Abstract
Low-risk myelodysplastic syndromes (MDS), including refractory anemia and sideroblastic anemia, are characterized by increased apoptotic death of erythroid progenitors. The signaling pathways that elicit this pathologic cell death in MDS have, however, remained unclear. Treatment with erythropoietin in combination with granulocyte colony-stimulating factor (G-CSF) may synergistically improve the anemia in patients with MDS, with a concomitant decrease in the number of apoptotic bone marrow precursors. Moreover, we have previously reported that G-CSF inhibits Fas-induced caspase activation in sideroblastic anemia (RARS). The present data demonstrate that almost 50% of erythroid progenitor cells derived from patients with MDS exhibit spontaneous release of cytochrome c from mitochondria with ensuing activation of caspase-9, whereas normal erythroid progenitors display neither of these features. G-CSF significantly inhibited cytochrome c release and suppressed apoptosis, most noticeably in cells from patients with sideroblastic anemia. Furthermore, inhibition of caspase-9 suppressed both spontaneous and Fas-mediated apoptosis of erythroid progenitors in all low-risk MDS cases studied. We propose that the increased sensitivity of MDS progenitor cells to death receptor stimulation is due to a constitutive activation of the mitochondrial axis of the apoptotic signaling pathway in these cells. These studies yield a mechanistic explanation for the beneficial clinical effects of growth factor administration in patients with MDS, and provide a model for the study of growth factor–mediated suppression of apoptosis in other bone marrow disorders.
Introduction
The myelodysplastic syndromes (MDS) constitute a heterogeneous group of clonal stem cell disorders characterized by ineffective hematopoiesis, various degrees of pancytopenia, and a risk of progression to acute myeloid leukemia.1 Low-risk myelodysplastic syndromes, including refractory anemia (RA) and RA with ringed sideroblasts (RARS), are defined as MDS with a low probability of progression to leukemia and with a relatively favorable outcome.2 Anemia and transfusion dependency are the main clinical problems for these patients.
Increased apoptosis of bone marrow precursors is a hallmark of MDS,3-5 and is thought to underlie the ineffective hematopoiesis evidenced in individuals with MDS. Indeed, apoptosis mediated via the death receptor, Fas, and its ligand has been suggested to serve as an important pathogenic mechanism in MDS.6-9However, a clear-cut correlation between the level of expression of these apoptosis regulators and the degree of bone marrow apoptosis, or cytopenia, has not been demonstrated. In addition to extrinsic, death receptor–mediated induction of apoptosis, intrinsic signaling pathways that depend on mitochondrial events, including the release of apoptogenic factors such as cytochrome c, also exist.10,11 Interestingly, signs of mitochondrial pathology are commonly seen in MDS, including the characteristic accumulation of iron in the mitochondrial matrix of ringed sideroblasts,12 as well as the occurrence of mitochondrial DNA mutations.13 14 However, the potential involvement of these organelles in apoptosis signaling in MDS has not been evaluated previously.
Recombinant hematopoietic growth factors are frequently used in the clinical management of patients with MDS to alleviate anemia and neutropenia. Hence, administration of granulocyte colony-stimulating factor (G-CSF) in combination with erythropoietin (EPO) improves hemoglobin levels in low-risk MDS, with a concomitant decrease in the number of apoptotic bone marrow precursors.4,15-17Furthermore, we have shown that G-CSF can inhibit Fas-mediated apoptosis in RARS, and that G-CSF treatment of CD34+ cells improves erythroid colony growth in patients with RARS with severe anemia.18 However, the mechanism underlying the spontaneous apoptosis of MDS bone marrow progenitors, and the protective effect of G-CSF in these cells, has remained elusive. In the present study, we report for the first time the constitutive mitochondrial release of cytochrome c in MDS progenitor cells, and show that this event is effectively blocked by G-CSF, thus providing a mechanistic explanation for the beneficial clinical effects of this growth factor.
Patients, materials, and methods
Patients and controls
We included 12 patients with RA (5 RA, 7 refractory cytopenia with multilineage dysplasia [RCMD]) and 14 with RARS (8 RARS, 6 RCMD with or without RS) with a median age of 75 years (range, 53-87 years) and 72 years (range, 37-85 years), respectively.19 There were 4 patients with RA/RCMD who carried the 5q− aberration. Since the total number of patients did not allow for a statistical calculation based on the new classification, we hereafter refer only to the French-American-British (FAB) classification. All patients were untreated at the time of the study. The diagnostic procedure was performed according to the criteria defined by the Scandinavian MDS Group.15 Median duration of disease was 18 months (range, 0-180 months). There were 9 patients who were transfusion-dependent, while the rest had stable anemia. Normal bone marrow samples were obtained from 14 healthy volunteers. Informed consent was obtained from both patients and controls, and the study followed the guidelines of the research ethical committee at Karolinska Institutet.
Bone marrow aspiration and MNC cultures
Bone marrow needle aspiration and mononuclear cell (MNC) isolation and cultivation were performed as previously described.18 20 MNCs were cultured in medium alone or in the presence of agonistic anti–Fas antibodies (clone CH-11; 1 μg/mL) (Medical & Biological Laboratories, Nagoya, Japan), the selective caspase-9 inhibitor, LEHD-fmk (20 μM; Enzyme System Products, Livermore, CA), or G-CSF (100 ng/mL) (Neupogen; Amgen, Twelve Oaks, CA). 3H-thymidine incorporation (Amersham Pharmacia, Buckinghamshire, England) was used to measure proliferation in MNC cultures.
CD34+ cell separation and colony formation assays
CD34+ cells were separated from MNCs using the MiniMacs system (Miltenyi Biotec, Bergisch Gladbach, Germany) as previously described.18 20 The purity of CD34+separated cells was assessed by flow cytometry using anti–CD34 monoclonal antibodies (BD Biosciences, San Jose, CA), and was found to be more than 95% in all cases. Phenotype and erythroid maturation were analyzed at days 4, 7, 11, and 14 using CD36 (ImmunoTech, Marseille, France) and GpA (DAKO, Copenhagen, Denmark) antibodies to define immature, early erythroid, and late erythroid cells, respectively, while CD45 was used to define nonerythroid cells.
Erythroblast cultures
To obtain erythroid progenitor cells of a defined stage of differentiation, we slightly modified a previously described method.21 CD34+ cells were cultured (0.1 × 106/mL) for 14 days in Iscove medium (Sigma, St Louis, MO) supplemented with 15% BIT9500 serum substitute (containing bovine serum albumin, bovine pancreatic insulin, and iron-saturated human transferrin; Stem Cell Technologies, Vancouver, BC, Canada) and recombinant human interleukin 3 (rh-IL-3) (10 ng/mL; PeproTech House, London, England), rh-IL-6 (10 ng/mL; PeproTech House), rh-stem cell factor (rh-SCF; 25ng/ml; Medical & Biological Laboratories), 1% penicillin and streptomycin (Invitrogen, Paisley, Scotland), and 1%l-glutamine (Invitrogen). At day 2 and day 4, medium was replenished as above to sustain the culture at the same cell concentration. Erythropoietin (2 IU/mL; Roche, Basel, Switzerland) was added to the medium at day 7, and fresh medium supplemented as above (plus EPO) was added at days 9 and 11. At day 4, 42% of cells were CD34+CD36−, while 24% were CD34+CD36+, and 12% were positive only for CD36 (median values). At day 7, 22% were CD34+CD36−, 14% were CD34+CD36+, 26% CD36+, and 5% CD36+GpA+. The median percentages of CD36+ and GpA+ cells at day 11 were 88% and 61%, respectively, while the corresponding numbers at day 14 were 92% and 77%. As the present study focused on early erythroid maturation, we used mainly cells obtained at 0 to 7 days of culture. In addition, to estimate the presence of ringed sideroblasts, Perls Prussian blue staining was performed on cytospins prepared at the same timepoints. However, very few ringed sideroblasts were observed during the 14 days of erythroblast culture (data not shown).
Caspase activation
Caspase-3–like, caspase-8, and caspase-9 activities in hematopoietic progenitor cells were determined at the indicated timepoints using a continuous fluorometric assay modified from Nicholson et al.22 Details of the assay have been described elsewhere.20 Appropriate peptide substrates (Asp-Glu-Val-Asp aminocoumarin [DEVD-AMC], Ile-Glu-Thr-Asp aminocoumarin [IETD-AMC], and Leu-Glu-His-Asp aminocoumarin [LEHD-AMC], respectively) were obtained from Peptide Institute (Osaka, Japan). Data are depicted as picomols AMC release per minute per 106 cells.
Cytochrome c release
Translocation of cytochrome c from mitochondria to cytosol in hematopoietic stem cells was determined at the indicated timepoints using a previously established method for the concomitant localization of mitochondria and cytochrome c in single cells.23 Briefly, cells were stained with MitoTracker Red CMXRos (Molecular Probes, Eugene, OR), cytocentrifuged onto glass slides, and fixed and permeabilized in paraformaldehyde and Triton X-100. Cells were then stained with an anti–cytochrome cantibody (BD Biosciences), followed by a fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse antibody (Sigma). Images were analyzed on a Leica DM RXA digital confocal microscope, and further processed using the Slide Book 3.0.9.0 analysis software (Intelligent Imaging, Denver, CO).
DNA fragmentation
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL)–based determination of DNA fragmentation was performed according to the manufacturer's instructions (ApopTag; Oncor, Gaithersburg, MD), as previously described.20
Statistical analysis
Statview 5.0 analysis software (SAS Institute, Cary, NC) was used for analysis of quantitative data. Results are presented as mean values plus or minus SD, or as median values plus ranges, when appropriate. Paired and unpaired t tests were used for comparison of related and unrelated samples. Comparison of 3 groups was made by ANOVA (analysis of variance; factorial measures). Pvalues less than .05 were considered statistically significant.
Results
Spontaneous cytochrome c release in MDS progenitors
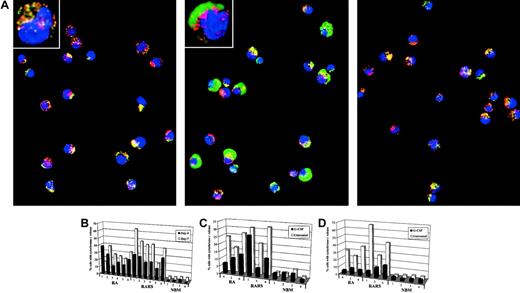
To explore the role of mitochondria in the ineffective erythroid maturation in MDS we measured release of the mitochondrial intermembrane space protein, cytochrome c, in early erythroblasts from 6 patients with RA (2 RA, of which 1 had 5q−, 4 RCMD, of which 3 had 5q−), 6 patients with RARS (4 RARS, 2 RCMD-RS), and 4 normal bone marrows (NBM). Cells were taken from erythroblast cultures at day 4 and day 7 and cytochrome c release was measured by digital confocal microscopy, with concomitant staining for mitochondria using MitoTracker. As seen in Figure1A, cytochrome c release occurred in MDS patient cells, yet was completely absent in NBM. Cytochrome c release was markedly increased in MDS cells at day 4 (median, 23%; range, 12%-39%) compared with NBM (median, 1.5%; range, 1%-4%), and increased further at day 7 (median, 35%; range, 15%-64%) (NBM median, 4.5%; range, 4%-5%) (Figure1B). The increase at day 7 was more pronounced in the RARS cultures (median, 45%; range, 20%-64%). Of note, translocation of cytochrome c was evident in early (day 4) progenitor cells that still retained the CD34 antigen, and occurred in the absence of morphologic signs of mitochondrial iron accumulation (data not shown). Moreover, DNA fragmentation, as assessed by TUNEL, was undetectable at this stage (data not shown), thus indicating that the release of cytochrome c is an early event that precedes nuclear apoptotic changes.
Spontaneous mitochondrial release of cytochrome
c in MDS progenitors: inhibition by G-CSF. (A) Mitochondria were stained with MitoTracker (red fluorescence), and cytochrome c localization was revealed by indirect immunofluorescence (green fluorescence). The punctate yellow pattern denotes mitochondrial localization of cytochrome c, and the diffuse green pattern indicates cytochrome c that has been released into the cytosol. Data are derived from normal bone marrow (NBM; left panel), RARS progenitors (middle panel), and RARS progenitors incubated in the presence of G-CSF (100 ng/mL; right panel), at day 7 of culture. Original magnification × 40; insets, × 100. (B) Release of cytochrome c from mitochondria of erythroid progenitors at day 4 and day 7, as determined by digital confocal microscopy. For each position, 500 nucleated cells were counted and each bar represents a single patient. Samples were obtained from patients with RA and patients with RARS, respectively, as well as from NBM. Each group could be further subdivided according to the new World Health Organization (WHO) classification. Hence, there were 2 patients with RA, one of whom carried the 5q− aberration, 4 patients with RCMD, 3 of whom had the 5q− aberration, 4 patients with RARS, and 2 with RCMD-RS. (C) Cytochrome c release in CD34+ (day 4) erythroid precursors incubated in the presence or absence of G-CSF (100 ng/mL). (D) Experimental procedure same as in panel C, for CD36+ (day 7) erythroid progenitors.
Spontaneous mitochondrial release of cytochrome
c in MDS progenitors: inhibition by G-CSF. (A) Mitochondria were stained with MitoTracker (red fluorescence), and cytochrome c localization was revealed by indirect immunofluorescence (green fluorescence). The punctate yellow pattern denotes mitochondrial localization of cytochrome c, and the diffuse green pattern indicates cytochrome c that has been released into the cytosol. Data are derived from normal bone marrow (NBM; left panel), RARS progenitors (middle panel), and RARS progenitors incubated in the presence of G-CSF (100 ng/mL; right panel), at day 7 of culture. Original magnification × 40; insets, × 100. (B) Release of cytochrome c from mitochondria of erythroid progenitors at day 4 and day 7, as determined by digital confocal microscopy. For each position, 500 nucleated cells were counted and each bar represents a single patient. Samples were obtained from patients with RA and patients with RARS, respectively, as well as from NBM. Each group could be further subdivided according to the new World Health Organization (WHO) classification. Hence, there were 2 patients with RA, one of whom carried the 5q− aberration, 4 patients with RCMD, 3 of whom had the 5q− aberration, 4 patients with RARS, and 2 with RCMD-RS. (C) Cytochrome c release in CD34+ (day 4) erythroid precursors incubated in the presence or absence of G-CSF (100 ng/mL). (D) Experimental procedure same as in panel C, for CD36+ (day 7) erythroid progenitors.
Mitochondria-dependent signaling in MDS progenitors
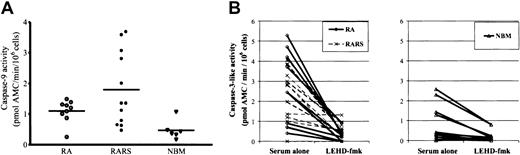
Pro–caspase-9 is activated in a cytochromec–dependent manner downstream of mitochondria, and active caspase-9, in turn, cleaves and activates caspase-3.24 To further explore the importance of mitochondrial signaling, we measured caspase-9 activation in MNCs from 9 patients with RA, 12 with RARS, and 6 with NBM. After 18 hours of in vitro culture, spontaneous caspase-9 activity, determined by the hydrolysis of the specific peptide substrate, LEHD-AMC, was almost undetectable in NBM (0.47 ± 0.31 pM AMC/min/105 cells), moderately elevated in RA (1.10 ± 0.37 pM AMC/min/105 cells) and clearly elevated in RARS (1.80 ± 1.20 pM AMC/min/105 cells) (Figure2A). The difference was significant between RARS and NBM (P = .005), but not between RA and NBM or between RARS and RA. Moreover, spontaneous caspase-3–like activity, determined by DEVD-AMC cleavage, was higher in RARS as compared with NBM (Figure 2B), thus corroborating our previous observations.20 Similar results were now obtained in RA cultures. Hence, in freshly isolated MNCs, DEVDase activity was 5- to 7-fold higher in RARS (1.19 ± 0.96 pM AMC/min/105 cells) and in RA (0.92 ± 0.89 pM AMC/min/105 cells), as compared with NBM (0.18 ± 0.22 pM AMC/min/105 cells) (RARS vs NBM, P = .005 and RA vs NBM,P = .04) (Figure 2B).
Central role of caspase-9 in MDS bone marrow cell apoptosis.
(A) Spontaneous caspase-9 activity was determined in MNCs derived from RA, RARS, and NBM at 18 hours of in vitro culture. Caspase-9 activity was significantly elevated in RARS (P < .05). Elevated levels of caspase-9 activity were also apparent in RARS cells at 8 hours of incubation (data not shown). The difference between RA and NBM was not statistically significant (P = .17). (B) Cells from 22 patients and 10 controls were incubated for 4 hours in the presence or absence of the selective caspase-9 inhibitor, LEHD-fmk (20 μM). Caspase-9 inhibition significantly decreased caspase-3–like activity in both RA and RARS (P < .0001). A similar inhibition of caspase-3–like activity was observed in NBM cultures (P < .05).
Central role of caspase-9 in MDS bone marrow cell apoptosis.
(A) Spontaneous caspase-9 activity was determined in MNCs derived from RA, RARS, and NBM at 18 hours of in vitro culture. Caspase-9 activity was significantly elevated in RARS (P < .05). Elevated levels of caspase-9 activity were also apparent in RARS cells at 8 hours of incubation (data not shown). The difference between RA and NBM was not statistically significant (P = .17). (B) Cells from 22 patients and 10 controls were incubated for 4 hours in the presence or absence of the selective caspase-9 inhibitor, LEHD-fmk (20 μM). Caspase-9 inhibition significantly decreased caspase-3–like activity in both RA and RARS (P < .0001). A similar inhibition of caspase-3–like activity was observed in NBM cultures (P < .05).
We then incubated myelodysplastic and control MNCs in the presence of the irreversible inhibitor of caspase-9, LEHD-fmk (20 μM). A significant reduction of spontaneous caspase-3–like enzyme activation was seen in MDS cultures (P < .0001), as well as in cultures from NBM (P = .02). No significant differences were observed between RA and RARS cultures (Figure 2B). Importantly, LEHD-fmk failed to block Fas-induced cleavage of IETD-AMC, indicative of caspase-8 activity, thus providing evidence for the specificity of this inhibitor (data not shown). These data underscore the importance of mitochondria-dependent signaling for spontaneous apoptosis of bone marrow progenitor cells.
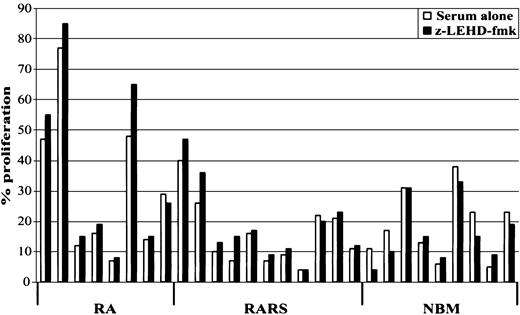
We next investigated whether spontaneous caspase-9 activation negatively affects stem cell proliferation in MDS. For this purpose, myelodysplastic and normal MNCs were cultured in the presence or absence of LEHD-fmk, and proliferation was estimated after 24 hours of incubation. As shown in Figure 3, proliferation increased in 7 out of 8 patients with RA (mean increase 14.7% ± 13%; P = .07), and in 9 out of 11 patients with RARS (mean increase 24.3% ± 33%; P = .02). No significant changes were evident in NBM cultures.
Caspase-9 inhibition promotes hematopoietic cell proliferation in MDS.
MNCs derived from RA, RARS, and NBM were incubated in the presence or absence of the selective caspase-9 inhibitor, LEHD-fmk (20 μM), for 24 hours and cell proliferation was determined by3H-thymidine incorporation. Increased cell proliferation was observed in the majority of RA and RARS cultures, but not in NBM cultures. Data are expressed as means of 3 independent experiments.
Caspase-9 inhibition promotes hematopoietic cell proliferation in MDS.
MNCs derived from RA, RARS, and NBM were incubated in the presence or absence of the selective caspase-9 inhibitor, LEHD-fmk (20 μM), for 24 hours and cell proliferation was determined by3H-thymidine incorporation. Increased cell proliferation was observed in the majority of RA and RARS cultures, but not in NBM cultures. Data are expressed as means of 3 independent experiments.
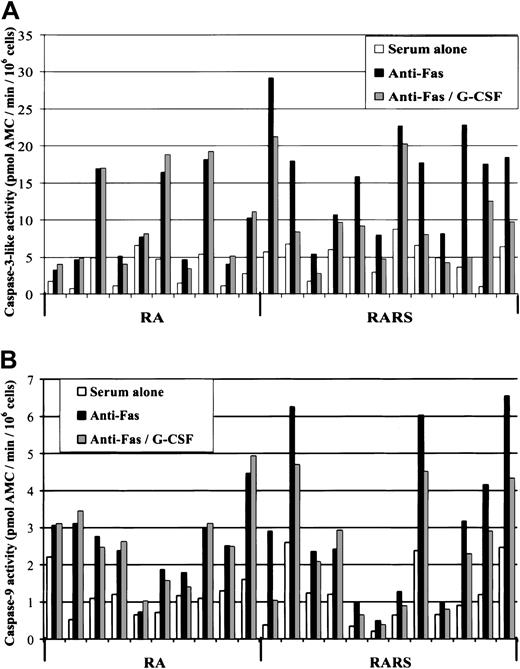
Fas-triggered mitochondrial signaling in MDS
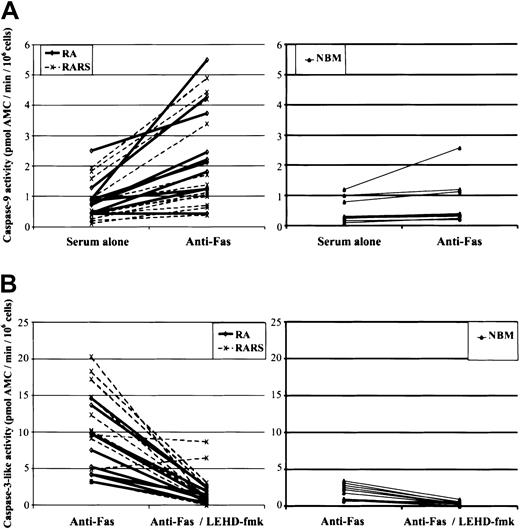
We previously showed that low-risk MDS progenitor cells are hypersensitive to Fas ligation.18 To gauge the role of mitochondria in Fas-mediated apoptosis, we assessed caspase-3–like, caspase-8, and caspase-9 activities in MNCs obtained from 10 patients with RA, 12 with RARS, and 10 with NBM. As expected, agonistic anti–Fas antibodies (1 μg/mL) triggered caspase-8, the apical caspase in the death receptor–initiated proteolytic cascade, in both MDS (P < .0001) and NBM cultures (P = .04). Fas ligation also induced caspase-3–like activity in RARS (mean increase 9.3 ± 5; P < .0001) and RA (5.1 ± 2.9;P = .0004) as well as in NBM (1.0 ± 0.7;P = .001). Furthermore, a significant increase in the activation of caspase-9 was seen in RARS (mean increase 1.26 ± 1.06;P = .002) and in RA (1.59 ± 1.32;P = .004) but not in NBM (0.25 ± 0.41;P = .09) (Figure 4A). Importantly, LEHD-fmk significantly reduced caspase-3–like activity in Fas-triggered RARS and RA cells (P = .0003 for both groups) (Figure 4B). Taken together, these data serve as evidence for the involvement of the mitochondrial axis in death receptor (ie, Fas)–induced apoptosis of MDS progenitors.
Fas-triggered mitochondria-dependent signaling in MDS.
(A) MNCs obtained from 10 patients with RA, 12 with RARS, and 10 with NBM were subjected to anti–Fas antibody treatment (1 μg/mL) for 4 hours, and caspase-9 activation was then evaluated. Fas ligation induced a significant increase in caspase-9 activity in RARS (P = .002) and in RA (P = .004), but not in NBM cells (P = .09). (B) Caspase-3–like activity was determined in MNCs from 10 patients with RA, 12 with RARS, and 10 with NBM incubated for 4 hours in the presence or absence of LEHD-fmk (20 μM). Fas-induced caspase-3–like activity was more prominent in RARS (P = .002) and in RA (P < .05) as compared with NBM, and this activity was suppressed by the inhibition of caspase-9 (P = .003) in both subgroups of MDS. Each line represents data obtained from one patient.
Fas-triggered mitochondria-dependent signaling in MDS.
(A) MNCs obtained from 10 patients with RA, 12 with RARS, and 10 with NBM were subjected to anti–Fas antibody treatment (1 μg/mL) for 4 hours, and caspase-9 activation was then evaluated. Fas ligation induced a significant increase in caspase-9 activity in RARS (P = .002) and in RA (P = .004), but not in NBM cells (P = .09). (B) Caspase-3–like activity was determined in MNCs from 10 patients with RA, 12 with RARS, and 10 with NBM incubated for 4 hours in the presence or absence of LEHD-fmk (20 μM). Fas-induced caspase-3–like activity was more prominent in RARS (P = .002) and in RA (P < .05) as compared with NBM, and this activity was suppressed by the inhibition of caspase-9 (P = .003) in both subgroups of MDS. Each line represents data obtained from one patient.
G-CSF prevents spontaneous and Fas-induced apoptosis in MDS
Having established the involvement of mitochondria in both spontaneous and Fas-triggered apoptosis of MDS progenitors, we next analyzed the response of these cells to the recombinant human growth factor, G-CSF. G-CSF (100 ng/mL) significantly reduced apoptosis, determined by the degree of caspase-3–like activity at 4 hours, in Fas-triggered MNCs derived from patients with RARS (P = .004), but not in RA cells (P = .16). After prolonged incubation (8 hours), the antiapoptotic effect of G-CSF was further enhanced in RARS (P = .0005), but this effect remained absent in the RA samples (Figure5A). G-CSF did not significantly affect apoptosis in NBM cultures (data not shown). Similar results were obtained when cells were probed for Fas-induced caspase-8 activity. Hence, G-CSF significantly reduced IETD-AMC cleavage in RARS (4 hours,P = .002 and 8 hours, P = .0002), while no effect was observed in RA or in NBM. Finally, G-CSF significantly reduced the activation of caspase-9, as evidenced by LEHD-AMC cleavage, in RARS cultures (8 hours, P = .005), whereas no effect was observed in RA cultures (Figure 5B).
G-CSF blocks Fas-induced caspase activation in MDS bone marrow cells.
(A) MNCs from patients with RA and patients with RARS were incubated for 8 hours in the presence or absence of G-CSF (100 ng/mL) and subsequently assessed for caspase-3–like activity. This caspase activation was diminished by G-CSF in RARS (P = .0005), while no significant effect was observed in RA (P = .21). G-CSF did not influence caspase-3–like activity in NBM cultures (data not shown). (B) Cells incubated as above were also assessed for caspase-9 activity. A significant inhibitory effect of G-CSF was seen in patients with RARS (P = .005), but not in patients with RA.
G-CSF blocks Fas-induced caspase activation in MDS bone marrow cells.
(A) MNCs from patients with RA and patients with RARS were incubated for 8 hours in the presence or absence of G-CSF (100 ng/mL) and subsequently assessed for caspase-3–like activity. This caspase activation was diminished by G-CSF in RARS (P = .0005), while no significant effect was observed in RA (P = .21). G-CSF did not influence caspase-3–like activity in NBM cultures (data not shown). (B) Cells incubated as above were also assessed for caspase-9 activity. A significant inhibitory effect of G-CSF was seen in patients with RARS (P = .005), but not in patients with RA.
In view of the potent inhibition of caspase-9 activity by G-CSF, we then investigated whether G-CSF could prevent cytochromec release. Indeed, addition of the growth factor drastically inhibited the translocation of cytochrome c in both RA and RARS cultures, at day 4 (early erythroid differentiation) as well as at day 7 (intermediate stage erythroblasts) (Figure 1A,C-D). These data thus indicate that G-CSF exerts its protective effect in MDS progenitors at the level of, or upstream of, mitochondrial release of cytochrome c, with subsequent inhibition of downstream caspases.
Discussion
Mitochondria are key regulators of apoptosis,10 but the role of these organelles in MDS progenitor cell apoptosis has not been assessed before. In the present study we demonstrate the spontaneous translocation of cytochrome c from mitochondria to cytosol in MDS hematopoietic progenitor cells, and we show that this redistribution of cytochrome c can be prevented by G-CSF. Our previous studies have shown that treatment with EPO alone or in combination with G-CSF yields erythroid improvement in patients with MDS.25 Furthermore, G-CSF is particularly useful in the RARS subgroup, with 50% of patients responding to the combination of growth factors versus only about 10% to EPO alone.15 The current data demonstrate that G-CSF protects against cytochromec release in early progenitor cells from both sideroblastic and refractory anemia patients. However, the antiapoptotic effect of G-CSF in mononuclear bone marrow cells was significantly more pronounced in patients with RARS, and these findings thus serve as a tentative explanation for the utility of this growth factor in the clinical management of patients with MDS.
RARS cells are hypersensitive to ligation of the death receptor, Fas. However, we have shown that inhibitors of Fas signaling fail to prevent spontaneous apoptosis of MDS progenitor cells,20suggesting that Fas-initiated apoptosis may not be the sole cause of the aberrant apoptosis in RARS. Strong inhibition of spontaneous apoptosis by antagonistic anti–Fas antibodies was observed in one patient with RARS in that study, but we later discovered that the profound anemia at the time of the experiment was due most likely to pneumonia and an activated rheumatoid arthritis, and that the RARS only produced a moderate, non–transfusion-dependent anemia. A recent report verified the increased Fas-sensitivity of CD34+-derived erythroid progenitors in MDS, and showed that an antagonistic Fas-Fc chimeric protein was able to reduce spontaneous apoptosis of these cells.26 However, the MDS subgroup(s) analyzed in that specific study was not disclosed. Thus, it remains possible that Fas-triggering is central in secondary anemia,20,27 and in some, but not all subgroups of MDS.26 Nevertheless, the current data show that inhibition of caspase-9 completely abrogated Fas-triggered activation of caspase-3, thus providing strong evidence that the Fas signal is amplified by, or mediated via, mitochondria in MDS progenitor cells. These findings concur with the previous demonstration of mitochondrial amplification of death receptor signaling both in vitro and in vivo.28-30 Moreover, the existence of mitochondria-dependent signaling pathways may serve to explain the sensitivity of certain cell types to Bcl-2–mediated inhibition of Fas-induced cell death.31
We have provided evidence that erythroid apoptosis in MDS is initiated at the stem cell level, since inhibition of caspase-3, an effector caspase, promoted erythroid colony growth in CD34+ cells derived from patients with MDS.20 In line with these findings, caspase-3 activation was detected in mononuclear bone marrow cells from both RA and RARS patients in the present study. Moreover, striking differences were found between myelodysplastic patients and controls when the percentage of early erythroid progenitors displaying cytochrome c release was analyzed. Hence, cytochromec release was evident in approximately 50% of the cells in the MDS cultures, while almost no release of cytochrome ccould be seen in normal controls. In RA, translocation of cytochromec was similar in the early stage erythroblasts at day 4 and in the intermediate stage erythroblasts at day 7. In RARS, however, the percentage of cells with cytochrome c release increased from day 4 to day 7, which may correspond to the higher degree of spontaneous caspase-9 activation in the more mature mononuclear cells in this subgroup of patients. We also found that inhibition of caspase-9 reduced the degree of spontaneous caspase-3 activation, and restored proliferation in MDS cultures. Taken together, these findings indicate a critical role for mitochondrial signaling in the spontaneous apoptotic process in low-risk MDS.
A classical observation in RARS is the perinuclear clustering of iron-overloaded mitochondria in so-called ringed sideroblasts (ie, erythroblasts).32,33 In view of the present findings, it appears reasonable to speculate that this pathologic pattern of iron distribution could promote the constitutive release of cytochromec. However, such iron-induced damage would have to occur before iron accumulation is morphologically visible, as very few ringed sideroblasts developed during the 14 days of erythroblast culture in the present study. Alternatively, cytochrome c release may be an early sign of a process that eventually leads to a defect transportation and/or incorporation of iron into hemoglobin. Indeed, previous work has suggested that the key problem in RARS is not a defect in hemoglobin synthesis, but rather a defect involving mitochondrial iron transport.34 In addition, we also observed cytochrome c release in RA cultures, including a conspicuous mitochondrial discharge of cytochrome c in cells from a patient with 5q− syndrome. Since iron-overloaded mitochondria are not usually seen in this subgroup of MDS, these findings support the view that cytochrome c release is a primary event which in RARS precedes iron accumulation, and in RA mediates erythroid apoptosis by a different pathway, not associated with iron-overloading. A multistep process underlying the final myelodysplastic phenotype may also explain the differential reactions of RA and RARS mononuclear cells to G-CSF.
It is well known that hematopoietic precursor cells undergo apoptosis upon withdrawal of the relevant CSF.35 Moreover, G-CSF has been shown to block constitutive apoptosis of peripheral blood neutrophils obtained from healthy controls.36,37 More recent studies have disclosed that G-CSF down-regulates Bax expression,38 or alternatively, that G-CSF prevents translocation of Bax to mitochondria in mature neutrophils.39 Similarily, Dewson et al40 have shown that IL-5 prevents the redistribution of Bax and the release of cytochrome c in eosinophils undergoing spontaneous apoptosis. Finally, Villunger et al41 have provided evidence that G-CSF promotes survival of neutrophils largely through a Bcl-2–controlled pathway, that is, through a pathway regulated by the mitochondrion and its associated factors. The latter observations are thus in line with our finding that G-CSF acts at the level of, or upstream of, mitochondrial release of cytochrome c in MDS progenitor cells. The present study is, to the best of our knowledge, the first to provide a potential mechanistic explanation for the antiapoptotic effect of G-CSF in hematopoietic stem cells.
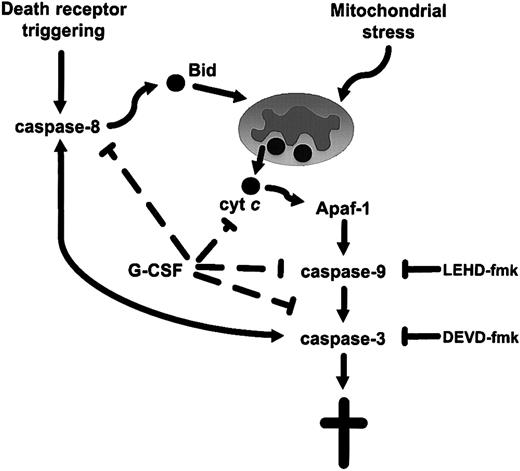
To conclude, we provide evidence for intrinsic or mitochondria-dependent apoptosis in MDS progenitor cells, and show that this aberrant cell death is reversed by G-CSF. As mentioned above, previous studies of MDS cells have documented an increased sensitivity to ligation of death receptors, including Fas and the tumor necrosis factor receptor (TNFR).7,9,42 Indeed, recent studies have demonstrated an increased sensitivity to tumor necrosis–related apoptosis-inducing ligand (TRAIL)/APO-2 ligand-mediated apoptosis in MDS, but not in normal bone marrow.43 44 The current data suggest that this increased susceptibility to apoptosis is caused by downstream events sensitizing cells to the receptor-mediated signal. We thus propose a model for apoptosis in low-risk MDS in which the increased sensitivity to ligation of death receptors is dependent on constitutive mitochondrial signaling with ensuing cytochrome c release and downstream activation of caspase-9 (Figure 6). The pathogenetic mechanism underlying this hyperactivation of MDS mitochondria merits further investigation, and such studies may ultimately provide a key to future, antiapoptotic therapeutic approaches in MDS. The present study may also serve as a paradigm for the dissection of stem cell apoptosis and growth factor–mediated survival of progenitor cells in other human bone marrow pathologies.
A schematic model of apoptosis signaling in low-risk MDS progenitor cells.
The model proposes that the increased sensitivity to ligation of death receptors evidenced in MDS progenitor cells is due to the sensitization of these cells by constitutive activation of the mitochondrial axis. Triggering of death receptors, such as Fas, or direct targeting of mitochondria, results in the release of cytochrome c and downstream activation of caspase-9 via apoptotic protease-activating factor 1 (Apaf-1)–mediated apoptosome formation. G-CSF exerts its antiapoptotic—and hence its erythrogenic—effect by interfering with this mitochondria-dependent pathway.
A schematic model of apoptosis signaling in low-risk MDS progenitor cells.
The model proposes that the increased sensitivity to ligation of death receptors evidenced in MDS progenitor cells is due to the sensitization of these cells by constitutive activation of the mitochondrial axis. Triggering of death receptors, such as Fas, or direct targeting of mitochondria, results in the release of cytochrome c and downstream activation of caspase-9 via apoptotic protease-activating factor 1 (Apaf-1)–mediated apoptosome formation. G-CSF exerts its antiapoptotic—and hence its erythrogenic—effect by interfering with this mitochondria-dependent pathway.
The authors wish to thank Professor Robert Howe (Minnesota University) for reviewing morphology slides according to the World Health Organization classification, and for helpful scientific input. We also thank Professor Sten Orrenius (Karolinska Institutet) for continuous support and illuminating discussions.
Prepublished online as Blood First Edition Paper, September 5, 2002; DOI 10.1182/blood-2002-06-1774.
Supported by grant nos. 3689-B01-07XBC (E.H.L.) and 3820-B01-06XAC (B.Z.) from the Swedish Cancer Society, grant nos. 01:164 (E.H.L.) and 01:188 (B.Z.) from the Stockholm Cancer Society, and grant no. 03X-2471 (B.Z., B.F.) from the Swedish Research Council. B.F. is a Swedish Society for Medical Research scholar.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eva Hellstrom-Lindberg, Department of Medicine, Division of Hematology, Huddinge University Hospital, Karolinska Institutet, S-141 86 Stockholm, Sweden; e-mail:eva.hellstrom-lindberg@medhs.ki.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal