Abstract
Recently it has been shown that the 2 populations of blood dendritic cells (DCs), termed plasmacytoid (pcDCs) and myeloid (myDCs), are reduced in HIV-1 infection. This study aimed to determine whether these 2 populations are targets for HIV-1 infection and whether their ability to stimulate T-lymphocyte proliferation is affected. Highly purified populations of myDCs and pcDCs were isolated from the blood of antiretroviral treatment–naive patients and assessed for the level of HIV provirus by polymerase chain reaction (PCR). We show that both populations are targets for HIV-1 infection as indicated by the presence of provirus in 12 of 14 pcDC and 13 of 14 myDC samples tested. A proportion of this provirus is integrated in myDCs. The ability of both myDCs and pcDCs from HIV-1–infected patients to stimulate allogeneic T-lymphocyte proliferation in a 6-day mixed leukocyte reaction was severely impaired, but was not mediated by secondary infection of T lymphocytes. Thus, in addition to depletion, both myeloid and plasmacytoid DCs are infected and show impaired functional capacity. These findings suggest that infection, depletion, and dysfunction of dendritic cells may contribute to the immunosuppression associated with HIV-1 disease.
Introduction
Primary adaptive immune responses are initiated by dendritic cells (DCs) through the presentation of antigen to T lymphocytes. DCs are also important stimulators of innate immune responses.1,2 DCs constitute about 1% of the mononuclear cell population of blood and can be divided into 2 major subpopulations that are phenotypically and functionally distinct.3,4 Myeloid DCs (myDCs) express CD11c, CD13, CD33, and the receptor for granulocyte-macrophage colony-stimulating factor (GM-CSF) and secrete interleukin 12 (IL-12). They are precursors of classical antigen-presenting cells, including Langerhans cells and dermal and interstitial DCs.5 By contrast, plasmacytoid DCs (pcDCs) are CD11c-; lack myeloid markers but express CD68, CD36, IL-3 receptor–α (IL-3Rα), immunoglobulin-like transcript 3 (ILT3); and produce high amounts of interferon-α (IFN-α),6,7 a potent inhibitor of HIV-1 replication.8 Unlike myDCs, which migrate to the tissues and intercept invading pathogens before migrating to the lymph nodes, pcDCs migrate directly from blood to the secondary lymphoid tissue where they differentiate into cells originally termed plasmacytoid T cells on the basis of their extensive endoplasmic reticulum.9,10
It has long been known that during HIV-1 infection there is a progressive decline in CD4 T-lymphocyte numbers, and in the later stages of disease there is loss of HIV-specific cytotoxic T-lymphocyte activity.11 Recently, we and others have reported that there is also a progressive loss in the number of blood DCs.12, 13, 14, 15 However, to fully understand the role of DCs in the pathogenesis of HIV-1, it is important to determine whether the 2 DC populations are targets for HIV-1 infection and whether their ability to stimulate T lymphocytes in infected individuals is affected. Evidence of infection would be important as it would provide a mechanism to explain DC loss and might facilitate infection of T lymphocytes during antigen presentation. Down-regulation of T-lymphocyte stimulatory capacity together with reduced numbers would severely suppress cell-mediated immunity and contribute to the immunosuppression seen in HIV-1 infection.
Several studies demonstrate that blood DCs are susceptible to HIV infection in vitro,16,17 but the question of infection in vivo has remained controversial.18, 19, 20, 21 Similarly, there are conflicting reports regarding the ability of DCs from HIV-1–infected patients to stimulate T-lymphocyte proliferation.18,20, 21, 22 Some have found an impairment in DC function18 while others showed no difference between the ability of DCs from HIV-infected patients and from controls to stimulate allogeneic T lymphocytes.20 The differentiation of DCs into myDC and pcDC subpopulations has only recently been described,4,10 and thus discrepancies among earlier studies may reflect the inadvertent isolation of one or the other population. Furthermore, until the recent development of monoclonal antibodies that specifically recognize the 2 populations of blood DCs, it has proved difficult to purify DCs from relatively small volumes of blood without contaminating T lymphocytes.
In this study, we describe the isolation of highly purified myDCs and pcDCs from HIV-1–infected patients and show for the first time that both subpopulations are infected with HIV-1 and that some myDCs contain integrated provirus. We also show that both DC populations are severely impaired in their ability to stimulate T-lymphocyte proliferation. These findings suggest that the immunosuppression seen during HIV-1 infection is, at least in part, due to effects of the virus on DC populations.
Patients and methods
Patients and blood samples
A total of 31 blood samples were collected from 26 HIV-1–infected males who were at different stages of disease progression and were not receiving antiretroviral therapy. Fourteen samples were used to investigate the level of HIV-1 within the DC populations, and 17 were used in functional studies. Five patients gave 2 samples each, 1 for each part of the study. Patients were selected from those attending an HIV clinic at the Chelsea and Westminster Hospital (London, United Kingdom). HIV-1 RNA virus loads range from 368 to more than 500 000 copies per milliliter (median, 68 433), and CD4 T-lymphocyte cell counts from 13 to 884/μL (median 170). Ethical approval and informed consent were obtained prior to blood donation. Approximately 50 mL blood was collected in EDTA (ethylenediaminetetraacetic acid) Vacutainers (Becton Dickinson, Oxford, United Kingdom). Blood was separated over Ficoll-Histopaque (Sigma Aldrich, Poole, United Kingdom) within 5 hours of collection. Interface cells were removed and resuspended in RPMI (HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] modification) medium (Sigma Aldrich) supplemented with 2% fetal calf serum (FCS) and stored overnight at 4°C. Control samples for functional studies were obtained from 6 healthy HIV-1- volunteers.
Cell purification
Plasmacytoid DCs were separated from peripheral blood mononuclear cells (PBMCs) by means of the BDCA-4 isolation kit (Miltenyi Biotech, Bisley, United Kingdom), and the myDCs were then separated from the BDCA-4- cells by means of the BDCA-1 isolation kit (Miltenyi Biotech) according to the manufacturer's instructions. To increase purity further, the cells were incubated at 4°C to 8°C for 30 minutes in 10 μL each of anti-CD3, anti-CD14, and anti-CD19 conjugated to immunomagnetic beads (Dyna-beads; Dynal, Oslo, Norway), and labeled cells were removed with a magnet. Isolated cells were counted and assessed for purity by flow cytometry prior to functional assays or polymerase chain reaction (PCR). T lymphocytes were isolated by means of immunomagnetic beads conjugated to anti-CD3 (Miltenyi Biotech).
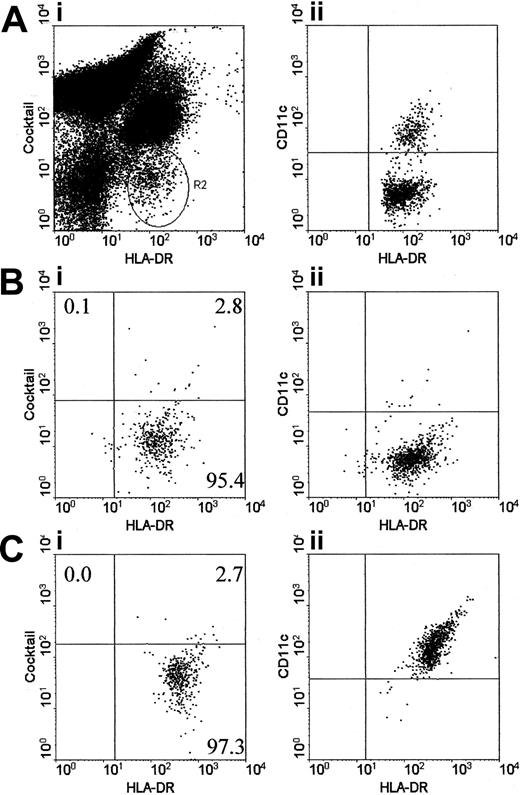
Assessment of purity of isolated DC populations
Three-color flow cytometric analysis was performed with a fraction (5% to 10%) of the isolated DC populations. These populations were labeled with phycoerythrin (PE)–conjugated anti-CD3 (BD Pharmingen, Oxford, United Kingdom), anti-CD14 (Pharmingen), anti-CD16 (Pharmingen), and anti-CD19 (Becton Dickinson); CyChrome anti–human leukocyte antigen–DR (anti–HLA-DR) (Pharmingen); and fluorescein isothiocyanate (FITC) anti-CD11c (Dako, Glostrup, Denmark). Approximately 1000 cells were acquired within 24 hours of staining and analyzed on a Becton Dickinson FACScaliber by means of CellQuest software (Clearwater, FL). DCs were identified as previously described12 by labeling for HLA-DR and the absence of staining with the PE-conjugated antibody cocktail. DCs were differentiated into the myDC and pcDC subpopulations on the basis of their expression of CD11c.
Quantitation of HIV-1 proviral DNA
Purified DCs, containing fewer than 1% contaminating T lymphocytes, and T lymphocytes were digested for 2 hours at 50°C with proteinase K (100 μg/mL) in 20 mM Tris (tris(hydroxymethyl)aminomethane), pH 7.6, containing 0.5% sodium dodecyl sulfate (SDS) and 5 mM EDTA. DNA was extracted with phenol and chloroform and precipitated with ethanol in the presence of 0.3 M sodium acetate. Provirus load was estimated by a semiquantitative nested PCR on limiting dilutions of DNA with the use of primers from the polymerase gene.23 Five-fold dilutions of DNA were performed starting with 5000 cell equivalents of DNA. The end point was taken as the lowest DNA dilution at which the PCR reaction was positive. The external primers were 5′-CATGGGTACCAGCACAAAAGG and 5′-TCTACTTGTCCATGCATGGCTTC, and the internal primers were 5′-GGAGGAAATGAACAAGTAGATAAATTAGTCAG and 5′-TCACTAGCCATTGCTCTCCAATT. Identical conditions were used for first- and second-round PCR reactions except that the cycle number was increased from 30 to 40 for the second-round PCR. Reactions were performed in 25 μL vol PCR buffer (Advanced Biotechnologies, Epsom, United Kingdom) with the use of 0.625 U Taq (Advanced Biotechnologies), 250 μM deoxynucleoside triphosphate (dNTP), 200 nM each primer, 1.5 mM MgCl2, and 0.01% Triton X-100. DNA was denatured for 4 minutes at 94°C and then amplified for 30 or 40 cycles at 94°C for 30 seconds, 55°C for 2 minutes, and 72°C for 2 minutes.
Detection of integrated HIV
Integrated HIV was detected by means of an Alu–long terminal repeat (Alu-LTR) nested PCR technique.24, 25, 26, 27 The Alu primer sequence was 5′-TCCCAGCTACTGGGGAGGCTGAGG, and the LTR sequence for the first reaction was 5′-AGGCAAGCTTTATTGAGGCTTAAGC. Reactions were performed in 25 μL vol PCR buffer containing 200 nM each primer, 2.5 mM MgCl2, 0.01% Triton X-100, 250 μM dNTP, and 0.625 U Taq polymerase. The DNA (5000 or 1000 cell equivalents) was denatured for 4 minutes at 94°C and then amplified for 35 cycles: 94°C for 30 seconds, 66°C for 40 seconds, and 72°C for 3 minutes. The amplified product was diluted to 500 μL with water, and 1 μL was used as template for the second-round reaction. The MgCl2 concentration was reduced to 1.5 mM for the second-round reaction with the use of the following LTR primers: 5′-CTGTGGATCTACCACACACAAGGCTAC and 5′-GCTGCTTATATGTAGCATCTGAGGGC. DNA was denatured for 4 minutes at 94°C and then amplified for 40 cycles: 30 seconds at 94°C, 30 seconds at 65°C, and 40 seconds at 72°C. To control for the possibility that a positive reaction was due to amplification of nonintegrated virus in the second-round reaction, parallel reactions were performed in which the Alu primers were omitted from the first-round PCR.
Mixed leukocyte reaction
Responder cells for the mixed leukocyte reaction (MLR) were prepared from buffy coats of HIV-1- individuals (National Blood Transfusion Service, London, United Kingdom) by centrifuging PBMCs for 30 minutes at 300g over a 50% Percoll density gradient. Pellet cells, enriched for T lymphocytes and depleted of DCs and monocytes, were cryopreserved until required. Responder cells prepared as described give consistently low levels of background proliferation. All MLR experiments used pellet cells from 1 of 2 donors. MLRs were performed in round-bottom 96-well microtiter plates in RPMI 1640 buffered with NaHCO3, supplemented with 100 IU/mL penicillin, 0.1 mg/mL streptomycin, 2 mM L-glutamine, and 10% male AB plasma (all from Sigma Aldrich). Triplicate doubling dilutions of purified DCs were cocultured with 105 responder cells for 6 days. On day 5, the culture supernatant was removed from each of the wells and frozen for cytokine and protein 24 (p24) analysis by cytokine bead array and enzyme-linked immunosorbent assay (ELISA), respectively. Fresh medium was added to the wells, and cells were pulsed with 50 μL (0.5 μCi [0.0185 MBq]) tritiated thymidine. The incorporation that occurred during a further 16 hours of culture was assessed by a beta plate reader.
p24 ELISA
We measured p24 in the culture supernatants using an HIV-1 p24 monoclonal antibody sandwich ELISA (Beckman Coulter, High Wycombe, United Kingdom) according to the manufacturer's instructions. Owing to limited sample volumes, it was not possible to conduct the assays in duplicate; however, assays were performed on MLRs with 2 different concentrations of DCs for each patient.
Cytokine bead array
Cytokine production in the culture supernatants was measured by means of the human T-helper 1 (TH1)/TH2 cytokine bead array kit II (Pharmingen) according to the manufacturer's instructions. Concentrations of IL-2, IL-4, IL-10, and IFN-γ were measured by flow cytometry.
Statistical analysis
The statistical significance of differences between controls and patients was evaluated with the Mann Whitney U test. P ≤ .05 was considered to represent significance. The Pearson correlation was used for regression of plasma RNA viral load and proviral DNA load within T lymphocytes and DCs.
Results
Purity of isolated DC populations
Generally between 4 × 104 and 1 × 105 myDCs and pcDCs were isolated from each patient. They were assessed for purity by labeling with the PE-conjugated cocktail of antibodies to CD3, CD14, CD16, and CD19; CyChrome-conjugated HLA-DR; and FITC CD11c (Figure 1A). DC purity from HIV-1- controls exceeded 95%. Purity of patient DCs ranged from 80% to 99%. For samples on which the level of provirus was measured, 8 of 14 plasmacytoid and 10 of 14 myeloid preparations were more than 95% pure, and of these, 4 pcDCs and 6 myDCs were more than 98% pure. The top left quadrant of the fluorescent-activated cell sorter (FACS) profiles (Figure 1Bi,Ci), where the T lymphocytes should be located, accounted for less than 1% of the population in all samples analyzed for provirus. Virtually all contaminating cells in the DC preparations are lineage-cocktail positive and express CD11c, a marker not expressed by T lymphocytes, and thus are probably monocytes that have been shown to contain very low levels of HIV-1 provirus in infected patients. For example, one study found detectable virus in monocytes from 2 of 14 HIV-1–infected patients,28 and a more recent study found HIV provirus in an average of 1 in 3700 monocytes.29 We therefore concluded that the few contaminating monocytes could not account for the levels of provirus detected in the isolated DC populations. In addition, monocyte-derived DCs from HIV-1–infected patients are not infected with HIV-1.21,30 For MLR assays, 3 of 17 pcDC preparations and 3 of 17 myDC preparations contained 1% to 5% contaminating T lymphocytes. The remaining preparations contained fewer than 1% contaminating T lymphocytes.
Estimation of DC numbers in blood and assessment of the purity of isolated pcDCs and myDCs. DCs were identified in peripheral blood mononuclear cells by their lack of labeling for a cocktail of CD3, CD14, CD16, and CD19, but positive labeling for HLA-DR (panel Ai; R2). The DCs were subsequently separated into 2 populations on the basis of labeling for CD11c (panel Aii). Following isolation with BDCA-1 and BDCA-4, the plasmacytoid (panel B) and myeloid (panel C) DCs were highly purified. Contaminating T lymphocytes are located in the upper left quadrant. For all infection studies, only DC preparations that contained fewer than 1% contaminating T lymphocytes were used. For functional studies, the majority of DC preparations had less than 1% contaminating T lymphocytes; however, 3 myDC and 3 pcDC preparations had 1% to 5% contaminating T lymphocytes. The percentage of cells within each quadrant is shown.
Estimation of DC numbers in blood and assessment of the purity of isolated pcDCs and myDCs. DCs were identified in peripheral blood mononuclear cells by their lack of labeling for a cocktail of CD3, CD14, CD16, and CD19, but positive labeling for HLA-DR (panel Ai; R2). The DCs were subsequently separated into 2 populations on the basis of labeling for CD11c (panel Aii). Following isolation with BDCA-1 and BDCA-4, the plasmacytoid (panel B) and myeloid (panel C) DCs were highly purified. Contaminating T lymphocytes are located in the upper left quadrant. For all infection studies, only DC preparations that contained fewer than 1% contaminating T lymphocytes were used. For functional studies, the majority of DC preparations had less than 1% contaminating T lymphocytes; however, 3 myDC and 3 pcDC preparations had 1% to 5% contaminating T lymphocytes. The percentage of cells within each quadrant is shown.
HIV infection of DCs and T lymphocytes
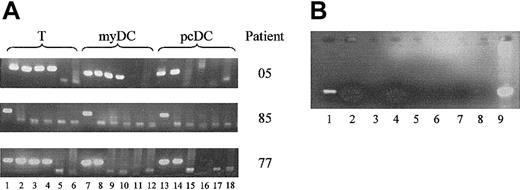
Previously, we have shown that both populations of DCs express CD4, CCR5, and CXCR4 and are susceptible to HIV-1 infection in vitro.31 We now asked whether these cells become infected in vivo. Using highly purified populations of DCs, we performed limiting-dilution nested PCR to determine the level of HIV-1 provirus within the myDCs and pcDCs from 14 HIV-1 patients who were naive to antiretroviral drug therapy. To ensure the level of proviral DNA detected could not be accounted for by the small numbers of contaminating T lymphocytes, the level of proviral DNA in purified T lymphocytes was also measured. Proviral DNA was detected in 12 of 14 pcDC, 13 of 14 myDC, and all T-lymphocyte samples (Table 1; Figure 2A). Only 1 patient had more proviral DNA in the pcDCs than the myDCs (pt11); the remainder had higher or similar levels in the myDCs. In 5 patients, higher levels of provirus were detected in the T lymphocytes than in either DC population, and in 2 individuals, proviral DNA was higher in the myDCs than the T lymphocytes. There was no correlation between the level of proviral DNA in the DC populations and the plasma viral load; however, there was a correlation between the viral load in the T lymphocytes and the plasma viral load (r = 0.51, P = .052). Several of the samples were analyzed for proviral DNA more than once, and identical levels of provirus were found, suggesting the results were reproducible. The level of proviral DNA detected in the DCs could not be attributed to the low numbers of contaminating infected T lymphocytes.
Level of proviral DNA and integrated virus found within myDCs, pcDCs, and T lymphocytes
. | HIV-1 plasma RNA virus load . | CD4 T cells, per μL . | Contaminating T cells, % . | . | Lowest no. of cells giving positive signal . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients . | . | . | pcDCs . | myDCs . | pcDCs . | myDCs . | T cells . | |||
| pt09 | 20 762 | 615 | BDL | BDL | < 5 000 | 5 000 | 5 000 | |||
| pt77 | 380 857 | 83 | 0.38 | 0.99 | 1 000 | 1 000* | 40 | |||
| pt78 | 62 032 | 651 | 0.19 | 0.12 | 5 000 | 1 000 | 1 000 | |||
| pt85 | 51 666 | 48 | BDL | BDL | 5 000 | 1 000 | 5 000 | |||
| pt95 | 14 028 | 308 | 0.41 | 0.29 | < 5 000 | < 5 000 | 200 | |||
| pt96 | 68 433 | 157 | 0.94 | 0.23 | 5 000 | 200* | 1 000 | |||
| pt97 | 174 782 | 110 | 0.94 | BDL | 1 000 | 200 | 200 | |||
| pt98 | 1 600 | 271 | BDL | BDL | 5 000 | 1 000 | 200 | |||
| pt05 | 500 000 | 114 | BDL | BDL | 1 000 | 40 | 40 | |||
| pt07 | 280 500 | 193 | 0.39 | BDL | 5 000 | 5 000 | 5 000 | |||
| pt06 | 84 531 | 191 | BDL | BDL | 1 000 | 1 000 | 1 000 | |||
| pt02 | 4 238 | 884 | 0.29 | 0.08 | 5 000 | 5 000 | 5 000 | |||
| pt10 | 390 158 | 213 | 0.88 | 0.46 | 200 | 200* | 40 | |||
| pt11 | 105 092 | 174 | 0.33 | BDL | 200 | 1 000* | 40* | |||
. | HIV-1 plasma RNA virus load . | CD4 T cells, per μL . | Contaminating T cells, % . | . | Lowest no. of cells giving positive signal . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients . | . | . | pcDCs . | myDCs . | pcDCs . | myDCs . | T cells . | |||
| pt09 | 20 762 | 615 | BDL | BDL | < 5 000 | 5 000 | 5 000 | |||
| pt77 | 380 857 | 83 | 0.38 | 0.99 | 1 000 | 1 000* | 40 | |||
| pt78 | 62 032 | 651 | 0.19 | 0.12 | 5 000 | 1 000 | 1 000 | |||
| pt85 | 51 666 | 48 | BDL | BDL | 5 000 | 1 000 | 5 000 | |||
| pt95 | 14 028 | 308 | 0.41 | 0.29 | < 5 000 | < 5 000 | 200 | |||
| pt96 | 68 433 | 157 | 0.94 | 0.23 | 5 000 | 200* | 1 000 | |||
| pt97 | 174 782 | 110 | 0.94 | BDL | 1 000 | 200 | 200 | |||
| pt98 | 1 600 | 271 | BDL | BDL | 5 000 | 1 000 | 200 | |||
| pt05 | 500 000 | 114 | BDL | BDL | 1 000 | 40 | 40 | |||
| pt07 | 280 500 | 193 | 0.39 | BDL | 5 000 | 5 000 | 5 000 | |||
| pt06 | 84 531 | 191 | BDL | BDL | 1 000 | 1 000 | 1 000 | |||
| pt02 | 4 238 | 884 | 0.29 | 0.08 | 5 000 | 5 000 | 5 000 | |||
| pt10 | 390 158 | 213 | 0.88 | 0.46 | 200 | 200* | 40 | |||
| pt11 | 105 092 | 174 | 0.33 | BDL | 200 | 1 000* | 40* | |||
Provirus DNA detected by 5-fold limiting-dilution PCR with DNA from 5 000, 1 000, 200, 40, or 8 cells per reaction. The lowest concentration of cells giving a positive PCR band is indicated. Fewer than 5 000 indicates undetectable proviral DNA. The level of contaminating T lymphocytes was determined by flow cytometric analysis as shown in Figure 1A. Integrated virus was measured by means of an Alu-LTR nested PCR technique, with the use of the equivalent of 5 000 cells. All samples were assessed for level of integrated virus. pt indicates patient; BDL, below detection limit (ie, that no T lymphocytes were detected in the top left-hand quadrant of the cocktail/HLA-DR plot).
Integrated virus was detected in this sample.
PCR analysis for provirus in DCs and T lymphocytes. (A) Analysis of HIV provirus load by limiting-dilution nested PCR on purified T lymphocytes, myDCs, and pcDCs. Results from 3 patients of 14 tested are shown. Lanes 1, 7, and 13 were amplified with 5000 cell equivalents of DNA; lanes 2, 8, and 14 were amplified with 1000 cell equivalents of DNA; lanes 3, 9, and 15 were amplified with 200 cell equivalents of DNA; lanes 4, 10, and 16 were amplified with 40 cell equivalents of DNA; lanes 5, 11, and 17 were amplified with 8 cell equivalents of DNA; and lanes 6, 12, and 18 were amplified with 1.6 cell equivalents of DNA. (B) DNA preparations from myDCs of 2 patients, (patient 1, lanes 1, 2, 5, 6; patient 2, lanes 3, 4, 7, 8) analyzed for integrated provirus. Lanes 1 through 4 employed Alu primers in the first-round reaction. In lanes 5 through 8, Alu primers were omitted from first-round reaction. Lanes 1, 3, 5, and 7 were amplified with 5000 cell equivalents of DNA. Lanes 2, 4, 6, and 8 were amplified with 1000 cell equivalents of DNA. Lane 9 is an unnested PCR product obtained using the second-round LTR primers and DNA extracted from H9 cells infected with HIV-1 IIIB.
PCR analysis for provirus in DCs and T lymphocytes. (A) Analysis of HIV provirus load by limiting-dilution nested PCR on purified T lymphocytes, myDCs, and pcDCs. Results from 3 patients of 14 tested are shown. Lanes 1, 7, and 13 were amplified with 5000 cell equivalents of DNA; lanes 2, 8, and 14 were amplified with 1000 cell equivalents of DNA; lanes 3, 9, and 15 were amplified with 200 cell equivalents of DNA; lanes 4, 10, and 16 were amplified with 40 cell equivalents of DNA; lanes 5, 11, and 17 were amplified with 8 cell equivalents of DNA; and lanes 6, 12, and 18 were amplified with 1.6 cell equivalents of DNA. (B) DNA preparations from myDCs of 2 patients, (patient 1, lanes 1, 2, 5, 6; patient 2, lanes 3, 4, 7, 8) analyzed for integrated provirus. Lanes 1 through 4 employed Alu primers in the first-round reaction. In lanes 5 through 8, Alu primers were omitted from first-round reaction. Lanes 1, 3, 5, and 7 were amplified with 5000 cell equivalents of DNA. Lanes 2, 4, 6, and 8 were amplified with 1000 cell equivalents of DNA. Lane 9 is an unnested PCR product obtained using the second-round LTR primers and DNA extracted from H9 cells infected with HIV-1 IIIB.
Integration of HIV provirus in DCs
Previous studies have shown that in HIV-1–infected asymptomatic individuals, only about 1% of provirus detected in resting and activated CD4 T lymphocytes is integrated32 and thus able to initiate full productive infection. We therefore looked for evidence of integrated provirus in DCs using a nested PCR technique that employs primers to the human Alu repeat sequence and the HIV LTR in the first-round PCR reaction.24,27,32 With the use of 5000 cell equivalents of DNA, integrated provirus was detected in myDCs in 4 of 14 patients, and in 1 of these patients, integrated virus was also detected in the T lymphocytes (Figure 2B; Table 1). Integrated virus was not detected in T lymphocytes from any other patient or in any of the pcDC preparations. Thus, although the provirus load was generally higher in the T-lymphocyte population, integrated virus was more frequently detected in the myDCs. Such data suggest that a greater fraction of provirus in myDCs is integrated than in T lymphocytes.
Impaired ability to stimulate allogeneic T lymphocytes
Having shown that the DCs were infected with HIV and that myDCs harbor integrated virus, albeit at low levels, we wanted to know whether the ability of DCs to stimulate T-lymphocyte proliferation was affected. Freshly isolated myDCs and pcDCs were used to stimulate allogeneic PBMCs depleted of monocytes and DCs.
Both myDCs and pcDCs from controls were potent stimulators of T-lymphocyte proliferation; however, the myDCs tended to stimulate higher T-lymphocyte proliferation than the pcDCs. The median maximum stimulation indices for pcDCs and myDCs in controls were 61.67 (range, 10.93-139.67) and 93.65 (range, 44.97-135.49), respectively (Figure 3A). In HIV-1–infected patients, both myDCs and pcDCs were severely impaired in their ability to stimulate allogeneic T-lymphocyte proliferation; median maximum stimulation indices for pcDCs and myDCs from 17 patients were 11.98 (range, 0.98-35.44) and 27.32 (range, 1.55-75.21), respectively (P < .01, Mann-Whitney U test, Figure 3A). This was seen at all concentrations of DCs used (Figure 3B-C). For both populations, there was no correlation between plasma RNA viral load and the proliferation induced. The degree of major histocompatibility complex (MHC) mismatch between stimulator DCs and responder T cells is likely to vary among individuals, causing some differences in the proliferation of the T lymphocytes. However, both patients and controls were recruited in a cross-sectional manner, and thus it is unlikely that differences in the proliferation were due to differences in the MHC phenotype of the 2 groups.
Impaired allostimulatory properties of DCs derived from HIV-1–infected patients. Highly purified myDCs and pcDCs at increasing concentrations were cocultured for 6 days with 105 allogeneic T lymphocytes. Incorporation of [3H]thymidine was measured for the last 16 hours of culture. The stimulation indices were determined by dividing the proliferation induced in the presence of DCs by the proliferation seen in culture with no DCs added (background). In all experiments, the backgrounds were fewer than 600 counts per minute. The maximum stimulation index for each of 17 HIV-1–infected patients (▦) was compared with 6 uninfected controls (□, panel A). Medians, 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars) are shown. Median stimulation indexes of pcDCs (panel B) and myDCs (panel C) from controls and patients at all concentrations of DCs are shown. Titrating progressively increasing numbers of DCs into the MLR produced different dose-response curves in controls (panel D) and patients (panel E). *P < .05, **P < .01, Mann Whitney U test.
Impaired allostimulatory properties of DCs derived from HIV-1–infected patients. Highly purified myDCs and pcDCs at increasing concentrations were cocultured for 6 days with 105 allogeneic T lymphocytes. Incorporation of [3H]thymidine was measured for the last 16 hours of culture. The stimulation indices were determined by dividing the proliferation induced in the presence of DCs by the proliferation seen in culture with no DCs added (background). In all experiments, the backgrounds were fewer than 600 counts per minute. The maximum stimulation index for each of 17 HIV-1–infected patients (▦) was compared with 6 uninfected controls (□, panel A). Medians, 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars) are shown. Median stimulation indexes of pcDCs (panel B) and myDCs (panel C) from controls and patients at all concentrations of DCs are shown. Titrating progressively increasing numbers of DCs into the MLR produced different dose-response curves in controls (panel D) and patients (panel E). *P < .05, **P < .01, Mann Whitney U test.
Loss of DC stimulatory capacity could reflect down-regulation of MHC class II; however, no difference in HLA-DR expression was observed between patients and controls (data not shown). Interestingly, the effect of titrating progressively increasing numbers of DCs into the MLR produced different dose-response curves in patients and controls (Figure 3D-E). Patients showed maximum proliferation at lower DC concentrations than controls. The maximum proliferation from controls was obtained at the highest concentration of DCs tested, 16 000 for both pcDCs and myDCs. By contrast, for patients, the concentration of DCs that yielded the highest proliferation was 4000 pcDCs and 2000 myDCs, with higher numbers of DCs inducing little or no response.
Five patients gave 2 blood samples each, 1 for assessment of the level of infection of DCs and the other for functional analysis. There was no correlation between level of proviral DNA detected in the DC populations and the ability to stimulate allogeneic T lymphocytes in an MLR (Table 2).
Viral load in DCs and ability to stimulate T-lymphocyte proliferation
. | . | CD4 cell count . | Stimulation index . | . | Lowest no. of cells giving positive signal . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients . | Virus load . | . | pcDCs . | myDCs . | pcDCs . | myDCs . | T cells . | |||
| pt06 | 84 531 | 191 | 27.4 | 37.8 | 1 000 | 1 000 | 1 000 | |||
| pt11 | 105 092 | 174 | 29.8 | 32.5 | 200 | 1 000 | 40 | |||
| pt07 | 280 500 | 615 | 8.1 | 42.3 | 5 000 | 5 000 | 5 000 | |||
| pt10 | 390 158 | 213 | 12.0 | 27.3 | 200 | 200 | 40 | |||
| pt05 | 500 000 | 114 | 16.0 | 21.3 | 1 000 | 40 | 40 | |||
. | . | CD4 cell count . | Stimulation index . | . | Lowest no. of cells giving positive signal . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients . | Virus load . | . | pcDCs . | myDCs . | pcDCs . | myDCs . | T cells . | |||
| pt06 | 84 531 | 191 | 27.4 | 37.8 | 1 000 | 1 000 | 1 000 | |||
| pt11 | 105 092 | 174 | 29.8 | 32.5 | 200 | 1 000 | 40 | |||
| pt07 | 280 500 | 615 | 8.1 | 42.3 | 5 000 | 5 000 | 5 000 | |||
| pt10 | 390 158 | 213 | 12.0 | 27.3 | 200 | 200 | 40 | |||
| pt05 | 500 000 | 114 | 16.0 | 21.3 | 1 000 | 40 | 40 | |||
Five patients attended clinic and donated 50 mL blood on 2 separate occasions. One sample was used to assess the level of infection of DCs; the other was used in a mixed leukocyte reaction for assessment of function. In these patients, there was no correlation between the amount of virus detected within the DC populations and the ability to stimulate an MLR.
Impaired DC function does not correlate with p24 levels
Since it is reported that clusters of DCs and T-lymphocytes support high levels of HIV replication,33 we postulated that the observed suppression of MLR responses was due to secondary productive infection of CD4 T lymphocytes by HIV-1 as they become activated. To test this hypothesis, p24 was measured at day 5 in MLR culture supernatants by ELISA. Assays were performed on cultures containing 2 different concentrations of DCs, including 1 that stimulated the highest levels of T-lymphocyte proliferation. Surprisingly, despite the detection of HIV-1 provirus in virtually every DC preparation analyzed, p24 was found in only 3 of 64 supernatants tested. Two of the p24+ cultures were stimulated with pcDCs (3.4 pg/mL and 4.6 pg/mL) and one with myDCs (62.65 pg/mL). The absence of virus in the majority of MLR culture supernatants suggests that the reduced T-lymphocyte proliferation was not in fact due to secondary infection of responding T lymphocytes as they become activated.
pcDCs and myDCs from infected patients do not stimulate different cytokine profiles
It has been reported that myDCs and pcDCs stimulate TH1 and TH2 responses, respectively,3 and thus the reduced TH1 responses seen in HIV-1 infection34 may reflect alteration in the 2 DC subsets. To examine this possibility, we analyzed culture supernatants of MLRs stimulated by patient myDCs and pcDCs. In HIV-1- controls, there was no overall difference in cytokine production in culture supernatants stimulated with myDCs and those stimulated with pcDCs. The high levels of IFN-γ but low levels of IL-4 and IL-10 from controls in the culture supernatants suggest that both myDCs and pcDCs are able to stimulate TH1 responses in vitro. In cultures stimulated with pcDCs from HIV-1–infected patients, the levels of IFN-γ, IL-2, and IL-10 were lower than in controls. T lymphocytes cultured with patient DCs had lower levels of IFN-γ than controls (P < .01, Mann Whitney U; Table 3). This may be a reflection of the low levels of T-lymphocyte proliferation that were observed in these patients. IL-2 and IL-10 production in myDCs and IL-4 production in both myDCs and pcDCs were not different in patients and controls, despite reduced proliferation. There was no correlation between the stimulation index and cytokine production (data not shown).
Cytokine profiles from MLRs stimulated by myDCs and pcDCs
. | HIV-1 plasma virus load . | . | . | Cytokine levels in myDC MLRs, pg/mL . | . | . | . | . | . | Cytokine levels in pcDC MLRs, pg/mL . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls/patients . | . | myDCs, no. . | SI . | IL-2 . | IFN-γ . | IL-4 . | IL-10 . | pcDCs, no. . | SI . | IL-2 . | IFN-γ . | IL-4 . | IL-10 . | ||||||
| c01 | NT | 32 000 | 45.0 | 20.7 | 3 441.2 | 6.8 | 12.6 | 16 000 | 10.9 | 6.5 | 216.4 | 1.8 | BDL | ||||||
| c02 | NT | 32 000 | 80.7 | 8.6 | 847.5 | 4.7 | 14.4 | 4 000 | 41.5 | 11.3 | 263.2 | 5.4 | 4.7 | ||||||
| c03 | NT | 16 000 | 126.3 | 4.2 | 337.0 | 3.9 | 2.4 | 8 000 | 94.6 | 3.8 | 481.6 | 4.9 | 5.1 | ||||||
| c04 | NT | 16 000 | 135.5 | 4.1 | 299.3 | 2.3 | 9.4 | 16 000 | 139.7 | 5.3 | 1 590.1 | 3.0 | 9.4 | ||||||
| c05 | NT | 16 000 | 68.6 | 3.4 | 157.8 | 4.5 | 7.2 | 16 000 | 68.6 | 5.2 | 1 514.9 | 3.5 | 7.2 | ||||||
| c06 | NT | 8 000 | 106.6 | 3.4 | 147.1 | 2.2 | 4.5 | 8 000 | 54.8 | 6.3 | 1 396.1 | 2.7 | 4.5 | ||||||
| Median, controls | NA | NA | 93.7 | 4.2 | 318.2 | 4.2 | 3.1 | NA | 61.7 | 5.8 | 938.8 | 3.3 | 4.9 | ||||||
| pt16 | 368 | 2 000 | 28.0 | 0.0 | 53.8 | 0.0 | BDL | 2 000 | 11.2 | 1.5 | 119.9 | 2.5 | 2.7 | ||||||
| pt33 | 2 841 | 4 000 | 14.6 | 4.1 | 99.4 | 2.9 | BDL | 4 000 | 31.8 | 5.6 | 1 151.7 | 2.3 | 4.5 | ||||||
| pt03 | 12 525 | 2 000 | 11.6 | 7.6 | 2.4 | 1.6 | BDL | 4 000 | 23.3 | 9.0 | 51.6 | 4.6 | 3.4 | ||||||
| pt18 | 12 960 | 2 000 | 18.1 | 2.8 | 84.2 | 3.2 | 2.7 | 2 000 | 14.7 | 0.0 | 151.3 | 0.0 | BDL | ||||||
| pt07 | 280 500 | 8 000 | 42.3 | 7.6 | 211.0 | 5.6 | BDL | 4 000 | 8.1 | 3.6 | 47.4 | 3.0 | BDL | ||||||
| pt99 | 41 755 | 2 000 | 4.2 | 5.9 | 12.4 | 2.8 | BDL | 2 000 | 2.9 | 0.0 | 14.2 | 3.5 | BDL | ||||||
| pt96 | 68 433 | 2 000 | 28.2 | NT | NT | NT | NT | 4 000 | 2.8 | NT | NT | NT | NT | ||||||
| pt12 | 81 954 | 1 500 | 68.0 | 1.9 | 121.8 | 2.2 | 3.3 | 3 000 | 35.4 | 4.2 | 691.9 | 2.7 | 2.2 | ||||||
| pt06 | 84 531 | 8 000 | 37.8 | 3.7 | 59.5 | 3.4 | 3.5 | 16 000 | 27.4 | 5.5 | 135.0 | 4.3 | 3.2 | ||||||
| pt13 | 85 434 | 1 500 | 1.5 | 0.0 | 6.9 | 3.2 | BDL | 1 500 | 15.2 | 0.0 | 20.8 | 2.8 | BDL | ||||||
| pt11 | 105 092 | 4 000 | 32.5 | 3.6 | 106.0 | 3.6 | 2.3 | 4 000 | 29.8 | 4.3 | 324.1 | 4.3 | 2.2 | ||||||
| pt88 | 190 920 | 6 000 | 67.1 | 2.9 | 230.3 | 3.7 | 4.0 | 2 000 | 1.0 | 0.0 | 2.4 | 2.7 | BDL | ||||||
| pt87 | 207 486 | 6 000 | 26.8 | 5.2 | 175.9 | 2.9 | 7.9 | 2 500 | 6.0 | 0.0 | 17.2 | 0.0 | BDL | ||||||
| pt10 | 390 158 | 4 000 | 27.3 | 4.8 | 45.4 | 2.3 | 2.7 | 2 000 | 12.0 | 0.0 | 13.6 | 0.0 | BDL | ||||||
| pt45 | 417 773 | 2 000 | 7.5 | 0.0 | 16.6 | 2.2 | 2.4 | 2 000 | 2.7 | 0.0 | 20.8 | 3.2 | 4.0 | ||||||
| pt92 | 441 000 | 1 500 | 75.2 | 7.4 | 742.2 | 3.5 | 7.5 | 4 000 | 26.2 | 5.3 | 623.1 | 3.5 | 4.5 | ||||||
| pt05 | 500 000 | 6 000 | 21.3 | 4.4 | 42.5 | 0.0 | BDL | 6 000 | 16.0 | 3.6 | 36.4 | 3.3 | BDL | ||||||
| Median, patients | 85 434 | NA | 27.3* | 3.9 | 71.9* | 2.9 | 2.3 | NA | 14.7* | 2.5* | 49.5* | 2.9 | 1.1* | ||||||
. | HIV-1 plasma virus load . | . | . | Cytokine levels in myDC MLRs, pg/mL . | . | . | . | . | . | Cytokine levels in pcDC MLRs, pg/mL . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls/patients . | . | myDCs, no. . | SI . | IL-2 . | IFN-γ . | IL-4 . | IL-10 . | pcDCs, no. . | SI . | IL-2 . | IFN-γ . | IL-4 . | IL-10 . | ||||||
| c01 | NT | 32 000 | 45.0 | 20.7 | 3 441.2 | 6.8 | 12.6 | 16 000 | 10.9 | 6.5 | 216.4 | 1.8 | BDL | ||||||
| c02 | NT | 32 000 | 80.7 | 8.6 | 847.5 | 4.7 | 14.4 | 4 000 | 41.5 | 11.3 | 263.2 | 5.4 | 4.7 | ||||||
| c03 | NT | 16 000 | 126.3 | 4.2 | 337.0 | 3.9 | 2.4 | 8 000 | 94.6 | 3.8 | 481.6 | 4.9 | 5.1 | ||||||
| c04 | NT | 16 000 | 135.5 | 4.1 | 299.3 | 2.3 | 9.4 | 16 000 | 139.7 | 5.3 | 1 590.1 | 3.0 | 9.4 | ||||||
| c05 | NT | 16 000 | 68.6 | 3.4 | 157.8 | 4.5 | 7.2 | 16 000 | 68.6 | 5.2 | 1 514.9 | 3.5 | 7.2 | ||||||
| c06 | NT | 8 000 | 106.6 | 3.4 | 147.1 | 2.2 | 4.5 | 8 000 | 54.8 | 6.3 | 1 396.1 | 2.7 | 4.5 | ||||||
| Median, controls | NA | NA | 93.7 | 4.2 | 318.2 | 4.2 | 3.1 | NA | 61.7 | 5.8 | 938.8 | 3.3 | 4.9 | ||||||
| pt16 | 368 | 2 000 | 28.0 | 0.0 | 53.8 | 0.0 | BDL | 2 000 | 11.2 | 1.5 | 119.9 | 2.5 | 2.7 | ||||||
| pt33 | 2 841 | 4 000 | 14.6 | 4.1 | 99.4 | 2.9 | BDL | 4 000 | 31.8 | 5.6 | 1 151.7 | 2.3 | 4.5 | ||||||
| pt03 | 12 525 | 2 000 | 11.6 | 7.6 | 2.4 | 1.6 | BDL | 4 000 | 23.3 | 9.0 | 51.6 | 4.6 | 3.4 | ||||||
| pt18 | 12 960 | 2 000 | 18.1 | 2.8 | 84.2 | 3.2 | 2.7 | 2 000 | 14.7 | 0.0 | 151.3 | 0.0 | BDL | ||||||
| pt07 | 280 500 | 8 000 | 42.3 | 7.6 | 211.0 | 5.6 | BDL | 4 000 | 8.1 | 3.6 | 47.4 | 3.0 | BDL | ||||||
| pt99 | 41 755 | 2 000 | 4.2 | 5.9 | 12.4 | 2.8 | BDL | 2 000 | 2.9 | 0.0 | 14.2 | 3.5 | BDL | ||||||
| pt96 | 68 433 | 2 000 | 28.2 | NT | NT | NT | NT | 4 000 | 2.8 | NT | NT | NT | NT | ||||||
| pt12 | 81 954 | 1 500 | 68.0 | 1.9 | 121.8 | 2.2 | 3.3 | 3 000 | 35.4 | 4.2 | 691.9 | 2.7 | 2.2 | ||||||
| pt06 | 84 531 | 8 000 | 37.8 | 3.7 | 59.5 | 3.4 | 3.5 | 16 000 | 27.4 | 5.5 | 135.0 | 4.3 | 3.2 | ||||||
| pt13 | 85 434 | 1 500 | 1.5 | 0.0 | 6.9 | 3.2 | BDL | 1 500 | 15.2 | 0.0 | 20.8 | 2.8 | BDL | ||||||
| pt11 | 105 092 | 4 000 | 32.5 | 3.6 | 106.0 | 3.6 | 2.3 | 4 000 | 29.8 | 4.3 | 324.1 | 4.3 | 2.2 | ||||||
| pt88 | 190 920 | 6 000 | 67.1 | 2.9 | 230.3 | 3.7 | 4.0 | 2 000 | 1.0 | 0.0 | 2.4 | 2.7 | BDL | ||||||
| pt87 | 207 486 | 6 000 | 26.8 | 5.2 | 175.9 | 2.9 | 7.9 | 2 500 | 6.0 | 0.0 | 17.2 | 0.0 | BDL | ||||||
| pt10 | 390 158 | 4 000 | 27.3 | 4.8 | 45.4 | 2.3 | 2.7 | 2 000 | 12.0 | 0.0 | 13.6 | 0.0 | BDL | ||||||
| pt45 | 417 773 | 2 000 | 7.5 | 0.0 | 16.6 | 2.2 | 2.4 | 2 000 | 2.7 | 0.0 | 20.8 | 3.2 | 4.0 | ||||||
| pt92 | 441 000 | 1 500 | 75.2 | 7.4 | 742.2 | 3.5 | 7.5 | 4 000 | 26.2 | 5.3 | 623.1 | 3.5 | 4.5 | ||||||
| pt05 | 500 000 | 6 000 | 21.3 | 4.4 | 42.5 | 0.0 | BDL | 6 000 | 16.0 | 3.6 | 36.4 | 3.3 | BDL | ||||||
| Median, patients | 85 434 | NA | 27.3* | 3.9 | 71.9* | 2.9 | 2.3 | NA | 14.7* | 2.5* | 49.5* | 2.9 | 1.1* | ||||||
The cytokines detected by cytometric bead array in supernatants of mixed leukocyte reactions stimulated with myDCs and pcDCs for 5 days. Samples chosen for analysis were those that generated the highest proliferation. Cytokine levels are in picograms per milliliter. Median values are in bold. SI indicates stimulation index; c, control; BDL, below detection limit; NA, not available; NT, not tested.
P < .01 Mann Whitney U test.
Discussion
Loss of circulating myeloid and plasmacytoid DCs is likely to be an important factor in the decline of acquired and innate immune responses in HIV-1 infection. Investigating the underlying mechanisms involved and possible alterations in DC function have been hampered until recently by the difficulties in isolating DC subpopulations from small volumes of blood. Previous studies of DCs in HIV-infected patients have used relatively crude preparations of the whole DC population, which may have inadvertently selected for one or the other DC population and could explain discrepancies among investigations. Now, using recently developed techniques for isolating the myeloid and plasmacytoid DCs, we have achieved up to 98% purity with less than 1% T-lymphocyte contamination. HIV proviral DNA was found in both myDCs and pcDCs at levels that could not be accounted for by low numbers of contaminating infected T lymphocytes. Although the DC provirus load was generally lower than that in the total T-lymphocyte population, our data may underestimate the number of DCs that become infected. Unlike T lymphocytes, which constantly recirculate, DCs in the blood either are en route to the tissues to become sentinels for antigen capture as Langerhans cells or interstitial DCs or are pcDCs that migrate to the lymph nodes, where they secrete IFN-α. Thus, infected resting T lymphocytes will continue to recirculate whereas infected DCs may become inaccessible for assay as they migrate out of the blood. This proposal is supported by the observation that in vitro HIV-1 activates DCs to release IFN-α35 and by studies showing that IFN-α secretion by pcDCs occurs in the lymph node.7 Entry of HIV-1 into resting CD4 T lymphocytes gives rise to a DNA provirus, but unless the T lymphocyte becomes activated, the proviral DNA does not integrate and initiate productive virus infection.36 Thus, only 1% or less of HIV-1 proviral DNA detected in CD4 T lymphocytes is integrated.32 The low levels of integrated viral DNA found in the T lymphocytes in this study are in agreement with previous reports. Despite lower levels of proviral DNA in the myDCs than in the T lymphocytes, we were able to detect integrated virus in 4 of 14 samples as opposed to 1 of 14 in the T lymphocytes. These findings suggest that myDCs could potentially become sites of virus production and transmit virus to T lymphocytes. These data also provide one mechanism to explain quantitative DC loss. In a previous in vitro study, we showed that pcDCs were readily susceptible to productive infection by HIV-1,31 and thus it was surprising that we were unable to detect integrated virus in this population of cells. It may be that on interaction with the virus the cells are rapidly recruited into the lymph nodes.
Loss of DCs alone would lead to a decline in T-lymphocyte responses, but here we show that the situation is further exacerbated because the capacity of DCs from HIV-infected patients to stimulate T lymphocytes is severely reduced. Restoration of the functional capacity of DCs during treatment of HIV is important given the findings that the numbers were not comprehensively restored in patients commencing highly active antiretroviral therapy during primary infection.15 Interestingly, the kinetics of allogeneic T lymphocyte proliferation mediated by patient DCs was different than in controls. Maximum T-lymphocyte proliferation was observed at lower DC concentrations than in controls, with little or no proliferation at the higher concentrations. Further studies will be required to determine whether this reflects production of factors that suppress T-lymphocyte proliferation, although the levels of the immunosuppressive cytokine IL-10 detected in the supernatants of patients were not elevated. MHC class II expression on DCs is not reduced in infected patients; neither does it seem likely that the defect in T-lymphocyte stimulatory properties is due to a failure to up-regulate the CD86 costimulatory molecule.14 Additionally, there was little evidence to suggest that suppression was due to secondary infection of T lymphocytes activated in the MLR since virus p24 was undetectable in most of the culture supernatants. The very low numbers of DCs isolated from patients precluded an analysis of p24 over the time course of the culture, and thus virus production may have peaked at an earlier time point. However, it seems unlikely that virus produced earlier would not be detectable on day 5. We cannot totally exclude the possibility that the defects observed were due to levels of virus that were below the detection range of the p24 assay (7 pg/mL). This possibility will be examined in future studies by performing functional assays in the presence of antiretroviral drugs. Reduced stimulatory capacity of measles virus–infected DCs has been shown to be mediated by apoptosis of both T lymphocytes and DCs.37,38 HIV-1 induces apoptosis of T lymphocytes,39,40 and in addition macrophages infected with HIV in vitro have been shown to induce apoptosis.41,42 Thus, T-lymphocyte apoptosis induced by DCs is one potential explanation for the present findings. Reduced proliferation correlated with lower IFN-γ production but no alteration in IL-4 release, suggesting that changes in DCs do not explain the reduced TH1 responses associated with disease progression. The cytokine data presented represent the total amounts secreted and are not directly correlated to number of T cells in the culture. It would be interesting in future studies to determine the number of T cells in MLR cultures and perform intracellular cytokine analyses to more closely relate the findings to the number of cytokine-producing T cells.
This work shows for the first time that myDCs, required for the initiation of the adaptive immune response, and pcDCs, the major source of IFN-α, a potent viral inhibitor, are targets for HIV-1 infection in vivo. In addition, the finding that DCs from HIV-1–infected patients are severely impaired in their ability to stimulate T-lymphocyte proliferation has implications for our understanding of the mechanisms involved in the pathogenesis of HIV-mediated disease.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-10-3189.
Supported by a grant from the Medical Research Council, United Kingdom, grant no. G9814980.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Thanks to the nursing staff, particularly Glenn Sontag, and patients of the St Stephen's Centre, London, United Kingdom, who have made this study possible.

![Figure 3. Impaired allostimulatory properties of DCs derived from HIV-1–infected patients. Highly purified myDCs and pcDCs at increasing concentrations were cocultured for 6 days with 105 allogeneic T lymphocytes. Incorporation of [3H]thymidine was measured for the last 16 hours of culture. The stimulation indices were determined by dividing the proliferation induced in the presence of DCs by the proliferation seen in culture with no DCs added (background). In all experiments, the backgrounds were fewer than 600 counts per minute. The maximum stimulation index for each of 17 HIV-1–infected patients (▦) was compared with 6 uninfected controls (□, panel A). Medians, 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars) are shown. Median stimulation indexes of pcDCs (panel B) and myDCs (panel C) from controls and patients at all concentrations of DCs are shown. Titrating progressively increasing numbers of DCs into the MLR produced different dose-response curves in controls (panel D) and patients (panel E). *P < .05, **P < .01, Mann Whitney U test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/11/10.1182_blood-2002-10-3189/5/m_h81134375003.jpeg?Expires=1767709051&Signature=hzr5QMxaswYBlL5YzYDtuZp~GKnMOoX01cZ~1Oz~~~Cy6-Unjk4hujp5XZAVVCIGgi-ao~oeSYChTQeYHr6F-dn9PQEKn~ZXvUzIpThbpd8MS92OAVk1nHQBAqNBz0pN5cVZ3x7f4ObKsWAyLX-~R~Srked60kNOUhxYY4FEatuOgZ9~0Hj-VNw-T55Akwrq7uDOzE8NOxIefhsYtsSC~o1KM8Dd-oBRZ0sG1SQi9yWxswQIFOrREXazmR4HYbvROR5yGkWOl3AHNyrCS10t5lBcvP0lqVdUvQwvlLkzKU7cEa6atc-~8wiLXxAonXsawca9jCyLDP8AIu4NfPaprQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal