Abstract
Since serum tryptase levels are elevated in some patients with myeloproliferative disorders, we examined their utility in identifying a subset of patients with hypereosinophilic syndrome (HES) and an underlying myeloproliferative disorder. Elevated serum tryptase levels (> 11.5 ng/mL) were present in 9 of 15 patients with HES and were associated with other markers of myeloproliferation, including elevated B12 levels and splenomegaly. Although bone marrow biopsies in these patients showed increased numbers of CD25+ mast cells and atypical spindle-shaped mast cells, patients with HES and elevated serum tryptase could be distinguished from patients with systemic mastocytosis and eosinophilia by their clinical manifestations, the absence of mast cell aggregates, the lack of a somatic KIT mutation, and the presence of the recently described fusion of the Fip1–like 1 (FIP1L1) gene to the platelet-derived growth factor receptor α gene (PDGFRA). Patients with HES and elevated serum tryptase were more likely to develop fibroproliferative end organ damage, and 3 of 9 died within 5 years of diagnosis in contrast to 0 of 6 patients with normal serum tryptase levels. All 6 patients with HES and elevated tryptase treated with imatinib demonstrated a clinical and hematologic response. In summary, elevated serum tryptase appears to be a sensitive marker of a myeloproliferative variant of HES that is characterized by tissue fibrosis, poor prognosis, and imatinib responsiveness.
Introduction
Hypereosinophilic syndrome (HES) is a term applied to a heterogeneous group of disorders defined by (1) the presence of eosinophilia (> 1500 eosinophils/mm3 for at least 6 months) that remains unexplained despite a comprehensive evaluation for known causes of eosinophilia and (2) evidence of organ dysfunction directly attributable to the eosinophilia or otherwise unexplained in the clinical setting.1-3 Although the etiology of HES is unknown, the recent identification of subgroups of patients with HES and clonal populations of eosinophils4 or lymphocytes5,6 suggests that patients with HES can be separated into at least 2 major subgroups: those with a primary disorder of myelopoiesis and those with secondary eosinophilia due to overproduction of eosinophilopoietic cytokines by a clonal population of lymphocytes. Using currently available techniques, however, clonal populations of cells are not readily identifiable in a majority of patients with HES.
The clinical manifestations and responses to therapy in patients with HES are highly variable and likely reflect, at least in part, the underlying etiology of the disorder in a given patient. This has become even more evident with the recent description of Fip1–like 1 (FIP1L1)–platelet-derived growth factor receptor α (PDGFRA), a constitutively activated tyrosine kinase created by an interstitial deletion on chromosome 4q12, which is associated with imatinib responsiveness in some patients with HES.7 Several clinical markers of poor prognosis in HES have been identified, including the lack of a response to steroids, a markedly elevated white blood cell count,8 and a normal immunoglobulin E (IgE) level, although the relationship of these markers to disease pathogenesis has not been explored.
Some patients with HES have features of an underlying myeloproliferative disorder, including the presence of increased early myeloid precursors in the peripheral blood and bone marrow, and elevated serum B12 levels. In contrast to patients with chronic myeloid leukemia, however, these patients exhibit no evidence of cytogenetic abnormalities or increased numbers of blasts. Since serum tryptase levels are elevated in some myeloproliferative disorders, we examined the utility of serum tryptase levels in identifying the subset of HES patients with a primary myelopoietic disorder. The clinical and laboratory features of this group of patients were compared with those of patients with HES and normal serum tryptase levels, and to patients with systemic mastocytosis and eosinophilia greater than 1500/mm3.
Patients and methods
Patient populations
All study participants were evaluated at the National Institutes of Health (NIH; Bethesda, MD) between 1994 and 2002 as part of research protocols designed to study the pathogenesis of eosinophilic disorders, helminth infections, or mastocytosis. Included in the analysis were 32 patients who had at least 2 documented absolute peripheral blood eosinophil counts greater than 1500/mm3. HES was defined as (1) peripheral blood eosinophilia greater than 1500/mm3 on 2 occasions at least 6 months apart; (2) no known etiology for the eosinophilia despite careful clinical evaluation; (3) evidence of end organ damage (histologic evidence of tissue infiltration by eosinophils and/or objective evidence of clinical pathology in any organ system that is temporally associated with eosinophilia and not clearly attributable to another cause); (4) no evidence of a clonal hematopoietic process as determined by cytogenetic analysis of unstimulated bone marrow cells (a minimum of 20 metaphases), polymerase chain reaction (PCR)–based T-cell receptor gamma and immunoglobulin heavy chain gene rearrangement analysis of peripheral blood mononuclear cells (PBMCs) and/or bone marrow aspirates,9 and flow cytometric analysis of T-cell surface markers (CD3, CD4, CD8, CD25, and HLA-DR); and (5) clinical and/or laboratory findings inconsistent with other idiopathic eosinophilic disorders, including Churg-Strauss vasculitis, chronic eosinophilic pneumonia, eosinophilic gastroenteritis, and episodic angioedema and eosinophilia.
All laboratory testing reported in this study was performed in the Clinical Pathology Departments at the NIH Clinical Center, with the exception of serum total tryptase levels, which were measured at the Mayo Medical Labs (Rochester, MN). The normal range for serum tryptase used in this study (< 11.5 ng/mL) was provided by Mayo Medical Labs. Splenomegaly was assessed by physical examination and confirmed by computed tomography (CT). Pulmonary disease was classified as restrictive or obstructive on the basis of characteristic findings on pulmonary function testing (PFT), including spirometry, flow volume loops, and assessment of diffusion capacity. Interstitial infiltrates on chest CT were considered supportive evidence of restrictive disease but were not sufficient for classification in the absence of PFT abnormalities.
Mutational analysis of KIT
Bone marrow mast cells were enriched based on their surface CD25 expression.10 Bone marrow aspirate mononuclear cells were isolated by Ficoll-Hypaque density gradient (1.077). CD25+ cells were then separated using anti–human CD25–coated paramagnetic beads according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Purified mast cells were obtained first by anti-CD25 magnetic bead selection as described followed by flow cytometric sorting based on high CD117 expression. Total RNA was isolated from peripheral blood mononuclear cells, bone marrow CD25+ cells, or purified mast cells using RNeasy kit (Qiagen, Valencia, CA). Reverse transcription (RT)–PCR and restriction fragment length polymorphism analysis of the PCR products for Asp816Val KIT mutation were performed as described.11 In some patients, direct sequencing of the PCR fragments was performed using an ABI Prism 310 Genetic Analyzer (Applied Biosystems, Foster City, CA) and a Big Dye Terminator DNA sequencing kit (Applied Biosystems).
Flow cytometric analysis of mast cells
Bone marrow aspirates were obtained from patients following informed consent. The bone marrow aspirate mononuclear cell fraction containing mast cells was then separated using Histopaque (density = 1.077; Sigma, St Louis, MO) gradient centrifugation, and contaminating red cells were lysed by incubation in 0.8% ammonium chloride solution (Stem Cell Technologies, Vancouver, BC, Canada) for 10 minutes. Mast cells in bone marrow aspirates were identified by flow cytometry as a CD117+, high side-scatter population as described.12 Briefly, bone marrow mononuclear cells were incubated in 100 microliter aliquots for 30 minutes at 4°C with a phycoerythrin conjugate of anti–human CD117 (Clone 104D2; Becton Dickinson, San Jose, CA) and a fluorescein isothiocyanate (FITC) conjugate of antihuman CD2, CD25, or CD35 (BD Pharmingen, San Diego, CA). The cells were then washed, resuspended in phosphate-buffered saline containing 0.1% bovine serum albumin, and analyzed on a flow cytometer (FACScan; Becton Dickinson).
Detection of FIP1L1-PDGFRA fusion
RNA was isolated from peripheral blood mononuclear cells using Trizol (Invitrogen). First-strand cDNA was synthesized from 2 μg total RNA using Superscript first-strand synthesis system (Invitrogen, Carlsbad, CA) with random primers. Fusion of FIP1L1 to PDGFRA was analyzed by nested PCR using primers FIP1L1-F1 (5′-acctggtgctgatctttctgat) and PDG-FRA-R1 (5′-tgagagcttgtttttcactgga) during the first PCR, and primers FIP1L1-F2 (5′-aaagaggatacgaatgggacttg) and PDGFRA-R2 (5′-gggaccggcttaatccatag) for the second PCR. Control RT-PCR for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed using the primers GAPDH-F (5′-tggaaatcccatcaccatct) and GAPDH-R (5′-gtcttctgggtggcagtgat). PCR products were cloned in pGEM-T-easy (Promega, Madison, WI), and cloned products were sequenced on an ABI system (Applied Biosystems).
Statistical analysis
Nonparametric comparisons of group means were made using the Mann-Whitney U test. Correlations between observations were determined by Spearman rank correlation coefficient. Contingency table analyses were performed by Fisher exact test. A P value of less than .5 was considered statistically significant for all tests.
Results
Detection of elevated serum tryptase levels in a subset of patients with HES
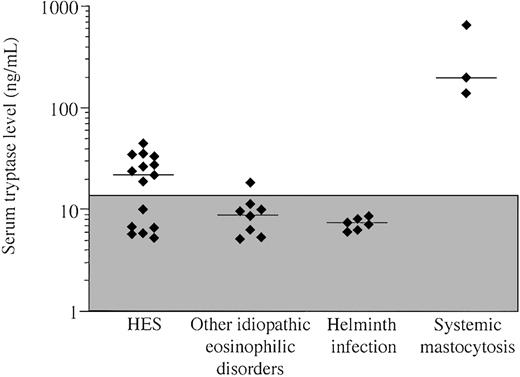
Serum tryptase levels were measured in 32 patients with peripheral eosinophil counts of 1500/mm3 or greater, including 15 patients with HES, 6 patients with documented helminth infection, 3 patients with systemic mastocytosis and eosinophilia, and 8 patients with other disorders associated with eosinophilia (Table 1). Elevated serum tryptase levels (> 11.5 ng/mL) were found in 9 of 15 patients with HES and both patients with systemic mastocytosis (Figure 1). One patient with eosinophilic gastroenteritis and an elevated peripheral blood eosinophil count also had a mildly elevated serum tryptase level of 18.7 ng/mL (Figure 1). Of note, serum tryptase levels in the 3 patients with systemic mastocytosis and eosinophilia were markedly increased (143, 205, and 675 ng/mL) compared with the patients with HES and elevated serum tryptase levels (median, 28 ng/mL; range, 19-44 ng/mL; P = .03).
Characteristics of eosinophilic patients
Patient group . | Hypereosinophilic syndrome . | Helminth infection . | Other eosinophilic disorders* . | Systemic mastocytosis . |
|---|---|---|---|---|
| Number of patients | 15 | 6 | 8 | 3 |
| Median age, y (range) | 36 (17-77) | 24 (16-60) | 35 (21-55) | 51 (47-61) |
| Sex, M/F | 9/6 | 1/5 | 4/4 | 2/1 |
| WBCs, × 109/L, median (range) | 13.3 (6.2-31.6) | 13.4 (5-14.6) | 10 (5.6-15.7) | 9.3 (8.3-19.7) |
| Eosinophils, × 109/L, median (range) | 5.927 (.707-27.55) | 4.409 (1.508-6.623) | 2.632 (.526-8.117) | 3.127 (1.598-4.492) |
| Basophils, × 109/L, median (range) | .018 (0-.072) | .02 (0-.044) | .056 (.008-.094) | .033 (.028-.079) |
| Serum IgE levels, kU/L, median (range) | 27 (0-4862) | 3081 (45-21 293) | 126 (15-6518) | 19 (13-24) |
| Serum B12 levels, pM, median (range) | 3896 (276-20311) | 283 (173-393) | 589 (455-791) | Not done |
Patient group . | Hypereosinophilic syndrome . | Helminth infection . | Other eosinophilic disorders* . | Systemic mastocytosis . |
|---|---|---|---|---|
| Number of patients | 15 | 6 | 8 | 3 |
| Median age, y (range) | 36 (17-77) | 24 (16-60) | 35 (21-55) | 51 (47-61) |
| Sex, M/F | 9/6 | 1/5 | 4/4 | 2/1 |
| WBCs, × 109/L, median (range) | 13.3 (6.2-31.6) | 13.4 (5-14.6) | 10 (5.6-15.7) | 9.3 (8.3-19.7) |
| Eosinophils, × 109/L, median (range) | 5.927 (.707-27.55) | 4.409 (1.508-6.623) | 2.632 (.526-8.117) | 3.127 (1.598-4.492) |
| Basophils, × 109/L, median (range) | .018 (0-.072) | .02 (0-.044) | .056 (.008-.094) | .033 (.028-.079) |
| Serum IgE levels, kU/L, median (range) | 27 (0-4862) | 3081 (45-21 293) | 126 (15-6518) | 19 (13-24) |
| Serum B12 levels, pM, median (range) | 3896 (276-20311) | 283 (173-393) | 589 (455-791) | Not done |
WBC indicates white blood cell count.
Eosinophilic gastroenteritis (n = 3), Churg Strauss vasculitis (n = 1), lymphoma (n = 1), episodic angioedema and eosinophilia (n = 1), and hypereosinophilia without end organ involvement (n = 2).
Serum tryptase levels in patients with marked eosinophilia due to HES, other idiopathic eosinophilic disorders, parasitic helminth infection, and systemic mastocytosis with eosinophilia. Each symbol represents the first serum tryptase value available for an individual patient. The median serum tryptase level for each group is indicated by a horizontal bar. The shaded box represents the normal range. Elevated serum tryptase levels are present in a subset of patients with HES and one patient with idiopathic eosinophilic gastroenteritis. As expected, the 3 patients with systemic mastocytosis have markedly elevated levels.
Serum tryptase levels in patients with marked eosinophilia due to HES, other idiopathic eosinophilic disorders, parasitic helminth infection, and systemic mastocytosis with eosinophilia. Each symbol represents the first serum tryptase value available for an individual patient. The median serum tryptase level for each group is indicated by a horizontal bar. The shaded box represents the normal range. Elevated serum tryptase levels are present in a subset of patients with HES and one patient with idiopathic eosinophilic gastroenteritis. As expected, the 3 patients with systemic mastocytosis have markedly elevated levels.
Association of elevated serum tryptase levels in patients with HES with features of myeloproliferative disease and poor prognosis
Patients with HES were divided into 2 groups based on the presence or absence of elevated serum tryptase levels (n = 9 and 6, respectively). As shown in Table 2, all 9 of the patients with elevated tryptase were male, in contrast to 1 of 6 patients with normal tryptase levels (P < .008). There was no difference in median age between the 2 groups. End organ manifestations of HES in patients with elevated tryptase included biopsy-proven endomyocardial fibrosis (n = 3), restrictive pulmonary disease (n = 4), mucosal ulcerations (n = 3), and splenomegaly (n = 7) (Table 3). None of these manifestations were identified in patients with HES and normal tryptase levels, who typically presented with obstructive pulmonary disease (n = 3), pruritic dermatitis (n = 6), and gastrointestinal complaints (n = 4). Of the 9 patients with HES and elevated serum tryptase, 3 died of complications of HES within 5 years of their diagnosis, in contrast to 0 of 6 patients with HES who had normal serum tryptase levels.
Characteristics of HES patients with increased and normal tryptase levels
Patient group . | Serum tryptase level 11.5 ng/mL or greater . | Serum tryptase level less than 11.5 ng/mL . | P . |
|---|---|---|---|
| Number of patients | 9 | 6 | |
| Median age, y (range) | 38 (28-77) | 36 (17-57) | NS |
| Sex, M/F | 9/0 | 1/5 | < .01 |
| WBC, × 109/L, median (range) | 18.1 (13.3-31.6) | 8.2 (6.2-16.4) | .04 |
| Eosinophils, × 109/L, median (range) | 8.352 (2.425-27.550) | 1.792 (.707-6.216) | < .01 |
| Basophils, × 109/L, median (range) | .001 (0-.072) | .026 (.009-.057) | NS |
| Splenomegaly | 7/9 | 0/6 | < .01 |
| Myeloid precursors on peripheral smear | 8/9 | 0/6 | < .01 |
| Bone marrow biopsy cellularity, greater than 50% | 7/9 | 1/4 | NS |
| Dysplastic mast cells* | 7/9 | 0/4 | .02 |
| Myelofibrosis† | 5/9 | 0/4 | NS |
| Median hemoglobin, g/dL (range) | 12.2 (6.9-13.8) | 13.8 (11-15) | .08 |
| Median platelet count, 109/L (range) | 165 (95-223) | 283 (192-531) | < .01 |
| Median serum IgE level, kU/L (range) | 15 (0-4862) | 137 (11-544) | .08 |
| Median serum B12 level, pM (range) | 4255 (2315-20 316) | 592 (276-838) | < .01 |
Patient group . | Serum tryptase level 11.5 ng/mL or greater . | Serum tryptase level less than 11.5 ng/mL . | P . |
|---|---|---|---|
| Number of patients | 9 | 6 | |
| Median age, y (range) | 38 (28-77) | 36 (17-57) | NS |
| Sex, M/F | 9/0 | 1/5 | < .01 |
| WBC, × 109/L, median (range) | 18.1 (13.3-31.6) | 8.2 (6.2-16.4) | .04 |
| Eosinophils, × 109/L, median (range) | 8.352 (2.425-27.550) | 1.792 (.707-6.216) | < .01 |
| Basophils, × 109/L, median (range) | .001 (0-.072) | .026 (.009-.057) | NS |
| Splenomegaly | 7/9 | 0/6 | < .01 |
| Myeloid precursors on peripheral smear | 8/9 | 0/6 | < .01 |
| Bone marrow biopsy cellularity, greater than 50% | 7/9 | 1/4 | NS |
| Dysplastic mast cells* | 7/9 | 0/4 | .02 |
| Myelofibrosis† | 5/9 | 0/4 | NS |
| Median hemoglobin, g/dL (range) | 12.2 (6.9-13.8) | 13.8 (11-15) | .08 |
| Median platelet count, 109/L (range) | 165 (95-223) | 283 (192-531) | < .01 |
| Median serum IgE level, kU/L (range) | 15 (0-4862) | 137 (11-544) | .08 |
| Median serum B12 level, pM (range) | 4255 (2315-20 316) | 592 (276-838) | < .01 |
NS indicates not significant.
More than 25% of mast cells in bone marrow biopsy are spindle-shaped.
Presence of antireticulin antibody staining on bone marrow biopsy.
End organ involvement in HES patients with and without elevated tryptase levels
Patient group . | Serum tryptase level 12 ng/mL or higher, n = 9 . | Serum tryptase level less than 12 ng/mL, n = 6 . |
|---|---|---|
| Endomyocardial fibrosis | 3 | 0 |
| Pulmonary | 4 | 3 |
| Obstructive | 0 | 3 |
| Restrictive | 4 | 0 |
| Skin | 7 | 6 |
| Pruritus/rash | 6 | 6 |
| Mucosal ulcerations | 3 | 0 |
| Gastrointestinal | 0 | 4 |
| Other* | 0 | 1 |
Patient group . | Serum tryptase level 12 ng/mL or higher, n = 9 . | Serum tryptase level less than 12 ng/mL, n = 6 . |
|---|---|---|
| Endomyocardial fibrosis | 3 | 0 |
| Pulmonary | 4 | 3 |
| Obstructive | 0 | 3 |
| Restrictive | 4 | 0 |
| Skin | 7 | 6 |
| Pruritus/rash | 6 | 6 |
| Mucosal ulcerations | 3 | 0 |
| Gastrointestinal | 0 | 4 |
| Other* | 0 | 1 |
One patient presented with serositis (pleuritis and pericarditis), small vessel vasculitis, and a cerebrovascular accident.
HES patients with elevated tryptase levels had increased median white blood cell and eosinophil counts and decreased platelet counts compared with patients with normal tryptase levels (Table 2). In addition, all of the patients with HES who had elevated serum tryptase, and none of those without elevated serum tryptase, had myelocytes and/or promyelocytes detectable on peripheral smear. Median hemoglobin and IgE levels were also decreased in the patients with HES and elevated serum tryptase, although these relationships did not reach statistical significance (P = .08).
Elevated serum B12 levels are seen in association with a variety of myeloproliferative diseases13 and have been described in patients with HES.14 Serum B12 levels were markedly increased in all of the patients with HES and elevated serum tryptase levels (median, 4255 pM; range, 1476-20 316 pM; normal values, 162-708 pM). In contrast, serum B12 levels were mildly elevated in only 2 patients with HES with normal serum tryptase levels (median, 592 pM; range, 276-838 pM).
Bone marrow biopsies from patients with HES with and without elevated serum tryptase levels were compared. The bone marrow biopsies of patients with HES and increased serum tryptase levels were more likely to be hypercellular with increased and left-shifted eosinophils (7/9 vs 1/4), have increased reticulin fibrosis (5/9 vs 0/4), increased atypical mast cells (spindle shaped) (8/9 vs 0/4), and increased small lymphocytes (4/9 vs 0/4) than biopsies from patients with HES and normal serum tryptase levels (Table 2; Figure 1).
Correlates of elevated serum tryptase in patients with HES
Although patients with HES and elevated serum tryptase levels had increased absolute eosinophil counts when analyzed as a group compared with patients with HES and normal tryptase levels, serum tryptase levels did not correlate with absolute eosinophil counts (P = .7; data not shown). In fact, serial tryptase levels continued to rise (from 27.1 to 35.6 to 47.5 ng/mL) in one patient who experienced progression of disease, despite a decrease in eosinophilia from 7500/mm3 to 1500/mm3 over a 7-month period. Basophils, a potential source of serum tryptase,15 were not increased in the peripheral blood of any of the patients studied and were similar in number in patients with HES with and without elevated serum tryptase levels (Tables 1, 2).
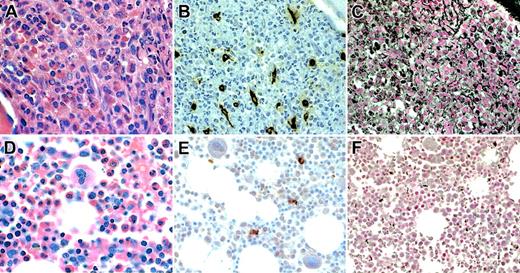
Both mast cells and myeloblasts have been reported to produce tryptase.16 Consequently, bone marrow biopsies from all study patients for whom sections were available were stained with antitryptase antibody. Increased numbers of spindle-shaped mast cells were seen in the biopsies of 8 of 9 patients with HES and elevated serum tryptase levels, although mast cell aggregates characteristic of systemic mastocytosis were notably absent (Figure 2). Mast cell morphology and numbers were normal in the biopsies of HES patients without elevated serum tryptase levels (n = 4).
Bone marrow biopsy findings in representative HES patients. Biopsy sections from patients with HES with (A-C) and without (D-F) elevated serum tryptase levels were stained with hematoxylin and eosin (A,D), antitryptase antibody (B,E), and antireticulin antibody (C,F). Although the bone marrow biopsy specimens from both patients show a dramatic increase in eosinophils and myeloid precursors (A,D), only the specimen from the patient with elevated serum tryptase shows marked hypercellularity (A), atypical spindle-shaped mast cells (B), and reticulin fibrosis (C).
Bone marrow biopsy findings in representative HES patients. Biopsy sections from patients with HES with (A-C) and without (D-F) elevated serum tryptase levels were stained with hematoxylin and eosin (A,D), antitryptase antibody (B,E), and antireticulin antibody (C,F). Although the bone marrow biopsy specimens from both patients show a dramatic increase in eosinophils and myeloid precursors (A,D), only the specimen from the patient with elevated serum tryptase shows marked hypercellularity (A), atypical spindle-shaped mast cells (B), and reticulin fibrosis (C).
HES with increased serum tryptase is a clinical syndrome that is distinct from systemic mastocytosis
The clinical manifestations of systemic mastocytosis include characteristic skin lesions (urticaria pigmentosa), malabsorption with weight loss, hepatomegaly with ascites, splenomegaly, and pathologic fractures.17 With the exception of mild splenomegaly, which was identified in 7 of 9 patients with HES and elevated tryptase levels, clinical findings typical of mastocytosis were notably absent in these patients. Furthermore, skin biopsies were performed in all patients with HES who had dermatitis or mucosal ulcerations and revealed no evidence of increased or atypical mast cells (data not shown).
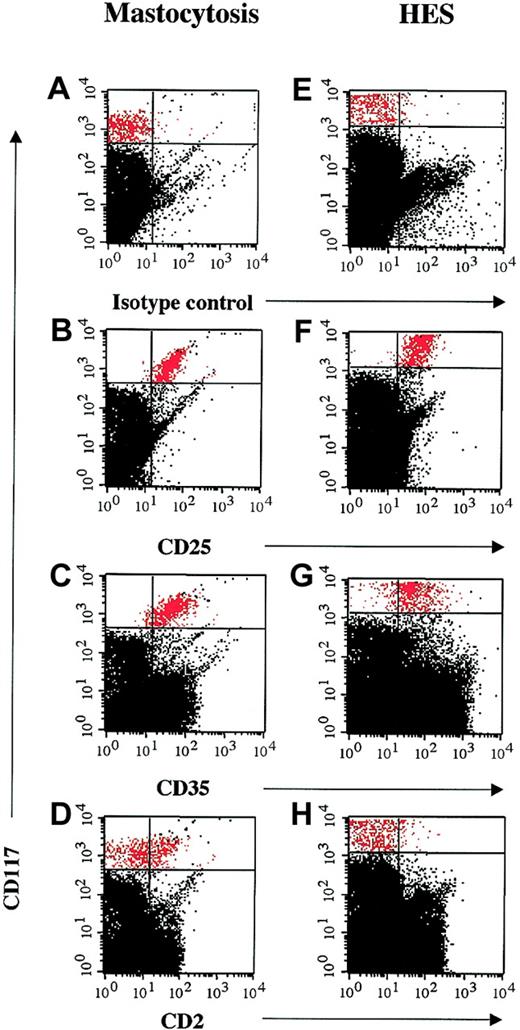
Since aberrant coexpression of CD2 and CD25 on the surface of bone marrow mast cells has been described as a feature characteristic of systemic mastocytosis,12,17 flow cytometry was performed on mast cells from the bone marrow aspirates of 4 patients with HES and elevated serum tryptase levels and 3 patients with systemic mastocytosis and marked eosinophilia. In all 4 patients with HES, CD25 expression was detected on CD117+CD34– bone marrow mast cells in the absence of CD2 expression (Figure 3). In contrast, bone marrow mast cells from the 3 patients with systemic mastocytosis and eosinophilia coexpressed CD2 and CD25.
Flow cytometric detection of CD25, but not CD2, expression on bone marrow mast cells from patients with HES and elevated serum tryptase levels. Multicolor flow cytometry results are shown for CD117+ high, side-scatter high bone marrow cells from a representative patient with systemic mastocytosis and eosinophilia (A-D) and a patient with HES and elevated tryptase (E-H). Cells were stained with FITC-conjugated mouse IgG1 control antibody (A,E), antihuman CD2 antibody (B,F), antihuman CD25 antibody (C,G), and antihuman CD35 antibody (D,H). CD117+ high, side-scatter high bone marrow mast cells are shown in red.
Flow cytometric detection of CD25, but not CD2, expression on bone marrow mast cells from patients with HES and elevated serum tryptase levels. Multicolor flow cytometry results are shown for CD117+ high, side-scatter high bone marrow cells from a representative patient with systemic mastocytosis and eosinophilia (A-D) and a patient with HES and elevated tryptase (E-H). Cells were stained with FITC-conjugated mouse IgG1 control antibody (A,E), antihuman CD2 antibody (B,F), antihuman CD25 antibody (C,G), and antihuman CD35 antibody (D,H). CD117+ high, side-scatter high bone marrow mast cells are shown in red.
Somatic KIT mutations at the amino acid position Asp816 are detectable in most adult patients with systemic mastocytosis.18 The presence of this 816 mutation in KIT was assessed by restriction fragment length polymorphism analysis of cDNA obtained from peripheral blood mononuclear cells (n = 1) or bone marrow mononuclear cells enriched for cells expressing CD25 on their surface (n = 3) from patients with HES and elevated serum tryptase. No codon 816 KIT mutations were detected by RT-PCR in cells from any of the 4 patients with HES (Table 4). The absence of a codon 816 KIT mutation was confirmed by direct sequencing of the cDNA in 2 of the patients with HES and elevated serum tryptase. In contrast, a codon 816 KIT mutation was present in unfractionated peripheral blood mononuclear cells (n = 2) or purified mast cells (n = 1) in all 3 of the patients with systemic mastocytosis and eosinophilia (Table 4).
Analysis of Asp816Val c-kit mutation in patients with mastocytosis and eosinophilia and hypereosinophilic syndrome with elevated serum tryptase
Patient . | c-kit mutation detected . | Source of RNA for analysis . | Method of analysis . |
|---|---|---|---|
| HES-1 | N | CD25+ bone marrow cells | RT-PCR followed by RFLP; direct sequencing |
| HES-2 | N | CD25+ bone marrow cells | RT-PCR followed by RFLP |
| HES-3 | N | CD25+ bone marrow cells | RT-PCR |
| HES-4 | N | Unfractionated PBMCs* | RT-PCR followed by RFLP; direct sequencing |
| SM-1 | Y | Unfractionated PBMCs | RT-PCR followed by RFLP |
| SM-2 | Y | Unfractionated bone marrow mononuclear cells | RT-PCR followed by RFLP |
| SM-3 | Y | Purified bone marrow mast cells | RT-PCR followed by RFLP |
Patient . | c-kit mutation detected . | Source of RNA for analysis . | Method of analysis . |
|---|---|---|---|
| HES-1 | N | CD25+ bone marrow cells | RT-PCR followed by RFLP; direct sequencing |
| HES-2 | N | CD25+ bone marrow cells | RT-PCR followed by RFLP |
| HES-3 | N | CD25+ bone marrow cells | RT-PCR |
| HES-4 | N | Unfractionated PBMCs* | RT-PCR followed by RFLP; direct sequencing |
| SM-1 | Y | Unfractionated PBMCs | RT-PCR followed by RFLP |
| SM-2 | Y | Unfractionated bone marrow mononuclear cells | RT-PCR followed by RFLP |
| SM-3 | Y | Purified bone marrow mast cells | RT-PCR followed by RFLP |
N indicates no; RFLP, restriction fragment length polymorphism; and Y, yes.
A bone marrow aspirate could not be obtained from this patient secondary to severe myelofibrosis.
Elevated tryptase levels are associated with imatinib responsiveness and expression of FIP1L1-PDGFRA in patients with HES
In view of the clinical and laboratory data supporting a myeloproliferative pathogenesis for HES with elevated tryptase and case reports demonstrating a response to imatinib mesylate therapy in some patients with HES, 6 patients with HES and elevated tryptase were treated with 400 mg imatinib mesylate daily. Symptoms resolved in all patients within a week of the initiation of imatinib therapy. Eosinophil counts normalized (< 750 eosinophil/mm3) in 5 of 6 patients within a week of the initiation of imatinib therapy. The sixth patient, whose eosinophil count had normalized on cyclosporin A and interferon therapy, had an initial increase in peripheral eosinophilia at 1 week, followed by a return to normal (< 750/mm3) by 3 weeks after the initiation of treatment. Of note, serum tryptase levels remained elevated in all 6 patients prior to the initiation of imatinib therapy despite treatment with steroids (n = 2), interferon (n = 2), hydroxyurea (n = 4), and cyclosporin (n = 1) and normalized within a week of imatinib therapy in all patients.
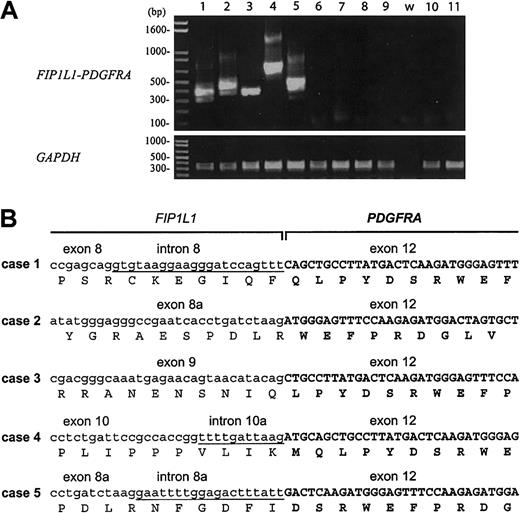
FIP1L1-PDGFRA is a constitutively activated tyrosine kinase created by an interstitial deletion on chromosome 4q12, which is associated with imatinib responsiveness in some patients with HES.7 The presence of this fusion was assessed by RT-PCR using RNA isolated from PBMCs from 5 patients with HES and elevated serum tryptase (4 of whom had demonstrated a clinical response to imatinib and 1 who died before imatinib was available), 4 patients with HES and normal serum tryptase levels, and 2 patients with familial eosinophilia, an autosomal dominant disorder that has been mapped to chromosome 5q31-33.19 The fusion was detected in RNA from all 5 of the patients with HES and elevated serum tryptase and in none of the patients with HES and normal serum tryptase or familial eosinophilia (Figure 4A). Sequence analysis confirmed that all breakpoints in PDGFRA occurred in exon 12 and that all of the fusions were in-frame (Figure 4B).
Detection of the FIP1L1-PDGFRA fusion in PBMCs from patients with HES and elevated tryptase. (A) RT-PCR analysis of RNA isolated from PBMCs from patients with HES and elevated serum tryptase (lanes 1-5), patients with HES and normal tryptase levels (lanes 6-9), and patients with a familial form of eosinophilia linked to chromosome 5q (lanes 10-11). The water control is indicated by the letter w. Only patients with elevated tryptase levels (1-5) were positive for the FIP1L1-PDGFRA fusion. Different PCR products are observed in the different cases, due to different breakpoints in the FIP1L1 gene (B). The different bands observed in each case represent splice variants (the sequence of 1 splice variant is shown in panel B), as observed previously.7 GAPDH was amplified as a control for cDNA quality. (B) cDNA and deduced amino acid sequence of FIP1L1-PDGFRA fusions from patients 1 to 5. Sequences derived from FIP1L1 are shown in lowercase; sequences derived from introns of FIP1L1 that are present in the fusion cDNA are underlined; sequences derived from PDGFRA are shown in uppercase in bold. Breakpoints in FIP1L1 are variable (in introns 8, 8a, 9, or 10), but all breakpoints in PDGFRA occur in exon 12. All fusions are in-frame. Exon numbering is according to Cools et al.7
Detection of the FIP1L1-PDGFRA fusion in PBMCs from patients with HES and elevated tryptase. (A) RT-PCR analysis of RNA isolated from PBMCs from patients with HES and elevated serum tryptase (lanes 1-5), patients with HES and normal tryptase levels (lanes 6-9), and patients with a familial form of eosinophilia linked to chromosome 5q (lanes 10-11). The water control is indicated by the letter w. Only patients with elevated tryptase levels (1-5) were positive for the FIP1L1-PDGFRA fusion. Different PCR products are observed in the different cases, due to different breakpoints in the FIP1L1 gene (B). The different bands observed in each case represent splice variants (the sequence of 1 splice variant is shown in panel B), as observed previously.7 GAPDH was amplified as a control for cDNA quality. (B) cDNA and deduced amino acid sequence of FIP1L1-PDGFRA fusions from patients 1 to 5. Sequences derived from FIP1L1 are shown in lowercase; sequences derived from introns of FIP1L1 that are present in the fusion cDNA are underlined; sequences derived from PDGFRA are shown in uppercase in bold. Breakpoints in FIP1L1 are variable (in introns 8, 8a, 9, or 10), but all breakpoints in PDGFRA occur in exon 12. All fusions are in-frame. Exon numbering is according to Cools et al.7
Discussion
The existence of a subset of patients with HES and features of myelodysplasia, including splenomegaly and increased vitamin B12 levels, has long been recognized.2 These features, as well as male sex, have also been associated with progression to endomyocardial fibrosis,20 although the mechanism underlying this link is poorly understood. As has been shown for serum B12 levels, elevated serum tryptase levels identify a subgroup of patients with other evidence of myeloproliferative disease, including splenomegaly, marrow hypercellularity, and the presence of early myeloid precursors on peripheral smear (Table 1). Interestingly, they are also associated with male sex, clinical manifestations of tissue fibrosis, poor prognosis, and the presence of the FIP1L1-PDGFRA fusion tyrosine kinase associated with imatinib responsiveness in HES.
Although serum total tryptase is produced predominantly by mast cells, other cell types, including basophils21 and myeloblasts,16 can produce tryptase. Eosinophil production of tryptase has not been described,21 and the lack of correlation between peripheral blood eosinophil counts and serum tryptase levels suggests that eosinophils are not the source of the elevated serum tryptase in patients with HES. This is further supported by immunohistochemical studies of the bone marrow biopsies from HES patients with elevated serum tryptase levels, which show tryptase expression in mast cells, but not in eosinophils or their precursors.
Since eosinophilia, at times marked, has been described in patients with systemic mastocytosis, (Table 1 and Horny et al22 ), an extensive evaluation, based on recently published consensus diagnostic criteria, was performed to exclude systemic mastocytosis in the subgroup of patients with HES who had elevated tryptase. Although multifocal dense infiltrates of mast cells were not detected in the bone marrow biopsies or other tissues in these patients, 3 of 4 minor criteria (> 25% of bone marrow mast cells are atypical or spindle-shaped, KIT+ mast cells coexpress CD2 and/or CD25, and serum tryptase is persistently > 20 ng/mL) were present in all patients.
Although the presence of 3 minor criteria would allow for a diagnosis of systemic mastocytosis using the currently accepted diagnostic criteria,17 several features distinguish patients with HES with elevated serum tryptase from those with systemic mastocytosis with eosinophilia suggesting that the 2 syndromes have different underlying etiologies and pathogenesis. First, in contrast to patients with systemic mastocytosis, who typically present with symptoms and signs related to mast cell infiltration of tissues and histamine release, patients with HES and elevated tryptase develop end organ damage characterized by eosinophilic infiltration of tissues and/or deposition of eosinophil granule proteins. Second, CD117+CD34 bone marrow mast cells from most patients with systemic mastocytosis coexpress CD2 and CD25,12 whereas CD2 was not detected on mast cells from any of the patients with HES and elevated tryptase. Third, a codon 816 KIT mutation was not detectable in bone marrow mononuclear cells enriched for CD25+ aberrant mast cells. This mutation is almost always detectable in lesional mast cells in patients with systemic mastocytosis and was present in mast cells from all 3 of the patients with systemic mastocytosis and eosinophilia. Finally, tryptase levels were significantly higher in all 4 patients with unexplained eosinophil counts greater than 1500/mm3 and systemic mastocytosis (diagnosed on the basis of characteristic clinical signs and symptoms and the presence of mast cell infiltrates in the bone marrow) than in the patients with HES and elevated serum tryptase (Figure 1).
There is substantial evidence that eosinophils and mast cells interact at sites of allergic inflammation,23 and recent studies have demonstrated that eotaxin, a chemokine central to the accumulation of eosinophils at sites of tissue inflammation, also acts on mast cells.24-26 Not only do mast cells secrete a wide variety of cytokines and chemokines involved in eosinophil production, activation, and survival (eg, interleukin-5 [IL-5], IL-3, and granulocyte-macrophage colony-stimulating factor [GM-CSF]),27 but eosinophils synthesize and release mast cell growth factors, including SCF and nerve growth factor.28 Furthermore, the eosinophil granule proteins, major basic protein (MBP) and eosinophil cationic protein (ECP), can activate mast cells to secrete histamine, tryptase, and prostaglandin PGD2, an effect that appears to be modulated by fibroblast-derived stem cell factor.29,30
Mast cell tryptase has been implicated in a wide variety of conditions associated with fibroproliferative tissue responses, including idiopathic bronchiolitis obliterans organizing pneumonia31 chronic inflammatory hepatobiliary disease,32 renal interstitial fibrosis,33 and male infertility.34 The role of tryptase in these conditions is supported by in vitro studies, which have demonstrated that mast cell tryptase can stimulate fibroblast proliferation through prostaglandin and COX2-mediated activation of protease-activated receptor 2.35
Patients with HES and elevated serum tryptase levels, but not those with normal tryptase levels, demonstrated clinical and pathologic evidence of tissue fibrosis, including endomyocardial fibrosis, restrictive pulmonary disease, and increased reticulin staining in the bone marrow, consistent with the hypothesis that mast cell tryptase, and not eosinophil products, is responsible for the tissue fibrosis seen in patients with the myeloproliferative form of HES.
In summary, we have described a subset of patients with a myeloproliferative form of idiopathic hypereosinophilic syndrome that is associated with elevated serum tryptase, mast cell dysplasia with a unique surface phenotype, and poor prognosis. This clinical subtype appears to correlate with the presence of a recently described fusion tyrosine kinase, FIP1L1-PDGFRα, that is a therapeutic target of imatinib in HES.7 Despite the fact that the laboratory findings in most of these patients fulfill the currently accepted diagnostic criteria for systemic mastocytosis, HES with elevated serum tryptase levels appears to be a syndrome that is distinct from systemic mastocytosis with eosinophilia, suggesting that these criteria will need to be refined. Stimulation of mast cells by eosinophils to produce tryptase may explain the observed relationship between this form of HES, tissue fibrosis, and a decreased response to therapy directed at lowering the eosinophil response without an effect on mast cell proliferation. This hypothesis is strengthened by the observation that the response to imatinib in this subset of patients is characterized not only by a decrease in eosinophilia, but also by the disappearance of atypical mast cells, normalization of serum tryptase levels, and resolution of myelofibrosis (data not shown). In conclusion, serum tryptase levels may be useful as a clinical marker of myeloproliferative HES, a predictor of imatinib responsiveness, and as a means to follow therapy in this subgroup of patients.
Prepublished online as Blood First Edition Paper, April 3, 2003; DOI 10.1182/blood-2003-01-0006.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Barbara Foster for her assistance with the mast cell flow cytometry; Drs Macdonald Horne, Barbara Karp, James Shelhamer, Maria Turner, and Paul Keiser who helped with the clinical characterization of the patients; Leigh Bernardino and Donna Jo McCloskey for their assistance with patient scheduling; and the many referring physicians and the study participants without whom this study would not have been possible.
Jan Cools is a postdoctoraal onderzoeker of the Fonds voor Westenschapdelijk Onderzoek-Vlaanderen.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal