Abstract
HIV-specific CD8+ T cells are prone to undergo apoptosis, and this may affect their ability to control HIV infection. Because CD8-mediated immune responses play a key role in controlling HIV infection, enhancing the survival and effector function of HIV-specific CD8+ T cells may augment their ability to control HIV virus. We show here that interleukin 15 (IL-15) potently inhibits spontaneous and CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells. IL-15 inhibits apoptosis in both CD45RA−CD62L− and CD45RA+CD62L− effector memory subpopulations of these cells. Furthermore, IL-15 greatly enhances the survival of HIV-specific CD8+ T cells in long-term cultures. Finally, IL-15 directly enhances activation, interferon γ (IFNγ) production, and direct ex vivo cytotoxicity of HIV-specific CD8+ T cells. Thus, IL-15 potently enhances the survival and effector function of HIV-specific CD8+ T cells and, therefore, may prove useful in augmenting the antiviral function of these cells.
Introduction
HIV-specific CD8+ T-cell responses play a key role in controlling HIV infection.1,2 However, these cells demonstrate an increased sensitivity to CD95/Fas-induced apoptosis and can be killed by HIV-infected cells.3 This sensitivity to CD95/Fas-induced apoptosis was found highest in effector memory CD45RA−CD62L− and CD45RA+CD62L− HIV-specific CD8+ T cells.3 This increased apoptosis of HIV-specific CD8+ T cells may be responsible for the skewed memory phenotype of these cells and may impair their function as serial killers.3,4 Inhibiting, therefore, the apoptosis of HIV-specific CD8+ T cells may prove important as a strategy to augment their anti-HIV activity. Interleukin 15 (IL-15) is a pluripotent cytokine that can enhance the generation and survival of murine memory CD8+ T cells both in vitro5,6and in vivo.5,7-13 IL-15 also modulates the effector function of CD8+ T cells, as it can increase mRNA expression of perforin, granzymes A and B, and interferon γ (IFNγ) in murine splenocytes14 and human peripheral blood mononuclear cells (PBMCs).15 IL-15 can also enhance activation and proliferation of PBMCs from HIV-infected individuals,16-18 the activity of natural killer cells,17,19 and the in vitro expansion of cytotoxic CD8+ T cells from HIV-infected individuals.20Finally, IL-15 has been shown to inhibit in vitro T-cell apoptosis12,18,21-23 and in vivo anti-Fas–induced apoptosis.22 These properties of IL-15 led us to investigate the effect of IL-15 on the apoptosis and effector function of HIV-specific CD8+ T cells.
We report here that IL-15 greatly reduces spontaneous and CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells. This apoptosis was inhibited in both CD45RA−C62L− and CD45RA+CD62L− effector memory populations. IL-15 did not inhibit anti-CD3–stimulated apoptosis. IL-15 markedly increased the survival of HIV-specific CD8+ T cells in long-term cultures. Finally, IL-15 increased the activation, IFNγ production, and direct ex vivo cytotoxicity of HIV-specific CD8+ T cells, thus demonstrating that it also augments effector function. Given its ability to inhibit apoptosis and increase effector function of HIV-specific CD8+ T cells, IL-15 may prove useful as a strategy to enhance the CD8+ T-cell response to HIV.
Patients, materials, and methods
Patients
Twenty-four HIV-infected individuals were included in this study who were HIV positive for at least 1 year (range, 3-22 years), the median CD4 count was 205 cells/μL (range, 18-851 cells/μL), the median viral load was 1466 RNA copies/mL blood (range, 36-340 000 copies/mL), 21 patients were asymptomatic, and 19 were on antiviral treatment. Peripheral blood was collected following approval of the Drexel University Institutional Review Board and obtaining informed consent.
Flow cytometry
PBMCs were isolated from blood of HIV-infected individuals by Ficoll-Hypaque density centrifugation (Amersham Pharmacia Biotech, Uppsala, Sweden). HIV-specific CD8+ T cells were identified by using tetramers of HLA class I A*0201-β2-microglobulin loaded with Gag p17 77-85 (SLYNTVATL) peptide and Pol 476-484 (ILKEPVHGV) peptide and tetramers of HLA class I A3-β2-microglobulin loaded with Nef 71-80 (QVPLRPMTYK) as previously described.24 The following antibody combinations were used: activation studies, anti-CD69-FITC/anti-CD8-PE-Cy5 with Gag- and Pol-specific tetramer–allophycocyanin (APC); apoptosis measurement, Annexin V–fluorescein isothiocyanate (FITC) (kind gift from Dr J. Tait, University of Washington, Seattle)/anti-CD8-phycoerythrin (PE)–Cy5 with Gag- and Pol-specific tetramer-APC, Annexin V–FITC/anti-CD62L-PE/anti-CD45RA-PE-CY5 with Gag- and Pol-specific tetramer-APC; long-term survival studies, Annexin V–FITC/A3-Nef-specific tetramer-PE/anti-CD8-PE-CY5 and Annexin V–FITC/anti-CD8-PE-CY5 with Gag- and Pol-specific tetramer-APC. All antibodies are from eBioscience, San Diego, CA. Briefly, 1 × 106 cells were stained with tetramers and antibodies in Hanks buffered saline solution (HBSS; Cellgro, Herndon, VA), 3% fetal bovine serum (FBS), 0.02% NaN3 for 30 minutes on ice; washed 2 times with HBSS, 3% FBS, and 0.02% NaN3; and fixed with 1% paraformaldehyde. For Annexin V staining 2.5 mM CaCl2 was added. Analysis was performed on a fluorescence-activated cell sorter (FACS)–Calibur (Becton Dickinson, San Jose, CA) using FlowJo software (TreeStar, San Carlos, CA).
Activation studies
PBMCs were cultured in RPMI 1640 supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin sulfate (Cellgro) at 37°C in a 5% CO2 incubator at 1 × 106 cells/mL per well in 24-well plates. For anti-CD3–induced activation, PBMCs were cultured in plates coated with 0.01 μg/mL monoclonal anti-CD3 antibody (OKT3)25 in the presence or absence of 5 ng/mL IL-15 (R&D Systems, Minneapolis, MN) for 2 hours and then transferred to uncoated plates for an additional 12 hours to allow the surface reappearance of the T-cell receptor (TCR)/CD3 complex and to permit tetramer staining. For antigen-specific stimulation, PBMCs were incubated with 1 μg/mL HIV-specific tetramer in the presence or absence of 5 ng/mL IL-15 for 3 hours.
Cytotoxicity assay
C1R-A2 (HmyC1R-A*201) cells (kind gift from Dr J. Frelinger, University of North Carolina, Chapel Hill) were loaded overnight at 37°C in a 5% CO2 incubator with HLA class I peptides (HIV Gag p17 77-85, HIV Pol 476-484), washed, and then radiolabeled with Na51CrO4 (NEN, Boston, MA) for 75 minutes at 37°C. CD8+ T cells were purified from freshly isolated PBMCs by negative selection by using RosetteSep for CD8+T-cell enrichment (StemCell Technologies, Vancouver, BC, Canada). CD8+ T-cell purity was more than 92% with the contaminating populations being CD3+CD4−CD8− T cells. Purified CD8+ T cells were incubated at an effector-to-target ratio of 50:1 with 5 × 103 C1R-A2 target cells in 96-well U-bottom plates for 6 hours at 37°C in the presence or absence of 5 ng/mL IL-15. Plates were then centrifuged, and supernatants (30 μL) were transferred to 96-well LumaPlates (Packard BioScience, Meriden, CT) and counted in a TopCount microplate scintillation counter (Packard BioScience). Specific cytotoxicity was determined by using the following formula: (cpm experimental release − cpm spontaneous release) × 100/(cpm maximal release − cpm spontaneous release).
Apoptosis studies
PBMCs were incubated at 1 × 106 cells/mL per well in 24-well plates in the presence or absence of 5 ng/mL IL-15, and spontaneous apoptosis was determined after 14 hours. For anti-CD3–stimulated apoptosis, PBMCs were cultured in the presence or absence of 5 ng/mL IL-15 in plates coated with 10 μg/mL anti-CD3 antibody (OKT3) for 2 hours and then transferred to uncoated plates for an additional 12 hours. For CD95/Fas-induced apoptosis, PBMCs were cultured in plates coated with 5 μg/mL monoclonal anti-CD95 antibody (immunoglobulin M [IgM], CH11; Immunotech, Brea, CA) in the presence or absence of 5 ng/mL IL-15 for 14 hours. Treatment-specific apoptosis was calculated by using the following formula: (% induced apoptosis − % spontaneous apoptosis) × 100/(100 − % spontaneous apoptosis).
For long-term survival studies, CD8+ T cells purified by negative selection (as described earlier) were cultured at 0.5 to 1 × 106 cells/well in the presence or absence of 5 ng/mL IL-15 for 7 days before cells were harvested, counted by using 0.1% Trypan Blue solution (Cellgro), and stained for apoptosis.
Intracellular IFNγ staining
IFNγ production by intracellular staining was determined after 6-hour stimulation of freshly isolated PBMCs from HIV-infected individuals with 10 μg/mL virus-specific peptide (HIV Gag p17 77-85, HIV Pol 476-484) in the presence or absence of 5 ng/mL IL-15 and in the presence of 10 μg/mL Brefeldin A (PharMingen). After cell surface staining with anti-CD8–APC and permeabilizing cells with Cytofix/Cytoperm (PharMingen, San Diego, CA), intracellular staining was performed with anti-IFNγ–FITC monoclonal antibody (eBioscience) as previously described.3 Cells were analyzed on a FACS-Calibur (Becton Dickinson) using FlowJo software (TreeStar) after fixation with 1% paraformaldehyde.
Statistical analysis
Statistical analysis was performed by using Mann-WhitneyU test, Student t test, nonparametric Wilcoxon signed rank test for paired samples, and Shapiro-Wilk W test for normality. P < .05 was considered significant. The JMP statistical analysis program was used (SAS, Cary, NC).
Results
IL-15 inhibits spontaneous and CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells
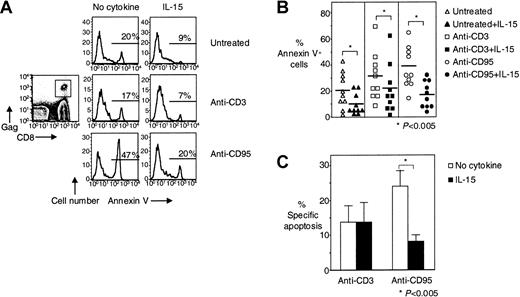
We have previously shown that HIV-specific CD8+ T cells are highly susceptible to CD95/Fas-mediated apoptosis and can be killed by HIV-infected cells.3 We have proposed that this apoptosis may be responsible for the skewed phenotype that we and others have observed for these cells.3,4 On the basis of the antiapoptotic properties of IL-15,9,12,18 21-23 we examined whether IL-15 can inhibit the apoptosis of HIV-specific CD8+ T cells. IL-15 (5 ng/mL) significantly reduced spontaneous cell death of HIV-specific CD8+ T cells by 52% from 21% ± 4.4% to 10% ± 2.5% (n = 10,P < .005) and CD95/Fas-induced apoptosis by 53% from 38% ± 5.0% for anti-CD95/Fas stimulation to 18% ± 3.3% for anti-CD95/Fas stimulation in the presence of IL-15 (n = 10,P < .005) (Figure 1A-B). When treatment-specific apoptosis was calculated, IL-15 significantly reduced specific CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells by 63% from 24% ± 4.7% to 9% ± 2.6% (P < .005) (Figure 1C). Although IL-15 appears to inhibit anti-CD3–stimulated apoptosis of HIV-specific CD8+ T cells (31% ± 5.8% in the absence of IL-15 and 22% ± 5.8% in the presence of IL-15, respectively, n = 10, P < .005) when treatment-specific apoptosis was calculated, no such effect was detectable, suggesting that the IL-15 effect seen in anti-CD3–stimulated culture was due mainly to inhibition of spontaneous apoptosis in these cultures (Figure 1C).
IL-15 inhibits spontaneous and CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells.
(A) Representative flow cytometry showing Annexin V staining of HIV-specific CD8+ T cells after stimulation of PBMCs with anti-CD3 antibody ± IL-15 and anti-CD95/Fas antibody ± IL-15 for 14 hours. Histograms depict HIV-specific CD8+ T cells gated first for lymphocytes by forward scatter (FSC) and side scatter (SSC) and then for HIV-specific CD8+ T cells by tetramer and CD8 staining. (B) Pooled data showing Annexin V binding of HIV-specific CD8+ T cells after stimulation of PBMCs with anti-CD3 antibody ± IL-15 and anti-CD95/Fas antibody ± IL-15 for 14 hours (n = 10). Horizontal lines depict means. (C) Percentage of treatment-specific apoptosis shown for CD3- and CD95/Fas-induced apoptosis in HIV-specific CD8+ T cells (n = 10). For treatment-specific apoptosis calculation see “Patients, materials, and methods.” Bars depict means ± standard errors. The P values were calculated by using Student t test for paired samples.
IL-15 inhibits spontaneous and CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells.
(A) Representative flow cytometry showing Annexin V staining of HIV-specific CD8+ T cells after stimulation of PBMCs with anti-CD3 antibody ± IL-15 and anti-CD95/Fas antibody ± IL-15 for 14 hours. Histograms depict HIV-specific CD8+ T cells gated first for lymphocytes by forward scatter (FSC) and side scatter (SSC) and then for HIV-specific CD8+ T cells by tetramer and CD8 staining. (B) Pooled data showing Annexin V binding of HIV-specific CD8+ T cells after stimulation of PBMCs with anti-CD3 antibody ± IL-15 and anti-CD95/Fas antibody ± IL-15 for 14 hours (n = 10). Horizontal lines depict means. (C) Percentage of treatment-specific apoptosis shown for CD3- and CD95/Fas-induced apoptosis in HIV-specific CD8+ T cells (n = 10). For treatment-specific apoptosis calculation see “Patients, materials, and methods.” Bars depict means ± standard errors. The P values were calculated by using Student t test for paired samples.
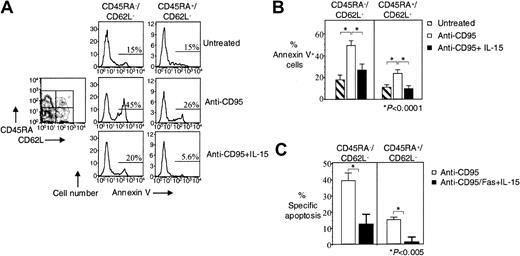
Previous studies have shown that HIV-specific CD8+ T cells lack the terminally differentiated CD45RA+CD62L− effector memory phenotype,3,4 and we have proposed that increased CD95/Fas-induced apoptosis of CD45RA−CD62L−and CD45RA+CD62L− HIV-specific CD8+ T cells may be responsible for this skewing.3 We, therefore, examined whether IL-15 can inhibit the apoptosis of these effector cells. In HIV-specific CD8+ T cells, IL-15 reduced CD95/Fas-induced apoptosis by 45% from 49% ± 5.1% to 27% ± 4.8% in the CD45RA−CD62L− effector memory subpopulation (n = 8, P < .0001) and by 58% from 24% ± 2.4% to 10% ± 1.9% in the CD45RA+CD62L− effector memory subpopulations (n = 8, P < .0001) (Figure2A-B). Treatment-specific apoptosis revealed the same significant reduction in CD95/Fas-induced apoptosis for both effector memory subpopulations in HIV-specific CD8+ T cells. IL-15 reduced specific CD95/Fas-induced apoptosis by 69% from 39% ± 4.6% to 12% ± 6.3% in the CD45RA−CD62L− effector memory population (n = 8, P < .005) and by 93% from 15% ± 1.6% to 1.0% ± 3.0% in the CD45RA+CD62L− effector memory population (n = 8, P < .005) (Figure 2C).
IL-15 decreases CD95/Fas-induced apoptosis of effector memory HIV-specific CD8+ T cells.
(A) Representative flow cytometry showing Annexin V staining of effector memory HIV-specific CD8+ T cells after stimulation of PBMCs with anti-CD95/Fas antibody ± IL-15 for 14 hours. Histograms show effector memory subpopulations of HIV-specific CD8+ T cells. Cells were gated for lymphocytes by FSC and SSC, for tetramer-specific CD8+ T cells by tetramer staining, and then based on CD45RA and CD62L expression. (B) Pooled data showing Annexin V binding of HIV-specific CD8+ T cells after stimulation of PBMCs with anti-CD95/Fas antibody ± IL-15 for 14 hours (n = 10). (C) Percentage of treatment-specific apoptosis shown for CD45RA−CD62L− and CD45RA+CD62L− effector memory HIV-specific CD8+ T cells (n = 10). For treatment-specific apoptosis calculation see “Patients, materials, and methods.” Bars depict means ± standard errors. The P values were calculated by using Student t test for paired samples.
IL-15 decreases CD95/Fas-induced apoptosis of effector memory HIV-specific CD8+ T cells.
(A) Representative flow cytometry showing Annexin V staining of effector memory HIV-specific CD8+ T cells after stimulation of PBMCs with anti-CD95/Fas antibody ± IL-15 for 14 hours. Histograms show effector memory subpopulations of HIV-specific CD8+ T cells. Cells were gated for lymphocytes by FSC and SSC, for tetramer-specific CD8+ T cells by tetramer staining, and then based on CD45RA and CD62L expression. (B) Pooled data showing Annexin V binding of HIV-specific CD8+ T cells after stimulation of PBMCs with anti-CD95/Fas antibody ± IL-15 for 14 hours (n = 10). (C) Percentage of treatment-specific apoptosis shown for CD45RA−CD62L− and CD45RA+CD62L− effector memory HIV-specific CD8+ T cells (n = 10). For treatment-specific apoptosis calculation see “Patients, materials, and methods.” Bars depict means ± standard errors. The P values were calculated by using Student t test for paired samples.
IL-15 enhances long-term survival of CD8+ T cells and HIV-specific CD8+ T cells
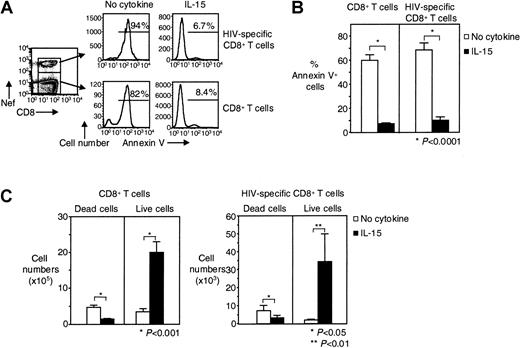
We then examined whether IL-15 can increase the in vitro survival of HIV-specific CD8+ T cells. We investigated the effect of IL-15 on purified CD8+ T cells from HIV-infected individuals that were cultured for 7 days in the presence or absence of IL-15. Without IL-15, spontaneous apoptosis was detected in 60% ± 4.8% of CD8+ T cells from HIV-infected individuals and in 69% ± 5.8% of HIV-specific CD8+ T cells. This apoptosis was significantly reduced to 7.3% ± 0.8% for total CD8+ T cells (88% inhibition) and to 10% ± 2.7% for HIV-specific CD8+ T cells (86% inhibition) when IL-15 was added to the culture (n = 8, P < .0001 for both) (Figure 3A-B). The same significant reduction in apoptosis was seen, when, instead of percentage of apoptosis, the absolute number of dead cells (Annexin V+) was calculated (Figure 3C). The absolute number of live cells significantly increased in the presence of IL-15 for both total CD8+ T cells (from 3.4 ± 0.9 × 105 to 20 ± 3.0 × 105 in the presence of IL-15, n = 8,P < .001) and HIV-specific CD8+ T cells (from 2.0 ± 0.5 × 103 to 34 ± 16 × 103 in the presence of IL-15, n = 8, P < .01) (Figure 3C). Given that 7.1 ± 0.5 × 105 CD8+ T cells and 14 ± 6.4 × 103 HIV-specific CD8+ T cells were put in culture, our findings suggest that IL-15 induced the proliferation of these cells in addition to inhibiting death. Spontaneous apoptosis was also inhibited when PBMCs instead of purified CD8+ T cells were cultured for 7 days in the presence of IL-15 (data not shown).
Long-term survival of HIV-specific CD8+ T cells is augmented by IL-15.
(A) Representative flow cytometry showing Annexin V staining of purified CD8+ T cells and HIV-specific CD8+ T cells after cultivation for 7 days ± IL-15. Histograms depict HIV-specific CD8+ T cells gated on tetramer and CD8 stains, CD8+ T cells gated on CD8 staining. (B) Pooled data showing percentages of dead (Annexin V+) CD8+ T cells and HIV-specific CD8+ T cells after 7 days in culture ± IL-15 (n = 8). (C) Pooled data showing absolute numbers of dead (Annexin V+) and live (Annexin V−) CD8+ and HIV-specific CD8+ T cells after 7 days in culture ± IL-15 (n = 8). Bars depict means ± standard errors. The P values were calculated by using Student t test for paired samples.
Long-term survival of HIV-specific CD8+ T cells is augmented by IL-15.
(A) Representative flow cytometry showing Annexin V staining of purified CD8+ T cells and HIV-specific CD8+ T cells after cultivation for 7 days ± IL-15. Histograms depict HIV-specific CD8+ T cells gated on tetramer and CD8 stains, CD8+ T cells gated on CD8 staining. (B) Pooled data showing percentages of dead (Annexin V+) CD8+ T cells and HIV-specific CD8+ T cells after 7 days in culture ± IL-15 (n = 8). (C) Pooled data showing absolute numbers of dead (Annexin V+) and live (Annexin V−) CD8+ and HIV-specific CD8+ T cells after 7 days in culture ± IL-15 (n = 8). Bars depict means ± standard errors. The P values were calculated by using Student t test for paired samples.
IL-15 augments CD3-induced and antigen-specific activation of HIV-specific CD8+ T cells
To determine whether IL-15 can enhance the activation of HIV-specific CD8+ T cells, PBMCs from HIV-infected individuals were stimulated in the presence or absence of 5 ng/mL IL-15 with either anti-CD3 antibody or HIV-specific tetramers, and the expression of the activation marker CD69 was measured. Stimulation with IL-15 alone for 14 hours significantly enhanced the percentage of CD69+ cells on HIV-specific CD8+ T cells. Although 3.7% ± 3.2% of unstimulated HIV-specific CD8+T cells were CD69+, this percentage increased to 17% ± 7.4% after the addition of IL-15 (n = 8,P < .01) (Figure 4A-B). CD3-induced activation was enhanced in the presence of IL-15 from 32% ± 10% to 65% ± 6.9% CD69+ HIV-specific CD8+ T cells (n = 8, P < .01) (Figure4A,B).
IL-15 increases anti-CD3–induced and antigen-specific activation of HIV-specific CD8+ T cells.
(A) Representative flow cytometry from one HIV-infected individual showing CD69 expression on Gag-specific CD8+ T cells after stimulation of PBMCs with anti-CD3 ± IL-15 for 14 hours. FACS plots show CD8+ T cells that were gated for lymphocytes by FSC and SSC and then for CD8+ T cells by CD8 staining. (B) Pooled data showing CD69 expression on HIV-specific CD8+ T cells from HIV-infected individuals after stimulation of PBMCs with anti-CD3 ± IL-15 for 14 hours (n = 8). Bars depict means ± standard errors. (C) Representative flow cytometry from one HIV-infected individual showing CD69 expression on Gag-specific CD8+ T cells after stimulation of PBMCs with Gag-specific tetramer ± IL-15 for 3 hours. FACS plots show CD8+ T cells first gated for lymphocytes by FSC and SSC and then by CD8 staining. (D) Pooled data showing CD69 expression on HIV-specific CD8+ T cells from HIV-infected individuals after stimulation of PBMCs with HIV-specific tetramer ± IL-15 for 3 hours (n = 5). Bars depict means ± standard errors. The P values were calculated by using nonparametric Wilcoxon signed rank test for paired samples for the graph in Panel B and Student t test for paired sample for the graph in Panel D.
IL-15 increases anti-CD3–induced and antigen-specific activation of HIV-specific CD8+ T cells.
(A) Representative flow cytometry from one HIV-infected individual showing CD69 expression on Gag-specific CD8+ T cells after stimulation of PBMCs with anti-CD3 ± IL-15 for 14 hours. FACS plots show CD8+ T cells that were gated for lymphocytes by FSC and SSC and then for CD8+ T cells by CD8 staining. (B) Pooled data showing CD69 expression on HIV-specific CD8+ T cells from HIV-infected individuals after stimulation of PBMCs with anti-CD3 ± IL-15 for 14 hours (n = 8). Bars depict means ± standard errors. (C) Representative flow cytometry from one HIV-infected individual showing CD69 expression on Gag-specific CD8+ T cells after stimulation of PBMCs with Gag-specific tetramer ± IL-15 for 3 hours. FACS plots show CD8+ T cells first gated for lymphocytes by FSC and SSC and then by CD8 staining. (D) Pooled data showing CD69 expression on HIV-specific CD8+ T cells from HIV-infected individuals after stimulation of PBMCs with HIV-specific tetramer ± IL-15 for 3 hours (n = 5). Bars depict means ± standard errors. The P values were calculated by using nonparametric Wilcoxon signed rank test for paired samples for the graph in Panel B and Student t test for paired sample for the graph in Panel D.
We next examined whether IL-15 could also enhance activation induced by antigen-specific stimulation of HIV-specific CD8+ T cells. As with the 14-hour treatments above, IL-15 alone for 3 hours increased CD69 expression from 1% ± 0.2% for unstimulated HIV-specific CD8+ T cells to 11% ± 8.6% after IL-15 treatment (n = 5); however, this increase was not significant (Figure 4C-D). Although stimulation of PBMCs with HIV-specific tetramer for 3 hours induced activation in only 6.0% ± 0.8% of HIV-specific CD8+ T cells (n = 5), IL-15 treatment significantly increased the percentage of HIV-specific tetramer-activated cells by more than 5-fold to 34% ± 3.4% (n = 5, P < .005) (Figure 4C-D).
IL-15 enhances peptide-induced intracellular IFNγ staining of HIV-specific CD8+ T cells
To investigate whether IL-15 could enhance the effector function of HIV-specific CD8+ T cells, PBMCs from HIV-infected individuals were stimulated with HIV-specific peptide in the presence or absence of IL-15, and then IFNγ production was determined by intracellular staining. In HIV-infected individuals, the frequency of HIV-specific CD8+ T cells measured by tetramer stain was 1.3% ± 0.4% (n = 5). Following stimulation with HIV-specific peptide, 1.0% ± 0.3% of CD8+ T cells produced IFNγ (n = 5). Addition of IL-15 had no effect on the frequency of IFNγ-producing cells, as 1.0% ± 0.4% of CD8+ T cells produced IFNγ after stimulation with HIV-specific peptide in the presence of IL-15 (n = 5) (Figure 5A). IL-15, therefore, does not increase the frequency of IFNγ-producing CD8+ T cells following stimulation with peptide. IL-15 does, however, increase significantly the mean fluorescence intensity (MFI) of IFNγ intracellular stain of peptide-stimulated HIV-specific CD8+ T cells. IL-15 increased the MFI from 54 ± 24 after peptide stimulation alone by 24% to 67 ± 29 (n = 5, P < .05) (Figure 5A).
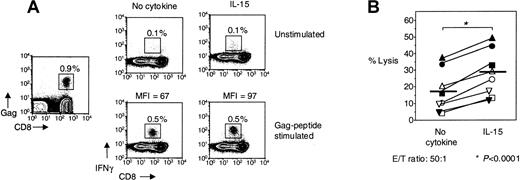
The effector function of HIV-specific CD8+ T cells is enhanced by IL-15.
(A) Representative flow cytometry from 1 HIV-infected individual showing IFNγ production by unstimulated PBMCs, treated with IL-15, Gag-specific peptide, or Gag-specific peptide + IL-15 for 6 hours, respectively. Gag-tetramer stain shown in plot on the left. FACS plots depict lymphocytes gated by FSC and SSC. MFI, mean fluorescence intensity. (B) Cytotoxicity by purified CD8+ T cells from HIV-infected individuals ± IL-15 against HIV-peptide–loaded targets in 6 hours direct ex vivo cytotoxicity assay (n = 8). An effector-to-target ratio of 50:1 is shown. Horizontal lines depict means. The P values were calculated by using Student t test for paired samples.
The effector function of HIV-specific CD8+ T cells is enhanced by IL-15.
(A) Representative flow cytometry from 1 HIV-infected individual showing IFNγ production by unstimulated PBMCs, treated with IL-15, Gag-specific peptide, or Gag-specific peptide + IL-15 for 6 hours, respectively. Gag-tetramer stain shown in plot on the left. FACS plots depict lymphocytes gated by FSC and SSC. MFI, mean fluorescence intensity. (B) Cytotoxicity by purified CD8+ T cells from HIV-infected individuals ± IL-15 against HIV-peptide–loaded targets in 6 hours direct ex vivo cytotoxicity assay (n = 8). An effector-to-target ratio of 50:1 is shown. Horizontal lines depict means. The P values were calculated by using Student t test for paired samples.
IL-15 increases ex vivo cytotoxicity of HIV-specific CD8+ T cells
To determine whether IL-15 can enhance the cytotoxic potential of HIV-specific CD8+ T cells, purified CD8+ T cells from freshly isolated PBMCs of HIV-infected individuals were used in a direct ex vivo cytotoxicity assay. IL-15 enhanced the percentage of target cell lysis in all 8 HIV-infected individuals examined (Figure5B). Whereas 18% ± 4.6% of HIV-peptide–loaded target cells were lysed by purified CD8+ T cells at an effector-to-target ratio of 50:1, this cytotoxicity was significantly enhanced by 60% to 29% ± 5.1% in the presence of IL-15 (n = 8,P < .0001) (Figure 5B). IL-15 affected only slightly nonspecific killing of unloaded target cells (3.6% ± 0.9% lysis with IL-15 compared with 1.5% ± 0.6% lysis without IL-15). This small increase in nonspecific cytotoxicity is probably due to IL-15 directly activating CD8+ T cells in the absence of TCR stimulation, something we have shown here.
Discussion
HIV-specific CD8+ T cells are highly susceptible to CD95/Fas-mediated apoptosis, and this may affect their survival and differentiation and ultimately their function as serial killers.3 As previously shown by our group and others, in HIV-specific CD8+ T cells the CD45RA−CD62L− effector memory subpopulation is increased, whereas the CD45RA+CD62L−effector memory subpopulation is decreased compared with the overall CD8+ T cells and cytomegalovirus (CMV)–specific CD8+ T cells in the same individual.3,4,26This lack of the terminally differentiated CD45RA+CD62L− effector memory phenotype, as we have previously proposed, may be due to increased CD95/Fas-induced apoptosis of CD45RA−CD62L− and CD45RA+CD62L− HIV-specific CD8+ T cells.3 The profound effect of IL-15 on survival of murine CD8+ T cells5,7-13 and on inhibition of apoptosis induced by different mechanisms9,12,22 raises the question whether IL-15 can enhance the survival and function of HIV-specific CD8+ T cells, something that may prove beneficial in HIV-infection, in which CD8+ T cells play a major role in controlling HIV.1,2 We, therefore, studied the effect of IL-15 on the apoptosis of HIV-specific CD8+ T cells and found that IL-15 greatly inhibits spontaneous and CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells, and this inhibition was seen for both effector memory populations. IL-15 had no inhibiting effect on anti-CD3–stimulated apoptosis, which is not increased for HIV-specific CD8+ T cells.3 Furthermore, IL-15 also markedly increased the survival of HIV-specific CD8+ T cells in 7-day cultures. The mechanism by which IL-15 inhibits spontaneous and CD95/Fas-induced apoptosis is not fully understood and may involve up-regulation of Bcl-2 or Bcl-xL expression in CD8+ T cells.12,27,28 Further studies are needed to address this question for HIV-specific CD8+ T cells. The inhibition of apoptosis of HIV-specific CD8+ T cells by IL-15 could presumably affect their effector memory phenotype and restore the skewed phenotype of these cells.3 However, we have not observed any changes in the effector memory phenotype of HIV-specific CD8+ T cells in our 14-hour experiments (Y.M.M. and P.D.K., unpublished observations, 2002), something not surprising if the skewed phenotype is due to in vivo deletion.3
As we show in this study, IL-15 not only enhances the survival of HIV-specific CD8+ T cells but also their activation and most importantly their effector functions such as cytotoxicity. These observations are in agreement with previous studies that have shown that IL-15 augments the proliferation of peptide-stimulated lymphocytes from simian immunodeficiency virus (SIV)–infected rhesus monkeys20 and recall antigen- and HIV-specific antigen-stimulated PBMCs from HIV-infected individuals.16,17 However, in contrast to these previous studies that focused on proliferation, our study directly examined effector function of HIV-specific CD8+ T cells. The effect of IL-15 on the activation of HIV-specific CD8+ T cells, we observed, does not necessarily translate to increased effector function of these cells, as it may only result in increased proliferation of effector cells as CD69 expression strongly correlates with proliferative responses.29,30 However, we find that, although IL-15 did not increase the frequency of IFNγ-producing cells following HIV-specific peptide stimulation, it did increase the mean fluorescence intensity (MFI) of the intracytoplasmic IFNγ stain of these cells by 24%. This finding suggests that IL-15 enhances IFNγ production, as previous studies have shown that MFI of intracytoplasmic IFNγ stain correlates with per cell IFNγ production by antigen-specific cells. This was demonstrated by showing that a decrease of MFI of intracytoplasmic IFNγ staining by 36% correlates with a 50% decrease per cell of IFNγ production.31 Therefore, the 24% increase in IFNγ MFI induced by IL-15 we found, we believe, reflects an increase in IFNγ production by HIV-specific CD8+ T cells. This increase of MFI was observed at 6 hours, 18 hours, and 24 hours, suggesting that it was not simply the result of IL-15 shifting the kinetic of activation and cytokine production forward (Y.M.M. and P.D.K., unpublished observations, 2002). Such increased IFNγ production by CD8+ T cells after treatment of mice with IL-15 has been shown to prolong the survival of mice in a malignant pleurisy model.32 Most importantly, we showed that IL-15 can enhance the cytotoxicity of HIV-specific CD8+ T cells. As previously described, PBMCs of SIV-infected monkeys and HIV-infected individuals cultured for 2 days in the presence of peptide and an additional 4 days in the presence of IL-15 showed enhanced cytotoxicity compared with PBMCs cultured without IL-15.20 However, enhanced cytotoxicity in these experiments was attributed to an increased expansion of peptide-specific CD8+ T cells in the presence of IL-15 rather than a direct increase in cytotoxicity. Our studies examining the effect of IL-15 in a direct ex vivo 6-hour cytotoxicity assay indicate that IL-15 can also directly augment effector function. Although IL-15 can increase granzyme B and perforin in CD8+ T cells,15 this is unlikely the mechanism behind the increased cytotoxicity we observe in our 6-hour assay, as IL-15 enhances granzyme B and perforin only after 48 hours with no effect even at 12 hours.15 We also do not believe that the apoptosis-decreasing effect of IL-15 plays a major role in the increased cytotoxicity seen in the presence of IL-15, because only low percentages of spontaneous apoptosis are seen in our 6-hour assay, and, most importantly, as we show here, activation-induced apoptosis is not inhibited by IL-15. Most likely, cytotoxicity is increased due to enhanced activation of HIV-specific CD8+ T cells, something we show to occur with IL-15 treatment.
Our study shows that IL-15 can potently inhibit the apoptosis of HIV-specific CD8+ T cells, increase their survival in long-term cultures, and augment their effector functions. The above findings suggest that IL-15 treatment may prove useful as a strategy to enhance CD8+ T-cell responses against viruses such as HIV by its ability to enhance the survival and effector function of HIV-specific CD8+ T cells and thus augment their ability to function as serial killers.
We thank Dr M. Gold and the staff of the Partnership Comprehensive Care Practice of the HIV/AIDS Medicine Division of Drexel University for patient recruitment.
Prepublished online as Blood First Edition Paper, September 19, 2002; DOI 10.1182/blood-2002-07-1957.
Supported by grants R01 AI46719 and R01 AI52005 from the National Institutes of Health (P.D.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter D. Katsikis, Department of Microbiology and Immunology, Drexel University College of Medicine, Drexel University, 2900 Queen Ln, Philadelphia, PA 19129; e-mail:katsikis@drexel.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal