed using either protocol. Between each condition, vessels were washed extensively and re-equilibrated for 30 minutes in KH buffer at 37°C. Oxygen tensions in the vessel baths perfused with “high” or “low” oxygen gas mixtures were determined using an oxygen electrode (Instech/YSI, Yellow Springs, OH). Oxygen concentrations were calibrated by addition of sodium dithionite to baths perfused with room air (37°C) and set to 195 μM O2 (calculated from oxygen solubility constants at 37°C for Krebs buffer). Perfusion with high oxygen gas increased concentrations to 902 μM. Perfusion with low oxygen gas decreased concentrations to 14.5 μM. The extent of Hb deoxygenation was determined by measurement of the oxygen-binding curve to SNOHb and direct measurement of Hb ligation states using visible spectroscopy. Oxygen-binding curves were determined as previously described.22 The degree of Hb deoxygenation was determined by addition of SNOoxyHb (3 μM heme) to the vessel bath perfused with the low oxygen gas mixture. Aliquots of SNOHb were taken using gas-tight syringes and transferred to a sealed cuvette in which the gas had been replaced with the 5% N2, 95% CO2 mixture and spectrum (500-700 nm) measured. The SNOHb solution was then exposed to room air (to form oxygenated Hb), sodium dithionite was added (to form fully deoxyHb), and the solution spectra measured.
Statistics
All results are reported as the mean ± SEM. Statistical analysis was performed using Origin Software (Northampton, MA). Differences between the groups were assessed by one-way analysis of variance (ANOVA).
Results
Effect of heme redox state on SNOHb-dependent relaxation
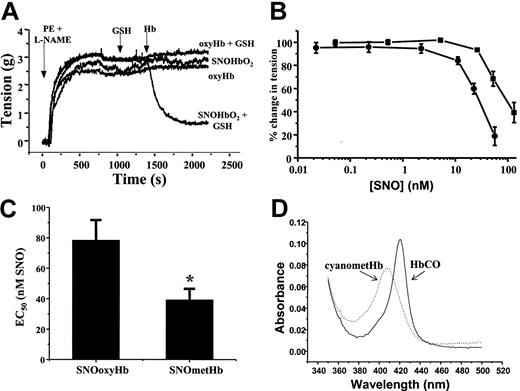
To investigate the mechanisms by which SNOHb elicits NO-dependent signaling, we compared vasodilatory effects of SNOoxyHb and SNOmetHb using isolated rat thoracic aorta. Figure 1A shows representative vessel tension traces measured as a function of time. Aortas were precontracted with both phenylephrine (PE) and the nitric oxide synthase inhibitor L-NAME, which was added to prevent vasoconstriction that occurs due to scavenging of endogenous ·NO by added oxyheme. Under these conditions, addition of SNOoxyHb alone, or oxyHb in the presence or absence of GSH, had no effect on vessel tone. Consistent with previous reports, however,4,17,24 in the presence of GSH, SNOHb stimulated vasorelaxation as indicated by a decrease in vessel tension (Figure 1A). Similar data were observed with SNOmetHb, although the kinetics of the relaxation process appeared to be faster with metheme (data not shown). To directly compare the vessel reactivity of SNOHb in the oxyferrous and ferric oxidation states, the efficiency of vessel relaxation mediated by these derivatives was determined. Figure 1B shows that both SNOoxyHb and SNOmetHb stimulate vessel relaxation in a dose-dependent manner, with SNOoxyHb being 2.2 ± 0.3-fold (mean ± SEM, n = 8) less efficient at promoting vasodilation compared with SNOmetHb. This is indicated by the right shift in the dose-response curve (Figure 1B) and determination of the fold difference in EC50 for relaxation (Figure 1C). To determine the heme oxidation and ligation state after stimulation of relaxation, aliquots were taken from the respective vessel baths and UV-Visible spectrum monitored. In the case of SNOoxyHb a typical spectrum of oxyHb was obtained that was transformed to the carbonmonoxy derivative upon bubbling of carbon monoxide through the solution (Figure 1D). In the case of SNOmetHb, a typical metHb spectrum (at pH 7.4) was obtained and was converted to the cyano-ferric complex spectrum upon addition of potassium cyanide (Figure 1D). These spectra and associated changes upon addition of selective ferrous and ferric ligands were identical to those obtained from SNOHb samples prior to the addition to the vessel baths and reactions with GSH. These data indicate that during the course of relaxation, both SNOoxyHb and SNOmetHb remain in their respective oxidation states and were still competent to bind ligands. Furthermore, no formation of the CO derivative was observed upon addition of CO to SNOmetHb, indicating that reduction of metHb to ferrous Hb was not occurring during the relaxation response (not shown). In the case of SNOoxyHb, therefore, the ferrous heme is still capable of scavenging and inhibiting ·NO-dependent vasodilation.
The heme redox state modulates SNOHb-mediated vasorelaxation. SNOHb with defined oxidation states and differing extents of S-nitrosation were added to isolated rat thoracic aorta and effects on tension determined as described in “Materials and methods.” (A) Representative vessel tension traces and demonstration of the ability of SNOoxyHb (1.4SNO per tetramer) to mediate relaxation responses in the presence of glutathione (GSH). Phenylephrine and L-NAME were added to vessel baths as indicated to induce a contraction. Once a steady tension had developed, GSH (100 μM) was added, followed by different Hb derivatives (1 μM in heme final concentration) as indicated. Addition of SNOoxyHb alone, GSH alone, or oxyHb + GSH had no effect on vessel tone, whereas SNOoxyHb + GSH stimulated vasodilatation. (B) Comparison of the dose response for relaxation induced by SNOoxyHb (2SNO per Hb tetramer, ▪) and SNOmetHb (1 SNO per Hb tetramer, •) expressed as the concentration of S-nitrosothiol (SNO) added. The range of heme concentrations over which a relaxation response was observed was approximately 10 to 300 nM for both SNOoxyHb and SNOmetHb. (C) The EC50s (SNO concentration at which SNOoxyHb and SNOmetHb stimulate 50% of the maximal relaxation response). Values represent means ± SEM (n = 8) and include data from SNOHb preparations in which the concentration of SNO varied from 0.5 to 2 SNO per Hb tetramer, *P < .03 versus SNOoxyHb group. Both panels B and C demonstrate that SNOmetHb is a more efficient vasodilator compared with SNOoxyHb. (D) The UV-Vis spectra of SNOoxyHb and SNOmetHb taken from the vessel chambers after relaxation had been stimulated. For SNOoxyHb, CO gas was bubbled through the solution (forming HbCO) and for SNOmetHb, potassium cyanide (50 μM) was added (forming cyanometHb) and spectra measured as shown. Experiments were performed on 8 different vessel preparations in KH buffer equilibrated with 95% O2,5%CO2, at 37°C.
The heme redox state modulates SNOHb-mediated vasorelaxation. SNOHb with defined oxidation states and differing extents of S-nitrosation were added to isolated rat thoracic aorta and effects on tension determined as described in “Materials and methods.” (A) Representative vessel tension traces and demonstration of the ability of SNOoxyHb (1.4SNO per tetramer) to mediate relaxation responses in the presence of glutathione (GSH). Phenylephrine and L-NAME were added to vessel baths as indicated to induce a contraction. Once a steady tension had developed, GSH (100 μM) was added, followed by different Hb derivatives (1 μM in heme final concentration) as indicated. Addition of SNOoxyHb alone, GSH alone, or oxyHb + GSH had no effect on vessel tone, whereas SNOoxyHb + GSH stimulated vasodilatation. (B) Comparison of the dose response for relaxation induced by SNOoxyHb (2SNO per Hb tetramer, ▪) and SNOmetHb (1 SNO per Hb tetramer, •) expressed as the concentration of S-nitrosothiol (SNO) added. The range of heme concentrations over which a relaxation response was observed was approximately 10 to 300 nM for both SNOoxyHb and SNOmetHb. (C) The EC50s (SNO concentration at which SNOoxyHb and SNOmetHb stimulate 50% of the maximal relaxation response). Values represent means ± SEM (n = 8) and include data from SNOHb preparations in which the concentration of SNO varied from 0.5 to 2 SNO per Hb tetramer, *P < .03 versus SNOoxyHb group. Both panels B and C demonstrate that SNOmetHb is a more efficient vasodilator compared with SNOoxyHb. (D) The UV-Vis spectra of SNOoxyHb and SNOmetHb taken from the vessel chambers after relaxation had been stimulated. For SNOoxyHb, CO gas was bubbled through the solution (forming HbCO) and for SNOmetHb, potassium cyanide (50 μM) was added (forming cyanometHb) and spectra measured as shown. Experiments were performed on 8 different vessel preparations in KH buffer equilibrated with 95% O2,5%CO2, at 37°C.
Effect of oxyHb on GSNO-, SNOoxyHb-, and SNOmetHb-dependent relaxation
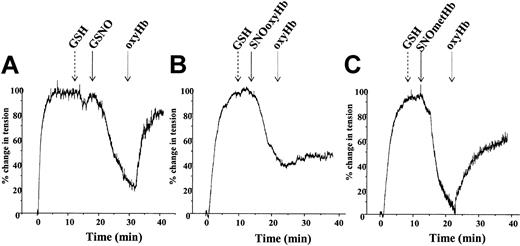
To test for a role of ·NO in mediating SNOHb-dependent relaxation, the effect of adding oxyHb or oxymyoglobin (oxyMb) to relaxed ring segments was tested. In these experiments contraction induced by addition of oxyheme is consistent with a role for ·NO, similar to that observed when endothelial-dependent relaxation is stimulated by acetylcholine or exogenous administration of nitrosovasodilators.36,37 Figure 2 shows representative traces for relaxation stimulated by GSNO (Figure 2A), SNOoxyHb (Figure 2B), and SNOmetHb (Figure 2C), respectively, in the presence of GSH and the subsequent addition of oxyHb (10 μM heme). Addition of oxyHb reversed relaxation stimulated by GSNO and SNOmetHb, consistent with a role for ·NO as the vasorelaxant stimulus. No addition of oxyHb led to a continuation of the relaxation response (as observed in Figure 1A). Similar effects were observed using oxyMb (from horse heart that lacks cysteine residues) to scavenge ·NO, and also with oxyHb in which the β93cys residues were alkylated with N-ethylmaleimide (NEM) (not shown) suggesting that the inhibitory effects of Hb are not due to reactions of NO-derived species with the β93cys residue. Addition of metHb or metMb had no affect on vessel tension, consistent with the relatively poor reactivity of ferric heme with ·NO. Interestingly, addition of oxyHb to vessels in which SNOoxyHb was used to elicit relaxation did not induce contraction (Figure 2, trace B), suggesting that ·NO is not playing a significant role in this reaction system. Similar results were obtained using oxyMb as the ·NO scavenging species. Figure 3 shows the extent to which either oxyHb or oxyMb reversed GSNO-, SNOoxyHb-, and SNOmetHb-dependent relaxation. Both oxyheme proteins completely reversed GSNO-dependent relaxation. In the case of SNOmetHb, approximately 60% of vasorelaxant response was inhibited, whereas only a small inhibition (5%) of SNOoxyHb-dependent relaxation was observed. These data indicate that SNOoxyHb and SNOmetHb promote vasodilation via different mechanisms. Furthermore, these data also suggest that with cell-free SNOoxyHb, GSNO does not play a significant role in the observed vasorelaxant responses.
Differential role of ·NO in GSNO-, SNOoxyHb-, and SNOmetHb-mediated vessel relaxation. Vessels were precontracted and tension was measured as described in “Materials and methods.” Relaxation was stimulated by addition of GSH (100 μM), followed by addition of either (A) GSNO (50 nM), (B) SNOoxyHb (1 μM heme, 250 nM SNO), or (C) SNOmetHb (1 μM heme, 250 nM SNO). Once relaxation exceeded 50% of maximal tension, oxyHb (10 μM) was added to scavenge ·NO and effects on vessel tension monitored. Panels A-C show representative traces. All experiments were conducted in KH buffer, equilibrated with 95% O2,5%CO2,at 37°C.
Differential role of ·NO in GSNO-, SNOoxyHb-, and SNOmetHb-mediated vessel relaxation. Vessels were precontracted and tension was measured as described in “Materials and methods.” Relaxation was stimulated by addition of GSH (100 μM), followed by addition of either (A) GSNO (50 nM), (B) SNOoxyHb (1 μM heme, 250 nM SNO), or (C) SNOmetHb (1 μM heme, 250 nM SNO). Once relaxation exceeded 50% of maximal tension, oxyHb (10 μM) was added to scavenge ·NO and effects on vessel tension monitored. Panels A-C show representative traces. All experiments were conducted in KH buffer, equilibrated with 95% O2,5%CO2,at 37°C.
Contractile effects of oxyHb and oxyMb on GSNO- and SNOmetHb-dependent, but not SNOoxyHb-dependent, vessel relaxation. Contractile effects of oxyHb (10 μM, ▪) or oxyMb (10 μM, ▦) on GSNO-, SNOoxyHb-, and SNOmetHb-mediated relaxation of rat thoracic aorta were determined as indicated in Figure 2. Contractile effects are expressed as the percentage reversal of the maximal contraction obtained with phenylephrine and L-NAME. Data represent mean ± SEM, n = 3. All experiments were conducted in KH buffer, equilibrated with 95% O2, 5% CO2,at 37°C.
Contractile effects of oxyHb and oxyMb on GSNO- and SNOmetHb-dependent, but not SNOoxyHb-dependent, vessel relaxation. Contractile effects of oxyHb (10 μM, ▪) or oxyMb (10 μM, ▦) on GSNO-, SNOoxyHb-, and SNOmetHb-mediated relaxation of rat thoracic aorta were determined as indicated in Figure 2. Contractile effects are expressed as the percentage reversal of the maximal contraction obtained with phenylephrine and L-NAME. Data represent mean ± SEM, n = 3. All experiments were conducted in KH buffer, equilibrated with 95% O2, 5% CO2,at 37°C.
Effect of SOD on GSNO-, SNOoxyHb-, and SNOmetHb-dependent relaxation
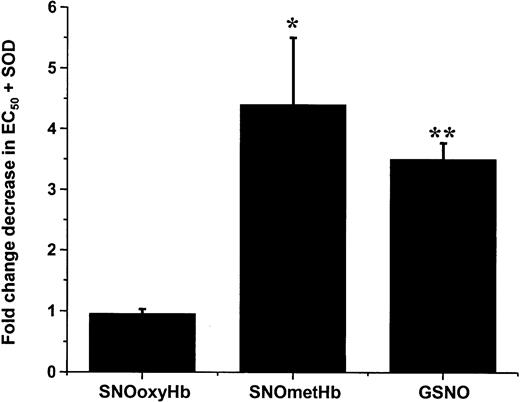
To further probe the nature of the NO derivative that mediates SNOHb-dependent relaxation, we investigated the effect of superoxide dismutase (SOD). By virtue of scavenging superoxide and hence preventing its reaction with ·NO, SOD has been shown to enhance vasorelaxation mediated by ·NO.37,38 We predicted, therefore, that SOD will enhance (ie, lower the EC50) relaxation for any vasorelaxant stimulus that involves formation of ·NO. Figure 4 shows the fold change in EC50 for GSNO, SNOoxyHb, and SNOmetHb relaxation in the presence of SOD. SOD enhanced GSNO- and SNOmetHb-dependent relaxation by approximately 4-fold but had no effect on SNOoxyHb-dependent responses. Taken together with Figures 2 and 3, these data indicate that GSNO and SNOmetHb elicit NO signaling by release of ·NO, whereas SNOoxyHb induces vasorelaxation by ·NO-independent mechanisms.
Differential effects of SOD on relaxation stimulated by SNOoxyHb, SNOmetHb, and GSNO. Vessels were precontracted and tension was measured as described in “Materials and methods.” Upon reaching a basal contractile tone, SOD (100 U/mL) or PBS vehicle control was added, followed by GSH (100 μM). Dose-dependent relaxation was then stimulated by addition of either SNOoxyHb (0-500 nM SNO), SNOmetHb (0-500 nM SNO), or GSNO (0-100 nM). The EC50s for relaxation with or without SOD were determined and the fold change in relaxation efficiency was calculated. SOD increased the relaxation efficiency of SNOmetHb and GSNO by approximately 3- to 4-fold but had no effect on SNOoxyHb-mediated responses. Values represent means ± SEM (n = 4-6) for SNOoxyHb and SNOmetHb. *P < .02 versus SNOoxyHb group and mean ± SEM (n = 3) for GSNO. **P < .001 versus SNOoxyHb group.
Differential effects of SOD on relaxation stimulated by SNOoxyHb, SNOmetHb, and GSNO. Vessels were precontracted and tension was measured as described in “Materials and methods.” Upon reaching a basal contractile tone, SOD (100 U/mL) or PBS vehicle control was added, followed by GSH (100 μM). Dose-dependent relaxation was then stimulated by addition of either SNOoxyHb (0-500 nM SNO), SNOmetHb (0-500 nM SNO), or GSNO (0-100 nM). The EC50s for relaxation with or without SOD were determined and the fold change in relaxation efficiency was calculated. SOD increased the relaxation efficiency of SNOmetHb and GSNO by approximately 3- to 4-fold but had no effect on SNOoxyHb-mediated responses. Values represent means ± SEM (n = 4-6) for SNOoxyHb and SNOmetHb. *P < .02 versus SNOoxyHb group and mean ± SEM (n = 3) for GSNO. **P < .001 versus SNOoxyHb group.
Effect of oxygen on vasodilation mediated by SNOHb
It has been argued that SNOHb-mediated relaxation is regulated by the allosteric state of the Hb, with deoxygenation allowing transfer of NO from SNOHb to GSH or AE-1.7,17,18 An important observation that led to this proposal was the enhanced vasodilatory activity of SNOHb at low oxygen tensions. However, interpretation of this data is complicated by the enhanced responsiveness of vessels to vasodilators at low oxygen tensions and, as reported previously, if these responses are normalized, the vasoactivity of SNOHb is not modulated by oxygen.24 To further investigate the mechanisms underlying the oxygen dependence and evaluate the nature of the vasorelaxant species from SNOHb under conditions where the “T” state of Hb is favored, vessel relaxation in response to SNOoxyHb, SNOmetHb, GSNO, and mechanistically distinct nitrosovasodilators DeaNonoate (which releases ·NO) and Angelis salt (AS, which releases the nitroxyl anion [NO–]) were conducted at low oxygen tensions. The oxygen tension in the vessel bath perfused with 95% N2, 5% CO2 decreased to 14.5 μM (11.7 mmHg). To determine the extent of deoxygenation that occurred under these conditions, the oxygen-binding curve of SNOHb was determined (Figure 5A). Consistent with previous findings, a sigmoidal-binding curve was obtained with a p50 of 9.3 mmHg at 37°C.22 An oxygen tension of 11.7 mmHg would be expected to cause approximately 40% deoxygenation. To determine this directly, using a gas-tight syringe SNOHb was transferred into a sealed cuvette in which the gas had been replaced with the 5%N2, 95% CO2 mixture and spectrum (500-700 nm) measured (Figure 5B, —). Figure 5B (- -) represents the spectrum of the corresponding SNOHb solution after exposure to air (ie, oxygenated Hb), and spectrum represented by the dotted line (···) represents the spectrum obtained after addition of sodium dithioinite (ie, deoxygenated Hb). The intermediacy of the spectrum of SNOHb obtained from the vessel bath corresponds to 50% deoxygenation of Hb and is consistent with the measured oxygen tensions and oxygen-binding curves. These data demonstrate that an “R” to “T” transition within the physiologic range is occurring under these experimental conditions. Figure 5C shows representative traces for SNOHb-dependent relaxation at high and low oxygen tensions. The left shift in dose response curve at low oxygen tensions demonstrates a more efficient relaxation response. Similar responses were observed for the other nitrosovasodilators tested. Table 1 shows that for all the NO donors tested, the EC50′s for relaxation decreased (ie, more efficient) at lower oxygen tensions.
Enhanced vasoactivity of SNOHb at low oxygen tensions. The degree of Hb deoxygenation that occurs in vessel baths perfused with 95% N2, 5% CO2 was determined by measurement of the oxygen tensions (see “Materials and methods”) and of the oxygen-binding curve to SNOHb at 37°C; V indicates fractional saturation (A). Panel B shows the visible spectra of SNOoxyHb (3 μM heme, 0.4SNO per tetramer) obtained from vessel baths perfused with 95% N2,5%CO2 (—), SNOoxyHb subsequently exposed to air (---), and after addition of sodium dithionite (....). Panel C shows concentration-dependent relaxation responses to SNOmetHb (1 SNO per Hb tetramer) in the presence of GSH (100 μM) under low oxygen (•) or high oxygen (▪) conditions. Fold changes in EC50 for relaxation were determined as described in “Materials and methods” and shown in Table 1.
Enhanced vasoactivity of SNOHb at low oxygen tensions. The degree of Hb deoxygenation that occurs in vessel baths perfused with 95% N2, 5% CO2 was determined by measurement of the oxygen tensions (see “Materials and methods”) and of the oxygen-binding curve to SNOHb at 37°C; V indicates fractional saturation (A). Panel B shows the visible spectra of SNOoxyHb (3 μM heme, 0.4SNO per tetramer) obtained from vessel baths perfused with 95% N2,5%CO2 (—), SNOoxyHb subsequently exposed to air (---), and after addition of sodium dithionite (....). Panel C shows concentration-dependent relaxation responses to SNOmetHb (1 SNO per Hb tetramer) in the presence of GSH (100 μM) under low oxygen (•) or high oxygen (▪) conditions. Fold changes in EC50 for relaxation were determined as described in “Materials and methods” and shown in Table 1.
Low oxygen tensions increase the efficiency of vasodilation stimulated by structurally and mechanistically distinct nitrosovasodilators
Nitrosovasodilator . | Fold decrease in EC50 for relaxation at low oxygen tensions . |
|---|---|
| SNOoxyHb | 3.7 ± 1.1 |
| SNOmetHb | 2.91 ± 1.3 |
| GSNO | 3.2 ± 0.8 |
| DeaNonoate | 14.7 ± 1.1 |
| Angelis salt | 3.3 |
Nitrosovasodilator . | Fold decrease in EC50 for relaxation at low oxygen tensions . |
|---|---|
| SNOoxyHb | 3.7 ± 1.1 |
| SNOmetHb | 2.91 ± 1.3 |
| GSNO | 3.2 ± 0.8 |
| DeaNonoate | 14.7 ± 1.1 |
| Angelis salt | 3.3 |
Concentration-dependent relaxation responses for SNOoxyHb, SNOmetHb, GSNO (0-100 nM), DeaNonoate (0-25 nM), and Angelis salt (AS, 0-200 nM) were determined as described in “Materials and methods” under either low oxygen or high oxygen conditions. For SNOHb, concentrations ranged from 10-300 nM heme with 1 SNO per tetramer. Fold changes in EC50 for relaxation were thus determined. Values represent the fold decrease in EC50 under hypoxic conditions relative to hyperoxic conditions. Data represent mean ± SEM (n = 3).
The concentrations of heme in the vessel bath that led to vasodilation were in the nM to μM range. Over these concentrations significant dimerization of Hb can occur (Kd approximately 3 μM heme). This in turn would affect deoxygenation and the “R” to “T” transition. To investigate the possible role of dimers, vessel dilation responses at high and low oxygen tensions were measured after addition of SNOoxyHb (0.5 SNO per tetramer) to yield final heme concentrations of 1 μM and 10 μM, respectively. At the latter concentration, tetrameric Hb will be more predominant than dimeric Hb. The relaxation efficiency increased at low oxygen tensions for both 1 μM (3.4% ± 1.2% to 16.2% ± 3.4%, high versus low oxygen, respectively) and 10 μM heme (16.9% ± 2.3% to 34.9% ± 7.6%, high versus low oxygen, respectively). Furthermore, inclusion of inositol hexaphosphate (100 μM), which stabilizes the “T” state and thus reduces dimerization, had no effect on the relaxation responses at 1 μM heme (3.8% to 18.5% at high versus low oxygen tensions, respectively). These data demonstrate that vasorelaxant responses at different oxygen tensions do not appear to be modulated by the Hb dimer-tetramer equilibrium.
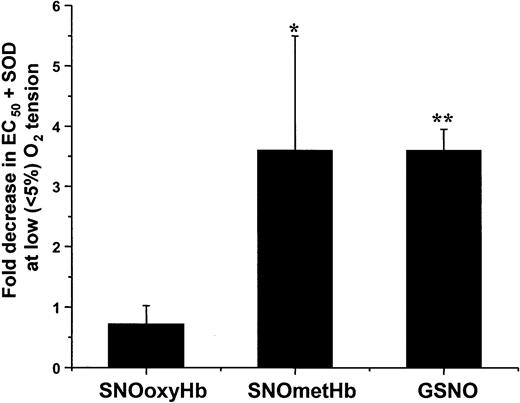
The effect of oxygen on the mechanisms of SNOHb-mediated relaxation was further tested by using SOD. Figure 6 shows that similar to responses at high oxygen, SOD decreased the EC50 for relaxation mediated by SNOmetHb or GSNO by 3- to 4-fold under low oxygen conditions but had no effect on the responses stimulated by SNOoxyHb.
Effect of SOD on relaxation stimulated by SNOoxyHb, SNOmetHb, and GSNO at low oxygen tensions. Vessels were exposed to low oxygen by perfusion of baths with 5% CO2, 95% N2 for 15 minutes prior to addition of relaxation stimuli. The effects of SOD (100 U/mL) on concentration-dependent relaxation stimulated by SNOoxyHb (0-500 nM SNO), SNOmetHb (0-500 nM SNO), or GSNO (0-100 nM) were then measured as described in the legend to Figure 4. Data represent the fold decreases in EC50 for relaxation observed in the presence of SOD. Data represent means ± SEM (n = 3-5). *P < .2 versus SNOoxyHb. **P < .001 versus SNOoxyHb group.
Effect of SOD on relaxation stimulated by SNOoxyHb, SNOmetHb, and GSNO at low oxygen tensions. Vessels were exposed to low oxygen by perfusion of baths with 5% CO2, 95% N2 for 15 minutes prior to addition of relaxation stimuli. The effects of SOD (100 U/mL) on concentration-dependent relaxation stimulated by SNOoxyHb (0-500 nM SNO), SNOmetHb (0-500 nM SNO), or GSNO (0-100 nM) were then measured as described in the legend to Figure 4. Data represent the fold decreases in EC50 for relaxation observed in the presence of SOD. Data represent means ± SEM (n = 3-5). *P < .2 versus SNOoxyHb. **P < .001 versus SNOoxyHb group.
Potential role of nitroxyl anion in SNOHb-mediated vasodilation
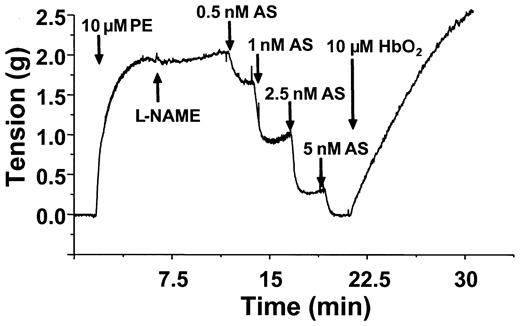
The data presented above point to a distinct NO-derived vasodilator being produced from SNOoxyHb interactions with GSH. One possible intermediate is the nitroxyl anion (NO–), which may be formed via a concerted 2-electron transfer reaction between GSH and the S-nitroso moiety of SNOHb (Equation 4).39,40 The other reaction product in this case is glutathionylated Hb, which has been previously detected in this reaction system.24 To probe whether NO– is playing a role in the relaxation responses elicited by SNOoxyHb, the effects of oxyHb on vasorelaxation mediated by the NO– donor Angelis salt was tested. Figure 7 shows representative time courses of relaxation induced by Angelis salt and effects of oxyHb. Angelis salt dose-dependently stimulated relaxation (as indicated by a decrease in tension). However, similar to the effects of relaxation mediated by GSNO and SNOmetHb, oxyHb reversed the relaxation induced by Angelis salt.
OxyHb reverses Angelis Salt–mediated relaxation. Vessels were precontracted and tension measured as described in “Materials and methods.” Relaxation (indicated by a decrease in tension) was stimulated by addition of GSH (100 μM), followed by increasing concentrations of Angelis salt (AS) as indicated. OxyHb (10 μM) was added as indicated and effects on vessel tension monitored. A representative vessel tracing from 3 separate experiments is shown. Experiments were conducted in KH buffer, equilibrated with 95% O2,5%CO2,at 37°C.
OxyHb reverses Angelis Salt–mediated relaxation. Vessels were precontracted and tension measured as described in “Materials and methods.” Relaxation (indicated by a decrease in tension) was stimulated by addition of GSH (100 μM), followed by increasing concentrations of Angelis salt (AS) as indicated. OxyHb (10 μM) was added as indicated and effects on vessel tension monitored. A representative vessel tracing from 3 separate experiments is shown. Experiments were conducted in KH buffer, equilibrated with 95% O2,5%CO2,at 37°C.
Discussion
Much interest surrounds the mechanisms by which SNOHb may modulate the vascular functions of NO. In the original hypothesis proposed by Stamler and colleagues, SNOHb is formed upon oxygenation of nitrosylHb (HbNO) in the lungs, and then, through reactions with thiols, promotes NO-dependent vasorelaxation or inhibition of platelet aggregation in the systemic circulation.3,5, 6, 7 Furthermore, this effect is regulated through an allosteric mechanism by oxygen such that NO transfer from SNOHb to the vasculature occurs only upon deoxygenation of Hb. This provides a coordinated mechanism by which the reactions between NO, oxygen, and Hb regulate blood flow and hence oxygen delivery. Despite its elegance, several aspects of this hypothesis, ranging from mechanisms of SNOHb formation to mechanisms by which SNOHb elicits vasodilation, have been disputed.10,16,21,24, 25, 26, 27, 28, 29
In this study we investigated the effect of heme redox state and oxygen on SNOHb-mediated vasodilation and the intermediates involved in this process. The increased efficiency by which erythrocytic or cell-free SNOHb stimulates vasodilation at low oxygen tensions is a central observation to the proposal that in vivo, deoxygenation of SNOHb is an important regulatory element of its biologic function. These data have been reproduced by others and in this study (Figure 5; Table 1). We have extended these latter observations and show increases in relaxation efficiency for Angelis salt, GSNO, and the ·NO-donor compound (DeaNonoate) at low oxygen. These nitrosovasodilator compounds were chosen for comparison since they are structurally and mechanistically distinct. Furthermore, none of these NO-donor compounds are subject to oxygen-dependent allosteric regulation. The increases in efficiency of the relaxation response for SNOHb, GSNO, DeaNonoate, and AS suggest therefore that the more efficient vasodilatory responses observed for SNOHb at low oxygen tensions is a function of the enhanced responsiveness of vessels to nitrovasodilators under hypoxic conditions. These data are consistent with and extend on those previously reported24,25 and negate such oxygen-dependent responses as providing conclusive evidence for an allosteric-based mechanism for SNOHb-mediated vasodilation. Furthermore, in the presence of GSH concentrations that are within the physiologic range in the red blood cell, “R” state SNOHb (ie, under oxygenated conditions) is still able to promote vasodilation. Taken together, these data suggest that Hb deoxygenation is not critical in mediating SNOHb-dependent vasodilation.
How the vasoactive mechanisms of SNOHb are modulated by the heme redox state is not known. SNOmetHb is more efficient at promoting vasodilation than SNOoxyHb, and similar data have been reported regarding the antiplatelet effects of SNOHb.6 The simplest interpretation of these data is the relatively poor reactivity of ferric heme, with ·NO allowing relaxation or antiplatelet responses to occur. In the case of SNOoxyHb, however, rapid scavenging of ·NO by the ferrous heme would effectively decrease the bioavailable concentration of the NO stimulus. However, both SNOoxyHb and SNOmetHb stimulate vasodilation, suggesting that both ·NO-dependent and ·NO-independent processes are activated upon addition of GSH to SNOHb. This may occur via previously described reaction pathways in which thiols have been shown to either transnitrosate or reduce RSNOs in 1 or 2 electron steps.39, 40, 41, 42 The oxidation state of SNOHb in vivo is not known, but recent insights suggest that SNOHb is more autoxidizable, suggesting it will be in the ferric oxidation state.43 Interestingly, electron transfer reactions between the heme and β93cys residue in Hb have been demonstrated.44,45 Modifying the β93cys modulates these processes, although the effects S-nitrosating this residue remain to be investigated in detail and may provide important insights into the mechanisms of SNOHb bioactivity.
It has been proposed that SNOHb elicits vasodilation in a manner that is not inhibitable by oxyheme, yet also prevents hypotensive shock by autoscavenging ·NO.17 Given the rapid kinetics of ·NO scavenging by heme and the significantly higher concentrations of ferrous heme relative to any ·NO released from SNOHb in the red blood cell, it is not clear how autocapture of NO released from SNOHb can regulate the vasodilation process. One proposed mechanism that allows SNOHb-mediated vasodilation to occur in the presence of oxyferrous heme is formation of GSNO. This can occur by a transnitrosation reaction, which is second order and reversible in nature (Equation 3).22,46 Since GSNO is not subject to rapid scavenging by oxyHb, it represents an attractive candidate to export a NO-dependent relaxation stimulus from the red blood cell. However, recent data show that deoxyHb reduces GSNO to ·NO, demonstrating that RSNOs can in fact react with ferrous heme groups.47 Furthermore, based upon the differential effects of oxyHb, oxyMb, and SOD on relaxation stimulated by SNOoxyHb compared with authentic GSNO, our data indicate this low molecular weight RSNO does not play an important role in the vasodilation stimulated by SNOoxyHb. In fact, in the context of vessel relaxation, GSNO formation from cell-free SNOoxyHb can be viewed a futile process, since oxyheme inhibits GSNO-mediated relaxation.
The effector of SNOoxyHb-mediated relaxation in the presence of GSH remains unknown. Clearly, ·NO is not involved, but an attractive candidate is the nitroxyl anion (NO–), which can be formed via a concerted 2-electron reduction of the nitrosonium moiety of SNOHb (Equation 4). In this scheme an additional reaction product is glutathionylated Hb, which has been detected previously in this reaction system.24
The mechanism by which NO– mediates vascular relaxation appears to be tissue specific, with both ·NO-independent and -dependent processes being reported.48 Data presented in Figure 7 show that oxyHb inhibits NO–-mediated relaxation, suggesting the intermediate formation of a species that reacts with ferrous heme. The identity of this species is not known but is likely to be ·NO formed via oxidation of NO– through specific interactions with redox centers in the vessel wall or with oxygen.49 The data presented in Figure 7 would not favor a role for NO– in the vasodilatory mechanisms of SNOoxyHb. However, it is also becoming apparent that the biologic responses to NO– depend on its excited state.50 The nature of NO– released from thiol-RSNO interactions is not known, but it is important to understand the role of this species as a mediator of the biologic functions of NO. At present the vasoactive mediator(s) from SNOoxyHb remains unknown and is currently being investigated.
Interestingly, SNOHb in the presence or absence of GSH does not stimulate vasodilation in the pulmonary vasculature nor reverse hypoxia-induced vasoconstriction (HPV). However, GSNO inhibits HPV via release of NO and dilation of the pulmonary vasculature.21,25 These discrepancies between the effects of GSNO and SNOoxyHb/GSH in the context of HPV are consistent with the differential vascular responses between GSNO and SNOoxyHb observed in this study. One possible explanation may lie in the differential responses of pulmonary and systemic vasculature to the vasodilatory stimulus released from SNOHb/GSH, which does not appear to be ·NO. Identification of this intermediate may shed insights not only into the mechanisms by which SNOHb modulates vasodilation but also to the responses of the pulmonary vasculature to NO donors during hypoxia.
Additional evidence for the lack of a role for ·NO in SNOoxyHb-dependent responses was obtained by the effect of SOD. Whereas SOD left-shifted the dose response curve for relaxation in response to GSNO and SNOmetHb (similar to that reported for ·NO38 ), SOD had no effect on the SNOoxyHb-dependent responses. Superoxide dismutase scavenges the superoxide radical (
In the second mechanism described by Jourd'heuil et al, GSH reduces the active site copper in SOD to the cuprous oxidation state, which in turn rapidly reduces RSNOs to ·NO (Equation 5).55 This latter mechanism appears to be important in these studies since SOD had similar effects on relaxation profiles to GSNO (and SNOmetHb) at high and low oxygen tensions (Figures 4 and 6, respectively), the latter of which does not support significant
In order to experimentally manipulate the redox state of heme and address the mechanistic issues surrounding the nature of the vasorelaxant stimulus released by SNOHb, the current studies used cell-free SNOHb. We recognize that erythrocytic and cell-free SNOHb may have different reactivities, with more recent studies proposing interactions with AE-1.18 Relaxation mediated via transnitrosation between SNOHb and AE-1 also was reported to occur upon deoxygenation of SNOHb. However, these observations also are subject to the arguments posed above regarding the effect of oxygen on vessel responsiveness. In addition, recent data indicate that AE-1 does not induce release of NO from SNOHb, underscoring the need for further investigation of the role of SNOHb as a regulator of the vascular functions of NO.43
Our data however suggest a thiol-dependent, NO-derived vasorelaxant species is released from SNOHb, which is not subject to inhibition by oxyheme. The biologic impact of this process remains to be defined but may play a role in the ability of erythrocytic SNOHb to mediate vasodilation. Physiologically, however, the low concentrations (< 100 nM) of SNOHb in vivo,26,27 the lack of biologically feasible mechanisms of SNOHb formation, and other arguments outlined elsewhere30 question a role for this species as a physiologic modulator of blood flow. However, S-nitrosation of Hb may be useful therapeutically as a component of Hb-based blood substitutes and may also have a role in inflammatory disease. Recent studies are encouraging since infusion of S-nitrosated Hb-based blood substitutes was not associated with hypertensive responses compared with the non–S-nitrosated controls.56 Furthermore, SNOHb is present at significantly higher concentrations (in the μM range) in endotoxemic rats.32 Coupled with studies showing that Hb can be S-nitrosated by NO derived from the inducible isoform of nitric oxide synthase, a potential role for SNOHb in inflammation is indicated.57 Data presented in this study indicate that the heme oxidation state, the presence of SOD, and thiols will be important determinants of the biologic effects of SNOHb administered therapeutically or formed during inflammation.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-12-3825.
Supported by funds from the National Institutes of Health (RO1HL70146, R.P.P.; RO1HL67930, C.R.W.) and from the Medical Scientist Training Program (T32 GM08361, J.H.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Wenxin Ma for technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal