Abstract
The outcome of acquired immunodeficiency syndrome–related lymphomas (ARLs) has improved since the era of highly active antiretroviral therapy, but median survival remains low. We studied dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) with suspension of antiretroviral therapy in 39 newly diagnosed ARLs and examined protein expression profiles associated with drug resistance and histogenesis, patient immunity, and HIV dynamics and mutations. The expression profiles from a subset of ARL cases were also compared with a matched group of similarly treated HIV-negative cases. Complete remission was achieved in 74% of patients, and at 53 months median follow-up, disease-free and overall survival are 92% and 60%, respectively. Following reinstitution of antiretroviral therapy after chemotherapy, the CD4+ cells recovered by 12 months and the viral loads decreased below baseline by 3 months. Compared with HIV-negative cases, the ARL cases had lower bcl-2 and higher CD10 expression, consistent with a germinal center origin and good prognosis, but were more likely to be highly proliferative and to express p53, adverse features with standard chemotherapy. Unlike HIV-negative cases, p53 overexpression was not associated with a poor outcome, suggesting different pathogenesis. High tumor proliferation did not correlate with poor outcome and may partially explain the high activity of dose-adjusted EPOCH. The results suggest that the improved immune function associated with highly active antiretroviral therapy (HAART) may have led to a shift in pathogenesis away from lymphomas of post–germinal center origin, which have a poor prognosis. These results suggest that tumor pathogenesis is responsible for the improved outcome of ARLs in the era of HAART.
Introduction
Although the survival of acquired immunodeficiency syndrome–related lymphoma (ARL) has improved 3-fold since the era of highly active antiretroviral therapy (HAART), its prognosis is poorer than similar lymphomas in HIV-negative patients.1-3 Unfortunately, the poor overall survival in such patients, combined with the toxicity associated with standard regimens, led to the use of less-aggressive therapy to reduce complications.2,4,5 We were interested in developing a therapeutic approach that balanced the competing needs of lymphoma treatment and AIDS management, and developed the dose-adjusted EPOCH (DA-EPOCH; etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) regimen for ARL. DA-EPOCH is an infusional regimen developed from in vitro studies showing that tumor cells are relatively less resistant to prolonged low concentration exposure to the natural product–derived agents vincristine, doxorubicin, and etoposide, compared with brief higher concentration exposure.6,7 Clinically, DA-EPOCH can overcome resistance to standard cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy, and a recent study of DA-EPOCH in 50 untreated HIV-negative patients with diffuse large B-cell lymphomas (DLBCLs) showed an overall and progression-free survival of 73% and 70%, respectively, at 5 years.6,8 The dose-adjustment strategy was developed to reduce hematopoietic toxicity while maintaining maximum dose intensity by normalizing drug doses to the absolute neutrophil nadir.8 We weighed the merits of antiretroviral therapy during DA-EPOCH on preserving immune function against adverse drug interactions that could reduce lymphoma cure.9-11 Because antiretroviral therapy will not avert chemotherapy-induced lymphocyte depletion, and the predicted HIV-dependent CD4+ cell loss during the 16-week period of antiretroviral therapy suspension is relatively small, we chose to suspend antiretroviral therapy during the lymphoma treatment.12,13
We were also interested in understanding to what extent ARL outcome, which is strongly associated with the CD4+ cell count at diagnosis, is due to the lymphoma pathobiology.1 To help assess this, we characterized biologic markers of chemotherapy resistance (p53, bcl-2, and MIB-1) and histogenesis (CD10, bcl-6, and bcl-2, MUM-1/IRF4) in ARLs treated with DA-EPOCH and compared the results to an historical group of similarly treated HIV-negative DLBCLs.14-18 A recent study using molecular profiling has confirmed the utility of biologic end points for predicting survival after chemotherapy for DLBCL, providing further evidence for their importance.18
Patients, materials, and methods
Patients
Eligible patients were HIV seropositive by Western blot, had aggressive B-cell lymphoma (World Health Organization classification), had adequate organ function unless due to tumor, and were untreated.19 Patients with infections preventing treatment or primary central nervous system lymphoma were ineligible. Patients were consecutively enrolled between April 1995 and August 2000. The National Cancer Institute's institutional review board approved the protocol and all patients gave written informed consent.
Treatment and assessment
EPOCH was administered for 6 cycles over day 1 through day 5 as a 96-hour continuous infusion of etoposide, doxorubicin, and vincristine (no cap), and oral prednisone with cyclophosphamide on day 5 as outlined in Table 1. To reduce the incidence of hematologic toxicity, the dose of cyclophosphamide on cycle 1 was based on the patient's CD4+ cell number at study entry (Table 1). Thereafter, the cyclophosphamide was adjusted up or down in increments of 187 mg/m2 (maximum dose 750 mg/m2) to achieve an absolute neutrophil nadir of approximately 500 cells/mm3. Vincristine was reduced for toxicity as previously described.6,8 No patients received antiretroviral treatment during DA-EPOCH. Antiretroviral treatment was begun immediately after the last DA-EPOCH cycle and complied with standards of care.
Dose-adjusted EPOCH
Drug . | Dose . | Route . | Treatment days . |
|---|---|---|---|
| Infused agents* | |||
| Etoposide | 50 mg/m2/day | CIV | 1,2,3,4 (96 hours) |
| Doxorubicin | 10 mg/m2/day | CIV | 1,2,3,4 (96 hours) |
| Vincristine† | 0.4 mg/m2/day | CIV | 1,2,3,4 (96 hours) |
| Bolus agents | |||
| Cyclophosphamide (cycle 1) | |||
| CD4+ cells ≥ 100/mm3 | 375 mg/m2/day | IV | 5 |
| CD4+ cells < 100/mm3 | 187 mg/m2/day | IV | 5 |
| Cyclophosphamide dose-adjustment (after cycle 1)‡ | |||
| nadir ANC > 500/μL | ↑ 187 mg above previous cycle | — | — |
| nadir ANC < 500/μL or platelets < 25 000/μL | ↓ 187 mg below previous cycle | — | — |
| Prednisone | 60 mg/m2/day | PO | 1,2,3,4,5 |
| Filgrastim | 5 μg/kg/day | SC | 6 → ANC > 5000/μL (past nadir) |
| Next cycle§ | Day 21 |
Drug . | Dose . | Route . | Treatment days . |
|---|---|---|---|
| Infused agents* | |||
| Etoposide | 50 mg/m2/day | CIV | 1,2,3,4 (96 hours) |
| Doxorubicin | 10 mg/m2/day | CIV | 1,2,3,4 (96 hours) |
| Vincristine† | 0.4 mg/m2/day | CIV | 1,2,3,4 (96 hours) |
| Bolus agents | |||
| Cyclophosphamide (cycle 1) | |||
| CD4+ cells ≥ 100/mm3 | 375 mg/m2/day | IV | 5 |
| CD4+ cells < 100/mm3 | 187 mg/m2/day | IV | 5 |
| Cyclophosphamide dose-adjustment (after cycle 1)‡ | |||
| nadir ANC > 500/μL | ↑ 187 mg above previous cycle | — | — |
| nadir ANC < 500/μL or platelets < 25 000/μL | ↓ 187 mg below previous cycle | — | — |
| Prednisone | 60 mg/m2/day | PO | 1,2,3,4,5 |
| Filgrastim | 5 μg/kg/day | SC | 6 → ANC > 5000/μL (past nadir) |
| Next cycle§ | Day 21 |
Data are for cycle 1, except where noted in “Cyclophosphamide dose-adjustment.” — represents not applicable.
Etoposide, doxorubicin, and vincristine can be admixed in the same solution. Etoposide, doxorubicin, and vincristine are never dose-adjusted for hematologic toxicity.
Vincristine dose should never be routinely capped.
Dose based on previous cycle absolute neutrophil count (ANC) nadir (CBC BIW); maximum cyclophosphamide dose 750 mg/m2.
Begin day 21 if ANC ≥ 1000/μL and platelets ≥ 50 000/μL.
In this study, we were interested in assessing the effects of interleukin 12 (IL-12) on immune reconstitution and lymphoma control. IL-12 has been shown to promote the TH-1 functional subtype of CD4+ cells, which is significantly diminished in HIV-infected patients.20,21 In vitro, IL-12 restores some HIV-associated depressed immunologic functions, suggesting that defective IL-12 production may play a pathogenic role in the immunodeficiency of HIV infection.22 IL-12 has also shown significant antitumor effects in a murine model system that was dependent on CD8 cytotoxic T cells.23 To assess the clinical effects of IL-12, 20 consecutive patients who achieved complete response were randomized to receive 300 ng/kg IL-12 twice weekly by subcutaneous injection for 3 months or no further therapy. Peripheral blood samples were serially obtained to assess immune function. This randomization had an 80% power and 2-sided α = .05 to detect a 1.3 standard deviation difference in mean Th-1/Th-2 ratios between the 2 arms.
Central nervous system prophylaxis for lymphoma, administered to the last 17 patients, was 12 mg intrathecal methotrexate on day 1 and day 5 of cycles 3 through 6. Patients with active central nervous system lymphoma received treatment according to the following paradigm: treatment— intrathecal or intraventricular methotrexate or methotrexate/cytarabine/prednisone twice weekly for 2 weeks beyond negative cytology and flow cytometry, for a minimum of 4 weeks; consolidation—once weekly for 6 weeks; maintenance—once monthly for 6 months. Patients without response to single agent methotrexate received cytarabine. Cranial radiation was used as clinically indicated in chemotherapy failures. No patients received radiation to any other sites. Pneumocystis pneumonia prophylaxis was administered during DA-EPOCH and discontinued posttreatment if the CD4+ cells were more than 200/mm3. Mycobacterium avium complex prophylaxis was administered to patients with CD4+ cells less than 100/mm3 and discontinued after DA-EPOCH on recovery.
Initial evaluation included routine blood tests, brain and body imaging, bone marrow biopsy, and lumbar puncture. An evaluation was performed after cycles 4 and 6, and following treatment. Tumor responses were according to the International Workshop criteria.24 Complete response and complete response unconfirmed endpoints were combined.
Molecular and immunohistochemical analyses
Tumor immunohistochemical analyses were performed on available paraffin-embedded tissues using antibodies against CD20, CD10, MIB-1, bcl-2, bcl-6, p53 (Dako, Carpenteria, CA), and MUM-1/IRF-4 (gift from B. Falini). Epstein-Barr virus (EBV) was detected by in situ hybridization against EBER-1 in the tumor cells. All immunohistochemical analyses were performed in the Laboratory of Pathology, National Cancer Institute, and scored as previously described by the same investigator (S. P.).17
After deparaffinization, the slides were submitted to antigen retrieval procedure (in 10 mM citrate buffer, pH 6.0, in a microwavable pressure cooker and microwaved for 40 minutes at 700 W) and immunohistochemistry was then performed on an automated immunostainer (Ventana Medical System, Tucson, AZ) according to the manufacturer's instructions. p53 was considered positive if more than 10% of the tumor cells had nuclear staining. A control slide was run together with the cases to confirm adequacy of the staining. For bcl-2, the staining intensity was compared with the control T cells present in the tumor samples. If the latter did not stain, the case was scored as unsatisfactory. Staining for bcl-2 was scored as follows: 0, tumor cells were negative, but interspersed reactive T cells were positive; 1, tumor cells stain lighter than T cells; 2, tumor cells stained equal to T cells; 3, tumor cells stained stronger than T cells. Tumor cells were considered positive for bcl-2 if they were scored more than or equal to 1. MUM-1 was considered positive if there was over 20% nuclear staining of neoplastic cells.17 MIB-1 staining was scored as a mean percentile of 200 tumor cells averaged over 3 high power fields (hpf; 40×), or in cases with minute amount of tissue as a mean percentile of all neoplastic cells and was reported as 0% to 100%.
Viral and immune analyses
Plasma HIV-1 mRNA viral loads were serially obtained, including day 1 and day 6 of each cycle in the first 8 patients. Viral loads were measured initially by nucleic acid sequence–based amplification (NASBA), and later by the Roche-Amplicor method.25 Both methods have similar sensitivity and dynamic range. HIV-1 reverse transcriptase genotyping was serially performed in 9 patients as previously described.26
T-lymphocyte subsets were serially determined by flow cytometry. In 8 patients, CD4+CD45RO+ (committed) and CD4+CD45RA+CD62L (naive) CD4+ subsets were serially measured. For patients on the IL-12 portion of the study, blood mononuclear cells were obtained at baseline, monthly times 3, and 6 to 12 months after DA-EPOCH for phytohemaglutinin (PHA)–stimulated cytokine production, including interferon gamma (Biosource International, Camarillo, CA) and interleukin 10 (Pierce Endogen, Rockford, IL), using commercial enzyme-linked immunosorbent assay kits.
Statistical analysis
The probabilities of overall survival and progression-free survival were calculated using the Kaplan-Meier method beginning from the on-study date until death, progression due to lymphoma, or last follow-up, as appropriate.27 Patients were censored for lymphoma progression-free survival if they developed second malignancies or died without lymphoma. In addition, all deaths were considered failures, regardless of cause. Disease-free survival was calculated from the on-study date until progression or last follow-up for patients who achieved a complete remission of disease. The significance of the difference between pairs of Kaplan-Meier curves was calculated using the Mantel-Haenszel procedure.28
Progression-free survival of patients with bcl-2–negative tumors in the present study was compared with patients with bcl-2–negative tumors from a National Cancer Institute study of DA-EPOCH in HIV-negative patients with DLBCL.8 Comparison of the distribution of characteristics between patients on the present study and those on the previous trial of DA-EPOCH were done with either the Fisher exact test or the chi-squared test as appropriate, and the results are presented without adjustment for multiple comparisons.
The Cox proportional hazards model was used to identify the effect of pretreatment prognostic factors on outcome and included central nervous system lymphoma, age, stage, Eastern Cooperative Oncology Group (ECOG) performance status, number of extranodal sites, lactate dehydrogenase (LDH), international prognostic index (IPI) score, and CD4+ count.29 Only those factors which were associated with marginal statistical signifi-cance or better (that is, with unadjusted P value < .05) were ultimately evaluated in the Cox model. In view of the limited number of patients in this study, the Cox models and associated P values were intended to be interpreted as hypothesis generating and suggestive of magnitude rather than absolute. All P values are 2-tailed and are denoted as P2.
Results
Patient characteristics
The median patient age was 40 years (Table 2). A performance status of 2 or higher was found in 33% of patients, extranodal disease was found in 79%, CD4+ cells less than 101/mm3 in 41%, and 59% of patients were considered high risk (2 or 3 score) by the age-adjusted IPI. Central nervous system lymphoma was found in 7 (18%) patients before treatment (involving the leptomeninges in 5 patients and the brain parenchyma in 2 patients), and in 2 patients during treatment. The principal histologic subtypes were DLBCL in 79% and Burkitt lymphoma in 18% of patients. Not unexpectedly, central nervous system lymphoma was significantly more frequent in patients with Burkitt histology compared with DLBCL and was present in 4 of 7 (57%) versus 5 of 31 (16%) patients (P2 = .04), respectively. Antiretroviral treatment at study entry included HAART in 18 (46%) patients, single or dual antiretroviral therapy in 10 (26%) patients, discontinued treatment in 2 (5%) patients, and antiretroviral naive in 9 (23%) patients.
Baseline characteristics and survival
Characteristic . | No. (%) . | Overall survival at 53 months (%) . | P2 . |
|---|---|---|---|
| Total patients | 39 (100) | 60 | — |
| Male sex | 36 (92) | NA | — |
| Median age (range) | 40 (31-57) | — | — |
| Performance status | |||
| ECOG | |||
| 0-1 | 26 (67) | 77 | .004 |
| 2-4 | 13 (33) | 26 | |
| Stage | |||
| I/II | 4/9 (33) | 85 | .04 |
| III/IV | 1/25 (67) | 47 | |
| LDH | |||
| normal | 9 (23) | 67 | .68 |
| less than normal | 30 (77) | 58 | |
| Extranodal sites | |||
| 0-1 | 27 (69) | 72 | .01 |
| 2 or more | 12 (31) | 33 | |
| Central nervous system | |||
| Lymphoma | |||
| Absent | 32 (82) | 70 | .0006 |
| Present | 7 (18) | 14* | |
| Age-adjusted international prognostic index score | |||
| 0/1 | 3/13 (41) | 81 | .03 |
| 2/3 | 13/10 (59) | 44 | |
| Histologic types | |||
| Diffuse large B cell† | 31 (79) | 66 | .22 |
| Burkitt | 7 (18) | 43 | |
| Primary effusion | 1 (3) | ||
| CD4+ cell count per mm3 | |||
| (median) | 198 | NA | |
| 3-100 | 16 (41) | 16 | .0001 |
| 101-1182 | 23 (59) | 87 | |
| HIV viral load log10 | |||
| Median | 4.4 | ||
| Range | 1.69-5.96 | ||
| p53 (n = 27) | |||
| Positive | 16 (59) | 75 | .57 |
| Negative | 11 (41) | 64 | |
| EBV (n = 30) | |||
| Positive | 10 (33) | 44 | .16 |
| Negative | 20 (67) | 71 |
Characteristic . | No. (%) . | Overall survival at 53 months (%) . | P2 . |
|---|---|---|---|
| Total patients | 39 (100) | 60 | — |
| Male sex | 36 (92) | NA | — |
| Median age (range) | 40 (31-57) | — | — |
| Performance status | |||
| ECOG | |||
| 0-1 | 26 (67) | 77 | .004 |
| 2-4 | 13 (33) | 26 | |
| Stage | |||
| I/II | 4/9 (33) | 85 | .04 |
| III/IV | 1/25 (67) | 47 | |
| LDH | |||
| normal | 9 (23) | 67 | .68 |
| less than normal | 30 (77) | 58 | |
| Extranodal sites | |||
| 0-1 | 27 (69) | 72 | .01 |
| 2 or more | 12 (31) | 33 | |
| Central nervous system | |||
| Lymphoma | |||
| Absent | 32 (82) | 70 | .0006 |
| Present | 7 (18) | 14* | |
| Age-adjusted international prognostic index score | |||
| 0/1 | 3/13 (41) | 81 | .03 |
| 2/3 | 13/10 (59) | 44 | |
| Histologic types | |||
| Diffuse large B cell† | 31 (79) | 66 | .22 |
| Burkitt | 7 (18) | 43 | |
| Primary effusion | 1 (3) | ||
| CD4+ cell count per mm3 | |||
| (median) | 198 | NA | |
| 3-100 | 16 (41) | 16 | .0001 |
| 101-1182 | 23 (59) | 87 | |
| HIV viral load log10 | |||
| Median | 4.4 | ||
| Range | 1.69-5.96 | ||
| p53 (n = 27) | |||
| Positive | 16 (59) | 75 | .57 |
| Negative | 11 (41) | 64 | |
| EBV (n = 30) | |||
| Positive | 10 (33) | 44 | .16 |
| Negative | 20 (67) | 71 |
— represents not applicable; NA, not available.
At longest follow-up of 2.0 years.
Includes immunoblastic and plasmablastic subtypes.
Treatment and outcome
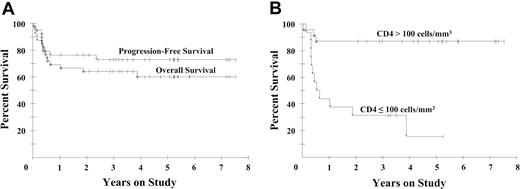
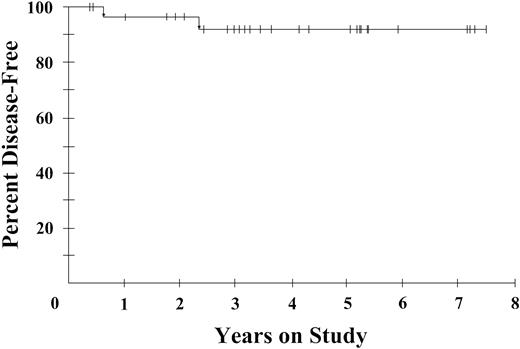
Of the patients enrolled in the study, 77% received all 6 planned cycles of DA-EPOCH. The administered dose intensity was 100% for doxorubicin and etoposide, 99% for vincristine, and 65% for cyclophosphamide. A complete response was achieved by 29 patients (74%; 95% confidence interval [CI]: 58-87) and a partial response was achieved by 5 patients (13%), for a total response rate of 87%. Among patients with CD4+ cells more than 100/mm3, 87% achieved a complete response, whereas 56% of patients with lower CD4+ cell counts achieved a complete response. At the median potential follow-up of 53 months, the progression-free survival is 73% and the overall survival is 60% (Figure 1A). Furthermore, when analyzed by CD4+ cell number (≤ or > 100 cells/mm3), the overall survival rates were 16% and 87%, respectively, at 53 months (Figure 1B). Notably, only 2 patients recurred after achieving a complete response, giving a 92% disease-free survival at 53 months (Figure 2). There was no difference in clinical outcome among patients randomized to receive IL-12 or no further therapy, with one relapsed patient in each arm.
Kaplan-Meier survival curves. (A) Overall and progression-free survival of 39 patients with AIDS-related lymphomas. At a median potential follow-up of 53 months, the overall survival probability is 60% and the progression-free survival probability is 73%. (B) Overall survival of 16 patients with low and 23 patients with high CD4+ cells (≤ and > 100/mm3, respectively). At 53 months, the overall survival rates are 16% and 87%, respectively.
Kaplan-Meier survival curves. (A) Overall and progression-free survival of 39 patients with AIDS-related lymphomas. At a median potential follow-up of 53 months, the overall survival probability is 60% and the progression-free survival probability is 73%. (B) Overall survival of 16 patients with low and 23 patients with high CD4+ cells (≤ and > 100/mm3, respectively). At 53 months, the overall survival rates are 16% and 87%, respectively.
Disease-free survival of 29 complete responders with AIDS-related lymphoma. At 53 months, the disease-free survival probability is 92%.
Disease-free survival of 29 complete responders with AIDS-related lymphoma. At 53 months, the disease-free survival probability is 92%.
Low CD4+ cells less than 101/mm3 (P2 = .002) and central nervous system involvement (P2 = .008) were the only factors simultaneously associated with decreased survival in a Cox model. When patients presenting with involvement of the central nervous system are excluded, 26 of 32 (81%) achieved a complete response and 79% were alive on the date of the analysis.
Toxicity was evaluable over 209 cycles. Neutropenia of less than 500 cells/mm3 occurred in 30% of cycles and was associated with fever in 13% of cycles. Grade 3 or 4 anemia and thrombocytopenia occurred in 17% and 21% of cycles, respectively. Serious constipation or stomatitis occurred in less than 3% of cycles, and grade 3 peripheral neuropathy occurred in 2 patients. No new opportunistic infections occurred during DA-EPOCH, although cytomegalovirus (CMV) retinitis developed in 2 patients and mycobacterium avium complex developed in 1 patient at 5, 13, and 8 weeks, respectively, after DA-EPOCH completion.
There were 5 patients who died in remission. There were 3 patients, all with CD4+ cells less than 100/mm3, who died from neurologic complications of central nervous system lymphoma treatment, which included progressive dementia with motor deterioration in 2 patients and radiation-induced cervical cord necrosis with a functional trans-section in 1 patient. One patient died from a refractory secondary Hodgkin lymphoma that occurred 6 years after successful treatment of the patient's ARL, and one patient died from pretreatment of AIDS complications with CMV colitis. One additional patient developed a secondary acute myelogenous leukemia and was censored at the time of allogeneic transplantation. All 10 patients not achieving a complete response died with lymphoma.
Effects on HIV and T-cell dynamics
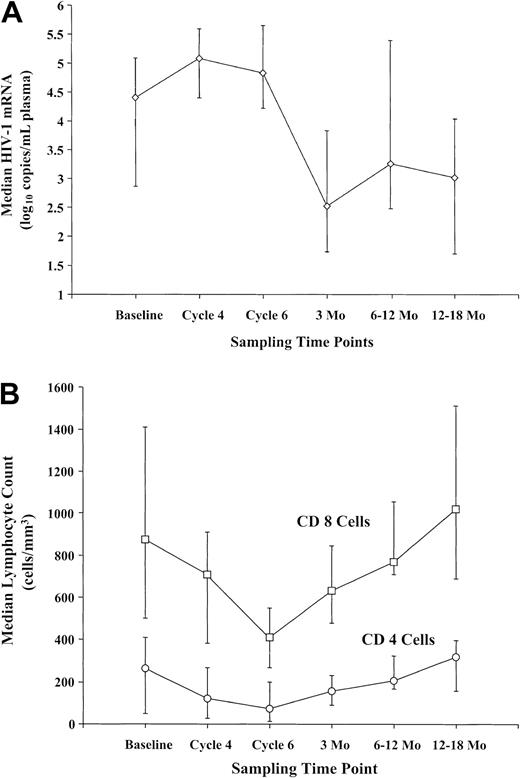
The viral load increased a median of 0.83 log10 (range: –0.28 to 4.12) during DA-EPOCH, with a smaller increase of 0.36 log10 (range: –0.46 to 0.85) in the 5 antiretroviral naive patients who completed treatment (Figure 3A). The viral loads plateaued between cycles 4 and 6, and at 3 months after restarting antiretroviral treatment they declined –0.61 log10 (P2 = .0005) below baseline. A similar pattern was observed in 7 of 8 patients with resistant HIV, defined as more than 5000 viral copies/mm3 plasma on 2 or more prior antiretroviral regimens, who were –0.47 log10 (P2 = .016) below baseline at 3 months. Interestingly, there was a strong trend (P2 = .06) for the viral loads to transiently decrement between day 1 and day 6 of each cycle during DA-EPOCH administration, suggesting an antiviral treatment effect (data not shown).
HIV viral loads and T-cell dynamic. (A) Mean change in plasma mRNA HIV viral loads in all 30 patients who completed treatment. Viral loads increased to an asymptote by cycle 4 and declined below baseline between 1 month and 3 months following completion of DA-EPOCH and reinstitution of antiretroviral treatment. (B) Mean change in CD4+ and CD8 T cells in 30 patients who were serially measured during and following treatment. T-cell subsets significantly declined to a nadir on cycle 6 of DA-EPOCH, with recovery to baseline between 12 months and 18 months following completion of DA-EPOCH and reinstitution of antiretroviral treatment.
HIV viral loads and T-cell dynamic. (A) Mean change in plasma mRNA HIV viral loads in all 30 patients who completed treatment. Viral loads increased to an asymptote by cycle 4 and declined below baseline between 1 month and 3 months following completion of DA-EPOCH and reinstitution of antiretroviral treatment. (B) Mean change in CD4+ and CD8 T cells in 30 patients who were serially measured during and following treatment. T-cell subsets significantly declined to a nadir on cycle 6 of DA-EPOCH, with recovery to baseline between 12 months and 18 months following completion of DA-EPOCH and reinstitution of antiretroviral treatment.
HIV reverse transcriptase resistance–related mutation sequences became undetectable in 5 patients studied during DA-EPOCH. In 3 patients, resistance mutations were undetectable during DA-EPOCH but recurred following antiretroviral treatment, and 2 patients only transiently lost their mutations during DA-EPOCH and developed new ones following a change in antiretroviral treatment.
CD4+ cells decreased a median of 189 cells/mm3 (range: +19 to –973) by cycle 6, followed by recovery to baseline within 6 to 12 months (Figure 3B). In a subset of 8 patients studied, memory cells (CD45RO) accounted for most of the early CD4+ cell recovery with naive cells (CD45RA) showing later recovery. CD8 cells also declined on cycle 6 by a median of 478 cells/mm3 (range: +1857 to –2232) with recovery by 12 months (Figure 3B). Among the 16 patients randomized to receive IL-12 or not, there were no differences in serum IL-10, interferon-γ or IL-2, rate of CD4+ cell recovery, or HIV viral loads (data not shown).
Immunohistochemical expression profiles of HIV-positive and HIV-negative diffuse large B-cell lymphomas
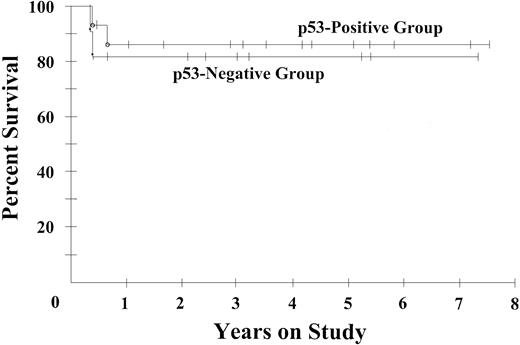
We analyzed biologic markers of drug resistance and histogenesis in the subset of 25 ARLs with DLBCL histologies and compared them with a matched historical group of 33 HIV-negative patients treated with DA-EPOCH at our institution.8 Both series had similarly distributed clinical prognostic features (Table 3). We assessed MIB-1, a marker of tumor proliferation, and p53 and bcl-2, because of their association with lower survival in HIV-negative patients with DLBCL treated with CHOP chemotherapy.14-17 The ARL cases were significantly more likely to be highly proliferative (MIB-1 > 80%), observed in 85% versus 58% of patients (P2 = .04), respectively, and to have p53 overexpression, a surrogate of gene mutation, observed in 64% versus 24% of patients (P2 = .004) (Table 3).17 However, there was no relationship between p53 overexpression and ARL progression-free (Figure 4) or overall survival (Table 2), and despite high tumor proliferation, the progression-free probability at 53 months was 73%, suggesting these are not negative prognostic factors with DA-EPOCH. Markers of histogenesis showed that fewer ARL cases expressed bcl-2 and MUM-1/IRF4, preferentially expressed in cells of post–germinal center B (GCB) origin, whereas they more often expressed CD10, preferentially expressed by GCB cells (Table 3).
Immunohistochemical analysis of diffuse large B-cell lymphoma
Characteristics . | HIV-positive series no. (%) . | HIV-negative series no. (%) . | P2 . |
|---|---|---|---|
| Total patients | 25 | 33 | — |
| Male sex | 21 (84) | 17 (52) | — |
| Median age, y (range) | 40 (31-57) | 49 (20-73) | — |
| Performance status > 1 | 5 (20) | 3 (9) | .26 |
| Stage III or IV | 15 (60) | 25 (76) | .20 |
| LDH greater than normal | 18 (72) | 23 (70) | .85 |
| More than 1 extranodal site | 10 (40) | 12 (36) | .78 |
| High risk age-adjusted International Prognostic Index Score (2 or 3) | 12 (48) | 17 (52) | .79 |
| Immunohistochemical markers* | |||
| MIB-1 ≥80% | 17 (85) | 19 (58) | .04 |
| bcl-2 positive | 3 (16) | 12 (41) | .06 |
| MUM-1 positive | 2 (14) | 7 (22) | .70 |
| CD10 positive | 10 (53) | 5 (19) | .02 |
| bcl-6 positive | 12 (60) | 10 (50) | .53 |
| p53 positive | 14 (64) | 8 (24) | .004 |
Characteristics . | HIV-positive series no. (%) . | HIV-negative series no. (%) . | P2 . |
|---|---|---|---|
| Total patients | 25 | 33 | — |
| Male sex | 21 (84) | 17 (52) | — |
| Median age, y (range) | 40 (31-57) | 49 (20-73) | — |
| Performance status > 1 | 5 (20) | 3 (9) | .26 |
| Stage III or IV | 15 (60) | 25 (76) | .20 |
| LDH greater than normal | 18 (72) | 23 (70) | .85 |
| More than 1 extranodal site | 10 (40) | 12 (36) | .78 |
| High risk age-adjusted International Prognostic Index Score (2 or 3) | 12 (48) | 17 (52) | .79 |
| Immunohistochemical markers* | |||
| MIB-1 ≥80% | 17 (85) | 19 (58) | .04 |
| bcl-2 positive | 3 (16) | 12 (41) | .06 |
| MUM-1 positive | 2 (14) | 7 (22) | .70 |
| CD10 positive | 10 (53) | 5 (19) | .02 |
| bcl-6 positive | 12 (60) | 10 (50) | .53 |
| p53 positive | 14 (64) | 8 (24) | .004 |
— represents not applicable.
Immunohistochemical analysis was performed in all patients with adequate tumor specimens. Due to technical factors or insufficient tissue, all analyses could not be performed in all available specimens.
Progression-free survival of 11 patients with p53-positive ARL and 16 patients with p53-negative ARL. Progression-free survival probabilities are 86% and 82%, respectively, at the median potential follow-up time of 53 months.
Progression-free survival of 11 patients with p53-positive ARL and 16 patients with p53-negative ARL. Progression-free survival probabilities are 86% and 82%, respectively, at the median potential follow-up time of 53 months.
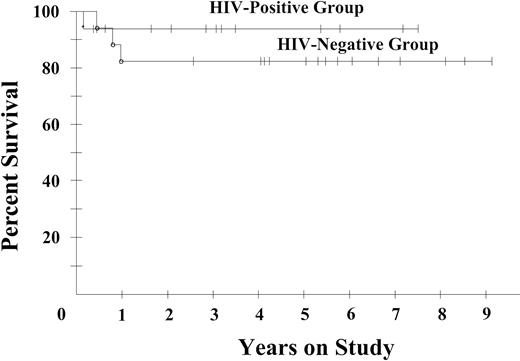
To help assess if the HIV-positive and -negative DLBCLs have similar treatment sensitivity, we compared the subset of patients with tumors not expressing bcl-2, which was the only marker of progression-free survival in our HIV-negative series.8 Within this biologic subset, the progression-free survival plateaus were 94% and 82%, respectively, for HIV-positive and -negative patients, suggesting equivalent treatment sensitivity (Figure 5).
Progression-free survival of bcl-2–negative diffuse large B-cell lymphomas in 16 HIV-positive (AIDS-related lymphoma) and in 17 HIV-negative (historical group) patients who were similarly treated with DA-EPOCH. Progression-free survival probabilities are 94% in the HIV-positive patients and 82% in the HIV-negative patients, at their respective median potential follow-up times of 53 months and 69 months.
Progression-free survival of bcl-2–negative diffuse large B-cell lymphomas in 16 HIV-positive (AIDS-related lymphoma) and in 17 HIV-negative (historical group) patients who were similarly treated with DA-EPOCH. Progression-free survival probabilities are 94% in the HIV-positive patients and 82% in the HIV-negative patients, at their respective median potential follow-up times of 53 months and 69 months.
Discussion
This study was designed to characterize the effects of antiretroviral therapy suspension and tumor biology on the outcome of ARL treated with DA-EPOCH, and to investigate the immune and clinical effects of IL-12 in a subset of patients who achieved a complete response. We found a 60% probability of survival without disease at 53 months and a 92% probability of durable remission at 53 months, and no additional benefit in patients randomized to receive IL-12. Among patients without involvement of the central nervous system, which is a treatment sanctuary site, there is a low incidence of systemic treatment failure with 79% of patients remaining alive and 70% actuarial survival at 53 months. Notably, all patients who died with Burkitt lymphoma had central nervous system lymphoma, and this accounted for their poorer outcome compared with DLBCL. In contrast to these results, 2 recent studies of standard CHOP-based chemotherapy and HAART in ARL with sufficient follow-up yielded overall survival rates of 40% at 2 years and 23% at 3 years, respectively.1,30 Although the improved results with DA-EPOCH could be due to patient selection, the present study had similar median CD4+ cell numbers but worse clinical prognostic features compared with the other studies.
Dose adjustment with antiretroviral suspension allowed full delivery of the infused agents, important for threshold concentrations, while minimizing clinical and immune toxicity. Treatment was well tolerated and the incidence of serious adverse effects was similar to standard CHOP chemotherapy.2 Although withholding antiretroviral treatment resulted in increased viral loads, they stabilized by cycle 4 with less than a log median increase, and then declined below baseline with resumption of antiretroviral therapy following DA-EPOCH. More importantly, following CD4+ cell loss during treatment, the dynamics of recovery were similar to those described in HIV-negative patients.12 These results suggest that withholding antiretroviral therapy does not significantly worsen AIDS progression and avoids potentially adverse affects on lymphoma treatment. Specifically, patients did not develop new HIV mutations, which could occur with poor antiretroviral therapy compliance during chemotherapy, and adverse pharmacokinetic interactions were avoided.9 An additional potential concern is raised by recent evidence that antiretroviral agents can inhibit lymphocyte apoptosis, and this could reduce chemotherapy efficacy.10
The pathobiology of ARL may account for the favorable results with DA-EPOCH. We had previously shown that, unlike reported with CHOP, DA-EPOCH is effective against highly proliferative lymphomas, which comprise 85% of the present series.8,14 Additionally, a recent study found 66% of untreated ARLs have multidrug resistance 1 (MDR-1), which correlated with poor clinical outcome.31 Infusion schedules can overcome MDR-1 in vitro, suggesting that DA-EPOCH or other infusional regimens like CDE (cyclophosphamide, doxorubicin, and etoposide) may be particularly beneficial in ARL.9 Most cases in our series were bcl-2–negative, which is a favorable prognostic feature in HIV-negative DLBCL, suggesting that HIV-positive patients should do well.15,17,18 To help assess if HIV infection altered the prognosis within this favorable bcl-2–negative group, we compared our patients to a control group of similarly treated HIV-negative patients and found a similar treatment outcome, indicating that, in the absence of central nervous system involvement, the HIV-positive cases are similar to good prognosis HIV-negative DLBCLs.8
We were also interested in evaluating the clinical impact of p53 overexpression because of its association with drug resistance in HIV-negative DLBCL.16,17 It is interesting that the frequency of p53 overexpression, a surrogate of gene mutation, was significantly higher in the HIV-positive compared with the HIV-negative DLBCL cases, a finding similar to other reports. In HIV-negative DLBCL, we and others have reported that approximately 20% of cases harbor a p53 mutation, whereas in HIV-positive patients, p53 mutations have been reported in 50% of Burkitt/Burkitt-like and 40% of DLBCL cases.16,17,32 Furthermore, similar to HIV-negative cases, p53 inactivating mutations in HIV-positive cases are associated with p53 overexpression by immunohistochemistry.17,32 We were surprised, however, to find no association between p53 overexpression and either progression-free or overall survival in our HIV-positive cases, suggesting that the pathogenesis of p53 is quite different in HIV-positive DLBCL, where it does not appear to convey a drug-resistant phenotype.8,16,17 It is also noteworthy that patients with EBV-positive DLBCL did relatively well even when compared with patients with EBV-negative tumors in this series.
These results raise important questions regarding the tumor pathogenesis of DLBCL in HIV-positive patients and its role in the improvement in clinical outcome of ARL since the HAART era. Interestingly, unlike HIV-negative DLBCL cases that are near equally distributed among GCB and post-GCB origin, consistent with our series and recent microarray results, the HIV-positive cases appear to derive primarily from GCB cells.33-35 Our results, however, must also be reconciled with earlier reports from the pre-HAART era that found the post-GCB phenotype in 50% and bcl-2 expression in 36% of cases, both of which are associated with a poor clinical outcome in HIV-negative patients.33-36 Notably, all of the cases with a post-GCB phenotype were immunoblastic DLBCLs, which occur significantly more often in patients with low CD4+ cell counts.35,37 In contrast, many patients in our series came from the post-HAART era and raise the hypothesis that the significant rise in CD4+ cell counts and improved immune function associated with HAART have led to a shift in pathogenesis away from lymphomas of post-GCB origin, with a concomitant improvement in treatment outcome and survival. These findings raise the interesting possibility that antigenic B-cell stimulation, associated with HIV infection, in the context of relatively preserved immune function, may disproportionately promote germinal center pathways of lymphomagenesis.38
These results also raise challenging questions about the role of HAART during the treatment of ARL. It is commonly held that HAART is necessary to prevent uncontrolled HIV replication and loss of immune function during chemotherapy, and a recent study suggested that response to HAART is essential to treat ARL with curative intent.39 However, we did not find that antiretroviral therapy is required during chemotherapy to achieve a high overall survival and posttreatment viral control and immune recovery. Of importance, only the CD4+ cell counts and involvement of the central nervous system at study entry were independent prognostic factors in our study, both of which are interrelated with the tumor pathobiology. Specifically, the frequency of post-GCB DLBCL, which is more often treatment resistant, is higher in immune-depleted patients, and multiple studies have shown a relationship between lymphoma pathobiology and central nervous system involvement.33,40,41 Notably, three-quarters of our patients who developed central nervous system involvement also had low CD4+ cell counts, indicating a major overlap between these 2 prognostic factors. These results suggest that tumor pathobiology at the time of clinical presentation is the important determinant of clinical outcome, consistent with recent findings in HIV-negative DLBCL, and not the immune environment during treatment.18 Our findings are not inconsistent with a recent report that showed an association between HAART response and improved ARL outcome, but we would offer an alternative to the author's hypothesis that this finding represents cause and effect.39 We believe it more likely that the HAART response serves as an indicator of the immune environment under which the tumor pathogenesis evolved.
Our results may have potential implications for future therapeutic strategies in ARL. Recently, a randomized study in HIV-negative DLBCL showed that rituximab, a monoclonal antibody against the CD20 antigen, significantly improved event-free and overall survival following CHOP chemotherapy, and has led to its general acceptance as standard therapy in DLBCL.24 However, we and others have shown that the benefit of rituximab is primarily restricted to bcl-2–expressing DLBCL, suggesting that it may have little value in most HIV-positive patients.42,43 On the other hand, the high tumor proliferation rate associated with ARL suggests that infusional regimens may be particularly important because of their potential increased efficacy in this setting.8
Our results suggest that ARLs in the HAART era may have favorable pathobiologic characteristics that are associated with a low incidence of chemotherapy resistance.Additionally, the highly proliferative nature of ARLs may account for the potential superiority of DA-EPOCH over standard treatment with CHOP regimens. ARL should be considered highly curable in the absence of central nervous system involvement, which remains a major barrier to cure.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-11-3589.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal