This study addressed several questions concerning age-related changes in human B lymphopoiesis. The relative abundance of pro-B, pre-B, immature, naive, and mature B cells among the CD19+lymphocyte fraction of human bone marrow was found not to change appreciably over the interval between 24 and 88 years of age. Moreover, proliferation of pro-B and large pre-B cells in adult marrow equaled that observed with fetal marrow specimens. Exceptionally low numbers of lymphocyte precursors were found in some marrow samples, and the values obtained were used to determine parameters that best reflect B lymphopoiesis. Cord blood always contained higher incidences of functional precursors than adult cells. However, sorted CD34+ Lin− CD10+ progenitors from cord blood and adult marrow had equivalent potential for differentiation in culture, and notable age-related changes were found in more primitive subsets. A recently described subset of CD34+CD38−CD7+ cord blood cells had no exact counterpart in adult marrow. That is, all adult CD34+Lin−CD7+CD10−cells expressed CD38, displayed less CD45RA, and had little B-lineage differentiation potential. The CD7+ fractions in either site contained progenitors for erythroid and natural killer (NK) lineages, and ones sorted from marrow expressed high levels of transcripts for the CD122 interleukin 2 (IL-2)/IL-15 receptor required by NK-lineage precursors. Dramatic changes in human B lymphopoiesis occur early in life, and more information is required to construct a probable sequence of differentiation events prior to the acquisition of CD10.
Introduction
Humoral immune responses are known to be compromised in the aged, and decreased numbers of B and T lymphocytes parallel increases in natural killer (NK) cells.1-3 Animal studies have revealed age-related changes in B-lymphocyte production within bone marrow (BM).4-6 Numbers of pro-B cells that can be resolved by flow cytometry are relatively stable with age, but pre-B cells decline substantially.4,7 Incorporation of bromodeoxyuridine (BrdU) into dividing cells indicates that B-cell production is diminished in old mice.7 This corresponds to the reduced entry of newly formed cells to the long-lived lymphocyte pool.7,8 Functional precursor activity may decline because of a diminished support capacity by marrow stromal cells.4 9 All of these observations indicate that peripheral B-lymphocyte populations in the aged may be replenished with fewer cells from marrow, with potential consequences for the repertoire of available antibody specificities. However, it is unclear if such findings are completely applicable to humans.
Animal studies have also been informative about molecular mechanisms and steps in B lymphopoiesis. Rare hematopoietic stem cells transit a number of “compartments” that are defined on the basis of cell surface markers. In reality, this represents a continuum where genes are expressed and repressed in a temporal, but not necessarily synchronous, fashion. Early precursors have multiple differentiation options and may express transcription factors in a way that does not reflect their ultimate fate.10,11 Cells with potential for differentiation to lymphoid, but not myeloid or erythroid, cells have been referred to as common lymphoid progenitors.12,13These Lin−c-kitloTdT+IL-7Rα+/loprecursors likely derive from early lymphoid progenitors in the very rare Lin−c-kithiSca-1hiCD27+fraction of BM cells.14,15 However, it is important to note that the differentiation pathways used during fetal life may be quite different from adult BM.16-18
Knowledge of early B-lymphocyte progenitors in humans lags behind that for mice, and there are important differences. Interleukin 7 (IL-7) is essential to this process in mice but not in humans, and no combination of cytokines has been identified that supports vigorous human lymphocyte formation in culture.19-21 Indeed, the most effective models for human B lymphopoiesis use murine stromal cells that provide uncharacterized stimuli.22 Cytokines and hormones have been identified that selectively inhibit B-lymphocyte formation in mice.13,23-27 It is important to know if lymphopoiesis is altered as humans age because such negative factors increase. Some investigative techniques are not appropriate with specimens of human marrow, and the yield of hematopoietic cells is variable. Cellularity decreases with age in some but not all human bones,28,29 and most studies have been performed on marrow aspirates contaminated by peripheral blood.30 31
Despite these species differences and technical difficulties, progress is being made in understanding the process in humans. For example, evidence indicates that CD10+CD19− cells may be the human equivalent of common lymphoid progenitors in the mouse, whereas candidates recently identified in umbilical cord blood (CB) are CD34+CD38−CD7+.32 33A major goal of this study was to determine if numbers and functions of such precursors decline with age because that could reflect important changes in human lymphopoietic capacity.
Expression of Rag genes in marrow of mature individuals might suggest that immunoglobulin genes are being rearranged.34,35 Unfortunately, little information has been available about proliferation36,37 or differentiation activity in humans, and it is possible this slows with age. Thus, although the incidence of lymphocytes has been known to decline between birth and puberty, there was an insufficient understanding of changes that may occur subsequently.31,35 38
Ratios of precursor subsets in human marrow, proliferative indices, and differentiation were evaluated in the present study with the goal of identifying age-related changes. We now report that whereas B-cell precursors are depressed in some patients, the entire differentiation series is normally present throughout life and is mitotically active. However, surface marker changes occur early in life, and adult marrow has reduced potential for lymphocyte production in 2 model systems. Additional information about acquisition of CD7 and CD10 may prove helpful in understanding the sequence of early differentiation events in humans.
Materials and methods
Cell sources
The CB samples were obtained from placentas of healthy newborns collected at the Oklahoma University or St Anthony hospitals (Oklahoma City). Adult marrow samples were collected from patients between 24 and 88 years of age who were undergoing hip replacement surgery at the Bone and Joint Hospital (Oklahoma City, OK). Patient records with no identifying information were used to determine that some individuals had a recent history of treatment with anti-inflammatory drugs such as prednisone or naproxen. Most specimens came from otherwise healthy patients with no evidence of malignancy or immunosuppression. The Anatomic Gift Foundation (Laurel, MD) supplied femoral BM from fetuses at 16 to 24 weeks of gestation. Investigational Review Boards at Oklahoma Medical Research Foundation and The University of Oklahoma Health Sciences Center approved all procedures involving human specimens.
Animals
NOD/LtSz-scid/scid (nonobese diabetic/severe combined immunodeficiency [NOD/SCID]) mice were obtained from a breeding colony established at the Oklahoma Medical Research Foundation (OMRF; Oklahoma City, OK) from breeding pairs kindly provided by Dr Leonard D. Schultz (Jackson Laboratory, Bar Harbor, ME). Animals were housed in a restricted barrier facility under specific pathogen-free conditions.
Antibodies and cytokines
The following antibodies were either purified, biotinylated, or conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or allophycocyanin (APC): anti-CD38 (clone HIT2), anti-CD24 (clone ML5), anti-CD23 (clone M-L233), anti-CD45 (clone HI30), anti–Ki-67 (clone B56), anti-IgD (clone IA6-2) from BD-Pharmingen; (San Jose, CA), anti-CD34 (HPCA-2), anti-CD3 (clone UCHT1), anti-CD7 (clone 6B7), anti-CD8 (clone HIT8a), anti-CD14 (clone TUK4), anti-CD16 (clone 3G8), anti-CD10 (clone 5-1B4), anti-CD19 (clone SJ25-C1), anti-CD13 (clone TUK1), anti-CD27 (clone CLB-27/1), anti-CD33 (clone 4D3), anti-CD56 (clone NKI nbl-1), anti-CD64 (clone 10.1), anti-κ light chain (clone HP6062), anti-λ light chain (clone HP6054), and anti–glycophorin A (clone CLB-ery1) from Caltag Laboratories (Burlingame, CA). Fab′ fragments of goat antihuman IgM or antihuman IgD were from Southern Biotechnology Associates (Birmingham, AL). The biotinylated antibodies were revealed with streptavidin red 613 (Gibco BRL, Rockville, MD). Purified recombinant human (rh) erythropoietin (rhEPO), rh stem cell factor (rhSCF), rhFlt3 ligand, rhIL-15, and rh granulocyte colony-stimulating factor (rhG-CSF) were purchased from R & D Systems (Minneapolis, MN).
Cell isolation and staining procedure for fluorescence activated cell sorting analysis
Four-color immunofluorescence analysis was used for the identification of the different B-cell precursor populations in total nucleated BM cell suspensions from fetal and adult marrow. Briefly, single-cell suspensions were obtained by flushing or gently vortexing the BM. After staining, erythrocytes were lysed using the Becton Dickinson lysis buffer solution according to the manufacturer's instructions and CD45 antibody was used to assess the percentages of leukocytes in each sample. Intracellular staining was done by permeabilizing cells after surface staining. The cells were fixed with 1% paraformaldehyde in phosphate-buffered saline (PBS) and permeabilized with 70% ethanol at −20°C, for more than 30 minutes and washed twice with the staining buffer. They were then incubated with antihuman IgM for 30 minutes at room temperature. To establish the proliferative fraction, cells were incubated with the antibody anti-Ki67 (mib-1) for 1 hour on ice, as described by Zupo et al.39 Data acquisition and analyses were performed on a FACSCalibur (Becton Dickinson).
Isolation of CD34+/− cells and cell sorting
Mononuclear cells were collected from CB or adult BM by Ficoll/Hypaque (Lymphocyte Separation Medium; Cellgro-Mediatech, Herndon, VA) centrifugation. Enrichment for CD34+ cells was performed using the Direct CD34 Isolation Kit (Miltenyi Biotec, Auburn, CA). CD34+-enriched cells were stained with CD34-FITC, lineage markers (Lin, CD13, CD14, CD33, and CD64 for myeloid cells; glycophorin A for erythroid cells; CD19 for B cells; CD3 and CD8 for T cells; CD56 for NK cells)–PE, CD7-biotin followed by streptavidin red 613, and CD10-APC. CD34+Lin−CD10−CD7−, CD34+Lin−CD10−CD7+, and CD34+Lin−CD10+CD7−cells were sorted using a MoFlo (Cytomation, Fort Collins, CO). The sorted cells were subsequently subjected to flow cytometry, culture, and gene expression analyses. In some experiments, CD34+CD38− cells were sorted and cultured. In this case, mononuclear cells were incubated with mouse antihuman antibodies: CD3, CD13, CD14, CD16, CD33, CD56, and glycophorin A, and negatively selected using the BioMag goat antimouse IgG-coated beads (Perseptive Biosystems, Framingham, MA). The enriched cells were stained with CD34-FITC and CD38-PE. CD34+CD38−cells were determined using gating previously described.40Analysis after sorting revealed the purity of the sort populations to be more than 95%.
Transplantation of human cells into NOD/SCID mice
Magnetically enriched CD34+ cells from CB or fetal or adult BM were diluted in PBS with 0.1% bovine serum albumin (BSA fraction V; Sigma Chemical, St Louis, MO) and injected into the tail vein of sublethally irradiated (100 cGy from a 137Cs source) 8- to 12-week-old male or female NOD/SCID mice. Then, 5 × 106 CD34− CB mononuclear cells were coinjected as a source of accessory cells.41 42 No chimerism was ever obtained in mice given transplants of the accessory cells alone.
LDAs
The frequencies of B-cell progenitors in CB and adult BM were determined by plating CD34+CD38− cells in limiting dilution assays (LDAs). Between 20 and 180 wells containing pre-established MS-5 stromal cell layers (a kind gift of Dr J. Mori, Niigata University) were plated with 3, 5, 10, 15, 20, 30, 50, 75, 100, 150, or 200 cells each using the automated cell deposition unit of the MoFlo. Cells were cultured in α-minimal essential medium (MEM; Cellgro-Mediatech) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT) and a combination of rhSCF (100 ng/mL) and rhG-CSF (10 ng/mL).21 Wells were inspected every week for the presence of new clones. Positive wells were harvested after 5 to 7 weeks of culture and analyzed by flow cytometry for CD19+cells. The frequencies of precursors were calculated by linear regression analysis on the basis of Poisson distribution as the reciprocal of the concentration of test cells that gave 37% negative cultures.
Multilineage coculture system and methycellulose culture
Sorted CD34+Lin−CD10−CD7−, CD34+Lin−CD10−CD7+, and CD34+ Lin−CD10+CD7−cells were cultured at 1 × 104 cells/6 mL/25-cm2 tissue culture flask (Corning, Corning, NY) with MS-5 stromal cells. Culture media was α-MEM containing 10% FCS, rhSCF (100 ng/mL), rhG-CSF (10 ng/mL), rhFlt-3 ligand (100 ng/mL), and rhIL-15 (10 ng/mL). The cultures were maintained with weekly whole medium changes and the generation of myeloid, B, and NK cells was evaluated at the indicated periods. Methylcellulose cultures were performed in 35-mm plates (Corning) using MethoCult GF H4534 medium (Stem Cell Technologies, Vancouver, BC, Canada) containing rhSCF, rh granulocyte-macrophage colony-stimulating factor (rhGM-CSF), and rhIL-3 along with 3 U/mL rhEPO (R & D Systems). All cultures were maintained at 37°C in a humidified incubator with 5% CO2 in air. Differential colony counts were scored after 10 to 14 days by morphologic characteristics using an inverted microscope and confirmed by staining individual colonies with Wright or May-Grünwald-Giemsa stain.
RT-PCR
Sorted cells were put in lysis buffer (Ambion, Austin, TX) and mRNA was extracted using Poly-A column (Ambion) according to the manufacturer's instructions. cDNA was prepared from mRNA treated with DNase I using oligo-dT and Moloney murine leukemia virus reverse transcriptase (RT; Gibco BRL). Semiquantitative polymerase chain reaction (PCR) was done to measure relative differences in transcript levels of target cDNAs against levels of the reference gene GAPDH. The PCR was done in 100 μL containing PCR buffer (Takara Biomedical, Osaka, Japan), 1.5 mM MgCl2, 200 μM deoxyadenosine triphosphate (dATP), deoxyguanosine triphosphate (dGTP), deoxythymidine triphosphate (dTTP), 50 μM deoxycytidine triphosphate (dCTP), and 50 pmol of each primer. For quantification, 0.5 μCi (0.0185 MBq) [α32P] dCTP (Amersham, Arlington Heights, IL) was included in each reaction tube. Samples were denatured in a DNA thermocycler (Perkin-Elmer, Norwalk, CT) for 10 minutes at 95°C. To increase specificity, 2.5 U Taq DNA polymerase (Takara) was added to each sample during this initial denaturation. Samples were then cycled for 1 minute at 94°C, 1 minute annealing 60°C, 1.5 minutes extension at 72°C with a final extension of 7 minutes at 72°C. Aliquots were removed at cycles 25, 28, 31, and 34 for glyceraldehyde phosphate dehydrogenase (GAPDH) and cycles 32, 35, and 38 for all others to ensure that PCR remained within the exponential range of amplification. Then, 5-μL aliquots were denatured in a formamide loading buffer and applied to a 6% polyacrylamide gel containing 7 M urea. Incorporation of [alpha32P] dCTP into PCR product bands was quantified from dry gels using a PhosphoImager (Molecular Dynamics, Sunnyvale, CA).
Primers were as follows: GAPDH sense: 5′-TCCAAAATCAAGTGGGGCGAT-3′, GAPDH antisense: 5′-TTCTAGACGGCAGGTCAGGTC-3′, 475-bp expected product; RAG1sense: 5′-CCTGAGTCCTCTCATTGCTGAGAG-3′, RAG1 antisense: 5′-AGGGCATGATGATCGCCATACT-3′, 681-bp product; RAG2sense: 5′-CTAATGAAGAGCAGACAACATTCA-3′, RAG2 antisense: 5′-TAGGACTCTTTGGGGAGTGTGTAG-3′, 422-bp product; EBF sense: 5′-CCGGGCTCACTTTGAGAAGCAG-3′, EBF antisense: 5′-CAGGGAGTAGCATGTTCCAGAT-3′, 638-bp product; Pax5 sense: 5′-CTCGGTGAGCACGGATTCGGCC-3′, Pax5 antisense: 5′-GCGGCAGCGCTATAATAGTAG-3′, 621-bp product; CD122 sense: 5′-GCGTGGCTCGGCCACCTC-3′, CD122 antisense: 5′-GACGATGAGGGGAAGGGCGAAGA-3′, 211-bp product, Id-1 as described.43
Statistical analysis
All results are shown as mean values ± SD. Differences between groups were assessed using the Student ttest.
Results
B lymphopoiesis persists throughout adult human life
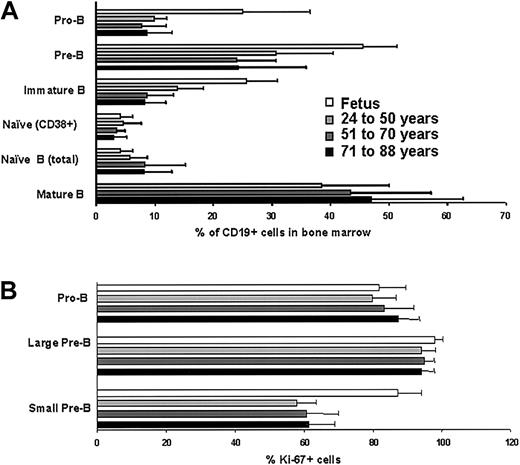
It has been reported that absolute numbers of B-lymphocyte precursors in human marrow decline with normal age and particularly during adolescence.38 Therefore, we calculated percentages of CD19+sIg− (surface Ig) lymphocytes and CD19+sIg+ B cells relative to total numbers of nucleated cells in our adult marrow samples (data not shown). As in the previous study,38 sample-to-sample variations were considerable, but we saw no substantial or consistent depletion of these lymphocyte populations as a function of age in adults. We then extended previous studies31,35 describing B-lineage lymphocyte precursors in adult human marrow (Figure1A). Pro-B cells (CD34+CD10+CD19+), pre-B cells (CD34−CD19+sIg− or CD19+cμ+sIg−) and immature B cells (sIgM+CD24hiIgD−CD10+CD38+) represented relatively constant percentages of the CD19+lymphocytes over a 24- to 88-year age range. The ratios between these populations and the presence of naive (sIgMhiIgD+CD24hiCD10+/−CD38+/−CD27−) B cells would all be consistent with stable B lymphopoiesis. Mature recirculating B cells (sIgM+IgD+CD24loCD38−) were previously shown to comprise a large fraction of marrow lymphocytes.35 We found no mature B cells in fetal bones, but there was no substantial influence of adult age on this population (Figure 1A). Mature B cells in marrow were also CD27+, a characteristic of somatically mutated memory B cells in peripheral blood.44 Most female marrow donors were receiving estrogen replacement therapy, and we found no sex-related differences in this analysis.
Relative frequencies and mitotic activity of B-lineage precursors in BM do not change during adult life.
(A) The incidences of pro B cells (CD34+CD10+CD19+), pre-B cells (CD19+sIgM−cμ+), immature B (IgM+CD24hiCD10+IgD−), naive B (IgM+CD24hiIgD+CD38+/−), and mature B (IgM+CD24loIgD+CD38−) cells were determined by flow cytometry and shown as percentages of total viable CD19+ lymphocytes (%) ± SDs. (B) Expression of the Ki-67 nuclear proliferation antigen was determined by flow cytometry, using low-angle light scatter to resolve large and small lymphocytes. The data represent average percentages of pro-B cells (CD34+CD19+) and pre-B cells (CD19+sIgM−cμ+) ± SDs. Differences between groups were not statistically significant (P > .05).
Relative frequencies and mitotic activity of B-lineage precursors in BM do not change during adult life.
(A) The incidences of pro B cells (CD34+CD10+CD19+), pre-B cells (CD19+sIgM−cμ+), immature B (IgM+CD24hiCD10+IgD−), naive B (IgM+CD24hiIgD+CD38+/−), and mature B (IgM+CD24loIgD+CD38−) cells were determined by flow cytometry and shown as percentages of total viable CD19+ lymphocytes (%) ± SDs. (B) Expression of the Ki-67 nuclear proliferation antigen was determined by flow cytometry, using low-angle light scatter to resolve large and small lymphocytes. The data represent average percentages of pro-B cells (CD34+CD19+) and pre-B cells (CD19+sIgM−cμ+) ± SDs. Differences between groups were not statistically significant (P > .05).
Blood cell production results from massive division within marrow, and we assessed expression of the Ki-67 nuclear proliferation antigen in B-lineage lymphocyte precursors (Figure 1B). Again, we found no age-related changes in this parameter and male-versus-female values were comparable. A similar analysis was recently performed with fetal marrow specimens,45 and the results are included here for comparison (Figure 1B). With the exception of small pre-B cells, which are more mitotically active during embryonic life, B-cell precursors were remarkably similar with respect to Ki-67 expression. We conclude that actively proliferating precursors are present within the lymphocyte fraction of human marrow until at least the eighth decade.
Analysis of marrow from exceptional patients reveals useful indices of B-cell production
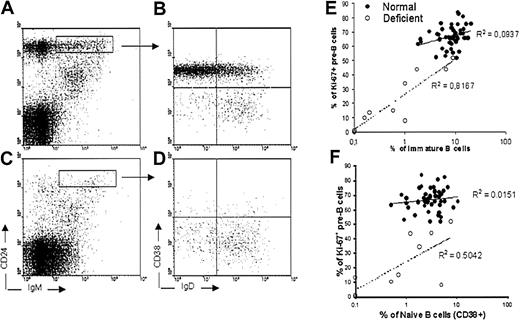
The present data would be consistent with stable B lymphopoiesis occurring over a large age range. However, it is difficult to know the degree to which lymphopoietic marrow is replaced by fat as a function of age and to control for sample-to-sample variations with respect to peripheral blood contamination and location. Therefore, it would be helpful to have other parameters for assessing marrow activity. Relatively constant proportions of B-lineage lymphocytes were found in most of the specimens we examined (Figure 1A). Whenever immature (IgM+CD24hiIgD−CD10+CD38+) B cells were present, marrow samples contained mitotically active pre-B (CD19+sIgM−) cells identified with the Ki-67 antibody. However, exceptionally low numbers were found in samples obtained from some patients. There were insufficient numbers of these individuals and information about specific treatments to formally assign them to groups. However, the degree of sample-to-sample variation in these specimens provided an opportunity to seek correlations (Figure 2). In these individuals, incidences of cycling pre-B cells predicted the presence of immature lymphocytes (P = .0001), consistent with a close precursor-product relationship between them. Naive (IgM+CD24hiIgD+CD38+CD10+/−D27−) B cells did not closely correlate with proliferating pre-B cells (P = .1035), although they are the progeny of immature B cells. We emphasize the usefulness of Ki-67 expression because simple percentages of pre-B cells did not correspond as closely with numbers of newly formed lymphocytes (data not shown). The findings suggest that the size of the proliferating pre-B cell pool determines numbers of newly formed B cells.
Profiles of immature and naive B cells in some deficient marrow specimens reveal a correlation between cycling pre-B cells and immature B cells.
Fluorescence-activated cell sorter (FACS) profiles of immature (IgM+CD24hiCD38+IgD−) and naive (IgM+CD24hiCD38+/−IgD+) B cells from a healthy patient (A-B) are compared to a deficient one (C-D). Immature B cells were also CD10+ (not shown). The index of cycling pre-B cells in exceptional patient samples correlated with production of immature B cells (E), and less closely with naive B cells (F). Coefficients of determination (R2) are indicated.
Profiles of immature and naive B cells in some deficient marrow specimens reveal a correlation between cycling pre-B cells and immature B cells.
Fluorescence-activated cell sorter (FACS) profiles of immature (IgM+CD24hiCD38+IgD−) and naive (IgM+CD24hiCD38+/−IgD+) B cells from a healthy patient (A-B) are compared to a deficient one (C-D). Immature B cells were also CD10+ (not shown). The index of cycling pre-B cells in exceptional patient samples correlated with production of immature B cells (E), and less closely with naive B cells (F). Coefficients of determination (R2) are indicated.
Early developmental age-related changes in populations resolved by flow cytometry
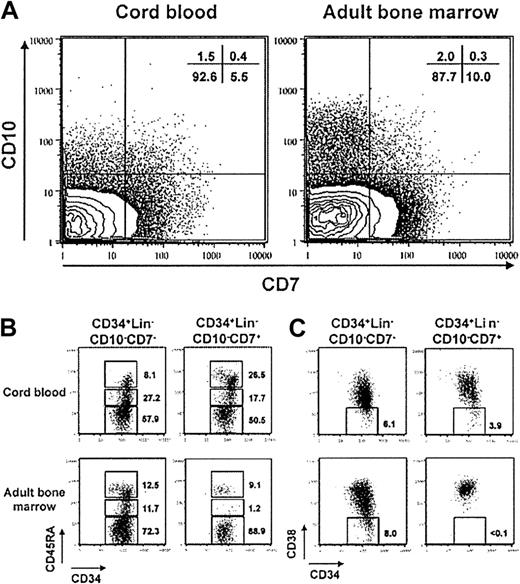
Earlier reports demonstrated that CD7 could be used to discriminate hematopoietic stem cells from more differentiated precursors of NK and T lymphocytes.46,47 It is also noteworthy that CD7 is rarely coexpressed with CD10 or CD19, and evidence indicates that CD7+ cells in CB represent common lymphoid progenitors.33 However, comparisons made by flow cytometry suggested that CB might not be representative of differentiation pathways used in adult marrow. Adult CD34+Lin− cells contained a more conspicuous subset of CD7−CD10+ progenitors as well as more CD7+CD10− cells (Figure3A). Additional differences were found by comparing sorted CD7+ and CD7− subsets of CD34+Lin−CD10− cells from the 2 sources (Figure 3B-C). It has been suggested that CD45RA is acquired before CD10,32 and we found that nearly half of the CB CD7+ cells expressed moderate to high levels (Figure 3B). In contrast, only 10% of the comparable subset from adult marrow was CD45RA+. As noted above, the CD38− subset of the CD34+Lin−CD7+ fraction of CB has been recently found to contain multilymphoid but not myeloerythroid progenitors.33 It is remarkable that cells with that combination of markers do not exist in adult marrow (Figure 3C). Note that the CD7− subsets of fetal and adult populations were similar with respect to CD45RA and CD38 expression. Thus, the composition of hematopoietic cell subsets or surface marker display in CB differs substantially from adult BM.
Early lymphohematopoietic progenitors in adult BM differ from ones in CB with respect to surface marker characteristics.
(A) CD7/CD10 profiles are shown for CD34+Lin−cells in CB and adult BM. CD34+Lin−CD10−CD7− and CD34+Lin−CD10−CD7+cells were sorted from CB and adult BM and stained with CD45RA-PE (B) or CD38-PE (C). These data are representative of 3 independently sorted samples.
Early lymphohematopoietic progenitors in adult BM differ from ones in CB with respect to surface marker characteristics.
(A) CD7/CD10 profiles are shown for CD34+Lin−cells in CB and adult BM. CD34+Lin−CD10−CD7− and CD34+Lin−CD10−CD7+cells were sorted from CB and adult BM and stained with CD45RA-PE (B) or CD38-PE (C). These data are representative of 3 independently sorted samples.
Transplantation experiments reveal age-related changes in differentiation potential
Immunodeficient NOD/SCID mice were prepared for transplantation with low-dose irradiation (“Materials and methods”) and then injected with highly enriched suspensions of CD34+cells (Table 1). Previous studies documented an extraordinary differentiation potential of CB stem cells in this model,48-50 so we compared one log less CD34+ cells from this source to ones isolated from fetal and adult marrow. The latter represented pools of CD34+cells obtained from specimens ranging from 35 to 82 years of age in one experiment and 62 to 77 years of age in a second transplantation. Flow cytometry was performed on aliquots of the suspensions to determine numbers of CD34+CD38− cells injected because of reports that these cells are particularly effective for engraftment of NOD/SCID mice51 (Table 1). It has been shown that undefined accessory cells can influence engraftment of human cells in mice.41 42 Therefore, we added 5 × 106CD34− CB mononuclear cells to all samples of CD34+ cells before transplantation, and the recipients were assessed 7 weeks later. No human cells were detectable in animals that received only the CD34− cells, but easily discernible populations of CD45+CD19+ lymphocytes were found in virtually all recipients of CD34+ cells, regardless of donor age (Table 1). Adult precursors were less effective than those obtained from the other 2 sources with respect to numbers of lymphocytes produced, suggesting a possible consequence of the early developmental age-related changes noted.
Adult human BM contains B-lymphocyte precursors revealed by transplantation in immunodeficient NOD/SCID mice
| Cells injected . | No. of cells Injected . | No. of CD34+CD38− cells injected* . | No. of engrafted/injected mice (%) . | Percentage of hCD45+CD19+ cells in BM of chimeras . |
|---|---|---|---|---|
| Fetal BM CD34+ | 2.9-3 × 106 | 28.5-30 × 103 | 5/6 (83) | 1.54 (± 1.28) |
| CB CD34+ | 5 × 105 | 4 × 103 | 11/11 (100) | 1.02 (± 0.86) |
| Adult BM CD34+ | 2 × 106 | 39.6-72 × 103 | 5/5 (100) | 0.22 (± 0.16) |
| CB CD34− | 5 × 106 | <0.01 | 0/5 | <0.02 |
| Cells injected . | No. of cells Injected . | No. of CD34+CD38− cells injected* . | No. of engrafted/injected mice (%) . | Percentage of hCD45+CD19+ cells in BM of chimeras . |
|---|---|---|---|---|
| Fetal BM CD34+ | 2.9-3 × 106 | 28.5-30 × 103 | 5/6 (83) | 1.54 (± 1.28) |
| CB CD34+ | 5 × 105 | 4 × 103 | 11/11 (100) | 1.02 (± 0.86) |
| Adult BM CD34+ | 2 × 106 | 39.6-72 × 103 | 5/5 (100) | 0.22 (± 0.16) |
| CB CD34− | 5 × 106 | <0.01 | 0/5 | <0.02 |
Numbers of CD34+CD38− cells were determined by flow cytometry, using small aliquots of each preparation of CD34+ enriched cells. These results were obtained in 2 very similar but independent experiments.
Culture experiments reveal age-related differences in expansion and differentiation potential of lymphocyte progenitors
Homing to the marrow, resistance to xenotransplantation barriers, and many other factors could influence the degree of chimerism observed in the NOD/SCID model. Furthermore, culture experiments could permit detailed analysis of small cell numbers. Therefore, we performed an LDA in which graded numbers of CD34+CD38− cells were seeded onto monolayers of murine MS-5 stromal cells and stimulated with SCF and G-CSF (Table 2). The frequency of precursors with the potential for clonal expansion varied from 3.6% to 9.78% in marrow suspensions obtained from healthy 24- to 88-year-old donors. In separate experiments, the cloning efficiency of umbilical CB CD34+CD38− cells ranged from 11.11% to 14.29% using the same culture conditions. In this case, the B-cell progenitor frequency was 6.67% (range, 5.56%-8.33%). Furthermore, in about 40% of the wells more than 50% of the cells produced by CB CD34+CD38− cells were CD19+, and this was never the case with adult BM. Analysis of pooled clones revealed that differentiation did not proceed efficiently beyond the CD19+ stage in such cultures and very few cytoplasmic or surface μ+ cells were recovered.
Adult human BM contains functional B-lineage precursors that can be enumerated in limiting dilution cultures of CD34+CD38− cells
| Source of CD34+CD38−cells . | Clonogenic frequency, % (CI) . | Frequency of cells able to give rise to CD19+ cells, % (CI) . |
|---|---|---|
| CB | 12.5 (11.11-14.29) | 6.67 (5.56-8.33) |
| Adult BM, 24 y | 6.09 (4.84-13.53) | 1.16 (0.77-1.96) |
| Adult BM, 44 y | 9.78 (7.78-19.01) | 1.04 (0.67-1.79) |
| Adult BM, 45 y | 3.69 (2.78-6.56) | 0.05 (<0.01-0.12) |
| Adult BM, 70 y | 4.5 (3.82-8.83) | 0.18 (0.07-0.31) |
| Adult BM, 88 y | 3.6 (3.24-4.26) | 0.15 (0.02-0.32) |
| Source of CD34+CD38−cells . | Clonogenic frequency, % (CI) . | Frequency of cells able to give rise to CD19+ cells, % (CI) . |
|---|---|---|
| CB | 12.5 (11.11-14.29) | 6.67 (5.56-8.33) |
| Adult BM, 24 y | 6.09 (4.84-13.53) | 1.16 (0.77-1.96) |
| Adult BM, 44 y | 9.78 (7.78-19.01) | 1.04 (0.67-1.79) |
| Adult BM, 45 y | 3.69 (2.78-6.56) | 0.05 (<0.01-0.12) |
| Adult BM, 70 y | 4.5 (3.82-8.83) | 0.18 (0.07-0.31) |
| Adult BM, 88 y | 3.6 (3.24-4.26) | 0.15 (0.02-0.32) |
CI indicates confidence interval.
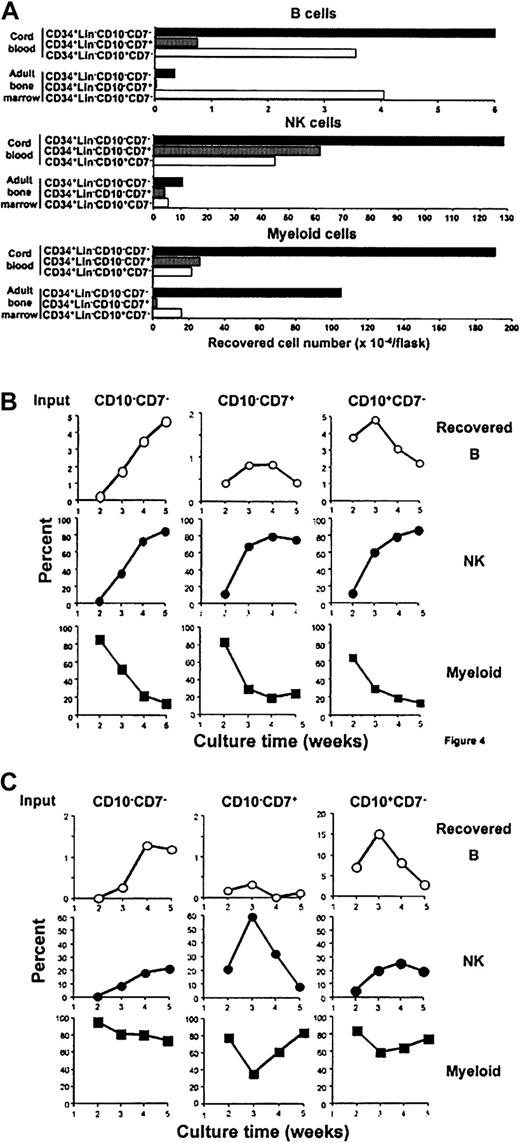
Culture conditions were then developed that allowed simultaneous differentiation of CD19+ B- lineage, CD13/CD33+myeloid-lineage, and CD56+ NK-lineage cells (“Materials and methods”). Absolute numbers of CD19+ cells produced were equal to or more than those obtained with other methods we have tried and NK-lineage cells were always efficiently produced when IL-15 was present. The method was used for a side-by-side comparison of fetal/neonatal-versus-adult marrow precursors. Three-week cultures initiated with 104CD34+Lin−CD7−CD10−CB precursors yielded much larger numbers of B-lineage lymphocytes than those prepared with equivalent cells from adult BM (Figure4A). Interestingly, the lymphoid potential of CD34+Lin−CD7−CD10+cells from the sources was comparable. The CD34+Lin−CD7+CD10−subset was efficient only when derived from CB. All subsets of CB yielded approximately 10 times the number of CD56+NK-lineage cells than obtained in cultures of their adult counterparts.
Early lymphohematopoietic progenitors in CB and adult BM differ with respect to expansion and differentiation potential.
CD34+Lin−CD10−CD7−, CD34+Lin−CD10−CD7+, and CD34+Lin−CD10+CD7−cells were sorted from CB or adult BM and cultured with MS-5 stromal cells (1 × 104 cells/flask in each fraction). Yields of CD19+ B–, CD56+ NK–, and CD13+/CD33+ myeloid–lineage cells in 3-week cultures are shown (A). The B-, NK-, and myeloid-lineage compositions were also determined weekly in cultures initiated with fractions sorted from CB (B) or adult BM (C). Similar results were obtained in 2 independent side-by-side comparisons between CB and adult BM.
Early lymphohematopoietic progenitors in CB and adult BM differ with respect to expansion and differentiation potential.
CD34+Lin−CD10−CD7−, CD34+Lin−CD10−CD7+, and CD34+Lin−CD10+CD7−cells were sorted from CB or adult BM and cultured with MS-5 stromal cells (1 × 104 cells/flask in each fraction). Yields of CD19+ B–, CD56+ NK–, and CD13+/CD33+ myeloid–lineage cells in 3-week cultures are shown (A). The B-, NK-, and myeloid-lineage compositions were also determined weekly in cultures initiated with fractions sorted from CB (B) or adult BM (C). Similar results were obtained in 2 independent side-by-side comparisons between CB and adult BM.
Myeloid potential was highest in the CD34+Lin−CD7−CD10−fractions and dramatically down-regulated in CD7+ or CD10+ subsets, regardless of source. These results are consistent with clonal assays conducted with methylcellulose cultures. With 2 analyses of CB, most nonlymphoid progenitors were present in the CD7− subset (710.8 ± 74.6 colonies/1000 cells). However, it is noteworthy that an average of 170.8 ± 50.8 colonies/1000 cells were also obtained when the CD7+fraction was plated and 95% of them were erythroid. Similarly, the CD34+ Lin−CD7−CD10−fraction of adult marrow contained most (an average of 60% in 3 experiments) of the granulocyte/macrophage progenitors (granulocyte/macrophage colony-forming units [CFU-GMs]). As with CB cells, 98% of the colonies obtained with the CD34+Lin−CD7+ fraction were erythroid (erythroid burst-forming units [BFU-Es]). Insufficient numbers of the rare CD7+CD10+ subset of adult marrow precluded their analysis in these cultures.
Weekly examination revealed that myeloid progeny appeared quickly and then declined in cultures initiated with the CD34+ Lin−CD7−CD10−fraction of CB, whereas myelopoiesis was more persistent in cultures of their adult counterparts (Figure 4B-C). Progressive production of B- and NK-lineage cells was consistent with the primitive nature of progenitors in this fraction. Whereas transient B-lineage differentiation was found with the CD34+Lin−CD7+CD10−fraction, this was never the case with the comparable adult marrow subset. The adult population also yielded a wave of NK-lineage cells as contrasted with sustained production from neonatal cells.
Neonatal or adult CD10+ fractions contained B-lineage precursors that differentiated within 2 weeks in culture, and CD56+ NK-lineage cells reached peak values 1 week later. This analysis revealed large age-related changes in the potential of highly purified CB and adult BM progenitors to expand in culture.
CD10 and CD7 expression denote commitment to B and NK lineages
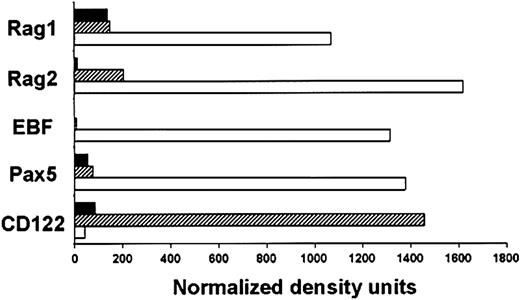
These findings highlight the importance of learning more about the B-lineage precursors in human marrow. Therefore, adult subsets sorted for use in the culture experiments as described were also subjected to RT-PCR analysis (Figure5). Transcripts associated with B-lymphocyte lineage differentiation (RAG1, RAG2, EBF, and Pax5) were markedly up-regulated in the CD34+Lin−CD10+CD7−population relative to the more primitive CD34+Lin−CD10−CD7−subset. Furthermore, levels of the Id-1 transcriptional repressor were reduced in that fraction (data not shown). In contrast, expression of the CD122 receptor for IL-15/IL-2 required by NK-lineage cells was strongly associated with display of CD7. Although transcripts for RAG1 and RAG2 were detectable in CD7+ cells, this fraction was deficient in the EBF transcription factor required for B lymphopoiesis. Very similar results were obtained in 3 independent experiments. These results complement those obtained with culture experiments and suggest that acquisition of CD10 or CD7 corresponds to substantial progression in the B or NK lineages, respectively.
B-lineage characteristics are established by the CD10+ stage in adult BM.
Expression of lineage-related genes was examined in the CD34+Lin−CD10−CD7−(▪), CD34+Lin−CD10−CD7+ (▨), and CD34+Lin−CD10+CD7−(■) fractions sorted from adult BM. RT-PCR was used to amplify transcripts for the indicated genes, and the results were normalized according to GAPDH expression.
B-lineage characteristics are established by the CD10+ stage in adult BM.
Expression of lineage-related genes was examined in the CD34+Lin−CD10−CD7−(▪), CD34+Lin−CD10−CD7+ (▨), and CD34+Lin−CD10+CD7−(■) fractions sorted from adult BM. RT-PCR was used to amplify transcripts for the indicated genes, and the results were normalized according to GAPDH expression.
Discussion
This study was informative about several aspects of human B lymphopoiesis. Ratios between precursors and immature B cells within marrow and an indication of mitotic activity were found to be remarkably constant throughout adult life. Exceptional marrow samples from some patients were deficient in lymphocyte precursors, providing a unique opportunity to see how the abundance of particular subsets corresponds to newly formed B cells. Although the potential for lymphoid differentiation, as assessed by culture and transplantation assays was retained in most individuals, the lymphocyte yield from adult progenitors was much less than that obtained from CB. Furthermore, we documented age-related changes in subsets resolved on the basis of CD7 and CD10 expression. Analysis of highly purified progenitors revealed that erythroid and NK differentiation potential might diverge from B-lineage potential as cells acquire one or the other of these markers.
There are many technical problems associated with analysis of human marrow specimens, and it is difficult to estimate absolute numbers of B-cell precursors. As one example, mature recirculating B cells have been identified that have a distinctive density of CD24 and sIgM.35 Absolute numbers of these cells per unit marrow or body weight are not known, but they progressively dilute B-cell precursors and newly formed B cells. However, it is fortunate that other measurements reflect the lymphopoietic activity in that organ. As in previous studies31,35 the ratios between B-cell precursors and newly formed B cells can be determined, and we confirm that they are relatively constant. Moreover, the proliferative activity of B-cell precursors was surprisingly stable with age. In a separate study, we evaluated precursors in fetal marrow and found that an exceptionally large fraction of small pre-B cells in that site are Ki-67+.45 This is one of several distinctive features of fetal lymphopoiesis. Once the process is established in adult marrow, the proliferation index of B-cell precursors remains unchanged until at least 88 years of age.
The striking deficiency of B-cell precursors in some of the marrow specimens suggests that influences of therapy and environmental stress on B lymphopoiesis merit further study. One previous report recorded delayed recovery of B cells after marrow transplantation in patients receiving cortisone.52 Most of our deficient samples came from patients who were being treated with anti-inflammatory drugs and we will show elsewhere that human lymphocyte progenitors are very sensitive to glucocorticoids. Here we used the variation provided by these unusual specimens to make inferences about precursor-product relationships within marrow. The best correlation was found between cycling pre-B cells and immature B cells. The Ki-67 proliferation antigen provided a particularly useful index, and simple pre-B cell percentages did not correlate as well. Whenever percentages of proliferating pre-B cells were beneath 50%, immature (IgM+IgD−CD24hiCD10+CD38+) B cells were scarce. The correlation between percentages of naive CD38+ B cells and cycling pre-B cells was not as good as the relationship between pre-B cells and immature B cells (Figure 2). Cells with a naive phenotype might randomly leave marrow to enter the peripheral B-cell pool, as has been suggested for newly formed B cells in mice.53
It was important to learn if cells with surface characteristics of B-cell precursors actually had the potential to give rise to lymphocytes. Human CD34+CD38− cells have been determined in other studies to be responsible for engraftment of NOD/SCID mice, but uncharacterized accessory cells among the CD34− fraction of hematopoietic cell suspensions can influence the outcome.41,42,51 To minimize this parameter, we added a constant number of CB CD34− mononuclear cells to each of the transplant samples, and human CD19+ cells were easily detectable in marrow of all mice receiving transplants. As others have shown, stem cells within CB are exceptionally efficient for engraftment of immunodeficient mice.48-50 We estimate that 7 weeks after transplantation there were 111 ± 98 human CD19+ cells present in each mouse femur per injected CD34+CD38− CB cell. This value is 10- to 100-fold higher than we obtained with precursors isolated from adult marrow.
Limiting dilution cultures initiated with highly purified CD34+CD38− cells provided an independent means of assessing differentiation potential and one that should be less influenced by contaminating accessory cells. CB precursors had a higher cloning efficiency in vitro than adult marrow–derived cells, and more of the responding wells generated CD19+ lymphocytes (Table2). This result is compatible with observations made in another study, using a different culture system54 and suggests the possibility of an intrinsic difference between neonatal and adult progenitors.
Because the proportion of hematopoietic marrow relative to fat progressively declines with age in long bones, total numbers of newly formed B cells might be reduced in some locations.28However, the cellularity of marrow in other sites is remarkably constant throughout life, and it will be important to determine the total body output of new B cells.29 This important issue awaits other types of analyses comparable to those recently used to assess thymic activity in adult humans.55-57 Many studies suggest that hematopoietic stem cells retain the potential for replenishing lymphocytes throughout life.58,59 However, there are changes with respect to incidence, homing potential, and function on a per-cell basis. Additionally, there can be age-related declines in the ability of environmental cells within marrow to support lymphopoiesis.4 Genetic polymorphisms influence stem cell numbers and senescence to a substantial degree.59 60 For that reason, our culture assays were conducted with cells from individual marrow donors. Although flow cytometry revealed proportions of lymphocyte precursors in most marrow specimens to be remarkably constant, functional precursor frequencies among CD34+CD38− cells were highly variable (Table2).
Flow cytometry experiments revealed that the composition and patterns of surface marker expression differ substantially between CB and adult BM. The interesting CD34+CD38−CD7+CD10−subset recently described in cord blood33 has no direct counterpart in adults. Furthermore, the CB CD34+CD38−CD7−CD10−subset differed substantially from the comparable adult population with respect to expression of CD45RA. Given these changes, we elected to sort 3 populations for side-by-side comparisons in culture studies. CD10+CD19− cells comprise a very small proportion (2.5% ± 1.1%) of the 1% of marrow mononuclear cells that express CD34+30,32 (Figure 3). Most of these CD34+CD10+CD19− cells also express TdT30,32,61 and data not shown) and already have D-JH rearrangements.34 Previous reports32,34 suggest that this fraction is a rich source of early B-lineage precursors. Our culture analyses substantiate that conclusion and it is noteworthy that CB and adult progenitors were equivalent in potential for B-lineage differentiation (Figure 4). PCR analysis of CD34+Lin−CD7−CD10+cells from adult BM revealed substantial expression of 4 genes required for B lymphopoiesis (Figure 5). However, it is also important to stress that CD10+ cells have some myeloid potential that can be appreciated in long-term cultures but not in clonal assays32 (Figure 4; data not shown). Thus, a completely lymphoid-restricted progenitor has not been isolated from adult human marrow.
The question arises whether B lineage–associated events begin prior to the CD10+ stage and CD7+ cells are interesting in this regard. One recent study found that a CD34+CD38−CD7+ fraction of CB contains what might be the human equivalent of common lymphoid progenitors described in mice.12,33 We confirm that CB CD34+CD7+CD10−CD19−cells can rapidly generate small numbers of CD19+ cells in culture (Figure 4; data not shown). Unlike the previous report, we detected some nonlymphoid, and especially erythroid, precursors in this population. This may be because we did not additionally select for CD38− cells in CB. However, as noted, there are no CD38−CD7+ cells in adult human marrow and the total CD34+Lin−CD7+CD10−fraction of marrow was a poor source of B-lineage progenitors (Figures3-4). PCR analysis of carefully sorted cells substantiated the culture results and showed a considerable enrichment for cells expressing the CD122 receptor for IL-15/IL-2 required for NK-lineage differentiation (Figure 5). It was previously reported that the CD34+CD7+ fraction of adult marrow contains precursors of NK cells.46 Thus, CD7 expression may characterize an intermediate stage between stem cells, erythroid cells, and pro-B cells in neonates, but be more closely associated with NK lineage–restricted precursors in adult marrow.
To summarize, several distinctive adult characteristics of B-lymphocyte precursors may be acquired during the neonatal or adolescent period. The primitive CD34+Lin−CD10−CD7−fraction retains differentiation potential throughout life, but it is much less efficient than CB cells with the same phenotype. Cells bearing CD10 include many early B-lineage precursors and ones in neonates and adults appear to have equivalent differentiation potential. All subsequent stages of B-lineage differentiation are represented in constant ratios in marrow and numbers of proliferating pre-B cells closely correlate with numbers of newly formed B cells. The search must continue for lymphoid-restricted progenitors in human marrow and our understanding of early differentiation events is incomplete.
Prepublished online as Blood First Edition Paper, August 29, 2002; DOI 10.1182/blood-2002-03-0896.
Supported by grants AI 20069 and AI 45864 from the National Institutes of Health. P.W.K. holds the William H. and Rita Bell Chair in biomedical research.
M.I.D.R. and T.Y. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul W. Kincade, Immunobiology and Cancer Program, Oklahoma Medical Research Foundation, 825 NE 13thSt, Oklahoma City, OK 73104; e-mail:kincade@omrf.ouhsc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal