This phase 2 trial was performed to evaluate the safety and efficacy of the chimeric monoclonal anti-CD20 antibody rituximab in patients with relapsed lymphocyte-predominant Hodgkin lymphoma or other CD20+ subtypes of Hodgkin disease (HD). Eligibility criteria required expression of the CD20 antigen on more than 30% of malignant cells. Fourteen patients were treated with 4 weekly intravenous infusions of rituximab (375 mg/m2). All patients had at least one prior chemotherapy (median, 2). The median time from first diagnosis was 9 years. Adverse events, such as rhinitis, fever, chills, and nausea, were usually transient and of mild to moderate grade, allowing outpatient treatment in most cases. All patients completed treatment and were eligible for a response. The overall response in 14 assessable patients was 86%, with 8 complete remissions and 4 partial remissions, and 2 patients with progressive disease. At a median follow-up of 12 months, 9 of 12 responders were in remission. The median duration of response has not been reached yet (20+ months). We conclude that rituximab is both safe and effective in a subgroup of CD20+ patients with HD.
Introduction
Lymphocyte predominant Hodgkin disease (LPHD) accounts for 3% to 8% of all Hodgkin cases.1 In contrast to classical Hodgkin disease (HD), LPHD has characteristic morphologic, biologic, and clinical features. Typically, LPHD presents as early-stage disease with slow progression and excellent initial response to conventional treatment. Although 96% of all patients with LPHD experience a complete remission (CR) upon first-line treatment, many relapses occur years after the initial diagnosis. A recent European-American retrospective analysis1 revealed that 21% of 219 patients with LPHD relapsed during a median observation period of 6.8 years, while the relapse rate was only 5% for 115 patients with lymphocyte-rich classical HD after similar treatment. Results of retrospective studies suggest that freedom from treatment failure and overall survival of LPHD patients were not significantly improved by intensification of polychemotherapy or radiotherapy.1,2 In addition, transformation to aggressive non-Hodgkin lymphoma (NHL), including T-cell–rich B-cell lymphoma, has been reported.3-5 Therefore, new therapeutic alternatives for patients with LPHD and patients with relapsed or refractory classical HD are warranted. One possibly very tempting selective new approach would be the use of a specific monoclonal antibody (MoAb) directed against surface antigens present on malignant Hodgkin Reed-Sternberg cells (H-RS). Our group has put substantial effort into the development of new CD30-based immunoreagents.6Promising studies with bispecific molecules such as the anti-CD30/anti-CD64 reagent H22xKi-47 or the Ki-4 J 131 radioimmunoconjugate8 are currently ongoing. After the advent of the chimeric anti-CD20 MoAb rituximab, the evaluation of this reagent in patients with HD is yet another interesting approach. Treatment of patients with relapsed follicular NHL using rituximab at standard dose (4 × 375mg/m2) has been associated with response rates of more than 50%.9 Only mild and reversible side effects were reported for the majority of patients.10 Long-term toxicity after rituximab has not been observed,11 and retreatment is feasible.12
While only the minority of cases of classical HD cells are CD20+, all patients with LPHD express this B-cell marker in high density on their malignant cell population. Due to small numbers of patients with LPHD or other forms of CD20+ HD, so far there is only very limited information on the feasibility and effects of rituximab in this patient population.13 14 Thus, the German Hodgkin Lymphoma Study Group (GHSG) conducted a phase 2 clinical trial to evaluate rituximab in relapsed or refractory CD20+HD patients. Here, we report data from this international multicenter phase 2 study in patients with LPHD or other CD20+ subtypes of HD at first or higher relapse.
Patients, materials, and methods
Patients
This multicenter phase 2 trial was initiated by the GHSG in May 1999. As of March 2002, 14 patients were enrolled by 5 different centers. The study was carried out in accordance with the Declaration of Helsinki (Hong Kong Amendment, 1989). The protocol met the requirements of the European Good Clinical Practise Guidelines (July 1990) and received approval by the institutional review board at each study site. Eligibility criteria included CD20+ Hodgkin lymphoma at first or higher relapse or progressive disease after at least one standard treatment. LPHD as well as CD20+classical HD or transformed HD cases were included. Lesions were classified as “CD20+” when the CD20 antigen was expressed on more than 30% of malignant cells. All histologic slides were reviewed by an independent expert panel consisting of 6 reference pathologists. For enrollment into the study, patients also had to meet the following requirements: have a bidimensionally measurable disease, at least one lesion larger than 1.0 cm in its greatest diameter, and a World Health Organization performance status of 0, 1, or 2. In addition, patients had to be at least 18 years of age, neither pregnant nor lactating, using accepted birth control methods, and had to have a life expectancy of 3 months or longer. Patients with major organ dysfunction or active infections were excluded from the study. Prior treatment with rituximab was also an exclusion criterion. Concurrent therapeutic use of corticosteroids was not allowed.
Treatment
Patients received 375mg/m2 of the anti-CD20 MoAb rituximab once weekly for 4 weeks given as intravenous infusion in saline solution. The drug, supplied by Hoffmann–La Roche (Basel, Switzerland), was administered at an initial dose rate of 50 mg/hour for the first hour and gradually escalated to a maximum of 400 mg/hour (300 mg/hour for the first infusion only). Acetaminophen and antihistamines at standard doses were administered one hour before each infusion. A concomitant infusion of saline solution was given during the first antibody infusion.
Patient monitoring
Patients were monitored for safety and antitumor effects using regular medical history, physical examination, and laboratory studies including complete and differential blood count and standard chemistry performed at baseline; at weeks 1, 2, 3, 4, and 5; and at 6 weeks and 3 months after completion of the last infusion. Toxicity was evaluated using the National Cancer Institute's Adult Toxicity Criteria (February 1988 Guidelines). Evaluation of disease assessment included physical examination and computed tomography (CT) or magnetic resonance imaging at baseline, 3 months after the end of treatment, every 3 months for 2 years, and then every 6 months thereafter. A bone marrow biopsy was performed at baseline and at confirmation of CR, if positive at baseline. Data were documented according to the guidelines of the GHSG.
Flow cytometry
Flow cytometry was used to detect the number of CD20+ lymphocytes in the peripheral blood of patients at baseline as well as after 1 week, 3 months, 6 months, and 1 year after the fourth rituximab infusion. Flow cytometric phenotyping of mononuclear cells of the peripheral blood was performed after red blood cell lysing of blood samples (Lysing solution: Becton Dickinson, Heidelberg, Germany). The cells were incubated with fluorochrome (fluorescein isothiocyanate [FITC], phycoerythrin [PE], perchlorophyll [PerCP]) conjugated mouse anti–human MoAbs (anti-CD3, anti-CD4, anti-CD5, anti-CD8, anti-CD14, anti-CD16, anti-CD19, anti-CD20, anti-CD25, anti-CD45, anti-CD56, anti–HLA-DR [Becton Dickinson]), and appropriate isotype controls for 20 minutes at 4°C. After 2 washing procedures in phosphate-buffered saline containing 0.1% bovine serum albumin and 0.01% NaN3, samples were measured on a flow cytometer (FACSCalibur; Becton Dickinson) with a minimum of 10 000 mononuclear cells acquired for each staining. Analysis and calculation were performed using CellQuest and Attractors software (Becton Dickinson).
End points and response criteria
Patients were assessable for efficacy if they had completed at least 2 infusions of rituximab, satisfied all prestudy entry criteria, and met criteria for evaluation of response. Response criteria were those previously defined by the report of an international workshop to standardize response criteria for non-Hodgkin lymphomas (National Cancer Institute Sponsored International Working Group).15CR required that all lymph nodes visible on CT scans of neck, chest, abdomen, and pelvis be less than 1.0 cm in diameter, and bone marrow had to be histologically negative for lymphoma cell. Liver and spleen (if abnormal at baseline) must have returned to normal size. Partial remission (PR) was defined as a decrease of 50% or more from baseline in the sum of the products of the greatest perpendicular diameters of all lesions measured (SPD) without increase in size of any other lesion or no new lesions. Stable disease referred to patients who did not show at least a 50% decrease or increase in SPD, and progressive disease was considered as any single observation of an increase of 50% or more in SPD from nadir or the appearance of new lesions.16Since the course of the disease is slow, investigators were asked to document the history of their patients for at least 10 years. In case of progression of the disease at any time, patients were off study and received alternative treatment according to the individual decision of the responsible physician at each study site.
Statistical analysis
Duration of response was measured from the first infusion of rituximab and the first observation of response, respectively. These data were analyzed by the Kaplan-Meier product-limit method. Adverse events were investigated in relation to the study treatment. Any adverse event reported as probably or possibly related or of unknown relationship to the study drug was considered a related adverse event. Adverse events were further classified as having occurred during the treatment period (time interval between first rituximab infusion and 30 days after the fourth infusion) or follow-up (time interval between 31 days after the fourth infusion and 1 year after the first infusion).
Sample size
This phase 2 trial was designed to evaluate the feasibility of standard-dose rituximab in patients with LPHD and to document possible antitumor effects. Since the incidence of LPHD is only 0.1 to 0.2/100 000, a sample size of 14 patients was regarded as statistically reasonable by the Statistical Advisory Board of the GHSG. Patients were evaluable when completing at least 2 infusions of rituximab. For the efficacy analysis the best response achieved from the start of treatment to progressive disease was recorded. The response rates (overall objective and complete response rates) are reported in rates with 95% confidence intervals (Pearson Clopper intervals).
Results
Patient characteristics
As of March 2002, 14 patients with LPHD at initial presentation (13 males, 1 female) were treated according to the study protocol. The characteristics of these patients are listed in Table1. Median age was 39.5 years (range, 18-51 years). The median time since first diagnosis was 9 years (range, 0.5-21 years). All patients had at least one prior cytoreductive chemotherapy and/or radiotherapy, including autologous peripheral stem cell transplantation in 2 cases. At the time of study entry, all patients were in their first to third relapse (median, 2); 8 patients had stage I/II disease, 6 were suffering from B-symptoms (Table 1). Size of lesions ranged between 1.5 cm and 4.5 cm in their greatest diameter (Table 2).
Patient characteristics at study entry
| Patient no. . | Age, y . | Histology . | Ann Arbor stage . | Years after diagnosis . | Prior treatment . | No. of relapses . |
|---|---|---|---|---|---|---|
| 1 | 45 | LPHD | IA | 17 | Radiation | 3 |
| 2 | 45 | LPHD | IIA | 9 | Radiation | 2 |
| 3 | 30 | LPHD | IVB | 6 | COPP/ABVD | 3 |
| 4 | 35 | LPHD | IA | 3 | Radiation | 1 |
| 5 | 49 | LPHD | IIA | 9 | COPP/ABVD, radiation | 2 |
| 6 | 41 | LPHD | IIB | 17 | Radiation | 1 |
| 7 | 51 | T-cell–rich B-NHL | IVA | 8 | COPP/ABVD, splenectomy | 1 |
| 8 | 47 | LPHD | IIIA | 8 | CHOP, VIP-E | 1 |
| 9 | 41 | LPHD | IA | 21 | MOPP, radiation, ABVD | 2 |
| 10 | 23 | LPHD | IVB | 0.5 | BEACOPP | 1 |
| 11 | 34 | T-cell–rich B-NHL | IVB | 6 | COPP/ABVD, radiation, AVD, VIP-E, ABMT | 3 |
| 12 | 18 | HD | IVA | 1.5 | OPPA, COPP, MINE-R | 1 |
| 13 | 37 | LPHD | IIB | 3 | Radiation | 1 |
| 14 | 51 | HD | IIB | 17 | MOPP, COPP/ABVD, ABVD, ABMT | 3 |
| Patient no. . | Age, y . | Histology . | Ann Arbor stage . | Years after diagnosis . | Prior treatment . | No. of relapses . |
|---|---|---|---|---|---|---|
| 1 | 45 | LPHD | IA | 17 | Radiation | 3 |
| 2 | 45 | LPHD | IIA | 9 | Radiation | 2 |
| 3 | 30 | LPHD | IVB | 6 | COPP/ABVD | 3 |
| 4 | 35 | LPHD | IA | 3 | Radiation | 1 |
| 5 | 49 | LPHD | IIA | 9 | COPP/ABVD, radiation | 2 |
| 6 | 41 | LPHD | IIB | 17 | Radiation | 1 |
| 7 | 51 | T-cell–rich B-NHL | IVA | 8 | COPP/ABVD, splenectomy | 1 |
| 8 | 47 | LPHD | IIIA | 8 | CHOP, VIP-E | 1 |
| 9 | 41 | LPHD | IA | 21 | MOPP, radiation, ABVD | 2 |
| 10 | 23 | LPHD | IVB | 0.5 | BEACOPP | 1 |
| 11 | 34 | T-cell–rich B-NHL | IVB | 6 | COPP/ABVD, radiation, AVD, VIP-E, ABMT | 3 |
| 12 | 18 | HD | IVA | 1.5 | OPPA, COPP, MINE-R | 1 |
| 13 | 37 | LPHD | IIB | 3 | Radiation | 1 |
| 14 | 51 | HD | IIB | 17 | MOPP, COPP/ABVD, ABVD, ABMT | 3 |
LPHD indicates lymphocyte-predominant Hodgkin disease; HD, Hodgkin disease; NHL, non-Hodgkin lymphoma; CHOP, cyclophosphamide, doxorubicin, prednisone, vincristine; COPP, cyclophosphamide, vincristine, procarbazine, prednisone; ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; OPPA, doxorubicin, procarbazine, prednisone, vincristine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; VIP-E, etoposide, ifosfamide, cisplatin, epirubicin; MOPP, mechlorethamine, vincristine, procarbazine, prednisone; and ABMT, autologous bone marrow transplantation.
Response and site of disease at study entry
| Patient no. . | Site of disease at study entry, measurement . | Method of measurement . | Response . | Duration of response . |
|---|---|---|---|---|
| 1 | Cervical, 2 cm | CT scan | CR | 26+ |
| 2 | Cervical, 3.5 cm | CT scan | CR | 12+ |
| Axillary, 3.5 cm | ||||
| 3 | Axillary, 3 cm | CT scan | PR | 12 |
| Bone marrow | ||||
| Erythema nodosum | ||||
| 4 | Inguinal, 3 cm | Ultrasound, PET | CR | 24+ |
| 5 | Axillary left, 3.5 cm | CT scan | CR | 12 |
| Axillary right, 1.5 cm | ||||
| 6 | Iliacal, 3.5 cm | CT scan | CR | 12+ |
| Inguinal, 2.5 cm | ||||
| 7 | Liver | Biopsy, CT scan, MRT | CR | 12+ |
| Spleen | ||||
| Truncus coeliacus, 1.5 cm | ||||
| 8 | Cervical, 2.6 cm | CT scan | PR | 9+ |
| Axillary, 1.5 cm | ||||
| Mediastinal, 1.5 cm | ||||
| Paraaortal, 2 cm | ||||
| Inguinal, 1.5 cm | ||||
| 9 | Multiple inguinal, 2.6-4.5 cm | CT scan | PR | 9+ |
| 10 | Liver | CT scan | PD | NR |
| Spleen | ||||
| Bone marrow | ||||
| 11 | Submandibular, 1.5 cm | CT scan | CR | 15+ |
| Iliacal, 2 cm | ||||
| Supraclavicular, 2 cm | ||||
| Retroperitoneal, 2 cm | ||||
| Pulmonal | ||||
| Spleen | ||||
| 12 | Cervical, 2.0 cm | CT scan | PD | NR |
| Supraclavicular, 2.5 cm | ||||
| Infraclavicular, 3.0 cm | ||||
| Lung | ||||
| 13 | Cervical, 2.5 cm | CT scan | PR | 12+ |
| Submandibular, 2.0 cm | ||||
| Supraclavicular, 2.5 cm | ||||
| Mediastinal, 2.5 cm | ||||
| 14 | Cervical, 1.5 cm | CT scan | CR | 16* |
| Mediastinal, 2.0 cm |
| Patient no. . | Site of disease at study entry, measurement . | Method of measurement . | Response . | Duration of response . |
|---|---|---|---|---|
| 1 | Cervical, 2 cm | CT scan | CR | 26+ |
| 2 | Cervical, 3.5 cm | CT scan | CR | 12+ |
| Axillary, 3.5 cm | ||||
| 3 | Axillary, 3 cm | CT scan | PR | 12 |
| Bone marrow | ||||
| Erythema nodosum | ||||
| 4 | Inguinal, 3 cm | Ultrasound, PET | CR | 24+ |
| 5 | Axillary left, 3.5 cm | CT scan | CR | 12 |
| Axillary right, 1.5 cm | ||||
| 6 | Iliacal, 3.5 cm | CT scan | CR | 12+ |
| Inguinal, 2.5 cm | ||||
| 7 | Liver | Biopsy, CT scan, MRT | CR | 12+ |
| Spleen | ||||
| Truncus coeliacus, 1.5 cm | ||||
| 8 | Cervical, 2.6 cm | CT scan | PR | 9+ |
| Axillary, 1.5 cm | ||||
| Mediastinal, 1.5 cm | ||||
| Paraaortal, 2 cm | ||||
| Inguinal, 1.5 cm | ||||
| 9 | Multiple inguinal, 2.6-4.5 cm | CT scan | PR | 9+ |
| 10 | Liver | CT scan | PD | NR |
| Spleen | ||||
| Bone marrow | ||||
| 11 | Submandibular, 1.5 cm | CT scan | CR | 15+ |
| Iliacal, 2 cm | ||||
| Supraclavicular, 2 cm | ||||
| Retroperitoneal, 2 cm | ||||
| Pulmonal | ||||
| Spleen | ||||
| 12 | Cervical, 2.0 cm | CT scan | PD | NR |
| Supraclavicular, 2.5 cm | ||||
| Infraclavicular, 3.0 cm | ||||
| Lung | ||||
| 13 | Cervical, 2.5 cm | CT scan | PR | 12+ |
| Submandibular, 2.0 cm | ||||
| Supraclavicular, 2.5 cm | ||||
| Mediastinal, 2.5 cm | ||||
| 14 | Cervical, 1.5 cm | CT scan | CR | 16* |
| Mediastinal, 2.0 cm |
CT scan indicates computed tomography; PET, positron emission tomography; and NR, no response.
Death due to adenocarcinoma.
Histology
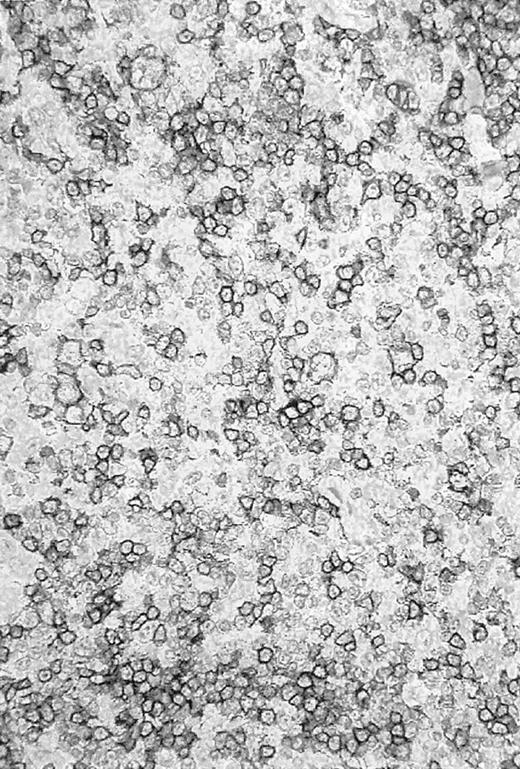
Re-evaluation of the histologic slides was performed by a panel of reference pathologists. The initial diagnosis of LPHD was confirmed in 10 of 14 cases. An example of a CD20+ tissue sample is given in Figure 1. The remaining cases were reclassified as HD transformed to T-cell–rich B-cell lymphoma2 or CD20+ classical HD.2
CD20+ tissue sample.
Photomicrograph of a histologic slide of a cervical lymph node infiltrated by a nodular/diffuse paragranuloma (lymphocyte predominant Hodgkin disease) confirmed by the pathology reference panel of the GHSG. Immunoperoxidase staining for L26 (CD20); original magnification, × 100.
CD20+ tissue sample.
Photomicrograph of a histologic slide of a cervical lymph node infiltrated by a nodular/diffuse paragranuloma (lymphocyte predominant Hodgkin disease) confirmed by the pathology reference panel of the GHSG. Immunoperoxidase staining for L26 (CD20); original magnification, × 100.
Adverse events
Infusion-related side effects were observed in 11 (79%) of 14. The most common events observed during the treatment period (22 days of treatment and 30 days of follow-up) included chills (71%), fever (50%), rhinitis (21%), nausea (21%), pruritus (21%), leukopenia (14%), and dizziness (14%) (Table 3). Most adverse events (94%) were mild to moderate (National Cancer Institute [NCI] toxicity grade 1 or 2). Usually, side effects occurred during the first infusion and resolved within one hour of the end of infusion. In 10 of 14 patients, continuous outpatient treatment was performed.
Toxicity grade during the treatment period
| . | No. of rituximab infusions = 583-150 . | |
|---|---|---|
| Grades 1 or 2 . | Grades 3 or 4 . | |
| Chills | 9 | 1 |
| Fever | 7 | 0 |
| Rhinitis | 3 | 0 |
| Pruritus | 3 | 0 |
| Nausea | 3 | 0 |
| Hypotension | 0 | 1 |
| Dizziness | 2 | 0 |
| Hyperventilation | 1 | 0 |
| Edema | 1 | 0 |
| Tachycardia | 2 | 0 |
| Vomiting | 1 | 0 |
| Paraesthesia | 1 | 0 |
| Hematological | 0 | 0 |
| Leukopenia | 2 | |
| Drop of CD20+ lymphocytes (counts not done in 4 patients) | 0 | 10 |
| . | No. of rituximab infusions = 583-150 . | |
|---|---|---|
| Grades 1 or 2 . | Grades 3 or 4 . | |
| Chills | 9 | 1 |
| Fever | 7 | 0 |
| Rhinitis | 3 | 0 |
| Pruritus | 3 | 0 |
| Nausea | 3 | 0 |
| Hypotension | 0 | 1 |
| Dizziness | 2 | 0 |
| Hyperventilation | 1 | 0 |
| Edema | 1 | 0 |
| Tachycardia | 2 | 0 |
| Vomiting | 1 | 0 |
| Paraesthesia | 1 | 0 |
| Hematological | 0 | 0 |
| Leukopenia | 2 | |
| Drop of CD20+ lymphocytes (counts not done in 4 patients) | 0 | 10 |
Number of adverse events during the treatment period. Symptoms were evaluated according to the National Cancer Institute's Adult Criteria.
The infusion of one patient had to be discontinued due to grade 3 toxicity. Her second rituximab dose was administered according to fractionated infusion schedule (one dose at two infusions on day 1 and 2) in order to minimize the severity of clinical adverse events.
Treatment-related grade 3 or 4 nonhematologic clinical events occurred in only one patient. This patient had LPHD stage IV, experiencing fever (grade 2), chills (grade 3), hyperventilation (grade 2), tachycardia (grade 2), edema (grade 2), and hypotension (grade 3) during her first rituximab infusion. Despite a very slow infusion rate, the symptoms only partly dissolved with concomitant medication, and her physicians decided to stop treatment after 100 mg rituximab had been infused. One week later the patient received another 100 mg rituximab on day 1, and the rest of the 375 mg/m2 dose on day 2. During both infusions, the patient still experienced grade 2 fever, chills, and nausea. Adverse events were absent during her second to fourth treatment cycle.
Severe hematologic effects were not observed during the treatment period in any patient. A mild leukopenia after the first infusion was noted in 2 patients (Table3) but did not result in any infectious complication. No hepatic or renal toxicity was noted. Counts of CD20+ lymphocytes were measured in 10 patients. In all these patients CD20 counts were normal at baseline and declined during the treatment period. CD20+ lymphocytes were not detectable in the blood of any of the patients 1 week and 3 months after the last rituximab infusion, respectively. One year after study entry all patients whose blood samples were monitored had normal CD20+ lymphocyte counts (data not shown).
Adverse events during the follow-up period were reported in only one patient who developed an adenocarcinoma of the right lung 11 months after treatment with rituximab. Retrospectively, this lesion was already present at study entry. The patient died 5 months later due to treatment-resistant pneumonia. Autopsy revealed a tracheoesophageal fistula adjacent to the lung tumor, without evidence of Hodgkin lymphoma. This 51-year-old patient had a 30-pack-per-year history of smoking. He had experienced an involved-field radiation for mediastinal involvement of HD at first diagnosis and had received high-dose chemotherapy with autologous peripheral stem cell transplantation after first relapse. The patient's history as well as the fact that the lesion was already present at baseline suggests that all the adverse events observed in this patient during follow-up were not related to rituximab.
Response to treatment
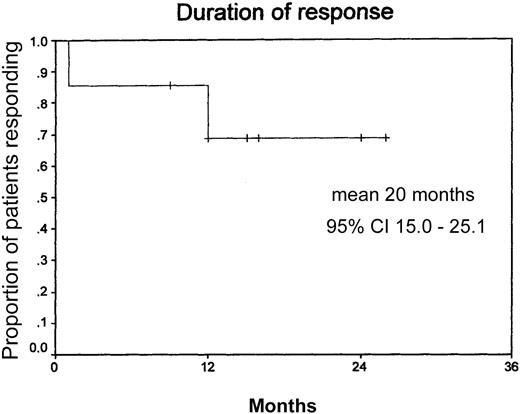
The overall response for all assessable patients (n = 14) was 86% (8 CR, 4 PR, 2 progressive disease) (Table4). One patient died in CR 16 months after end of therapy due to an adenocarcinoma of the lung. One patient with LPHD, stage IVB, reached a PR upon treatment with rituximab lasting for 12 months. Of the12 patients who responded to treatment, 9 (75%) are still in remission at a median follow-up of 12 months. The median duration of response has not been reached yet at 20+ months (Figure 2). Two patients, both stage IV, one with nodular paragranuloma and one with classical HD and 50% CD20+ tumor cells, had progressive disease after 2 and 4 cycles of rituximab, respectively. Both presented with an aggressive form of disease with early relapse after chemotherapy with BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone), OPPA (doxorubicin, procarbazine, prednisone, vincristine) followed by COPP (cyclophosphamide, vincristine, procarbazine, prednisone) and salvage therapy with MINE-R (mitoxantrone, ifosfamide, vinorelbine, etoposide, radiation), respectively (Table 1).
Response rate according to stage (Ann Arbor classification) at study entry
| . | No. of patients, % . | ||||
|---|---|---|---|---|---|
| Overall response . | Complete response . | Partial response . | No change . | Progressive disease . | |
| Total group, n = 14 | 86 | 57 | 29 | 0 | 14 |
| Stage I A/B, n = 3 | 100 | 67 | 33 | 0 | 0 |
| Stage II A/B, n = 5 | 100 | 80 | 20 | 0 | 0 |
| Stage III A, n = 1 | 100 | 0 | 100 | 0 | 0 |
| Stage IV A/B, n = 5 | 67 | 40 | 20 | 0 | 40 |
| . | No. of patients, % . | ||||
|---|---|---|---|---|---|
| Overall response . | Complete response . | Partial response . | No change . | Progressive disease . | |
| Total group, n = 14 | 86 | 57 | 29 | 0 | 14 |
| Stage I A/B, n = 3 | 100 | 67 | 33 | 0 | 0 |
| Stage II A/B, n = 5 | 100 | 80 | 20 | 0 | 0 |
| Stage III A, n = 1 | 100 | 0 | 100 | 0 | 0 |
| Stage IV A/B, n = 5 | 67 | 40 | 20 | 0 | 40 |
Duration of response.
Mean duration of response, 20 months (n = 14: censored, 10; events, 4). Median observation time, 12 months.
Duration of response.
Mean duration of response, 20 months (n = 14: censored, 10; events, 4). Median observation time, 12 months.
Discussion
The major results to emerge from this multicenter phase 2 trial initiated by the GHSG are as follows: rituximab given in standard doses (4 × 375 mg/m2) has low toxicity and high efficacy in patients with LPHD. Adverse events usually were related to the first infusion and of mild to moderate grade. Overall, 12 of 14 patients responded (8 CR, 4 PR), with 9 of 12 in continuous remission at a median follow-up of 12 months. The median duration of response has not been reached yet (20+ months).
To our knowledge, this is the first detailed report on rituximab in CD20+ HD. Keilholz et al13 were the first to communicate a single patient with relapsed LPHD treated with rituximab at standard dose. This patient presented with stage IV disease at the time of treatment and experienced a CR for 6 months. In abstract form, Lucas et al14 described response rates of 100% in 9 patients with LPHD. Patients were either relapsed or at first diagnosis. Six weeks after treatment with 4 × 375 mg/m2rituximab 6 patients presented with CR and 3 with PR. One patient relapsed 6 months after treatment, while the remaining 8 patients were in remission after a median observation period of 10 months. Younes et al17 speculated that eliminating CD20+bystander B cells from HD lesions might abort cytokine-mediated stimulation of H-RS cells. Using rituximab at standard doses, they treated 18 patients with relapsed classical HD irrespective of CD20 expression on their H-RS cells. Three patients with CD20−classical HD responded with 2 PR and 1 CR. Unfortunately, no data were collected in this study in an attempt to support their original hypothesis. Taken together, these data indicate immunophenotyping of histologic slides for assessing antigen expression on the H-RS cells is required before treatment of HD patients with rituximab.
In the present trial, 2 cases were diagnosed as CD20+transformed T-cell–rich B-cell lymphoma upon reference pathology. Several reports describe a higher probability of transformation of LPHD into secondary NHL,3-5 even for those patients who had not received cytotoxic treatment. A clonal relationship between transformed large-cell lymphoma and LPHD was established.18 Taken together, these findings suggest that a reevaluation of LPHD cases by expert pathologists is mandatory, particularly in patients with relapse. It could be speculated that application of the anti-CD20 antibody rituximab in patients with LPHD might prevent survival of malignant B-cell clones and subsequent transformation into aggressive NHL. It was also demonstrated recently that LPHD and classical HD are malignant B-cell lymphomas of germinal center origin.19The good efficacy of single-agent rituximab in LPHD as well as in follicular NHL might be explained by the similar germinal center origin of these 2 entities.
Rituximab has been shown to sensitize lymphoma cell lines toward cytotoxic drugs.20 Results of clinical studies in patients with aggressive B-cell NHL suggest an at least additive effect of rituximab and CHOP (cyclophosphamide, doxorubicin, prednisone, vincristine) chemotherapy.21 In a nonrandomized study combining CHOP and rituximab in patients with advanced-stage low-grade B-cell NHL, the response rate was 95% with CR in 55%.22In addition, 7 of 7 patients with follicular histology and complete remission were rendered bcl-2 negative. A more recent phase 3 study prospectively randomized 399 elderly patients with diffuse large B-cell lymphoma.23 Patients received either 8 cycles of CHOP or 8 cycles of CHOP plus rituximab (R-CHOP). The CR rate was significantly higher in the R-CHOP group (76% versus 63%, P = .0050). Disease progression during treatment was observed in 22% of patients in the CHOP group and 9% of patients in the R-CHOP group. With a median follow-up of 2 years, event-free and overall survival also were significantly higher in the R-CHOP group: at 2 years, 70% of patients treated with R-CHOP were alive, as compared with 57% of those treated with CHOP alone.
Clinical studies combining rituximab with standard chemotherapy such as ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or BEACOPP are thus warranted to fully investigate potential superadditive effects of a combined immuno/chemotherapy in CD20+ HD. This combined approach using rituximab in LPHD patients might allow reduction of the dose of the cytotoxic agents for patients with extended disease or relapse. Since the total number of patients with LPHD or other types of CD20+ HD is small and progression of this lymphoma is slow, additional studies are warranted to more precisely define the role of rituximab in LPHD.
In summary, our data suggest that the anti-CD20 MoAb rituximab is both safe and effective in patients with CD20+ LPHD.
The following individuals are members of the GHSG: V. Diehl, A. Engert, J. Wolf, R.-P. Müller, H. Eich, K. Müller-Hermelink, U. Paulus, M. Sieber, H. Bredenfeld, K. Breuer, L. Nogová, K. Wingbermühle, and K. Behringer.
A complete list of the members of the German Hodgkin Lymphoma Study Group appears in the “.”
Supported in part by the Deutsche Krebshilfe, grant KZ 70-2368 Di 8.
U.R. and H.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andreas Engert, Department I of Internal Medicine, University of Cologne, Cologne, 50924, Germany; e-mail:a.engert@uni-koeln.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal