Abstract
Resting platelet adhesion to inflammatory vascular endothelium is thought to play a causal role in secondary thrombus formation or microcirculatory disturbance after vessel occlusion. However, though adhesion receptors involved in platelet-matrix interactions have been extensively studied, the molecular mechanisms involved in platelet-endothelium interactions are incompletely characterized and have been mainly studied under static conditions. Using human platelets or platelets from wild-type and CD47–/– mice in whole blood, we demonstrated that at low shear rate, CD47 expressed on human and mouse platelets significantly contributes to platelet adhesion on tumor necrosis factor-α (TNF-α)–stimulated vascular endothelial cells. Using the CD47 agonist peptide 4N1K and blocking monoclonal antibodies (mAbs), we showed that CD47 binds the cell-binding domain (CBD) of endothelial thrombospondin-1 (TSP-1), inducing activation of the platelet αIIbβ3 integrin that in turn becomes able to link the endothelial receptors intercellular adhesion molecule 1 (ICAM-1) and αvβ3. Platelet CD36 and GPIbα are also involved because platelet incubation with blocking mAbs directed against each of these 2 receptors significantly decreased platelet arrest. Given that anti-CD47 treatment of platelets did not further decrease the adhesion of anti-CD36–treated platelets and CD36 is a TSP-1 receptor, it appears that CD36/TSP-1 interaction could trigger the CD47-dependent pathway. Overall, CD47 antagonists may be potentially useful to inhibit platelet adhesion on inflamed endothelium.
Introduction
The interaction of platelets with the vascular wall is an important step in hemostasis and the development of thrombotic lesions. Although adhesion receptors involved in platelet-subendothelial matrix interactions have been extensively studied,1-5 those involved in platelet-endothelium interactions, and particularly those involved in resting platelets to inflamed vascular endothelium (IVE), are incompletely characterized. However, this phenomenon is thought to occur during the occlusive, thromboembolic, reperfusion, and septic complication stages of atherosclerotic and diabetic vascular diseases.6
Normally, endothelial cells act as nonthrombogenic surfaces, but, with inflammatory stimuli such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), endothelial cells acquire a new phenotype, allowing resting platelets to adhere. Platelet adhesion follows the general principles of leukocyte extravasation: tethering and rolling on endothelial cells and rapid activation of integrins allowing platelet firm arrest. Several in vivo studies clearly demonstrated that platelet rolling on vessel wall mainly involved endothelial P-selectins,7 platelet P-selectin glycoprotein ligand-1 (PSGL-1),8 and glycoprotein Ibα (GPIbα; CD42b)9 as counterreceptors on platelets. In addition to the P-selectin–dependent mechanism, a pathway involving the von Willebrand factor (VWF) on stimulated endothelial cells and platelet GPIbα has been described.10 For firm adhesion, the receptors implicated are not yet totally identified. Few studies have been performed, and usually the adhesion molecules involved have been studied either on resting platelets or on activated endothelial cells, but rarely on both sides. In vivo experiments yielded evidence that platelet endothelial cell adhesion molecule 1 (PECAM-1; CD31) on endothelial cells may contribute to platelet adhesion at a site of injured but not denuded endothelium.11 In another in vivo study, endothelial cells rendered ischemic have been shown to acquire a procoagulant phenotype, characterized by fibrinogen accumulation that promoted resting platelet adhesion. Intercellular adhesion molecule 1 (ICAM-1; CD54) was identified as the receptor for fibrinogen on the endothelial side and the integrin αIIbβ3 on the platelet side.12 In vitro, it has been shown that endothelial cells undergoing apoptosis become proadhesive for nonactivated platelets. Platelet β1 integrins were demonstrated to be involved in this interaction, but their endothelial ligand is still unknown.13 With human umbilical vein endothelial cells (HUVECs) infected with herpesvirus14 or stimulated with IL-1,15 platelet adhesion was effectively inhibited by antibodies directed respectively against VWF secreted by endothelial cells and endothelial αvβ3 integrin (CD51). Therefore, integrins, especially β1 (CD29) and β3 integrins (CD61), are involved in platelet-IVE interactions.
CD47, or integrin-associated protein (IAP), is a 50-kDa glycoprotein expressed on all mammalian cells that has been initially described as a molecule physically associated with integrins and able to regulate their functions.2,3,16 So far, 2 ligands of CD47 have been identified: thrombospondin-1 (TSP-1) and signal regulatory protein α (SIRPα). In platelets, where CD47 has been described as physically associated with β13 and β32,16 integrins, TSP-1/CD47 interaction was demonstrated to activate the integrin αIIbβ3, which resulted in platelet spreading on immobilized fibrinogen,2 and to activate the integrin α2β1, involved in the early activation of platelets on adhesion to collagen.3 In a human melanoma cell line, the TSP-1/CD47 interaction was shown to activate αvβ3 integrin, which enhanced cell spreading on vitronectin.17
Because CD472,3,16 and the 3 integrins αIIbβ3, αvβ3, and α2β118 are expressed on platelets and because TSP-119 and SIRPα20 are expressed on endothelial cells, we tested the possibility that CD47 contributes to resting platelet adhesion on IVE. Although the capacity of platelets to adhere greatly depends on tensile strength generated by blood flow and the availability of plasma components,21 platelet-endothelium interactions have been mainly studied with washed platelets in static assays. We chose to investigate these interactions using whole blood under flow conditions, at low shear rate (100 seconds–1), which simulates blood flow in venous vessels.
Materials and methods
Reagents and antibodies
Recombinant human and mouse TNF-α and recombinant human ICAM-1 (rhICAM-1) were obtained from R&D Systems (Abingdon, United Kingdom). The 4N1K peptide corresponding to the cell-binding domain (CBD) of TSP-1 (KRFYVVMWKK) was purchased from Bachem (Voisins-le-Bretonneux, France), and the control peptide 4NGG (KRFYGGMWKK) was purchased from Mimotopes (Paris, France). In some experiments, thrombin was inactivated with 2 U/mL hirudin (Sigma-Aldrich, St Quentin Fallavier, France) for 10 minutes at room temperature.
Monoclonal antibody (mAb) B6H12, directed against CD47, was obtained from the American Type Culture Collection (ATCC, Rockville, MD), as was mAb 1F5 directed against CD20. Murine anti-TSP1 mAbs C6.7 and A2.5 directed, respectively, against the CBD or the binding site of heparan sulfates on TSP-1 were purchased from NeoMarkers (Union City, CA). mAbs SE5A5, directed against SIRPα1 and SIRPα2, was a kind gift of Dr H.-J. Büring (University of Tübingen, Germany). Anti-α2β1 (BHA2.1), anti-αvβ3 (LM609) directed against RGD binding sites,21 and anti-GPIbα (SZ2), which inhibits ristocetin-induced platelet aggregation,21 were from Chemicon (Temecula, CA). Anti-αIIbβ3 (P2), which inhibits platelet binding to fibrinogen,22 anti-GPIbα (clone SZ2), anti-VWF (clone 4F9), and anti-CD36 mAbs (clone FA6.152) were purchased from Immunotech (Marseille, France). Anti–ICAM-1 (B-H17) was kindly donated by Dr J. Widjenes (Diaclone, Besançon, France). Anti-CD31 mAb (clone WM59) was obtained from PharMingen (San Diego, CA). All these antibodies are mouse immunoglobulin G1 (IgG1) and blocking antibodies. They were used at 40 μg/mL.
Cells and mice
The transformed HUVEC line EA.hy926 was kindly provided by Dr C. J. Edgell (University of North Carolina at Chapel Hill)23 and was cultured in Dulbecco modified Eagle medium (DMEM) with 1 g/L glucose (Invitrogen, Grand Island, NY) supplemented with 20% fetal calf serum (FCS), 2 mM L-glutamine, and 10 mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid). HUVECs were purchased from BioWhittaker (Emerainville, France) and were cultured in the recommended EGM-2 BulletKit medium (BioWhittaker).
C56BL/6 mice deficient in CD47 were generated as previously described.24 Wild-type C57BL/6J mice were purchased from IFFA-CREDO (l'Arbresle, France). Mice aged 8 to 12 weeks were used.
Blood sampling and platelet preparation
For human platelet isolation, blood from healthy adult donors was obtained by venipuncture. Donors did not take any drugs for the previous 10 days. Approval for this study was obtained from the institutional review board of the French National Institute of Health and Medical Research; informed consent was provided according to the Declaration of Helsinki. Blood was drawn into propylene tubes containing heparin (final concentration, 20 U/mL) and was centrifuged at 120g for 25 minutes at room temperature to pellet erythrocytes and obtain platelet-rich plasma (PRP). Subsequently, platelets in the PRP containing 5 U/mL apyrase (Sigma-Aldrich) were stained with 0.5 μg/mL green calceine-acetoxymethyl ester (Molecular Probes, Eugene, OR) in the dark. In some experiments, the PRP was then incubated with different mAbs or peptides for 10 to 15 minutes at 37°C, and whole blood was reconstituted before perfusion in the flow chamber.
For mouse platelet isolation, wild-type or CD47–/– C57BL/6 mice were used as donors. Murine blood was collected from the retro-orbital venous plexus. Blood of each mouse was drawn into 2-mL propylene tubes containing heparin (final concentration, 20 U/mL). The blood of 10 mice in each group was pooled and centrifuged at 120g for 25 minutes at room temperature to pellet erythrocytes and obtain PRP. No labeling of mouse platelets was observed by adding calceine to the PRP, even at a concentration of 5 μg/mL, and a dramatic decrease in platelet adhesion occurred with washed platelets. Weak labeling was observed with mepacrine; thus, DiOC6 (3,3′-dihexyloxacarbocyanine iodide; Sigma-Aldrich) in PRP (final concentration, 2 μM) was used to label mouse platelets as previously published by Moog et al.1 Whole blood was then reconstituted with PRP containing fluorescent platelets and 5 U/mL apyrase, before perfusion in the flow chamber. Under such conditions, mouse platelets were well labeled, but the residual amount of free DiOC6 in whole blood was able to slightly label the endothelial cell monolayers during 3-minute blood perfusion, as may be seen in Figure 1B. This parasite fluorescence caused a weak background of approximately 7%, deduced from the percentage of the surface coverage measured in mouse platelet experiments.
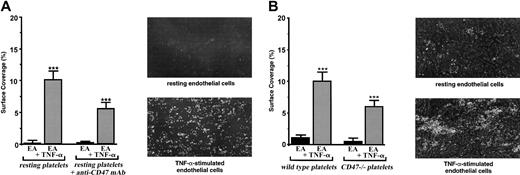
Involvement of platelet CD47 in the adhesion of resting platelets on the inflammatory vascular endothelium under flow conditions. EA endothelial cells were grown onto permanox Lab-Tek 1 chamber slides and stimulated or not stimulated with 25 ng/mL rhTNF-α for 18 hours. Then the slides were placed in a parallel plate flow chamber that produces a linear variable shear rate. (A) Human platelets were labeled with calceine in the PRP and were incubated or not incubated with 40 μg/mL anti-CD47 mAb B6H12 for 10 minutes at 37°C. Whole blood was reconstituted and perfused through the chamber at 37°C. Single-frame images were obtained after perfusion for 3 minutes at a shear rate of 100 seconds–1 on EA cells treated or not treated with TNF-α. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. These experiments are representative of at least 8 experiments performed using blood from different donors. For each single-frame image shown in the panels, the magnification of the objective was × 10. (B) Mouse platelets from 10 wild-type or 10 CD47–/– C57BL/6 mice were labeled with DiOC6 in the PRP (see “Materials and methods”). Whole blood was reconstituted and perfused through the chamber at 37°C. Single-frame images were obtained after perfusion for 3 minutes at a shear rate of 100 seconds–1 on EA cells treated or not treated with TNF-α. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. These experiments are representative of 4 different experiments. For each single-frame image shown in the panels, the magnification of the objective was × 10.
Involvement of platelet CD47 in the adhesion of resting platelets on the inflammatory vascular endothelium under flow conditions. EA endothelial cells were grown onto permanox Lab-Tek 1 chamber slides and stimulated or not stimulated with 25 ng/mL rhTNF-α for 18 hours. Then the slides were placed in a parallel plate flow chamber that produces a linear variable shear rate. (A) Human platelets were labeled with calceine in the PRP and were incubated or not incubated with 40 μg/mL anti-CD47 mAb B6H12 for 10 minutes at 37°C. Whole blood was reconstituted and perfused through the chamber at 37°C. Single-frame images were obtained after perfusion for 3 minutes at a shear rate of 100 seconds–1 on EA cells treated or not treated with TNF-α. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. These experiments are representative of at least 8 experiments performed using blood from different donors. For each single-frame image shown in the panels, the magnification of the objective was × 10. (B) Mouse platelets from 10 wild-type or 10 CD47–/– C57BL/6 mice were labeled with DiOC6 in the PRP (see “Materials and methods”). Whole blood was reconstituted and perfused through the chamber at 37°C. Single-frame images were obtained after perfusion for 3 minutes at a shear rate of 100 seconds–1 on EA cells treated or not treated with TNF-α. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. These experiments are representative of 4 different experiments. For each single-frame image shown in the panels, the magnification of the objective was × 10.
Flow chamber and in vitro flow studies
Platelet interaction with endothelial cells was studied at a shear rate of 100 seconds–1 using a flow chamber purchased from Immunetics (Cambridge, MA), described elsewhere.25 It was designed to allow stabilized laminar flow between 0.1 and 2 dynes/cm2. Whole blood was perfused through the chamber on a monolayer of endothelial cells or on immobilized peptide using a withdrawal syringe pump (Harvard Apparatus, Boston, MA). Monolayers of endothelial cells were obtained after 1 day of culture with 5 × 105 cells in Lab-Tek 1 chamber slides (Poly Labo, Strasbourg, France). Cells were either stimulated for 18 hours with 25 ng/mL TNF-α at 37°C or they were left unstimulated. The 4N1K- and 4NGG-coated coverslips were prepared as follows: 3 mL of 50 or 100 μM of each peptide in DMEM medium was incubated on a Lab-Tek 1 chamber slide for 12 hours at 4°C, washed 3 times with phosphate-buffered saline (PBS), saturated for 2 hours at room temperature with PBS/0.2% bovine serum albumin (BSA), and washed again with PBS before use. In some experiments 30 μg rhICAM-1 in 3 mL carbonate/bicarbonate buffer (pH 9.6) was added after the 4N1K peptide. Whole blood was perfused for 3 minutes. Hanks balanced salt solution (HBSS) medium (Gibco Laboratories) was perfused to remove blood cells and nonfirmly adherent platelets before quantification.
Platelet adhesion quantification
The flow chamber, mounted on an epifluorescence inverted microscope (Axiovert 25 B/W; Carl Zeiss, Oberkochen, Germany), allowed direct visualization in real time of the platelet adhesion process. The microscope was coupled to a numeric camera of high resolution (Axiocam; Carl Zeiss) directly linked to a Pentium 3 personal computer (SCS, Antony, France) equipped with acquisition software (Axiovision; Carl Zeiss), and 10 random fields were recorded per coverslip. Each image was subjected to computer-assisted analysis with the NIH-image 1.62/fat software (National Institutes of Health, Bethesda, MD). This program was used to calculate the percentage of the area covered by adhering platelets in a defined area (surface coverage) after background subtraction, setting the threshold value and binarization of each image. This technique implicates the use of an average value for adhesion of all platelets because it cannot discriminate between individual bound platelets and clumps of platelets. Nevertheless, mainly individual platelets or very small aggregates were visible (Figure 1A). Each experiment was performed at least 3 times.
Flow cytometry analysis
Endothelial cells (2 × 106 cells/mL) were incubated at room temperature in 200 μL DMEM containing the blocking mAb. Then the cells were stained with fluorescein isothiocyanate (FITC)–conjugated rabbit antimouse (RAM-FITC) immunoglobulins (DAKO, Glostrup, Denmark) for 30 minutes at room temperature in the dark. Between each step, cells were washed twice with DMEM. Controls included cells incubated with anti-CD20 (negative control) and anti-CD47 (positive control) mAbs plus RAM-FITC. After a wash, cells were analyzed on a flow cytometer (FACScan; Becton Dickinson, Mountain View, CA).
Statistics
For overall comparison between groups, nonparametric Kruskal-Wallis analysis of variance (ANOVA) was performed. For the detection of differences between groups, Wilcoxon testing was used. P < .05 was considered significant. Data are reported as means ± SEMs.
Results
Platelet CD47 is involved in the adhesion of resting platelets to the inflammatory vascular endothelium
To investigate a role for CD47 in platelet-endothelial cell interactions, we used an in vitro flow chamber assay that permits study of the dynamic aspects of platelet adhesion without altering endothelial cell integrity. When human platelets were allowed to flow in whole blood at 100 seconds–1 on a monolayer of resting vascular endothelial EA cells, as expected, no significant platelet adhesion was detected (Figure 1A). When endothelial EA cells were pretreated with TNF-α, platelets did adhere (P < .001). Under such experimental conditions, surfaces covered by platelets adhering to the endothelial monolayer varied between 8% and 12%. When the blocking anti-CD47 mAb B6H12 was added to human platelets at a saturating concentration before flow, platelets did not adhere on resting endothelial cells. They significantly adhered (P < .0001) on TNF-α–treated cells, but a decrease of approximately 45% in platelet attachment (P < .01) was observed (Figure 1A). The addition of the nonrelevant anti-CD20 mAb had no effect. Similar results were obtained with primary venous endothelial HUVECs (not shown). Cells from the transformed vascular endothelial EA line were chosen for the study because platelet adhesion on stimulated HUVECs resulted in large aggregates difficult to quantify. Because platelets and endothelial cells express CD47, we also studied the role of endothelial CD47 in this process. Pretreatment of the endothelial cells with the blocking anti-CD47 mAb B6H12 did not modify the adhesion of platelets (not shown), indicating that endothelial CD47 was not involved. Thus, these results disclose a role for platelet CD47 in the adhesion of platelets on IVE at low shear rate.
Therefore, it was of interest to compare the adhesion of platelets from CD47–/– and C57BL/6 mice (Figure 1B). Although human platelets were labeled with calceine, mouse platelets were labeled with DiOC6 before flow on the vascular endothelial cell monolayer stimulated or not stimulated with TNF-α (see “Materials and methods”). Under such experimental conditions, few mouse platelets arrested on resting endothelial cells when platelets from wild-type and CD47–/– mice significantly attached (P < .001) to TNF-α–stimulated cells. Nevertheless, though platelet concentration was similar in wild-type and CD47–/– mice, platelets from CD47–/– mice adhered significantly less (P < .05) than did platelets from wild-type mice. A decrease of approximately 40% in platelet attachment was observed with platelets from CD47-deficient mice (Figure 1B). Consequently, as observed with human platelets, these results demonstrate the involvement of platelet CD47 in the adhesion of resting platelets on IVE at low shear rate.
Platelet CD47 binds the CBD domain of endothelial TSP-1
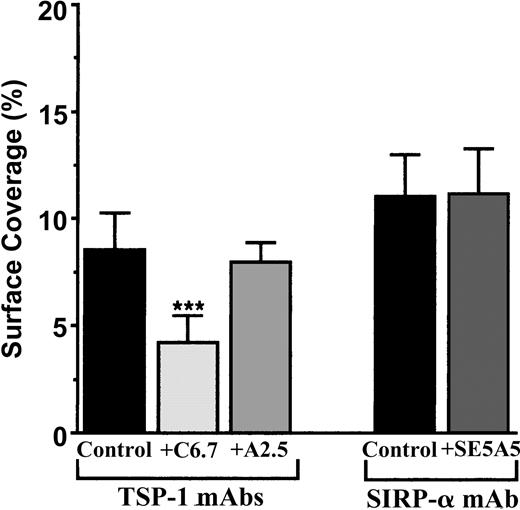
To study the CD47 pathway, we first looked for the endothelial counterreceptors of platelet CD47. Thus far, TSP-1 and SIRPα have been described to link CD47. Because vascular endothelial cells display these 2 ligands for CD47,19,20 TNF-α–activated endothelial monolayers were treated or not treated with known blocking mAbs directed against TSP-1 and SIRPα. Figure 2 shows that the treatment of vascular endothelial cells with the blocking anti–TSP-1 mAb C6.7, directed against the CBD of the molecule, inhibited the arrest of human platelets by approximately 50%. In contrast, the anti-TSP-1 mAb A2.A, directed against the proteoglycan heparan sulfate region of TSP-1, had no effect. When the mAb SE5A5, known to block the binding of SIRPα (SIRPα1 and SIRPα2) to CD47 was added, no effect on platelet arrest to IVE at low shear rate was observed (Figure 2). Consequently, the CBD of the endothelial TSP-1 is a ligand of platelet CD47 in platelet-IVE interactions under flow conditions, but we found no evidence for a role of SIRPα in those events.
Arrest of flow platelets in whole blood on TNF-α–treated vascular endothelial cells incubated with blocking mAbs directed against TSP-1 or SIRPα. EA endothelial cells were grown onto permanox Lab-Tek 1 chamber slides and stimulated with 25 ng/mL rh TNF-α for 18 hours. Then 40 μg/mL anti-TSP-1 C6.7 and A2.5 or anti-SIRPα SE5A5 mAbs were added or not added (control) to these cells for 10 minutes at 37°C. They were washed with PBS before being placed in the flow chamber. After platelet labeling in PRP with calceine, whole blood was reconstituted and perfused through the chamber at 37°C at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as the percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. This experiment is representative of 3 experiments performed using blood from different donors.
Arrest of flow platelets in whole blood on TNF-α–treated vascular endothelial cells incubated with blocking mAbs directed against TSP-1 or SIRPα. EA endothelial cells were grown onto permanox Lab-Tek 1 chamber slides and stimulated with 25 ng/mL rh TNF-α for 18 hours. Then 40 μg/mL anti-TSP-1 C6.7 and A2.5 or anti-SIRPα SE5A5 mAbs were added or not added (control) to these cells for 10 minutes at 37°C. They were washed with PBS before being placed in the flow chamber. After platelet labeling in PRP with calceine, whole blood was reconstituted and perfused through the chamber at 37°C at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as the percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. This experiment is representative of 3 experiments performed using blood from different donors.
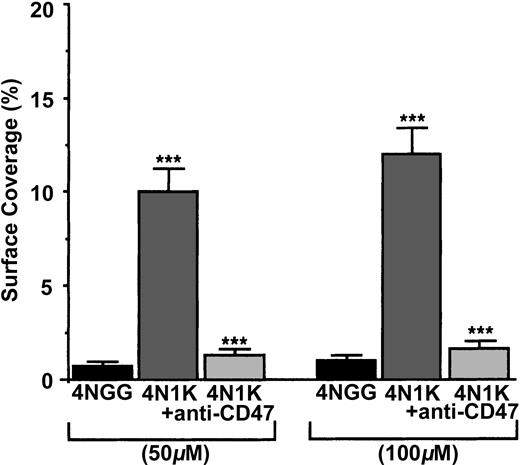
To check whether platelet CD47 was able to directly link the CBD of TSP-1, we measured the arrest of human platelets, treated or not treated with the blocking anti-CD47 mAb B6H12, on immobilized peptide 4N1K, derived from the CBD and on the immobilized control peptide 4NGG19,26 (Figure 3). When resting human platelets were allowed to flow on 50-μM– and 100-μM– coated 4N1K or 4NGG peptides, platelets arrested and adhered at the 2 concentrations on 4N1K peptide (approximately 10% and 12%, respectively, of the surface covered), but no arrest was observed on the 4NGG control peptide. When platelets were preincubated with the blocking anti-CD47 mAb B6H12, they no longer arrested on the 4N1K-coated surfaces. Because the CBD sequences of human and mouse TSP-1 are identical,26 we compared, under the same experimental conditions, the arrest of platelets from wild-type and CD47–/– mice on 100-μM–coated peptides. Platelets from wild-type C57BL/6 mice adhered to 100-μM–coated 4N1K peptide (surface coverage, approximately 13%-15%), but they did not adhere to 100-μM–coated 4NGG peptide (surface coverage, approximately 1%-2%). By contrast, platelets from CD47–/– mice arrested on neither 4NGG nor 4N1K immobilized peptides (surface coverage, approximately 1%-2%) (not shown). Thus, platelet CD47 is able to directly link the CBD of TSP-1.
Arrest of flow platelets in whole blood on immobilized 4N1K and 4NGG peptides. Permanox Lab-Tek 1 chamber slides were coated with 50 or 100 μM 4N1K (a CD47 agonist from the CBD of TSP-1) or 4NGG (control) peptides and were placed in the flow chamber. Platelets were first labeled with calceine in PRP and then incubated or not incubated with 40 μg/mL anti-CD47 mAb B6H12 for 10 minutes at 37°C. Whole blood was reconstituted and perfused through the chamber at 37°C and at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. This experiment is representative of 3 experiments performed using blood from different donors.
Arrest of flow platelets in whole blood on immobilized 4N1K and 4NGG peptides. Permanox Lab-Tek 1 chamber slides were coated with 50 or 100 μM 4N1K (a CD47 agonist from the CBD of TSP-1) or 4NGG (control) peptides and were placed in the flow chamber. Platelets were first labeled with calceine in PRP and then incubated or not incubated with 40 μg/mL anti-CD47 mAb B6H12 for 10 minutes at 37°C. Whole blood was reconstituted and perfused through the chamber at 37°C and at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. This experiment is representative of 3 experiments performed using blood from different donors.
Soluble 4N1K peptide triggers platelet arrest on inflamed endothelium
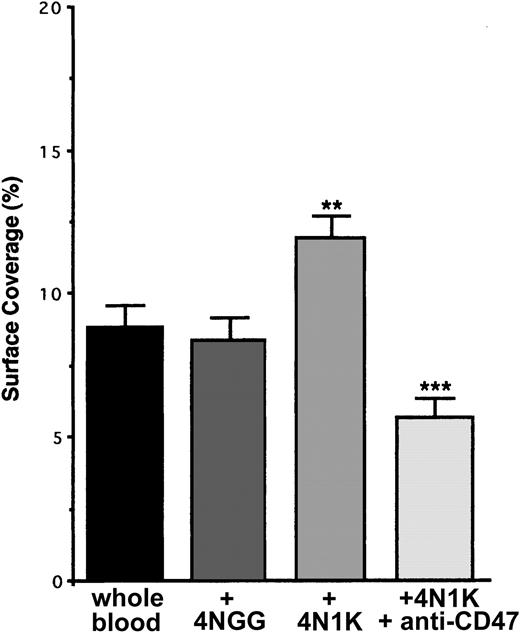
In many cell types and in platelets, CD47 has been described as physically linked to β13 and β32,16 integrins and, after its ligation with the CBD region of TSP-1, to stimulate their activation to a higher affinity/avidity state. To investigate whether this happened during the adhesion of resting platelets on inflammatory endothelium, we incubated human platelets in PRP with the 4N1K or 4NGG peptides in solution before flow. When platelets were preincubated with the peptide 4NGG, no modification in platelet arrest on TNF-α–stimulated EA cells was observed compared with basal arrest (Figure 4). Yet, when platelets were preincubated with the peptide 4N1K, an increase of approximately 40% to 50% in platelet adhesion was observed. This increase was completely abolished when platelets were first incubated in the presence of the anti-CD47 mAb B6H12 (Figure 4). Similar results were obtained with primary endothelial HUVECs (not shown).
Arrest of flow platelets incubated with or without the 4N1K peptide in solution on TNF-α–treated endothelial cells. EA endothelial cells were grown onto permanox Lab-Tek 1 chamber slides and stimulated with 25 ng/mL rhTNF-α for 18 hours. After platelet labeling in PRP with calceine, platelets were incubated for 15 minutes at 37°C with or without 100 μM 4N1K or 4NGG peptides in solution. In some experiments, platelets were first incubated for 10 minutes at 37°C with 40 μg/mL anti-CD47 mAb B6H12 before stimulation with the 4N1K peptide. Whole blood was then reconstituted and perfused through the chamber at 37°C and at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. **P < .01; ***P < .001. This experiment is representative of 3 experiments performed using blood from different donors.
Arrest of flow platelets incubated with or without the 4N1K peptide in solution on TNF-α–treated endothelial cells. EA endothelial cells were grown onto permanox Lab-Tek 1 chamber slides and stimulated with 25 ng/mL rhTNF-α for 18 hours. After platelet labeling in PRP with calceine, platelets were incubated for 15 minutes at 37°C with or without 100 μM 4N1K or 4NGG peptides in solution. In some experiments, platelets were first incubated for 10 minutes at 37°C with 40 μg/mL anti-CD47 mAb B6H12 before stimulation with the 4N1K peptide. Whole blood was then reconstituted and perfused through the chamber at 37°C and at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. **P < .01; ***P < .001. This experiment is representative of 3 experiments performed using blood from different donors.
Furthermore, when platelets from C57BL/6 mice were incubated with the 4N1K peptide before flow, an increase of approximately 45% in platelet arrest on the TNF-α–stimulated endothelial cell monolayers was observed compared with basal arrest. No modification was seen when platelets were preincubated with the 4NGG peptide. With platelets from CD47–/– mice, no difference in platelet arrest was observed between resting or 4N1K- or 4NGG-stimulated platelets (not shown). Therefore, these results disclose an activating effect of CD47 on platelet integrins in the interactions of platelets with IVE at low shear rate.
CD47 ligation activates the platelet αIIbβ3 integrin
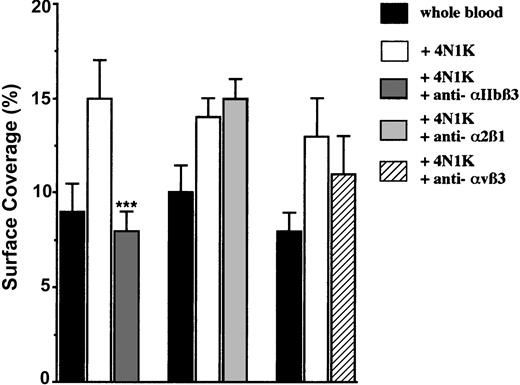
So far, CD47 has been described to be linked and to activate 3 distinct integrins on nucleated cells or on platelets: αIIbβ3,2,16 α2β1,3 and αvβ3.17 Because these 3 integrins are expressed on platelet surfaces, we investigated whether they could be involved in platelet adhesion to IVE. For this purpose, platelets were first activated with the 4N1K peptide in solution, and then they were incubated in the presence of known blocking mAbs directed against each of these integrins. The addition of the anti-α2β1 mAb had no effect. Although a slight decrease (approximately 18%) in platelet arrest was observed with the anti-αvβ3 mAb, a significant (P < .001) decrease (approximately 47%) was observed only with the anti-αIIbβ3 mAb (Figure 5). We found evidence that platelet CD47 ligation with a peptide derived from the CBD of TSP-1 induces the activation of the platelet αIIbβ3 integrin to a higher affinity/avidity state, which allows its interaction with endothelial receptors.
Arrest of 4N1K-activated platelets incubated with blocking mAbs directed against the integrins αIIbβ3, α2β1, and αvβ3 on TNF-α–treated endothelial cells. EA endothelial cells were grown onto permanox Lab-Tek 1 chamber slides and stimulated with 25 ng/mL rhTNF-α for 18 hours. After platelet labeling in PRP with calceine, platelets were incubated for 15 minutes at 37°C with or without 100 μM 4N1K peptide in solution followed by 10-minute incubation at 37°C with 40 μg/mL of either anti-αIIbβ3 mAb P2, anti-α2β1 mAb BHA2.1, or anti-αvβ3 mAb LM609. Whole blood was then reconstituted and perfused through the chamber at 37°C and at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. This experiment is representative of 3 experiments performed with blood from different donors.
Arrest of 4N1K-activated platelets incubated with blocking mAbs directed against the integrins αIIbβ3, α2β1, and αvβ3 on TNF-α–treated endothelial cells. EA endothelial cells were grown onto permanox Lab-Tek 1 chamber slides and stimulated with 25 ng/mL rhTNF-α for 18 hours. After platelet labeling in PRP with calceine, platelets were incubated for 15 minutes at 37°C with or without 100 μM 4N1K peptide in solution followed by 10-minute incubation at 37°C with 40 μg/mL of either anti-αIIbβ3 mAb P2, anti-α2β1 mAb BHA2.1, or anti-αvβ3 mAb LM609. Whole blood was then reconstituted and perfused through the chamber at 37°C and at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. This experiment is representative of 3 experiments performed with blood from different donors.
Endothelial ICAM-1 and αvβ3 integrin are involved in the CD47-dependent pathway
One study performed under static conditions reported that platelet αIIbβ3 interactions with endothelial ICAM-1, αvβ3, and GPIbα were involved in the adhesion of thrombin-activated human platelets to endothelial cells.21 Therefore, we first investigated, using flow cytometry, the expression of these 3 receptors on vascular endothelial cells stimulated or not stimulated with 25 ng/mL TNF-α for 18 hours. In agreement with Mutin et al,27 we confirmed that EA cells constitutively express ICAM-1 and αvβ3 and that, on TNF-α stimulation, the expression of αvβ3 was not modified when the expression of ICAM-1 was increased. We did not, however, detect GPIbα on resting or TNF-α–stimulated EA cells (not shown).
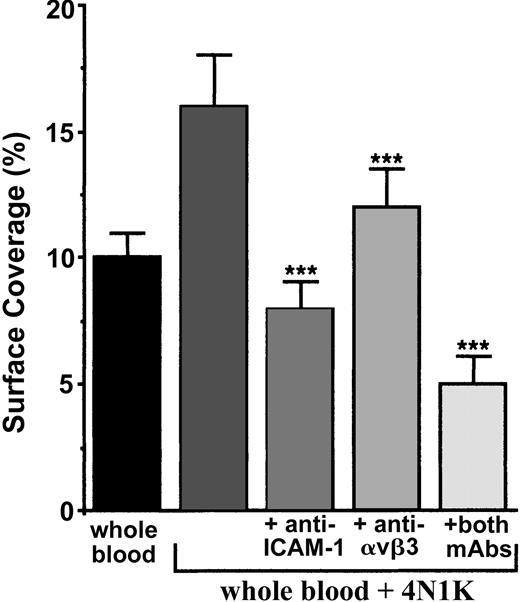
We first incubated TNF-α–stimulated cells with or without blocking mAbs directed against ICAM-1, the integrin αvβ3, or both. Next, the arrest of 4N1K-activated human platelets in whole blood was evaluated under flow (Figure 6). Platelet arrest was significantly diminished (P < .001) in the presence of anti–ICAM-1 (decrease of approximately 50%), anti-αvβ3 (decrease of approximately 25%), or both mAbs (decrease of approximately 68%). Nevertheless, it is noteworthy that we never could observe a total inhibition of platelet adhesion under flow. Thus, at least these 2 endothelial receptors are implicated in platelet-IVE interaction at low shear rate.
Arrest of 4N1K-activated platelets on TNF-α–treated endothelial cells incubated with blocking mAbs directed against ICAM-1, αvβ3 integrin, or both. EA endothelial cells were grown onto permanox Lab-Tek 1 chamber slides and stimulated with 25 ng/mL rhTNF-α for 18 hours. Then 40 μg/mL anti–ICAM-1 B-H17, anti-αvβ3 LM609, or both mAbs were added to these cells for 10 minutes at 37°C. They were washed with PBS before being placed in the flow chamber. After platelet labeling in PRP with calceine, platelets were incubated for 15 minutes at 37°C with or without 100-μM 4N1K peptide in solution. Whole blood was then reconstituted and perfused through the chamber at 37°C and at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. This experiment is representative of 3 experiments performed using blood from different donors.
Arrest of 4N1K-activated platelets on TNF-α–treated endothelial cells incubated with blocking mAbs directed against ICAM-1, αvβ3 integrin, or both. EA endothelial cells were grown onto permanox Lab-Tek 1 chamber slides and stimulated with 25 ng/mL rhTNF-α for 18 hours. Then 40 μg/mL anti–ICAM-1 B-H17, anti-αvβ3 LM609, or both mAbs were added to these cells for 10 minutes at 37°C. They were washed with PBS before being placed in the flow chamber. After platelet labeling in PRP with calceine, platelets were incubated for 15 minutes at 37°C with or without 100-μM 4N1K peptide in solution. Whole blood was then reconstituted and perfused through the chamber at 37°C and at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. This experiment is representative of 3 experiments performed using blood from different donors.
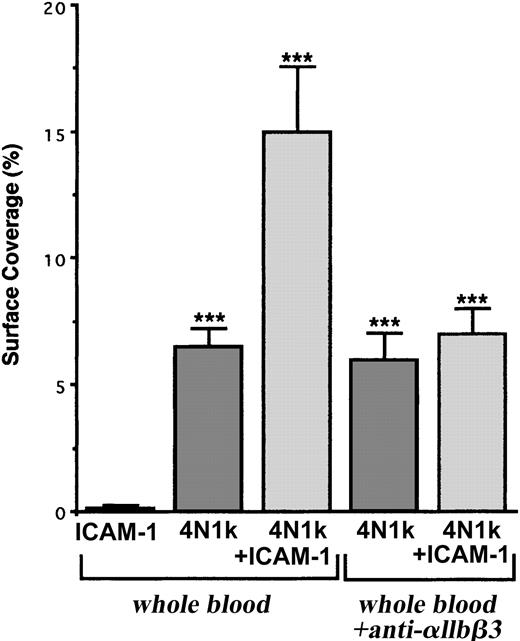
These results are strengthened by the fact that human resting platelets adhered more significantly to 4N1K + ICAM-1–coated peptides than 4N1K-coated peptide alone (surface coverage, approximately 15% and 7%, respectively) and did not adhere to ICAM-1 alone (Figure 7). Furthermore, this increase in platelet arrest was inhibited in the presence of the blocking mAb P2 directed against the integrin αIIbβ3 (Figure 7), confirming the involvement of this platelet integrin during resting platelet-IVE interactions under flow.
Arrest of flow platelets incubated with or without a blocking anti-αIIbβ3 mAb on immobilized peptides 4N1K ± ICAM-1. Permanox Lab-Tek 1 chamber slides were coated with 100 μM 4N1K ± 30 μg rhICAM-1 peptides (see “Materials and methods”) and were placed in the flow chamber. Platelets were first labeled with calceine in PRP and then were incubated or not incubated with 40 μg/mL anti-αIIbβ3 mAb P2 for 10 minutes at 37°C. Whole blood was reconstituted and perfused through the chamber at 37°C and at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. This experiment is representative of 3 experiments performed using blood from different donors.
Arrest of flow platelets incubated with or without a blocking anti-αIIbβ3 mAb on immobilized peptides 4N1K ± ICAM-1. Permanox Lab-Tek 1 chamber slides were coated with 100 μM 4N1K ± 30 μg rhICAM-1 peptides (see “Materials and methods”) and were placed in the flow chamber. Platelets were first labeled with calceine in PRP and then were incubated or not incubated with 40 μg/mL anti-αIIbβ3 mAb P2 for 10 minutes at 37°C. Whole blood was reconstituted and perfused through the chamber at 37°C and at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. This experiment is representative of 3 experiments performed using blood from different donors.
TSP-1–CD36 interaction would trigger the CD47-dependent pathway
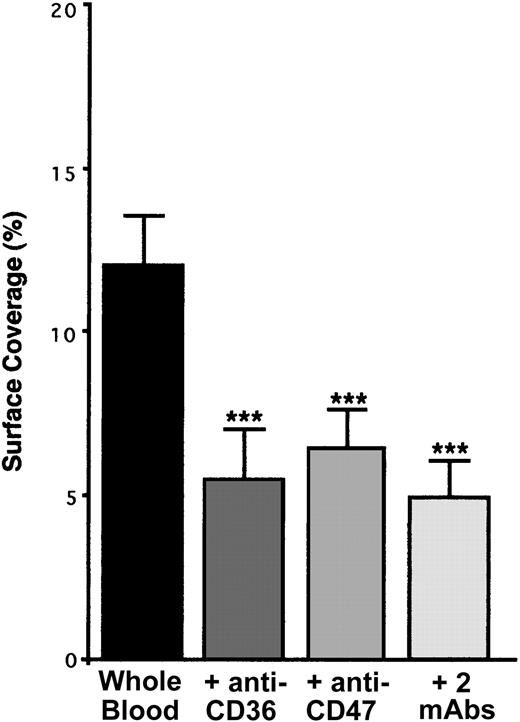
CD36 is a well-known TSP-1 ligand28-30 which is associated especially with the integrin αIIbβ3 on the surface of resting platelets.31 Because the incubation of platelets with a blocking mAb directed against CD36 resulted in a decrease in platelet arrest of approximately 55% (Figure 8), platelet CD36 is involved in our phenomenon. TSP-1–CD36 interaction has been demonstrated to be a 2-step process involving conformational changes in TSP-1.30 Thus, our results suggest that TSP-1–CD36 interaction could trigger conformational changes in endothelial TSP-1, leading to the exposure of its CBD domain and allowing binding with platelet CD47. This hypothesis is strengthened by the fact that the incubation of anti–CD36-treated platelets with a blocking anti-CD47 mAb did not further decrease (approximately 58%) platelet arrest on IVE (Figure 8).
Arrest of flow platelets incubated with or without blocking anti-CD36, anti-CD47, or the 2 mAbs successively on TNF-α–treated endothelial cells. EA endothelial cells were grown onto permanox Lab-Tek 1 chamber slides and stimulated with 25 ng/mL rh TNF-α for 18 hours. Platelets were first labeled with calceine in PRP and then were incubated or not incubated with 40 μg/mL anti-CD36 mAb FA6.152 or anti-CD47 mAb B6H12 for 10 minutes at 37°C or with the 2 mAbs successively. Whole blood was reconstituted and perfused through the chamber at 37°C and at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. This experiment is representative of 3 experiments performed using blood from different donors.
Arrest of flow platelets incubated with or without blocking anti-CD36, anti-CD47, or the 2 mAbs successively on TNF-α–treated endothelial cells. EA endothelial cells were grown onto permanox Lab-Tek 1 chamber slides and stimulated with 25 ng/mL rh TNF-α for 18 hours. Platelets were first labeled with calceine in PRP and then were incubated or not incubated with 40 μg/mL anti-CD36 mAb FA6.152 or anti-CD47 mAb B6H12 for 10 minutes at 37°C or with the 2 mAbs successively. Whole blood was reconstituted and perfused through the chamber at 37°C and at a shear rate of 100 seconds–1 for 3 minutes. Platelet adhesion, expressed as percentage of surface covered with platelets, is the average ± SEM of 10 random fields per coverslip. ***P < .001. This experiment is representative of 3 experiments performed using blood from different donors.
As mentioned, other endothelial receptors are involved. Furthermore, because no complete inhibition in platelet attachment to IVE was reached by blocking or deleting CD47, other platelet receptors may be implicated. Interactions involving endothelial VWF and platelet GPIbα10 were demonstrated to occur in mice at low shear rate (80-100 seconds–1). PECAM-1 was described as contributing to platelet adhesion at a site of injured but not denuded endothelium.11 Because this receptor performs its adhesive functions through PECAM-1/PECAM-1 homophilic interactions32,33 and because resting platelets express PECAM-1,34 this pathway may also be involved. No inhibition in platelet arrest was observed when platelets were incubated with a blocking anti-PECAM-1 mAb, when a decrease of approximately 68% was observed, when they were incubated with a blocking anti-GPIbα mAb (not shown). Thus, other pathway(s) cooperate with the CD47-dependent pathway in the adhesion of resting platelets to IVE in dynamic conditions.
Discussion
In thrombus formation associated with hemostasis or thrombotic disease, blood platelets first undergo rapid transition from a circulating state to an adherent state, followed by activation and aggregation. Under flow conditions in the bloodstream, this process potentially involves platelet-platelet, platelet-endothelium, and platelet-subendothelial matrix interactions. Specific adhesion receptors on platelets mediate these interactions by engaging counterreceptors on endothelial cells or noncellular ligands in the plasma or matrix. Although adhesion receptors involved in platelet-matrix interactions have been studied extensively, those involved in platelet-endothelium interactions are incompletely characterized and have been mainly studied in static conditions with washed platelets. Here we identified a CD47-dependent pathway acting in cooperation with other pathways involving platelet GPIbα, CD36, but not platelet PECAM-1. Using a flow chamber, we observed that at low shear rate, CD47 on human platelets in whole blood significantly contributes to platelet adhesion on TNF-α–stimulated endothelial cells. CD47 binds to the CBD of the endothelial TSP-1; it thus induces the activation of the platelet αIIbβ3 integrin, which, in turn, becomes able to link the endothelial receptors ICAM-1 and αvβ3. The main points of this study were confirmed with platelets from wild-type and CD47–/– mice.
Using blocking mAbs directed against the 2 known ligands of CD47—TSP-1 and SIRPα—we observed no inhibition of platelet arrest in the presence of anti-SIRPα or the mAb directed against the proteoglycan heparan region of TSP-1 when approximately 50% inhibition was obtained in the presence of a mAb directed against the CBD domain of TSP-1. Under flow conditions, the ligand of endothelial TSP-1 on platelets has never been clearly established. It was initially reported that CD47 has no functional role in platelets.35 Since then, under static conditions, platelet CD47 has been reported to be involved in platelet activation and aggregation3 and in platelet spreading on fibrinogen-coated surfaces.2 In all these phenomena, the CBD region of TSP-1 is reported to be the ligand for CD47. Tulasne et al,36 however, recently demonstrated that the C-terminal peptide of TSP-1 induced platelet aggregation through the FcR-γ–chain signaling pathway. An earlier study indicated that immobilized TSP-1 may induce platelet arrest and that shear rate influences this adhesion.37 Our study confirmed and completed this observation. We showed that platelets adhered to the immobilized 4N1K peptide (a peptide derived from the CBD region of TSP-1) and that this adhesion was inhibited in the presence of a neutralizing mAb directed against CD47, indicating a direct association between platelet CD47 and the CBD region of TSP-1 at low shear rate. Given that a deep decrease in platelet arrest was observed on immobilized 4N1K at high shear rate (1200 seconds–1) (not shown), it seems that this interaction can be established only at low shear rate and that it is not sufficient at high shear rate.
In platelets, CD47 has been described to be physically linked to β13 and β32,16 integrins and to form a signal-transducing complex, allowing their activation to a higher affinity/avidity state after its ligation with the CBD region of TSP-1 in static conditions. For instance, the incubation of washed platelets in the presence of the 4N1K peptide was shown to stimulate platelet spreading on fibrinogen-coated surfaces, to induce their aggregation through activation of the integrin αIIbβ3,2 and to synergize with collagen in α2β1-mediated platelet activation.3 Although 4N1K alone failed to induce platelet aggregation in PRP,38 we observed that the incubation of platelets in the presence of the 4N1K peptide in PRP before blood reconstitution resulted in a significant enhancement of platelet arrest on the TNF-α–stimulated endothelial cells. Because this increase was inhibited by a blocking anti-CD47 mAb in our experimental conditions, it likely indicates that platelet CD47 is able to positively regulate integrin activity not only in static but also in dynamic conditions. CD47 regulates the activity of integrins αIIbβ32 and α2β13 in platelets and αvβ3 in fibroblasts39 and melanoma cells.17 Although these 3 integrins are expressed on platelets, only the integrin αIIbβ3 appeared to be activated after incubation with the 4N1K peptide in our experimental model. This work was performed with whole blood; hence, we cannot exclude a possible activation of the integrin αIIbβ3 by factors such as thrombin, adenosine diphosphate (ADP), epinephrine, and thromboxane A2. However, their roles seem unlikely because the experiments were performed with heparinized blood in the presence of apyrase (an ADP scavenger) and because results were not modified in the presence of 2 U/mL hirudin (a thrombin inhibitor; not shown). Moreover, such an involvement of integrin αIIbβ3 has already been described in the interaction of ADP-activated platelets on noninflammatory endothelium under flow conditions40 and thrombin-activated platelets under static conditions.25
Using neutralizing mAbs, we next identified ICAM-1 and αvβ3 integrin as endothelial receptors involved in resting platelet-IVE interactions, with ICAM-1 as the predominant receptor. The anti–ICAM-1 mAb B-H17 used in our study was previously described41 to inhibit the ligation of ICAM-1 to the αLβ2 integrin (LFA-1; CD11a-CD18) and, the binding site involved in this interaction was also implicated in the binding of ICAM-1 to the fibrinogen.42 The fact that αIIbβ3 integrin serves as the main receptor for fibrinogen strongly suggests that resting platelets bind to TNF-α–stimulated endothelial cells by an αIIbβ3-dependent bridging mechanism. Fibrinogen is the bridge between endothelial ICAM-1/αvβ3 and platelet αIIbβ3 integrin. Likewise, as demonstrated by flow cytometry in the adherence of thrombin-activated platelets to HUVECs,21 the participation of fibronectin, vitronectin, and VWF as other bridges is probable. No complete inhibition of platelet adhesion was observed in the presence of the 2 mAbs directed against ICAM-1 and αvβ3. In the same way, blocking or deleting the CD47 pathway did not induce complete inhibition of platelet adhesion on IVE. Residual arrest of approximately 50% remained, indicating that other endothelial and platelet receptors would act in synergy. In vivo, rolling is indispensable before firm adhesion for normal platelet function because it serves to decelerate cell movement relative to flowing blood, allowing receptors with slower bond kinetics (ie, integrins) to engage adhesive ligands. Using intravital microscopy, endothelial P- and E-selectin7,8,43 and VWF10 have been demonstrated to mediate platelet rolling. Considering the short-term expression of selectin in vitro (a few hours), their participation in our experimental conditions seems unlikely. Nevertheless the pathway involving the VWF secreted on the luminal face of endothelial cells and the platelet GPIbα10 must be considered because the addition of a blocking mAb directed against platelet GPIbα decreases platelet adhesion of approximately 65% to 70%. In accordance with Perrault et al,44 we detected no expression of GPIbα on resting or TNF-α–stimulated EA cells, excluding its participation in our phenomenon. An in vivo study presented evidence that endothelial PECAM-1 contributed to platelet adhesion at a site of injured but not denuded endothelium.11 However, the addition of a blocking mAb directed against either endothelial or platelet PECAM-1 at a saturating concentration did not modify platelet arrest in our model. On the other hand, the presence of a blocking anti-CD36 mAb in the PRP significantly decreased platelet arrest, strongly suggesting that endothelial TSP-1 has at least 2 platelet receptors, CD36 and CD47.
Normally, endothelial cells act as nonthrombogenic surfaces. Inflammatory stimuli (TNF-α, IL-1β), however, induce phenotypical and conformational changes in the surface receptors of endothelial cells, allowing resting platelets to adhere. Using flow cytometry, we confirmed that EA endothelial cells express TSP-1, αvβ3, and ICAM-1. Eighteen hours of TNF-α stimulation induced an increase in ICAM-1 expression (not shown). Thus, considering the literature and our data, we can propose the following model. After endothelial activation under low shear conditions, the interaction between platelet GPIbα and VWF expressed on endothelial cells initiates platelet rolling,10 allowing platelet CD36 and endothelial TSP-1 ligation. CD36/TSP-1 interaction is a 2-step process.30 The sequence 139-155 region of CD36 binds first to a sequence localized in the type 1 repeated unit domain of TSP-1, triggering a conformational change in TSP-1 to reveal a second site in the C-terminal domain of TSP-1, which links the 93-110 region of CD36 with high affinity. Because the CBD of TSP-1 is localized in its carboxyl-terminal domain,26 we hypothesize that TSP-1/CD36 interaction induces the exposition of the CBD, allowing its ligation with platelet CD47, which results in platelet αIIbβ3 integrin activation. The αIIbβ3 integrin in its active form can then link endothelial αvβ3 and overexpressed ICAM-1 through fibrinogen bridges, as described, allowing firm adhesion of platelets. TSP-1 was also demonstrated to be nonadhesive in a Ca2+-depleted conformation when supporting platelet adhesion in a Ca2+-repleted conformation.37 Using a mAb that differentially labels these 2 conformations of TSP-1,45 we confirmed with flow cytometry that in our experimental conditions, EA cells express TSP-1 in Ca2+-repleted conformation, but no change was observed on TNF-α stimulation (not shown).
In conclusion, we disclosed a role for CD47 in the adhesion process of resting platelets to inflamed vascular endothelium at low shear rate. Such platelet-endothelium interaction is thought to play a causal role during atherogenesis46,47 and to represent the initial event leading to remodeling and reocclusion of the vasculature.6,48 It has also been demonstrated to contribute to several diseases of the central nervous system (autoimmune inflammatory diseases, stroke) by recruiting leukocytes.49 Overall, CD47 antagonists may be potentially useful to inhibit platelet adhesion on inflamed endothelium.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-11-3483.
Supported by grants from the Association pour la Recherche contre le Cancer, the Ligue nationale contre le Cancer, the Fondation pour la Recherche Médicale, and the Fondation de France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Monique Freund and François Lanza for technical suggestions, Dominique Gautier and Francis Autem for animal care, and Alexandre Rouquette for his kind assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal