Abstract
We constructed a chimeric molecule, composed of the T-cell receptor (TCR)–ζ chain fused to the extracellular domains of a prototypical allogeneic major histocompatibility complex (MHC) class I molecule, Dd, to assess whether such a construct could affect Dd allospecific responses in vitro and in vivo. To generate cytotoxic T lymphocytes (CTLs) expressing the construct, Dd-ζ was targeted to lymphocyte populations in transgenic mice by placing its expression under control of the CD2 promoter. In response to ligation of Dd, lymphocytes from transgenic mice expressing high levels of Dd-ζ are activated to proliferate and kill cells binding to Dd, despite the near total loss of CD8+ T cells in these mice. Thus, the Dd-ζ cytolytic cell was found not to be a conventional CD8+ CTL, but rather an unusual T lineage cell (CD3-CD5+Thy1.1+) that lacked αβ or γδ TCRs, as well as CD4 and CD8 coreceptors, but expressed surface markers strikingly similar to memory CTLs, including CD44, Ly-6C, and CD122. These cells originate in the thymus and potently veto responses to Dd in vitro. Lacking TCRs, these veto cells are unlikely to mediate graft-versus-host disease (GVHD) and thus may be useful as a cellular therapy for therapeutic deletion of alloreactive T cells in the settings of graft rejection and GVHD.
Introduction
Most T lymphocytes express the hypervariable, heterodimeric αβ T-cell receptor (TCR)1 that is noncovalently associated with CD3 subunits γ, δ, ϵ, and TCR-ζ, responsible for signal transduction.2, 3, 4, 5 The ζ chain exists as a homodimer within the CD3 complex,6 with each ζ chain containing 3 immunoreceptor tyrosine-based activation motifs (ITAMs). Cross-linking of TCRs initiates a signaling cascade mediated by Src family tyrosine kinases, resulting in phosphorylation of ITAMs, and subsequent T-cell activation.7, 8, 9, 10, 11
Studies in which the extracellular domains of molecules involved in immune system activation were fused to the ζ chain, thereby creating chimeras such as Fv, CD4, CD8, and interleukin-2 receptor α (IL-2Rα)–ζ,7, 8, 9,12, 13, 14 have shown that ζ chains, as monomers, and in the absence of the remainder of the CD3 complex, can transduce signals in T cells. For example, an IA-ζ chimeric construct was found sufficient for activation of both naive and memory CD4+ and CD8+ T cells and for their differentiation into Th1 and Th2 subsets. These functions were elicited by antibody (Ab)–induced cross-linking and were independent of the TCR or other CD3 complex components.15 Thus, a ζ chain chimera has the potential to act as a surrogate TCR. In addition to using ζ-immune receptor chimeras to explore the effects on cell signaling and lymphocyte development, ζ chimeras have been used to redirect killing activity, eliminating only those cells binding to the external domains of the chimeric molecule. For example, CD8+ T cells or natural killer (NK) cells expressing a CD4-ζ fusion protein13, 14, 15, 16 were activated by and specifically killed HIV gp120-expressing cells in vitro via a CD4-gp120 interaction, and cytotoxic T lymphocyte (CTL)–expressing Fv(αErbB-R2)-ζ–chimeric protein killed ErbB-R2–expressing target cells and retarded tumor cell growth in nude mice.
The development of specific immunotherapies to prevent graft rejection and graft-versus-host disease (GVHD) (reviewed in Yu et al17 ) offers the promise of specifically deleting only those immune responses directed to the allospecificities of the graft. An unexplored approach to the generation of alloantigen-specific tolerance is the development of an “enhanced” veto cell. Veto cells are antigen-presenting cells (APCs), which inactivate or kill cells binding to alloantigen on their surface.18 Veto activity involves direct cell-cell interaction, is most potently mediated by CD8+ CTLs,19,20 and results in anergy or deletion of allospecific T cells.18,21, 22, 23 The hallmark of veto cell interactions is unidirectional recognition: the TCR of the CTL recognizes the veto cell, but the specificity or even the existence of TCRs on a veto cell is irrelevant to veto activity, albeit veto activity has been shown to be augmented by concomitant TCR stimulation.24 Thus, we reasoned that providing TCR-like signaling to allogeneic major histocompatibility complex (MHC) on the surface of CD8+ CTLs might result in enhanced veto function. Here, we evaluate the immunologic effects of transgenic expression of a novel Dd-ζ fusion protein. We describe the generation and characterization of a novel memory CTL-like killer cell that lacks both TCR and CD8. Splenocytes from Dd-ζ transgenic mice exhibit veto function in vitro by potently and specifically inhibiting the in vitro generation of Dd-specific CTL responses. Future studies will consider the in vivo activity of these cells in models of graft rejection and GVHD.
Materials and methods
Dd-ζ construct
A chimeric protein, Dd-ζ, was created by joining the extracellular and transmembrane portions of the mouse H-2Dd molecule with the cytoplasmic portion of the mouse ζ chain (a kind gift from Alan Weissman, NCI, NIH, Bethesda, MD). The Dd extracellular and transmembrane domains were derived by polymerase chain reaction (PCR) from a Dd cDNA, pDdSEL.FIX34 (a kind gift from Dr Randy Ribaudo, Molecular Applications Group, Palo Alto, CA). The PCR reaction was performed using a forward primer that corresponded to the 5′ end of the Dd cDNA and a reverse primer that corresponded to the 3′ end of the Dd transmembrane domain and contained an EcoRI restriction cleavage site. The primers used in the PCR reaction of Dd are as follows: forward primer, 5′-TAAATTCTCGAGGGATCCCAGATGGGGGCG-3′; reverse primer, 5′-CTGCACGAATTCTCTCCTCCTCTTCATCAC-3′. The mouse ζ intracellular region was obtained by PCR from pGEM 7Zf+.25 The sequences of DNA primers used were the following: 5′-TCGTATGAATTCGCAAAATTCAGCAGGAGTGCAGAG-3′, which is present in the ζ intracellular domain and engineered in an EcoRI restriction cleavage site, and 5′-CTAGCTAGATCTAAGCTTGCATGCCTGCAGGTCGAC-3′, present in the polylinker sequence of pGEM 7Zf+, which is 3′ of the zeta cDNA. The Dd-ζ chimeric construct was created by subcloning the Dd PCR fragment into pSP72 (Promega, Madison, WI), then ligating the EcoRI site at the 5′ end of the zeta PCR fragment to the EcoRI site present at the 3′ end of Dd.
The Dd-ζ gene fusion and the full-length Dd cDNA were cloned into the human CD2 promoter/enhancer cassette, p29Δ2(Sal–)26, 27, 28 as follows: the EcoRI-SalI fragment of p29Δ2(Sal–) was removed by restriction enzyme digestion. Inserted into its place was either the Dd-ζ chimeric construct or a Dd cDNA, creating the constructs CD2–Dd-ζ (henceforth referred to as Dd-ζ) and CD2-Dd,28 respectively. Prior to ligation into the p29Δ2(Sal–) vector, the polyadenylation signal sequence was removed from the end of the Dd-ζ construct to allow the chimeric construct to use the poly (A) signal sequences derived from the p29Δ2(Sal–) vector.
Transfections
The Jurkat and EL-4 cell lines were transfected by electroporation with 10 μg linearized CD2-Dd or CD2–Dd-ζ plasmid and 1 μg linearized pFNeo, a plasmid carrying a neomycin resistance gene for selection,29 and cloned by limiting dilution. Transfectants used for study were selected according to surface expression of Dd assessed by immunofluorescence and flow cytometry.
Animals
Mice were bred in house or purchased from Taconic Farms (Germantown, NY). All procedures involving animals were performed after review and approval by the Center for Biologics Evaluation and Research (CBER) Institutional Animal Care and Use Committee.
Generation of transgenic mouse lines
Pronuclear injections were performed on fertilized FVB/N oocytes as described.30 Transgenic animals were produced using the CD2–Dd-ζ construct (strains 130 and 6657), the CD2-Dd construct (strain 4906), or a genomic Dd (MHC-Dd) gene (strain 3604) from the pDd1 plasmid.28 The MHC haplotype of FVB/N mice is H-2q.
Immunofluorescent staining and flow cytometry
Flow cytometry was performed as described.31 Cells to be stained were first incubated with unlabeled monoclonal antibody (mAb) to Fc receptors (2.4G2). Background staining was evaluated using fluorochrome-conjugated rat immunoglobulin G2a (IgG2a) mAb controls, with the exception of the allophycocyanin control, which is conjugated to a mouse IgG1 mAb. Cells were analyzed using Becton Dickinson Immunocytometry Systems (Mountain View, CA) fluorescence-activated cell sorter scan (FACScan) or FACSCalibur using CellQuest software. Cell sorting was done on a FACStarPlus. Other antibodies used for flow cytometry were obtained from BD PharMingen (San Diego, CA) and included anti-CD3ϵ (145-2C11), anti-Dd α3 (34-2-12), anti-Dd α1/α2 (34-5-8S), anti-CD45R/B220 (RA3-6B2), anti-CD4 (RM4.5), anti-CD8 (53-6.7), anti-CD5 (53-7.3), anti-CD44 (IM7), anti–Ly-6C (AL-21), anti-CD25 (7D4), anti-CD122 (TM-β1), anti-Thy1.1 (OX-7), anti–Pan-NK cells (DX5), anti–Ly-49AB6 (A1), anti–Ly-49A and Ly-49D (12A8), anti–Ly-49C and Ly-49I (5E6), anti–Ly-49D (4E5), and anti–Ly-49G2 (4D11).
In vitro assays for lymphocyte function
Mixed lymphocyte reaction (MLR) at a 1:1 stimulator-to-responder ratio32 and 51Cr release cytotoxicity assays were performed as described.33 Proliferation was evaluated by 3H-thymidine (Perkin Elmer, Boston, MA) uptake32 or by dilution of carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR).34 Approximately 1 × 107 cells to be labeled were pelleted and resuspended in 1 mL phosphate-buffered saline (PBS; Biofluids, Rockville, MD) containing 250 ng/mL CFSE and were incubated at 37°C in the dark with shaking for 15 minutes. The cells were then washed twice with RPMI 1640 (Biofluids) containing 10% fetal bovine serum (Hyclone, Logan, UT) (complete medium, CM) and stimulated as described for individual experiments.
Interleukin-2 (IL-2) assay
Untransfected Jurkat cells or Jurkat cells transfected with either CD2-Dd or CD2–Dd-ζ were treated with saturating concentrations of anti-Dd (34-2-12) or anti-CD3 (OKT3; ATCC, Manassas, VA) mAb for 30 minutes at 4°C. After the incubation period, the cells were washed twice and resuspended in CM containing 10 ng/mL phorbolmyristate acetate (PMA; Sigma, St Louis, MO). The cells were then plated at 1 × 105 cells/well in a 96-well flat-bottom plate that had been previously coated with a rabbit antimouse IgG antibody (Cappel/Organon Teknika, West Chester, PA). The plate was then centrifuged for 2 minutes at 100g. After centrifugation, the cells were incubated for 24 hours in a humidified CO2 incubator at 37°C. Cell-free supernatants were collected from the wells. The IL-2–dependent mouse cell line, CTLL-2 (ATCC), was used to determine the presence of IL-2 in the supernatants as described.35
Skin grafting
Mice were engrafted on the flank with tail skin from donor mice as described.36 Grafts were scored daily until rejected (> 80% loss of graft tissue).
Results
Dd-ζ chimeric construct
To construct a class I MHC molecule with enhanced signaling capabilities, we created a chimeric gene, consisting of the extracellular and transmembrane domains of the MHC class I Dd molecule, fused to the intracellular domain of the TCR signaling chain, ζ. The transmembrane domain of Dd was chosen because it lacked a cysteine, present in the transmembrane domain of ζ, and thereby limited the potential for excessive signaling that could result from dimerization of Dd-ζ with other Dd-ζ molecules, or with TCR components (Figure 1A). The Dd-ζ chimeric construct (Figure 1B), as well as a full-length Dd cDNA control,28 was inserted into a human CD2 promoter/enhancer cassette, p29D2(Sal–). These constructs will be referred to as Dd-ζ and CD2-Dd, respectively. A third construct, the genomic Dd (pDd1), is termed MHC-Dd.28 The CD2 promoter has been shown to drive lymphocyte-specific expression of transgenes in mouse systems.26,28 The Dd-ζ, CD2-Dd, and MHC-Dd constructs were transfected into cell lines and used to generate transgenic mice.
The Dd-ζ construct. (A) A chimeric protein containing the extracellular (EC) and transmembrane (TM) portions of the mouse H-2 Dd molecule associated with the intracellular (IC) portion of the mouse ζ chain. (B) A schematic illustrating the cloning of the Dd-ζ construct into the human CD2 promoter/enhancer cassette p29Δ2(Sal–). The Dd-ζ chimera was partially digested with BamH1, such that only the poly A tail was removed. An EcoR1-SalI fragment was removed from p29Δ2(Sal–) and replaced with the Dd-ζ chimera by blunt-end ligation.
The Dd-ζ construct. (A) A chimeric protein containing the extracellular (EC) and transmembrane (TM) portions of the mouse H-2 Dd molecule associated with the intracellular (IC) portion of the mouse ζ chain. (B) A schematic illustrating the cloning of the Dd-ζ construct into the human CD2 promoter/enhancer cassette p29Δ2(Sal–). The Dd-ζ chimera was partially digested with BamH1, such that only the poly A tail was removed. An EcoR1-SalI fragment was removed from p29Δ2(Sal–) and replaced with the Dd-ζ chimera by blunt-end ligation.
Expression and signaling capacity of Dd-ζ in cell lines
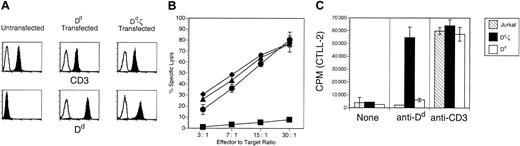
The Dd moiety of the Dd-ζ fusion protein was confirmed to be in the correct configuration by the positive staining of Dd-ζ–transfected Jurkat cells with the conformationally dependent 34.5.8 mAb37 (Figure 2A) and by the specific kill of Dd-ζ–expressing EL-4 cells by Dd-specific CTLs (Figure 2B). To evaluate the signaling capability of the Dd-ζ protein, stably transfected Jurkat cells expressing either the CD2-Dd or Dd-ζ construct were assessed for IL-2 production by cross-linking Dd with Ab. As shown in Figure 2C, cells expressing Dd-ζ had markedly enhanced secretion of IL-2, relative to cells expressing unmodified Dd. In fact, the level of IL-2 produced by Dd-ζ–expressing Jurkat cells was commensurate with that elicited through TCR stimulation. As all cell lines produced similarly high levels of IL-2 in response to TCR stimulation, these data indicate that the signaling cascade mediated by the fused ζ chain is intact and results in profound functional enhancement of lymphokine-secreting lymphocytes.
The signaling cascade mediated by Dd-ζ is intact in transfected Jurkat cells. (A) Flow cytometric analysis of Dd and Dd-ζ expression in transfected Jurkat cells. Open histograms represent isotype matched, fluorochrome-labeled control Ab. Closed histograms show staining with anti-CD3 (top panels) or anti-Dd (bottom panels). (B) Dd-ζ–transfected EL4 cells are killed by Dd-specific CTLs. EL4 cells that were untransfected (▪) or transfected with either the MHC-Dd (▴)or Dd-ζ (♦) constructs were used as targets for Dd-specific CTLs in a 51Cr release cytotoxicity assay. • indicates P815 cells. (C) IL-2 production by Dd-ζ–transfected Jurkat cells following cross-linking with anti-Dd mAb. Following incubation with anti-Dd (34-2-12) or anti-CD3 (OKT3) mAbs, the cells were treated with PMA and cross-linked with plate-bound anti-IgG. Supernatant fluid was tested in a proliferation assay for IL-2 content measuring 3H-thymidine uptake by CTLL-2 cells.
The signaling cascade mediated by Dd-ζ is intact in transfected Jurkat cells. (A) Flow cytometric analysis of Dd and Dd-ζ expression in transfected Jurkat cells. Open histograms represent isotype matched, fluorochrome-labeled control Ab. Closed histograms show staining with anti-CD3 (top panels) or anti-Dd (bottom panels). (B) Dd-ζ–transfected EL4 cells are killed by Dd-specific CTLs. EL4 cells that were untransfected (▪) or transfected with either the MHC-Dd (▴)or Dd-ζ (♦) constructs were used as targets for Dd-specific CTLs in a 51Cr release cytotoxicity assay. • indicates P815 cells. (C) IL-2 production by Dd-ζ–transfected Jurkat cells following cross-linking with anti-Dd mAb. Following incubation with anti-Dd (34-2-12) or anti-CD3 (OKT3) mAbs, the cells were treated with PMA and cross-linked with plate-bound anti-IgG. Supernatant fluid was tested in a proliferation assay for IL-2 content measuring 3H-thymidine uptake by CTLL-2 cells.
Dd-ζ transgenic mice
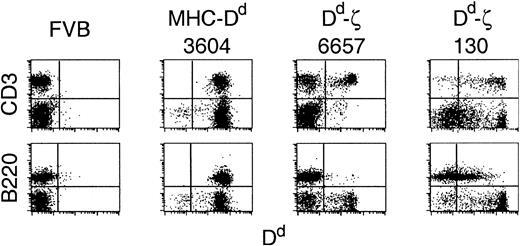
Two lines of transgenic mice were generated that showed distinct patterns of Dd-ζ expression. Lymphocytes from 6657 and 130 transgenic mice (FVB/N, H-2q background) showed a distinct expression pattern from that of MHC-Dd transgenic mice, in which the endogenous MHC promoter elicited Dd expression on all lymphocyte populations. The 6657 mice had a single, tightly defined peak of Dd expression that was restricted to a subpopulation of CD4+ and CD8+ T cells (Figure 3 and unpublished observations, June 3, 1997), whereas 130 lymphocytes exhibited a range of Dd expression that extended over 4 logs, with a substantial population of T and CD3–B220– cells expressing supraphysiologic levels. Furthermore, in 130 mice, transgene expression was also observed on a substantial population of B220+ cells (Figure 3). This expression pattern correlated with gene copy number, as the 6657 line had less than 10 gene copies per cell, whereas the 130 line had at least 10-fold more (unpublished observations, March 24, 1998).
Expression of Dd-ζ in transgenic mice. Fresh splenocytes from the indicated strains were stained with anti-CD3 or anti-B220 and anti-Dd (34-2-12) mAbs and evaluated by flow cytometry. Represented are the parental FVB strain, the 3604 strain (MHC-Dd), and 2 Dd-ζ homozygous transgenic strains, 6657 and 130.
Expression of Dd-ζ in transgenic mice. Fresh splenocytes from the indicated strains were stained with anti-CD3 or anti-B220 and anti-Dd (34-2-12) mAbs and evaluated by flow cytometry. Represented are the parental FVB strain, the 3604 strain (MHC-Dd), and 2 Dd-ζ homozygous transgenic strains, 6657 and 130.
Lymphocyte populations and immune responses to Dd in Dd-ζ transgenic mice
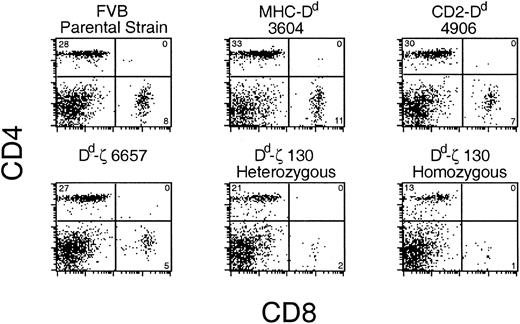
In Dd-ζ transgenic mice, there was a ζ dose-dependent loss of CD8+ and CD4+ T cells (Figure 4). In 130 homozygotes, CD8+ T cells were virtually absent, and CD4+ T cells were dramatically reduced.
Dd-ζ dose-dependent loss of T cells in transgenic mice. Fresh splenocytes from the indicated strains were stained with anti-CD4 and CD8 mAbs and evaluated by flow cytometry. The percentage of viable cells within each quadrant is indicated.
Dd-ζ dose-dependent loss of T cells in transgenic mice. Fresh splenocytes from the indicated strains were stained with anti-CD4 and CD8 mAbs and evaluated by flow cytometry. The percentage of viable cells within each quadrant is indicated.
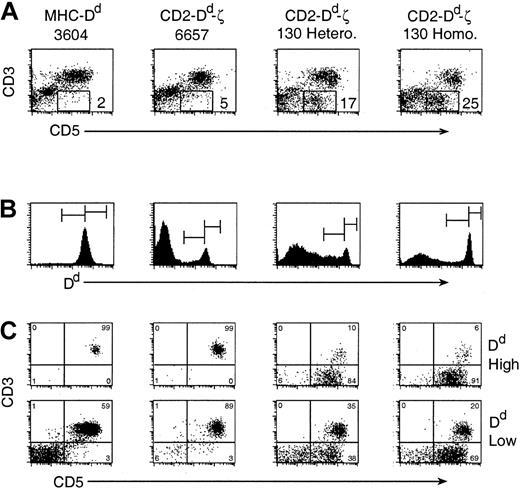
The substantial loss of single positive T cells appeared to be compensated for by the presence of a reciprocally large and distinct population of CD3–CD5+ cells expressing very high levels of Dd (Figure 5).
A large, distinct population of CD3-CD5+ cells exists in Dd-ζ transgenic mice. (A) Fresh splenocytes were stained with mAbs to CD3 and CD5 and examined by flow cytometry. The percentage of CD3–CD5+ cells for each strain is indicated. (B) In a similar experiment, cells were also stained with anti-Dd mAb (34-2-12), and the positive cells (histograms) were gated, as indicated, into Dd high and Dd low populations. (C) CD3 versus CD5 expression on the Dd high splenocytes (top panels) and Dd low expressing cells (bottom panels).
A large, distinct population of CD3-CD5+ cells exists in Dd-ζ transgenic mice. (A) Fresh splenocytes were stained with mAbs to CD3 and CD5 and examined by flow cytometry. The percentage of CD3–CD5+ cells for each strain is indicated. (B) In a similar experiment, cells were also stained with anti-Dd mAb (34-2-12), and the positive cells (histograms) were gated, as indicated, into Dd high and Dd low populations. (C) CD3 versus CD5 expression on the Dd high splenocytes (top panels) and Dd low expressing cells (bottom panels).
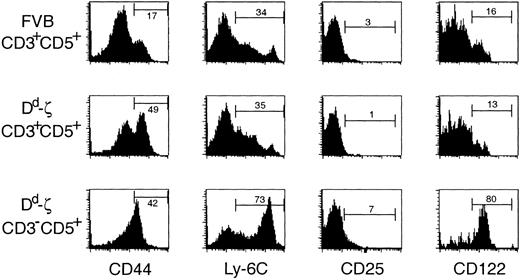
Although these CD3–CD5+Dd high cells did not express α/β or γ/δ TCR, CD4, or CD8, they appeared to be of T-cell lineage as they expressed Thy-1, failed to express hematolymphoid lineage-specific markers (CD34, B220, CD11b, CD19, DX5, or CD11c; unpublished observations, December 11, 1998), and, most intriguingly, expressed markers characteristic of memory CD8+ CTLs, including high levels of CD44, Ly-6C, and CD12238, 39, 40 (Figure 6).
CD3-CD5+ cells express markers characteristic of memory CTLs. Fresh, unstimulated splenocytes from mice were stained with antibodies to CD3, CD5, and one of the following activation/memory markers: CD44, Ly-6C, CD25 (IL-2Rα), and CD122 (IL-2Rβ) and evaluated by flow cytometry. Strains and cell populations gated are listed to the left of the indicated row. The percentage of viable gated cells expressing each activation/memory marker is indicated. The Dd-ζ cells were obtained from homozygous 130 strain mice.
CD3-CD5+ cells express markers characteristic of memory CTLs. Fresh, unstimulated splenocytes from mice were stained with antibodies to CD3, CD5, and one of the following activation/memory markers: CD44, Ly-6C, CD25 (IL-2Rα), and CD122 (IL-2Rβ) and evaluated by flow cytometry. Strains and cell populations gated are listed to the left of the indicated row. The percentage of viable gated cells expressing each activation/memory marker is indicated. The Dd-ζ cells were obtained from homozygous 130 strain mice.
The number of CD3–CD5+ cells correlated with the dose of the ζ transgene, with almost none found in the 6657 line, 17% in the spleens of 130 heterozygotes, and 25% in the spleens of 130 homozygotes (Figure 5A). As the combined percentages of CD3–CD5+ cells and CD4+/CD8+ T cells in the Dd-ζ mice approximated the expected percentage of splenic T cells (about 46%), it is tempting to speculate that development of single positive T cells was thwarted by a signaling event through the ζ chain in the thymus that generated CD3–CD5+ lymphocytes. Evidence to support this possibility is offered by the increased percentage of double negative (DN) thymocytes in Dd-ζ mice, which express CD44 and Ly-6C (Figure 7). In addition, many of the CD44+ and Ly-6C+ thymocytes bear high levels of CD5 and CD25, a phenotype suggestive of recent activation (Figure 7).41
Dd-ζ thymocytes show an increased percentage of DN cells expressing high levels of CD5. (A) Freshly isolated thymocytes from MHC-Dd (left column) or homozygous 130 Dd-ζ (right column) transgenic mice were evaluated by flow cytometry. The CD4–CD8–(DN) gated cells were examined for CD3 and CD5 expression. The percentage of viable gated cells is indicated in each quadrant. (B) Fresh thymocytes from panel A were gated on CD3–CD5high cells as indicated and evaluated for Dd, Thy1.1, CD25, CD44, or Ly-6C expression. MHC-Dd cells are represented with a thin solid line, Dd-ζ by a thick solid line, and control fluorochrome-labeled Ab by a dotted line.
Dd-ζ thymocytes show an increased percentage of DN cells expressing high levels of CD5. (A) Freshly isolated thymocytes from MHC-Dd (left column) or homozygous 130 Dd-ζ (right column) transgenic mice were evaluated by flow cytometry. The CD4–CD8–(DN) gated cells were examined for CD3 and CD5 expression. The percentage of viable gated cells is indicated in each quadrant. (B) Fresh thymocytes from panel A were gated on CD3–CD5high cells as indicated and evaluated for Dd, Thy1.1, CD25, CD44, or Ly-6C expression. MHC-Dd cells are represented with a thin solid line, Dd-ζ by a thick solid line, and control fluorochrome-labeled Ab by a dotted line.
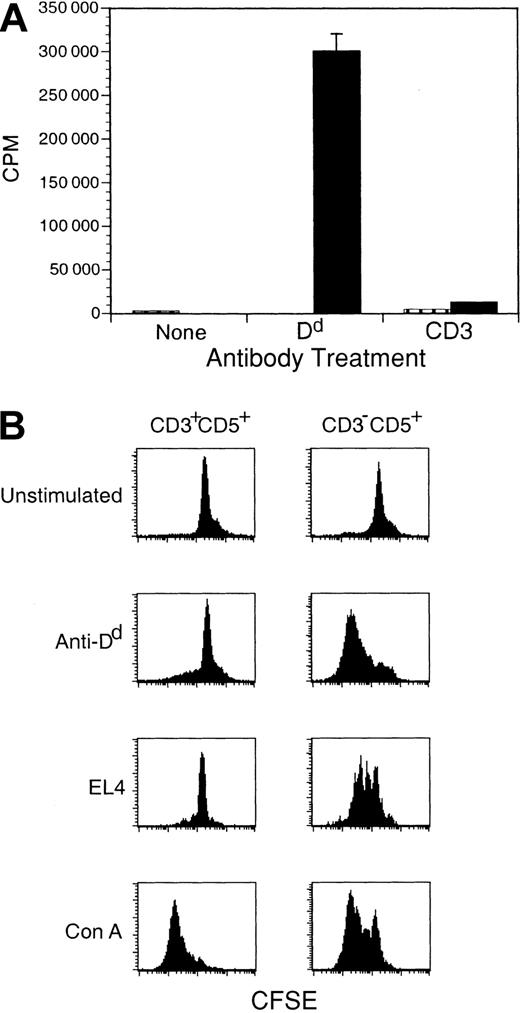
We next asked whether signaling through ζ could activate Dd-ζ lymphocytes that inhabited peripheral lymphoid tissue. Such cells evidenced robust proliferation following ligation with anti-Dd mAb and also proliferated when Dd was bound by other ligands such as Ly-49A, the NK inhibitory receptor expressed on EL4 cells that binds with TCR-like affinity to Dd (Figure 8).42, 43, 44 We confirmed that proliferation of Dd-ζ cells in response to incubation with EL-4 tumor cells was Ly-49A specific by the complete blockade of proliferation in such cultures with anti–Ly-49A mAb (unpublished results, March 26, 2001). Interestingly, although CD3–CD5+ cells proliferated vigorously in response to Dd ligation, CD3+CD5+ cells showed only a minimal response and also did not respond to cross-linking with anti-CD3 mAb but did respond to Con A, suggesting that they are anergic (Figure 8B).
Dd ligation alone stimulates proliferation of CD3-CD5+ cells from Dd-ζ transgenic mice. (A) Freshly isolated splenocytes from homozygous Dd-ζ (130 strain, ▪) or MHC-Dd (▥) mice were incubated with saturating amounts of anti-CD3 or anti-Dd (34-2-12), washed, and cross-linked with plate-bound anti-IgG. After 48 hours the plates were pulsed with 3H-thymidine and harvested 16 hours later. Proliferation was measured by 3H-thymidine uptake and expressed as counts per minute (CPM). (B) Fresh splenocytes (4 million cells) from Dd-ζ mice were CFSE labeled and incubated for 3 days with 10 μg/mL anti-Dd (34-5-8S), irradiated EL4 (0.5 million, 2000 rad), or 2.5 μg/mL concanavalin A (Con A). The cells were stained with Ab to CD3 and CD5, gated as indicated, and evaluated for CFSE loss. EL4-stimulated proliferation of Dd-ζ cells was almost completely blocked by anti–Ly-49A (unpublished observations, March 26, 2001).
Dd ligation alone stimulates proliferation of CD3-CD5+ cells from Dd-ζ transgenic mice. (A) Freshly isolated splenocytes from homozygous Dd-ζ (130 strain, ▪) or MHC-Dd (▥) mice were incubated with saturating amounts of anti-CD3 or anti-Dd (34-2-12), washed, and cross-linked with plate-bound anti-IgG. After 48 hours the plates were pulsed with 3H-thymidine and harvested 16 hours later. Proliferation was measured by 3H-thymidine uptake and expressed as counts per minute (CPM). (B) Fresh splenocytes (4 million cells) from Dd-ζ mice were CFSE labeled and incubated for 3 days with 10 μg/mL anti-Dd (34-5-8S), irradiated EL4 (0.5 million, 2000 rad), or 2.5 μg/mL concanavalin A (Con A). The cells were stained with Ab to CD3 and CD5, gated as indicated, and evaluated for CFSE loss. EL4-stimulated proliferation of Dd-ζ cells was almost completely blocked by anti–Ly-49A (unpublished observations, March 26, 2001).
Activation and cytolytic activity of CD3-CD5+ cells
Because elicitation of veto activity first requires TCR recognition of allogeneic MHC molecules on the veto cells, which then activates their lytic activity, we next assessed directly whether Dd-ζ cells could be activated by TCR engagement of Dd. Incubation of Dd-ζ cells with a Dd-restricted T-cell hybridoma, B4.2.3,45 revealed that the B4.2.3 cells activated the lytic activity of Dd-ζ cells (which killed EL-4 cells via binding of Dd to Ly-49A) but only in the presence of its cognate peptide (Figure 9A). This finding demonstrates that specific recognition of Dd by TCRs is required to activate Dd-ζ cells to generate cytolytic activity. We next asked whether the Dd-ζ killer cells could be activated by Dd allospecificT cells from Dd-primed FVB parental strain mice. First, we found that incubation of Dd-ζ cells with Dd-primed parental cells caused extensive proliferation of Dd-ζ lymphocytes (unpublished observations, April 19, 2000). Next, we found that Dd-ζ cells, activated by Dd-primed FVB, mediated high levels of kill of EL-4 target cells, and that the population mediating such kill was the CD3–CD5+ “memory” CTL population (Figure 9B-C). The identification of the CD3–CD5+ cell population as the killer cell population by positive selection (Figure 9B-C) was supported by several negative selection experiments that demonstrated that the killer cell was CD3–, CD8–, and CD4– but Thy-1+ and Ly-6C+ (unpublished observations, August 7, 2000, September 1, 2000, and October 6, 2000).
TCR recognition of Dd activates CD3-CD5+ Dd-ζ killer cells. (A) Dd-ζ killer cells are activated by a Dd-restricted T-cell hybridoma. A total of 3 million homozygous 130 strain Dd-ζ splenocytes were incubated for 2 days with 1 million paraformaldehyde-fixed B4.2.3 cells in the presence and absence of the cognate p18 peptide. The Dd-ζ cells were then tested for cytotoxicity against EL4 and P815 target cells in a 51Cr release assay. No killing of P815 target cells was observed. Error bars show the standard deviation (SD) for triplicate samples. ▪ indicates unstimulated cells; •, B4 stimulators; and ▴, B4 + p18 stimulators. (B) Spleen cells from 130 Dd-ζ transgenic mice were stimulated by incubation with irradiated Dd-primed splenocytes for 4 days and were left either unsorted or were sorted by FACS into CD3+CD5+ and CD3–CD5+ populations. The large dot plot on the left is before sorting and shows the sort gates. The 2 smaller plots on the right show the sort purity. Percentages of viable cells are indicated for each quadrant. (C) Unsorted (▪) and sorted (•, CD3+CD5+; ▴, CD3–CD5+) cells were tested in a 51Cr release assay for cytotoxic activity against EL4 (H-2b, Ly-49A+). Anti–Ly-49A blocked killing of EL4 target cells by unsorted Dd-ζ cells (unpublished observations, January 25, 2001). Specific lysis was not observed against syngeneic (H-2d) P815 target cells (unpublished observations, January 25, 2001). Error bars show the SD for triplicate samples.
TCR recognition of Dd activates CD3-CD5+ Dd-ζ killer cells. (A) Dd-ζ killer cells are activated by a Dd-restricted T-cell hybridoma. A total of 3 million homozygous 130 strain Dd-ζ splenocytes were incubated for 2 days with 1 million paraformaldehyde-fixed B4.2.3 cells in the presence and absence of the cognate p18 peptide. The Dd-ζ cells were then tested for cytotoxicity against EL4 and P815 target cells in a 51Cr release assay. No killing of P815 target cells was observed. Error bars show the standard deviation (SD) for triplicate samples. ▪ indicates unstimulated cells; •, B4 stimulators; and ▴, B4 + p18 stimulators. (B) Spleen cells from 130 Dd-ζ transgenic mice were stimulated by incubation with irradiated Dd-primed splenocytes for 4 days and were left either unsorted or were sorted by FACS into CD3+CD5+ and CD3–CD5+ populations. The large dot plot on the left is before sorting and shows the sort gates. The 2 smaller plots on the right show the sort purity. Percentages of viable cells are indicated for each quadrant. (C) Unsorted (▪) and sorted (•, CD3+CD5+; ▴, CD3–CD5+) cells were tested in a 51Cr release assay for cytotoxic activity against EL4 (H-2b, Ly-49A+). Anti–Ly-49A blocked killing of EL4 target cells by unsorted Dd-ζ cells (unpublished observations, January 25, 2001). Specific lysis was not observed against syngeneic (H-2d) P815 target cells (unpublished observations, January 25, 2001). Error bars show the SD for triplicate samples.
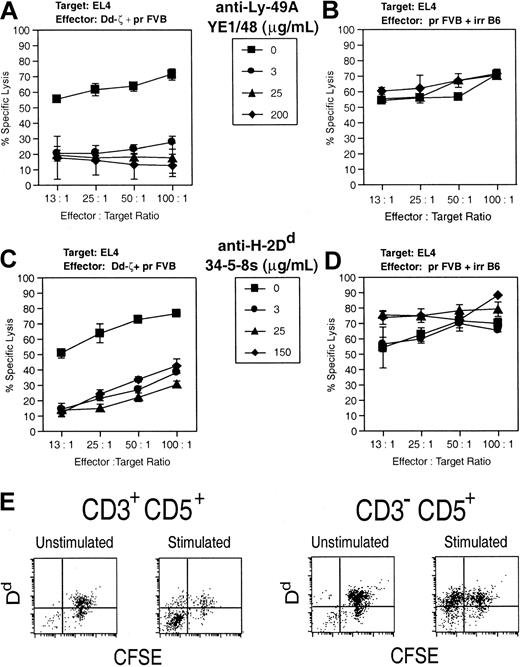
The killing of EL-4 cells by CD3–CD5+ memory CTLs was Dd specific, as the response was fully inhibited by Ab to both Dd and Ly-49A43,44 (Figure 10A-D). Thus, Dd-ζ killer cell activation and target cell recognition are both antigen specific and mediated through Dd-ζ.
Target cell killing by Dd-ζ cells via Dd–Ly-49A interaction. (A) Dd-ζ effector cells were generated from homozygous 130 splenocytes in a 4-day mixed lymphocyte culture (MLC) with irradiated, Dd-primed, FVB spleen cells and were tested in a 51Cr release cytotoxicity assay against EL4 target cells. The indicated amounts of anti–Ly-49A mAb were added to triplicate wells at the start of the cytotoxicity assay. (B) This panel shows the control for panel A in which anti–H-2b effectors are generated by stimulating unirradiated FVB splenocytes with irradiated C57BL/6J (H-2b) splenocytes. (C) Anti–H-2Dd mAb was tested for its ability to block Dd-ζ versus EL4 cytotoxicity assays using cells from the MLC described in panel A. (D) This panel shows a control cytotoxicity assay using the anti-H-2b effectors from panel B with anti-H-2Dd mAb added as in panel C. The effectors described earlier showed minimal kill in cytotoxicity assays using P815 (H-2d) target cells. This experiment was performed twice with similar results. Error bars show the SD for triplicate samples. (E) Following activation, CD3+CD5+ cells lose Dd expression, whereas CD3–CD5+ cells retain Dd expression. Splenocytes from Dd-ζ transgenic mice (130 strain) were CFSE labeled and left unstimulated or stimulated with irradiated (2000 rad) splenocytes from Dd-primed mice. After 4 days of incubation, the cells were harvested, stained with anti-CD3 and anti-CD5, and evaluated by flow cytometry. The cells were gated into CD3+CD5+ and CD3–CD5+ and examined for CFSE versus Dd expression.
Target cell killing by Dd-ζ cells via Dd–Ly-49A interaction. (A) Dd-ζ effector cells were generated from homozygous 130 splenocytes in a 4-day mixed lymphocyte culture (MLC) with irradiated, Dd-primed, FVB spleen cells and were tested in a 51Cr release cytotoxicity assay against EL4 target cells. The indicated amounts of anti–Ly-49A mAb were added to triplicate wells at the start of the cytotoxicity assay. (B) This panel shows the control for panel A in which anti–H-2b effectors are generated by stimulating unirradiated FVB splenocytes with irradiated C57BL/6J (H-2b) splenocytes. (C) Anti–H-2Dd mAb was tested for its ability to block Dd-ζ versus EL4 cytotoxicity assays using cells from the MLC described in panel A. (D) This panel shows a control cytotoxicity assay using the anti-H-2b effectors from panel B with anti-H-2Dd mAb added as in panel C. The effectors described earlier showed minimal kill in cytotoxicity assays using P815 (H-2d) target cells. This experiment was performed twice with similar results. Error bars show the SD for triplicate samples. (E) Following activation, CD3+CD5+ cells lose Dd expression, whereas CD3–CD5+ cells retain Dd expression. Splenocytes from Dd-ζ transgenic mice (130 strain) were CFSE labeled and left unstimulated or stimulated with irradiated (2000 rad) splenocytes from Dd-primed mice. After 4 days of incubation, the cells were harvested, stained with anti-CD3 and anti-CD5, and evaluated by flow cytometry. The cells were gated into CD3+CD5+ and CD3–CD5+ and examined for CFSE versus Dd expression.
The failure of CD3+CD5+ cells expressing Dd-ζ to mediate significant kill is attributable to the fact that nearly all such cells are CD4+ T cells, which generally fail to mediate efficient CTL activity and that, when activated, down-regulate the target Dd antigen (Figure 10E). The loss of Dd from the surface of activated CD3+ Dd-ζ T cells, and its retention on CD3– Dd-ζ T cells, suggests that factors necessary for internalization of TCR-associated signaling molecules may be absent in cells lacking the CD3 complex.46, 47, 48
Effects of Dd-ζ cells on allogeneic T-cell responses in vitro
The activation of Dd-ζ cells by alloreactive splenocytes led us to ask what effect the Dd-ζ cells might have on Dd allospecific responses in vitro. Could the activated Dd-ζ cells “veto” alloreactive CTLs generated in a mixed lymphocyte culture? As shown in Table 1, Dd-primed FVB (H-2q) responders cultured with irradiated Dd-ζ (FVB background) stimulators efficiently killed P815 (H-2Dd) cells and showed substantial but considerably lower levels of EL-4 (H-2b) cell kill, consistent with a cross-reactive CTL response.
Dd-ζ cells inhibit the generation of anti-Dd cytotoxicity in MLC
. | . | P815 . | . | . | EL4 . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Responder . | Stimulator . | 40:1* . | 20:1 . | 10:1 . | 40:1 . | 20:1 . | 10:1 . | ||||
| FVB pr† | Dd-ζ irr‡ | 100 | 100 | 100 | 67 | 31 | 23 | ||||
| FVB pr | Dd-ζ | 34 | 19 | 11 | 100 | 100 | 98 | ||||
| Dd-ζ | FVB pr irr | 33 | 28 | 23 | 100 | 100 | 100 | ||||
| FVB pr | MHC-Dd irr | 100 | 100 | 99 | 57 | 38 | 25 | ||||
| FVB pr | MHC-Dd | 100 | 100 | 85 | 49 | 37 | 22 | ||||
. | . | P815 . | . | . | EL4 . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Responder . | Stimulator . | 40:1* . | 20:1 . | 10:1 . | 40:1 . | 20:1 . | 10:1 . | ||||
| FVB pr† | Dd-ζ irr‡ | 100 | 100 | 100 | 67 | 31 | 23 | ||||
| FVB pr | Dd-ζ | 34 | 19 | 11 | 100 | 100 | 98 | ||||
| Dd-ζ | FVB pr irr | 33 | 28 | 23 | 100 | 100 | 100 | ||||
| FVB pr | MHC-Dd irr | 100 | 100 | 99 | 57 | 38 | 25 | ||||
| FVB pr | MHC-Dd | 100 | 100 | 85 | 49 | 37 | 22 | ||||
Stimulator and responder splenocytes were incubated in MLC at a 1:1 ratio for 5 days, harvested, and tested against 51Cr-labeled P815 or EL4 target cells. The Dd-ζ cells were obtained from homozygous 130 strain mice.
Chromium release cytotoxicity assay results are listed as the percentage of specific lysis at the indicated effector-to-target cell ratios.
Stimulators or responders marked “pr” were primed at least 4 weeks prior to use with an intraperitoneal injection of 20 million splenocytes from an MHC-Dd transgenic mouse.
Stimulators marked “irr” were irradiated at a dose of 2000 rad.
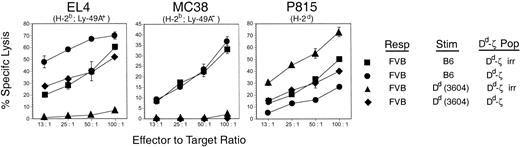
However, culture of Dd-primed FVB responders with unirradiated Dd-ζ cells dramatically reduced the virulent kill of H-2d targets by Dd-primed FVB cells while reciprocally enhancing the kill of EL-4 cells (98% lysis at 10:1 versus 23% lysis). We found that the enhanced kill of EL-4 cells in such cultures was attributable to the Dd-ζ CTLs, as culture of unirradiated Dd-ζ cells with irradiated-primed FVB (able to bind to Dd-ζ but unable to generate CTLs) yielded a similar high level of EL-4 cell killing (100% lysis at 10:1 versus 23%). In contrast to the suppression of the Dd allospecific CTL response by Dd-ζ cells, the response was minimally affected by Dd cells lacking a ζ tail (Table 1, bottom 2 rows). That the veto activity of Dd-ζ cells was antigen (Dd) specific is shown by the failure of Dd-ζ cells to veto H-2b allospecific responses (MC38 kill), while vetoing H-2d allospecific responses (P815 kill) (Figure 11). Dd-ζ cells were activated in these cultures, as shown by the enhanced kill of Ly-49A expressing EL4 targets in the H-2b-specific MLR when unirradiated Dd-ζ cells were present (Figure 11). These studies indicate that Dd-ζ splenocytes were activated by culture with T cells that bind to Dd and specifically killed or neutralized (vetoed) the generation of Dd allospecific T cells. Furthermore, Dd-ζ cells required activation, as they did not kill EL4 targets spontaneously, nor was the killing promiscuous, as they did not kill QII (H-2q lymphoma line) and Yac-1 cells (unpublished observations, October 25, 1999).
CTL inhibition by Dd-ζ splenocytes is Dd specific. FVB responder splenocytes were incubated with irradiated 3604 (Dd) or C57BL/6 (H-2b) stimulator cells and with irradiated or unirradiated homozygous 130 Dd-ζ splenocytes at a ratio of 1:1:1. The effector cells generated were then tested against EL4 (H-2b, Ly-49A+; left panel), MC38 (H-2b, Ly-49A–; middle panel), and P815 (H-2d, Ly-49A–; right panel) target cells in a 4-hour 51Cr-release cytotoxicity assay. Error bars show the SD for triplicate samples.
CTL inhibition by Dd-ζ splenocytes is Dd specific. FVB responder splenocytes were incubated with irradiated 3604 (Dd) or C57BL/6 (H-2b) stimulator cells and with irradiated or unirradiated homozygous 130 Dd-ζ splenocytes at a ratio of 1:1:1. The effector cells generated were then tested against EL4 (H-2b, Ly-49A+; left panel), MC38 (H-2b, Ly-49A–; middle panel), and P815 (H-2d, Ly-49A–; right panel) target cells in a 4-hour 51Cr-release cytotoxicity assay. Error bars show the SD for triplicate samples.
Evidence of veto activity in vivo
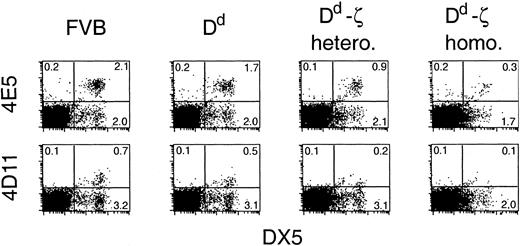
Evidence of in vivo effects on allogeneic responses by Dd-ζ mice was sought by evaluating the degree of tolerance to Dd skin grafts. The 130 homozygotes not only failed to reject Dd skin grafts, but they also failed to efficiently reject third-party skin grafts (unpublished observations). Thus, the near absent levels of CD8+ T cells and dramatic reduction in CD4+ T cells in homozygous 130 mice engendered a state of generalized immunosuppression, making evaluation of in vivo veto activity by this means problematic. Because Dd-ζ cells eliminated Dd allospecific CTLs in vitro, we asked whether they might eliminate other cells specific for Dd in vivo. We thus investigated the fate of NK cells (DX5+ cells)49 with specificity for Dd in Dd-ζ mice. DX5+ cells were dramatically reduced in homozygous Dd-ζ transgenic mice (50%), with the entire reduction accounted for by the combined loss of the 2 NK cell populations expressing receptors that recognize Dd with high affinity (Ly-49 family defined by 4E55 and 4D11 mAbs; Figure 12). Although binding of these mAbs in FVB mice has not been studied in detail, this strain is closely related to the 129 strain and likely has the 129 strain-related NK complex50, 51, 52, 53 for which these Abs have been studied extensively. Coupled with the observation that only a slight reduction (7.5%) of DX5+ cells was observed in MHC-Dd (Figure 12) and CD2-Dd transgenic mice (unpublished observations, February 22, 2000), as compared to parental FVB, these data strongly suggest that cells capable of binding to Dd may be specifically eliminated in Dd-ζ mice in vivo.
In vivo loss of NK cells that bind to Dd. Fresh splenocytes from mice of the indicated strains were evaluated by flow cytometry for expression of the NK cell marker DX5 and either 4E5 (Ly-49D) or 4D11 (Ly-49G2). FVB, MHC-Dd, and Dd-ζ splenocytes did not show detectable levels of A1 (Ly-49A) or 5E6 (Ly49-C&I) staining (unpublished observations, February 22, 2000). The Dd-ζ cells were obtained from 130 strain mice.
In vivo loss of NK cells that bind to Dd. Fresh splenocytes from mice of the indicated strains were evaluated by flow cytometry for expression of the NK cell marker DX5 and either 4E5 (Ly-49D) or 4D11 (Ly-49G2). FVB, MHC-Dd, and Dd-ζ splenocytes did not show detectable levels of A1 (Ly-49A) or 5E6 (Ly49-C&I) staining (unpublished observations, February 22, 2000). The Dd-ζ cells were obtained from 130 strain mice.
Discussion
Our intent in constructing an MHC-ζ fusion molecule was to generate CD8+ CTLs with enhanced veto activity directly from transgenic mice, given the inefficiency of transfection techniques using primary CD8+ T-cell populations. Instead of the anticipated result, we were met with an unexpectedly potent and interesting population of veto cells that lack TCR and CD8 coreceptor expression. Ironically, Dd-ζ transgenic mice expressing the highest levels of the transgene are almost totally deficient of the CD8+ T cells we sought, and early attempts to clone rare CD8+ T cells expressing Dd-ζ resulted in loss of transgene expression over time (unpublished observations, July 25, 1996).
The CD2 promoter/enhancer was used to direct expression of Dd-ζ to an early stage in thymocyte development. Because CD2 first appears on thymocytes at the CD25+ DN stage, prior to expression of the TCR, we think that the presence of Dd-ζ on these early thymocytes enabled the premature transmission of TCR-like signals following ligation of Dd by receptors such as TCR, CD8, and Ly-49, expressed on cell populations in the thymus. Evidence for strong signaling in the DN thymocyte population includes the expression of very high levels of CD5, up-regulation of CD44 and Ly-6C, and up-regulation of CD25 on a subpopulation of cells, indicating recent activation. Indeed, in healthy mice, CD5 levels in the thymus are strictly regulated, with low levels expressed on DN thymocytes, and high levels only expressed in cell populations that have been signaled through TCR: double positive (DP) and CD4 and CD8 single positive (SP) cells.41,54,55 Furthermore, the lack of TCRs on DN cells of Dd-ζ mice we think is attributable to the suppression of RAG gene expression, observed in cells following aberrant or premature signaling through ζ. Evidence supporting these possibilities comes from studies of mice transgenic for a full-length ζ chain (FL ζ) under the control of the CD2 promoter/enhancer.27 It was found that overexpression of FL ζ caused premature termination of RAG-1 and RAG-2 production, preventing productive rearrangements of TCR α and β, thus blocking entry into the DP pathway. The cellular populations in the FL ζ mice were very similar to those found in our mice, with increased numbers of CD5hi DN cells and reduced DP, CD4 SP, and CD8 SP populations, indicating a developmental block at the DN stage of thymic development.27
In the periphery, we found a large population of cells with a phenotype identical to Dd-ζ DN thymocytes, except that they lacked CD25 expression. These cells bear a striking resemblance to memory CTLs, and to homeostatically expanding cells (CD44+, Ly-6C+, and CD122+), with the exception that they lack TCRs and CD8.56, 57, 58 Further, consistent with memory CTLs and homeostatically expanding cells, most Dd-ζ CD3–CD5+ cells do not express the early activation markers CD69 or CD25, indicating past, but not current, activation. Like memory CTLs, which are more readily activated than naive cells, Dd-ζ CD3–CD5+ cells are rapidly activated to kill by means that usually fail to produce activation in normal T cells, including treatment with non–cross-linked Ab to receptor (Dd); stimulation with IL-2 alone; or stimulation by nonprofessional APCs, including Ly-49A+ tumor cells (EL4), and a Dd-restricted T-cell hybridoma (B2.4.3) (Figure 9). Veto function was demonstrated in vitro by Dd-ζ lymphocytes, which specifically inhibited generation of Dd allospecific CTL responses. In vivo, however, the near absence of CD8+ CTLs resulted in a state of generalized immunosuppression, making Dd-specific tolerance impossible to demonstrate in Dd-ζ transgenic mice. That these cells may veto cells binding with high affinity to Dd in vivo is suggested by the near absence of NK cell populations with highest affinity for Dd: 4E5+ (Ly-49D) and 4D11+ (Ly-49G2)50,51 in Dd-ζ mice but not in Dd transgenic mice lacking a signaling tail. Although decreased NK cell expression of a particular Ly-49 inhibitory receptor has been observed in mice expressing its specific high affinity MHC class I ligand,59 the diminished expression was shown to be due to down-modulation of Ly-49 and not to decreased NK cell numbers.60 In contrast, in the Dd-ζ mice, we observed a loss of NK cells, as assessed by an independent marker (DX5), and, specifically, the loss of cells expressing the high affinity Dd-binding Ly-49 receptors (4E5+ and 4D11+), with preservation of NK cells that do not express these Dd-binding Ly-49 receptors. It is thus tempting to speculate that the absence of these NK cells is due to their specific elimination in vivo by cytotoxic veto cells binding via Dd to Ly-49 receptors on the NK cell surface, although inappropriate signaling through a combination of Dd-ζ and Ly-49D or Ly-49G2 could potentially result in activation-induced death.61 An alternative possibility is that the supraphysiologic levels of Dd expressed by 130 mice might lead to impaired development of Dd-reactive NK cells.
Figure 9A shows that cognate peptide is required for activation of Dd-ζ cells by the unique TCR expressed by B4.2.3 hybridoma cells. This finding raises the question of whether Dd-ζ killer cells, expressing peptides of endogenous origin, can eliminate sufficient alloreactive T cells, whose peptide specificities may include peptides unique to an allograft, to have an effect on allograft rejection. Our data support substantial inhibition of alloreactivity by Dd-ζ cells against cells that may express distinct peptides in the context of H-2Dd (P815 mastocytoma). That we have observed a sizable but not complete elimination of alloreactive CTLs in these cultures suggests that some alloreactive CTLs may not recognize Dd plus self-peptide with sufficient affinity to activate the veto cells. Whether a substantial reduction in alloreactivity can be achieved in vivo, and, if so, whether it will inhibit allograft rejection, remain to be seen.
By expressing an allo-MHC molecule with signaling capability in transgenic mice, a population of unique veto killer cells was generated that lack TCRs and CD8 or CD4, but express Thy-1 and an array of memory CTL-like activation markers. These cells can be activated by allo-specific TCRs and can efficiently kill cells capable of recognizing the alloantigen on their surface. It is apparent that our CD3–CD5+ memory CTL-like cells would theoretically serve as ideal veto cells for the prevention of graft rejection, because they mediate potent veto activity but lack the equipment (TCRs) for mediating classical GVHD. However, the question arises as to whether such cells can be generated in healthy animals. Support for such a possibility includes studies from Sabapathy et al,62 who described a population of killer cells with phenotypic similarity to our CD3–CD5+ memory CTLs (CD3–CD5+Thy1+Ly-6C+) that killed tumor targets in vitro. However, the routine ability to generate such cells from different strains of mice is unclear; therefore, a gene therapy approach may be more successful. Such studies are under way.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-10-3265.
H.I.M. and S.A.H. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very grateful to Dr Allan Weissman for donation of the ζ construct and help in generating the fusion protein. We thank Karen Mason for able technical support, Howard Mostowski for cell sorting, and Anthony Ferrine and Tasha Gassaway for fine animal care. We are grateful to Dr Nicholas Restifo for kindly providing the MC38 cells and Dr David Margulies for the B4.2.3 hybridoma. We further thank Drs Elizabeth Shores and Eda Bloom for critical reading of the manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal