Abstract
Acute chicken leukemia retroviruses, because of their capacity to readily transform hematopoietic cells in vitro, are ideal models to study the mechanisms governing the cell-type specificity of oncoproteins. Here we analyzed the transformation specificity of 2 acute chicken leukemia retroviruses, the Myb-Ets– encoding E26 virus and the ErbA/ErbB-encoding avian erythroblastosis virus (AEV). While cells transformed by E26 are multipotent (designated “MEP” cells), those transformed by AEV resemble erythroblasts. Using antibodies to separate subpopulations of precirculation yolk sac cells, both viruses were found to induce the proliferation of primitive erythroid progenitors within 2 days of infection. However, while AEV induced a block in differentiation of the cells, E26 induced a gradual shift in their phenotype and the acquisition of the potential for multilineage differentiation. These results suggest that the Myb-Ets oncoprotein of the E26 leukemia virus converts primitive erythroid cells into proliferating definitive-type multipotent hematopoietic progenitors.
Introduction
The first leukemia-inducing oncogenes discovered are those transduced by acute avian leukemia viruses. These genes encode truncated or mutated forms of cellular proteins. Acute leukemia viruses are relatively common in chickens, probably reflecting this species' unusual susceptibility to the transforming effects of a variety of oncoproteins. More than 10 different isolates of acute avian leukemia viruses have been identified, each inducing a distinct type of leukemia within a matter of weeks in vivo and transforming cultured hematopoietic cells within a few days of infection.1 Cells transformed in vivo and in vitro closely resemble each other.2,3 Thus, for example, hematopoietic cells transformed by the Myb-encoding avian myeloblastosis virus (AMV) resemble monoblasts while cells transformed by the ErbB and ErbA proteins of the avian erythroblastosis virus (AEV) resemble erythroblasts both in the animal and in tissue culture.4-6Crude fractionation experiments using adherence and cytotoxic antibodies as selection methods suggested that these viruses block the differentiation of their respective target cells.7Additional experiments have shown that transformation specificity is a property mediated by the individual viral oncogene(s) and not by the specificity of the viral envelope.8
An exception to the rule is the avian acute leukemia E26 virus, which encodes a fused oncoprotein between the truncated cellular transcriptional activators c-Myb and c-Ets-1. This virus was originally described as an erythroleukemia virus, based on the expression in leukemic cells of the erythroid-specific histone H5 and the observation that E26-transformed hematopoietic colonies contained a small number of mature erythroid cells.9 In addition, myelomonocytic precursors (“myeloblasts”) were seen within the leukemic cell population of infected animals, and this cell type could be transformed in culture as well.2,3 The virus therefore transforms 2 different cell types and induces a mixed “erythroid”/myeloid leukemia. Subsequent studies have shown that the transformed “erythroid” cells actually resemble thromboblasts (the avian equivalents of megakaryocytes, which give rise to thrombocytes) and that they are, in fact, multipotent. Thus, they can be induced to differentiate into erythrocytes and thrombocytes by inactivation of the viral Ets and Myb proteins, respectively,10,11 and into eosinophils and myeloblasts (granulocyte/macrophage precursors12) by activation of the Ras and protein kinase C (PKC) pathways.12,13 In addition, E26-transformed cells (called MEPs for Myb-Ets–transformed progenitors) express cell surface antigens characteristic of both normal, definitive hematopoietic progenitors and thrombocytes.12 14-16 In summary, E26-transformed MEP cells can differentiate not only into myeloblasts but also into 3 additional hematopoietic lineages and thus resemble adult multipotent hematopoietic progenitors.
The mechanism of E26-mediated transformation has been studied in some detail. Earlier studies showed that the fusion between Myb and Ets proteins is essential for leukemogenesis because animals infected with a viral construct expressing Myb and Ets as separate proteins only rarely developed the disease. In the few animals that did develop leukemia, rearrangements in the viral genome were found to have taken place that led to an in-frame refusion of the oncoproteins. Similarly, cells transformed in vitro with the “split” Myb/Ets virus exhibited an erythroid phenotype and lacked the capacity for multilineage differentiation.17 To assess the relative contributions of the Ets and Myb moieties of the oncoprotein to the biology of the virus, mutants were produced that rendered either of these moieties temperature-sensitive for DNA binding.10,11,18,19 In aggregate, these studies suggested that a functional DNA-binding domain of the Myb portion of the fusion protein is necessary to block thrombocytic (and macrophage) differentiation of E26-transformed MEP cells,11,18 while an active Ets domain is required to block erythroid (and to a lesser extent eosinophil and myleolomonocytic) differentiation.10 19
These observations raised the question about the target cells of E26 virus: Does the virus transform multipotent cells, or does it transform a committed progenitor, imposing a new fate? In the present study we have investigated the mechanism of transformation specificity of E26 and AEV viruses by infecting sorted cells from day 2 precirculation chicken blastoderms. Surprisingly, our results indicate that E26 target cells correspond to committed primitive erythroid progenitors and that these are shared with those of AEV. E26 virus, but not AEV, thus appears to endow primitive erythroid cells with the capacity for multilineage differentiation, a property that is normally associated with definitive-type hematopoietic cells.
Materials and methods
Cells and tissue culture
Blastoderms were produced from fertilized White Leghorn eggs incubated for 2 days at 37°C as described earlier.12They were dissected under a stereomicroscope and appropriate stages separated into an embryo and a yolk sac portion. In some experiments the yolk sacs were dissected further along the midline into a posterior part and anterior parts. The corresponding fractions were pooled and the embryo pool dispersed by incubation with trypsin-EDTA (ethylenediaminetetraacetic acid) (Gibco, Carlsbad, CA). In some experiments the culture dishes (Falcon, Franklin Lakes, NJ) were precoated with collagen (100 μg/mL) as a substrate of very late antigen-2 (VLA-2) to facilitate the spreading of E26-transformed MEP cells. Protocols for virus infection and generation of transformed cells in methylcellulose-containing cultures were as described previously.1 E26 and AEV stocks used contained saturating amounts of transforming virus, as determined by dilution experiments (approximately 105 to 106colony-forming units per milliliter). Leukemic cells were obtained after intravenous inoculation of 1-day-old chicks. Blood smears were examined beginning 2 weeks after virus inoculation. Animals with a high proportion of leukemic cells were bled by heart puncture into a heparinized syringe and leukemic cells isolated by centrifugation at room temperature on “lymphocyte separation medium” (Eurobio, les Ulis, France). Staining of cells with neutral or acid benzidine or with Diff Quik (a May-Grünwald stain from Baxter, Duedingen, Switzerland) was as described previously.2 12
Antibodies, FACS analysis, and cell sorting
The antibodies used and their specificity were as follows: MEP17 recognizes VLA-2/CD49B and stains all leukocytes except mature erythroid and granulocytic cells.16,20 MEP21 recognizes thrombomucin, the avian equivalent of podocalyxin or PCLP-1.14,16,21 It is expressed on avian and murine multipotent hematopoietic progenitors14 and on a number of endothelial and epithelial tissues14,15 as well as on thrombocytes.16 MEP26 recognizes a 50- to 60-kDa protein expressed on erythroid cells and eosinophil precursors.16JS3 recognizes HEMCAM, an adhesion molecule expressed on immature primitive and definitive erythroid cells as well as on avian lymphoid precursors.22,23 JS4 recognizes an antigen of unknown nature expressed on immature (weakly) and mature primitive and definitive erythrocytes (highly).22 EOS47 recognizes melanotransferrin on avian eosinophils.16,24 MYL51/2 recognizes a 170-kDa antigen on chicken myelomonocytic cells.25 Cells were stained with the respective monoclonal antibodies, or anti-Src antibody as a negative isotype-matched control, followed by goat antimouse fluorescein isothiocyanate (FITC)–coupled antibodies (Dianova, Hamburg, Germany) as described previously.12 Flow cytometric analyses and cell sorts were performed using a FACScan Plus and a FACS Vantage machine, respectively (Becton Dickinson, Mountainview, CA).
PCR analyses of hemoglobins
RNA was isolated using the RNeasy kit (Qiagen) and reverse transcriptase–polymerase chain reaction (RT-PCR) performed on 1 μg total RNA per sample. Primer pairs 332-351 and 1575-1556 were used for chicken ε-globin transcripts26 and primer pairs 425-442 and 1432-1415 for chicken β-globin.27 The ε-globin was amplified at 60°C for 20 cycles and β-globin at 57°C for 23 cycles. Probes used for hybridization were obtained by cloning the respective PCR products. Exposure of the gels was for 10 minutes.
Results
E26 and AEV target cells reside in the yolk sac of precirculation chicken blastoderms
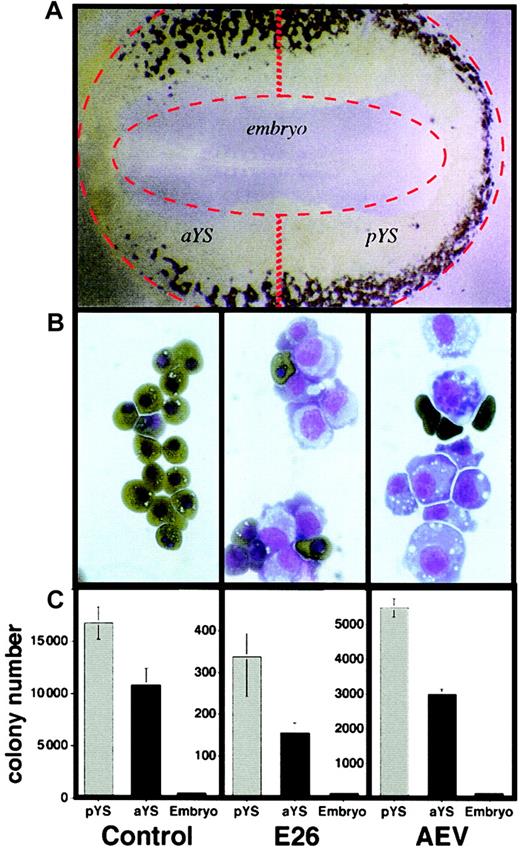
The first blood cells in chickens arise after about 20 hours of incubation within the yolk sac, where they are located in the lateral and posterior parts of the area vasculosa surrounding the embryo (stages 10 to 1228; Figure1A). After about another 5 hours of incubation, the blood islands fuse to form the vascular system and establish the blood circulation. Because earlier work showed that cell suspensions from 2-day-old chick blastoderms can readily be transformed by both E26 and AEV viruses,9 we wanted to determine whether the target cells for transformation by these viruses are located in the embryo proper or in the yolk sac. For this purpose, 2-day-old blastoderms (stages 9 to 11) were dissected into yolk sac and embryo, and the yolk sac was further dissected into anterior and posterior regions as shown in Figure 1A. Suspensions of these tissues were then infected with either E26 or AEV or mock-infected and seeded in methylcellulose-containing medium at 39°C. As illustrated in Figure 1B, colonies of mock-infected cells consisted of primitive erythrocytes at day 4, while colonies transformed by E26 and AEV consisted of a mixture of blastlike cells and some mature primitive erythrocytes. As shown in Figure 1C, mock-infected cells from posterior and anterior yolk sac yielded 17 000 and 11 000 erythroid colonies per 105 cells seeded, respectively, while cells from the embryo proper yielded 170 colonies, most of which were probably derived from yolk sac contaminants. In comparison, posterior and anterior yolk sac cells infected with E26 virus yielded 350 and 150 transformed colonies, respectively, while embryos yielded no transformed colonies. Similar data were obtained with AEV, except that for all fractions the yield of transformed colonies was about 15-fold higher than with E26 virus. Thus, relative to the total number of erythroid colony-forming cells obtained in the mock infections, about 2% of the total colonies were transformed by E26 virus while about 30% were transformed by AEV virus. We conclude that in stage 9 to 11 chick blastoderms most of the target cells for E26 and AEV viruses are contained within the yolk sac and not in the embryo and are mostly located in the posterior part of the yolk sac. This correlates with the sites where the highest numbers of blood islands can be observed and that generate the highest numbers of erythroid colony-forming cells.
In vitro transformation of chick blastoderm cells by E26 and AEV leukemia viruses.
(A) Stage 10 blastoderm (embryo with 10 somites) stained with acid benzidine. Blood islands are dark brown. Original magnification, × 2.5. (B) Colonies obtained 4 days after plating blastoderm suspensions in methylcellulose, stained with neutral benzidine and counterstained with May-Grünwald-Giemsa. Mature, hemoglobin-positive cells are dark brown; blastlike cells are light blue and purple. Left to right panels indicate control, E26-, and AEV-transformed cells, respectively. Original magnification, × 100. (C) Quantitation of colony formation of yolk sac and embryo pools, with and without infection by E26 and AEV leukemia viruses. Mock-infected normal colonies were scored after 4 days of incubation, E26-transformed colonies after 10 days, and AEV-transformed colonies after 6 days. Error bars represent values obtained from duplicate cultures.
In vitro transformation of chick blastoderm cells by E26 and AEV leukemia viruses.
(A) Stage 10 blastoderm (embryo with 10 somites) stained with acid benzidine. Blood islands are dark brown. Original magnification, × 2.5. (B) Colonies obtained 4 days after plating blastoderm suspensions in methylcellulose, stained with neutral benzidine and counterstained with May-Grünwald-Giemsa. Mature, hemoglobin-positive cells are dark brown; blastlike cells are light blue and purple. Left to right panels indicate control, E26-, and AEV-transformed cells, respectively. Original magnification, × 100. (C) Quantitation of colony formation of yolk sac and embryo pools, with and without infection by E26 and AEV leukemia viruses. Mock-infected normal colonies were scored after 4 days of incubation, E26-transformed colonies after 10 days, and AEV-transformed colonies after 6 days. Error bars represent values obtained from duplicate cultures.
Comparison of cell surface antigens between E26 and AEV leukemic cells and 2-day yolk sac cells
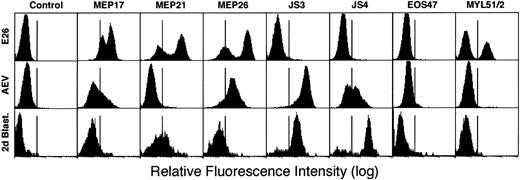
Before attempting to identify the specific target cells for the 2 virus strains, we compared the phenotypes of leukemic cells transformed by E26 and AEV viruses with those of normal 2-day blastoderm cells using antibodies that detect lineage-restricted cell surface antigens. Figure 2 shows fluorescence-activated cell sorter (FACS) profiles of leukemic cells isolated from the blood of diseased chicks infected 1 month earlier with E26 or AEV, respectively, using antibodies that detect lineage-restricted cell surface antigens. In the E26-infected animal, about two thirds of the leukemic cells displayed an “MEP”/thromboblast phenotype, expressing high levels of MEP17 (α2β1integrin20), MEP21 (also called PCLP-1, podocalyxin, thrombomucin14,15,21), and MEP2616 antigens. These cells were negative for the erythroid cell antigens JS3 (HEMCAM23) and JS4 as well as for the myeloid antigen MYL51/2 and the eosinophil antigen EOS47 (melanotransferrin24). The other third of the cells exhibit a “myeloblast” phenotype, being MYL51/2-positive but negative for all other antigens (with the exception of MEP17, which is weakly expressed in myeloblasts). In contrast, AEV leukemia cells exhibited an erythroid phenotype, expressing JS3, JS4, and weakly MEP26 antigen but not MEP21 and EOS47 antigens. These data confirm earlier observations showing that E26 leukemia cells resemble normal multipotent progenitor/thrombocytic cells while AEV cells resemble erythroid cells.10,11 16
FACS profiles of leukemic cells transformed by E26 and AEV compared with cells from 2-day-old blastoderms.
Leukemic cells were collected from chicks infected with the E26 and AEV viruses, respectively, and purified by Ficoll centrifugation. Blastoderm cells were obtained by dispersing the cells from a pool of day-2 blastoderms by vigorous pipetting and by removing tissue fragments by filtration.
FACS profiles of leukemic cells transformed by E26 and AEV compared with cells from 2-day-old blastoderms.
Leukemic cells were collected from chicks infected with the E26 and AEV viruses, respectively, and purified by Ficoll centrifugation. Blastoderm cells were obtained by dispersing the cells from a pool of day-2 blastoderms by vigorous pipetting and by removing tissue fragments by filtration.
To determine the cell surface phenotype of 2-day yolk sac cells, suspensions from dissected stage 9 to 11 blastoderms were stained with the above antibodies and analyzed by FACS. As shown in Figure 2, these cells were weakly positive for MEP17, MEP21, and MEP26 antigens, strongly positive for JS3 and JS4 antigens, and negative for EOS47 and MYL51/2 antigens. These results demonstrate that precirculation chick yolk sac cells strongly express erythroid markers but are essentially negative for the multipotent progenitor/thrombocytic marker MEP21. Thus, these primitive hematopoietic precursors more closely resemble AEV- rather than E26-transformed cells.
Yolk sac target cells of both E26 and AEV leukemia viruses correspond to primitive erythrocyte progenitors
To identify the target cells of E26 and AEV in the precirculation chick yolk sac, suspensions of 2-day blastoderm pools were stained with the erythroid-specific JS4 antibody and sorted into antigen-positive and -negative fractions. Reanalysis of the sorted fractions by FACS showed that the JS4+ cells were 99% and the JS4− cells were 98% pure. Benzidine staining revealed that 75% to 85% of the JS4+ cells expressed hemoglobin, while the JS4− cells contained less than 1% hemoglobin-positive cells. Next, cells from each fraction were mock-infected or infected with either E26 or AEV virus and seeded in 35-mm dishes containing methylcellulose. Colony formation was scored between days 3 and 10 of culture. In the uninfected samples, of 50 000 cells seeded, about 20 000 primitive erythrocyte colonies were obtained with the JS4+ cell fraction while only around 100 colonies were obtained with the JS4− fraction (primitive erythrocyte colonies could be recognized by their red color and consisted of 4 to 20 cells that grew in tight clusters that disintegrated within 4 to 5 days). In addition to the erythrocyte colonies, occasional single macrophages or macrophage clusters consisting of 2 to 10 cells could be detected in both fractions, with 2- to 3-fold higher proportions in the JS4− fraction (about 20 vs 45). Finally, thrombocyte colonies consisting of 4 to 16 small dispersed hemoglobin-negative cells could be seen in the JS4+ but not in the JS4− fraction (up to about 30 colonies). Because these colonies disintegrated within 2 to 3 days, their number could not be assessed accurately.
In the infected cultures a subset of colonies proliferated and became macroscopically visible after 1 week. No such large colonies were seen in the mock-infected cultures. A quantitative evaluation of the results (experiment nos. 1 and 2, Table1) shows that, as for the erythrocyte colonies seen in the uninfected controls, most of the E26- and the AEV-transformed colonies were obtained with cells from the JS4+ fraction, with a transformation efficiency of 1.5% to 2.5% for E26 and 10% to 20% for AEV relative to the number of normal erythroid colonies observed. In another experiment, cells were sorted using the JS3 antibody. Although the separation of the erythroid colony-forming cells was not as clean as with the JS4 antibodies (there was still a significant number in the JS3− fraction) qualitatively similar results were obtained (experiment no. 3; Table2). Together, these results indicate that most yolk sac target cells for both E26 and AEV viruses correspond to committed erythroid precursors.
E26- and AEV-transformed colonies obtained from yolk sac cells fractionated with JS4 monoclonal antibodies
| . | No. of normal or transformed colonies . | |
|---|---|---|
| JS4+fraction . | JS4− fraction . | |
| Experiment no. 1 | ||
| Mock-infected | 20 000, 18 000 | 100, 150 |
| E26-infected | 600, 500 | 12, 30 |
| AEV-infected | 3000, 2800 | 45, 100 |
| Experiment no. 2 | ||
| Mock-infected | 20 900, 15 600 | 65, 100 |
| E26-infected | 350, 330 | 17, 18 |
| AEV-infected | 3320, 3280 | 45 |
| . | No. of normal or transformed colonies . | |
|---|---|---|
| JS4+fraction . | JS4− fraction . | |
| Experiment no. 1 | ||
| Mock-infected | 20 000, 18 000 | 100, 150 |
| E26-infected | 600, 500 | 12, 30 |
| AEV-infected | 3000, 2800 | 45, 100 |
| Experiment no. 2 | ||
| Mock-infected | 20 900, 15 600 | 65, 100 |
| E26-infected | 350, 330 | 17, 18 |
| AEV-infected | 3320, 3280 | 45 |
Numbers represent normal (mock-infected) or transformed colonies (virus-infected) per 5 × 104 cells seeded in duplicate cultures. Colonies were scored after 3 to 4 days for the mock-infected controls, after 10 days for the E26-infected cultures, and after 6 days for the AEV-infected dishes.
E26- and AEV-transformed colonies obtained from yolk sac cells fractionated with JS3 monoclonal antibodies
| . | No. of normal or transformed colonies . | |
|---|---|---|
| JS3+fraction . | JS3− fraction . | |
| Experiment no. 3 | ||
| Mock-infected | 18 000, 14 000 | 500, 1100 |
| E26-infected | 300, 230 | 50, 20 |
| AEV-infected | 3000, 2800 | 400, 800 |
| . | No. of normal or transformed colonies . | |
|---|---|---|
| JS3+fraction . | JS3− fraction . | |
| Experiment no. 3 | ||
| Mock-infected | 18 000, 14 000 | 500, 1100 |
| E26-infected | 300, 230 | 50, 20 |
| AEV-infected | 3000, 2800 | 400, 800 |
For explanations, see legend to Table 1.
E26 target cells express hemoglobin
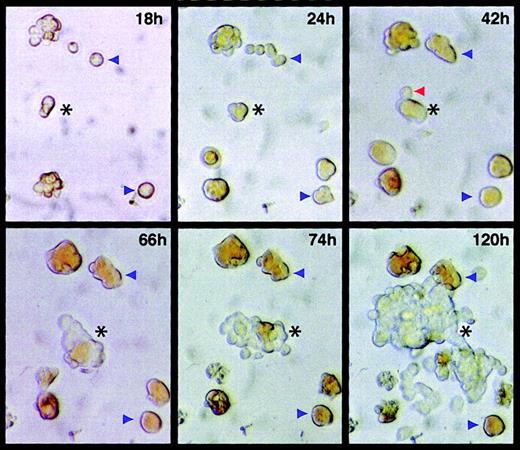
The finding that most JS4 antigen–positive cells express hemoglobin and that early E26-transformed colonies contain mature erythroid cells (Figure 1C) raises the possibility that the virus is capable of reprogramming committed primitive erythroid cells, conferring upon them an MEP phenotype. Indeed, in numerous assays we observed emergence of MEP-type cells from small primitive erythroid colonies. To study this possibility and to determine the time at which transformants can first be detected with E26 virus, cultures infected by the virus were photographed at different times after infection. For this purpose, JS4+ cells were sorted from 2-day blastoderms, infected with E26 virus, and seeded in methylcellulose cultures. The plates were pretreated with collagen, a ligand for the integrin α2β1, to facilitate the identification of the transformed cells (MEP cells, through expression of the MEP17 antigen, α2β1 integrin, become adherent to extracellular matrix, whereas normal erythroid cells do not.20 Thus, transformed cells can be identified by their ability to spread on collagen). At 18 hours after seeding of E26-infected cells, 2 fields were randomly selected, marked on the bottom of the plate, and photographs of the fields taken at various intervals for up to 120 hours. Selected images collected from field no. 1 are shown in Figure 3. Normal erythroid cells and colonies could be identified by their reddish coloration, indicative of hemoglobin expression. Most of the colonies stopped dividing after about 3 days and began to disintegrate (blue arrowheads in Figure 3). In contrast, one colony (black asterisk in Figure 3) continued to proliferate and contained well over 100 cells at 120 hours and too many to count at 150 hours. This E26-transformed colony consisted of a mixture of transformed cells, recognizable by their plastic adherence and grayish coloration, and of normal erythroid cells, recognizable by their reddish coloration (best seen in the images taken at 66, 74, and 120 hours). Tracing the origin of this colony to the 18-hour time point showed that it derived from a 2-cell cluster whose cells appeared to be hemoglobin-low or -negative (more obvious by direct microscopic inspection). This colony progressed into 3 weakly hemoglobin-positive cells at 24 hours and 5 partially hemoglobin-positive cells at 42 hours, one of which probably represents the first transformed cell (red arrowhead, Figure 3). From this point on the colony proliferated rapidly, containing 20 cells at 66 hours. These observations suggest that the target cells of E26 express hemoglobin and that they can become reprogrammed to acquire a hemoglobin-negative “MEP” phenotype. At the same time, some of the E26-infected cells continue to differentiate into erythrocytes, as had already been indicated by the appearance of benzidine-stained E26-transformed colonies isolated 4 days after infection (Figure 1B, middle panel).
Temporal sequence of cell transformation by E26 virus.
JS4+ cells were sorted by FACS and, after verifying that they were mostly single, they were infected with E26 virus and seeded in methylcellulose in 35-mm culture dishes that had been pretreated with collagen. After the cells had settled through the semisolid medium, starting at 18 hours after infection, micrographs were taken of the same field (marked on the bottom of the dish) at various intervals. The black asterisks in the frames taken at 18 and 24 hours indicate the cells from which the transformed colony visible in frames 66, 72, and 120 hours emerged. The red arrowhead in the 42-hour frame points to a cell that probably represents the first transformant in the nascent E26 colony. Blue arrowheads denote positions of normal erythroid colonies. Two erythroid “colonies” (seen on the upper and bottom left of the 18-hour frame) represent clusters formed during seeding. Two colonies in the 24-hour time frame appeared to form de novo resulted from cells that settled more slowly through the viscous medium after seeding of the cells in methylcellulose cultures. The same analysis was performed with another E26-transformed colony, with similar results. Original magnification for all panels, × 20.
Temporal sequence of cell transformation by E26 virus.
JS4+ cells were sorted by FACS and, after verifying that they were mostly single, they were infected with E26 virus and seeded in methylcellulose in 35-mm culture dishes that had been pretreated with collagen. After the cells had settled through the semisolid medium, starting at 18 hours after infection, micrographs were taken of the same field (marked on the bottom of the dish) at various intervals. The black asterisks in the frames taken at 18 and 24 hours indicate the cells from which the transformed colony visible in frames 66, 72, and 120 hours emerged. The red arrowhead in the 42-hour frame points to a cell that probably represents the first transformant in the nascent E26 colony. Blue arrowheads denote positions of normal erythroid colonies. Two erythroid “colonies” (seen on the upper and bottom left of the 18-hour frame) represent clusters formed during seeding. Two colonies in the 24-hour time frame appeared to form de novo resulted from cells that settled more slowly through the viscous medium after seeding of the cells in methylcellulose cultures. The same analysis was performed with another E26-transformed colony, with similar results. Original magnification for all panels, × 20.
E26-transformed clones undergo a gradual transition from an erythroid to an MEP phenotype
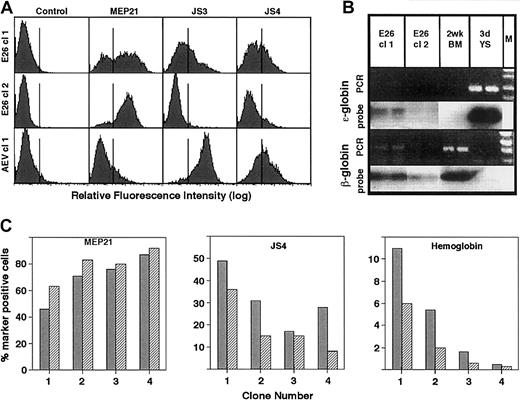
The presence of erythroid cells in the E26-transformed colonies could also be shown by FACS analysis. Thus, of 13 transformed clones derived from JS4+ yolk sac cells, analyzed at day 17 after infection, the average percentage of cells expressing MEP21 was 61.1% (SD ± 23.7%), JS4 antigen 29.5% (SD ± 18.0%), JS3 antigen 15.8% (SD ± 11.3%), and MYL51/2 3.0% (SD ± 2.1%). Two AEV-transformed clones analyzed in comparison revealed that they were essentially negative for MEP21 (7.5%) but highly positive for JS4 and JS3 antigens (67% and 95%, respectively). These results are shown in Figure 4A for 2 E26-transformed clones (chosen to illustrate the variations observed) and for 1 AEV-transformed clone. When examined for the type of hemoglobin produced, using semiquantitative PCR, E26-transformed colonies were found to express both ε-globin (an embryonic β-globin) and adult β-globin (Figure 4B). The fact that JS4+ erythroid cells were still seen in E26 clones that had grown to more than a million cells within 2 weeks after infection suggested that they retained their erythroid differentiation potential for more than 20 cell divisions. To determine whether at this stage the clones continued to drift toward an MEP phenotype, 4 of the E26-transformed colonies were monitored for MEP21 and JS4 expression after an additional week in culture. In addition, the clones were stained with benzidine at both time points to determine hemoglobin expression. As shown in Figure 4B, the proportion of MEP21+ cells increased in all clones, with a corresponding decrease in JS4-expressing cells. Correlating with this, the percentage of benzidine-positive cells in these colonies (which ranged from less than 1% to 10% at day 17 after infection) decreased during the same time period. These observations indicate that E26-transformed colonies consist of variable proportions of erythroid and MEP type cells and that during culture the colonies progressively lose their erythroid differentiation potential while acquiring MEP properties. Such a selection of cells with an MEP phenotype might also occur during leukemogenesis in vivo because E26 leukemia cells are typically MEP21+ and JS4− (Figure1A).
Characterization of E26- and AEV-transformed clones.
(A) JS4+ cells were infected with each of the 2 viruses, transformed colonies isolated after 6 days (AEV) or 10 days (E26), and expanded until day 17, when they were analyzed by FACS. The top 2 rows represent cells transformed by E26; bottom row, by AEV. (B) Hemoglobin expression in E26-transformed clones. PCR indicates ethidium bromide–stained reverse transcriptase–PCR products; probe, autoradiographs of bands hybridized to an ε-globin– or β-globin–specific probe. Samples (tested in duplicate) correspond to E26-transformed clones 1 and 2. Bone marrow cells from a 2-week-old chick and 3-day yolk sac were used as controls. M indicates marker DNA used to identifiy size of PCR fragments. (C) Histograms showing the proportion of cells positive for MEP21, JS4, and hemoglobin in 4 E26-transformed colonies at day 17 (dark gray bars) and day 24 (hatched bars) after infection. Clones were ordered according to their content of MEP21+ cells.
Characterization of E26- and AEV-transformed clones.
(A) JS4+ cells were infected with each of the 2 viruses, transformed colonies isolated after 6 days (AEV) or 10 days (E26), and expanded until day 17, when they were analyzed by FACS. The top 2 rows represent cells transformed by E26; bottom row, by AEV. (B) Hemoglobin expression in E26-transformed clones. PCR indicates ethidium bromide–stained reverse transcriptase–PCR products; probe, autoradiographs of bands hybridized to an ε-globin– or β-globin–specific probe. Samples (tested in duplicate) correspond to E26-transformed clones 1 and 2. Bone marrow cells from a 2-week-old chick and 3-day yolk sac were used as controls. M indicates marker DNA used to identifiy size of PCR fragments. (C) Histograms showing the proportion of cells positive for MEP21, JS4, and hemoglobin in 4 E26-transformed colonies at day 17 (dark gray bars) and day 24 (hatched bars) after infection. Clones were ordered according to their content of MEP21+ cells.
Discussion
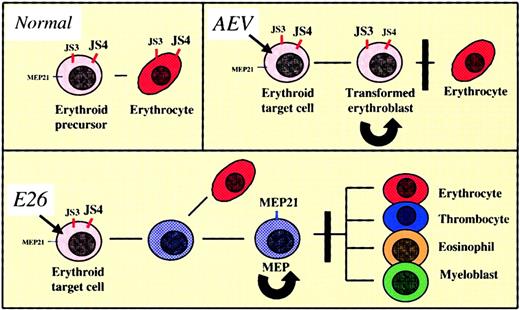
The schematic in Figure 5 summarizes how E26 transforms its target cells and how this differs from AEV transformation. Both viruses induce the proliferation of a subset of primitive erythroid progenitors and in the initial stages after infection colonies transformed by the 2 viruses are morphologically very similar, consisting of a mixture of blastlike cells and mature erythrocytes (Figure 1B). However, while AEV-transformed cells maintain an erythroid phenotype, cells infected by E26 virus gradually down-regulate erythroid cell surface antigens while up-regulating antigens characteristic of multipotent progenitors/thrombocytes, resulting in an MEP phenotype. In parallel to the antigenic changes the cells acquire multipotentiality.10-13 Thus, the mechanism of cell transformation by E26 appears to involve a Myb-Ets–induced cell fate change and thus differs from that seen with AEV. Whether these changes represent an induced “differentiation” or “dedifferentiation” of a committed precursor is not clear. Adopting the classical view that a monopotent progenitor is more differentiated than a multipotent progenitor, our observations would be compatible with a dedifferentiation. Alternatively, they could also represent forward differentiation because monopotent progenitors appear to arisebefore multilineage progenitors and hematopoietic stem cells during ontogeny.29 Ultimately, however, a distinction between the 2 processes might be semantic, because there is increasing evidence for the reversibility of differentiation among hematopoietic cells (reviewed by Graf30). For example, committed B-lineage cells have been shown to be reprogrammable to become macrophages by overexpression of the Rafoncogene.31
Model of cell transformation by E26 and AEV avian leukemia viruses.
For explanation, see “Discussion.” Black arrows indicate infection by virus; black lines, differentiation potential; curved arrows, self-renewal; black bars, block of differentiation. The cell surface antigens expressed are indicated in red (erythroid markers) or blue (multipotent progenitor/thrombocytic markers). Highly expressed antigens are indicated by large letters; weakly expressed antigens by smaller letters.
Model of cell transformation by E26 and AEV avian leukemia viruses.
For explanation, see “Discussion.” Black arrows indicate infection by virus; black lines, differentiation potential; curved arrows, self-renewal; black bars, block of differentiation. The cell surface antigens expressed are indicated in red (erythroid markers) or blue (multipotent progenitor/thrombocytic markers). Highly expressed antigens are indicated by large letters; weakly expressed antigens by smaller letters.
Another interpretation of our data is that E26 target cells represent rare definitive multilineage progenitors present in precirculation yolk sac, especially in view of the low transformation efficiency observed. Although studies in the mouse have indicated the presence of small numbers of definitive progenitors in the precirculation yolk sac,32 several arguments speak against this possibility: (1) Definitive multilineage, hematopoietic progenitors in the chick emerge only after establishment of circulation, in day 2.5 to 3.0 yolk sac,14 and have been shown to be recent immigrants from intraembryonic sites (see also below). Thus, the use of precirculation yolk sac precludes the possibility of such target cells in the assay. (2) Most of the E26 target cells are contained within the erythroid cell fraction of the precirculation yolk sac (as judged by their marker expression and differentiation potential). In contrast, multilineage progenitors in the postcirculation yolk sac are MEP21+, JS4−, and hemoglobin-negative.14 (3) E26-transformed colonies, especially when analyzed early after infection, contain mature erythroid cells, and MEP cells can be induced to differentiate into erythrocytes after inactivation of the Ets oncoprotein.10 33 However, these colonies did not contain any myeloid cells, as would be expected if the target cells corresponded to multipotent progenitors.
The low transforming efficiency observed with E26 relative to AEV might be explained by an inefficient reprogramming of committed erythroid cells by the Myb-Ets oncoprotein. Consistent with this interpretation is the finding that superinfection of AEV-transformed erythroblasts with E26 virus induces cell death and that E26 infection of yolk sac cells can induce an accelerated disintegration of normal erythroid colonies. In addition, we have observed that thets1.1 mutant of E26 (encoding a protein with a point mutation in Ets, Myb-Etsts) transforms yolk sac cells at similar efficiencies as AEV in cells infected at the permissive temperature (data not shown). This might be due to the fact that Myb-Etsts–transformed cells exhibit an immature erythroid phenotype, expressing hemoglobin and JS4 but no MEP21 antigen.10
Studies with chick-chick and chick-quail chimeras have suggested that during development the first hematopoietic cells arise in the yolk sac and that these are subsequently replaced by a definitive population of stem cells from intraembryonic sites (splanchnopleura and paraaortic foci).34-40 In addition to their separate sites of origin, primitive yolk sac progenitors differ from adult hematopoietic progenitors in that they are largely restricted to erythroid differentiation and undergo complete erythrocyte differentiation in only 1 to 2 days versus 1 to 2 weeks. Thus, the fact that E26 transforms primitive erythroid progenitors, giving rise to multilineage progenitors, raises the possibility that the virus induces a transition from primitive to definitive-type hematopoiesis. Whether this transition requires the induction of cell cycling is not known. Even in the absence of oncogenic transformation, there is evidence to suggest that primitive hematopoietic progenitors can undergo a shift toward definitive progenitors if normal patterns of transcription factor expression are perturbed. This has been shown most strikingly in mice lacking a single allele of the transcription factor AML-1, resulting in the precocious emergence of long-term repopulating (definitive) hematopoietic stem cells in the yolk sac and aorta-gonad-mesonephros (AGM) region.41
Studies with AEV have shown that the v-ErbA and v-ErbB oncoproteins each contribute to the induction of cell proliferation and differentiation arrest of erythroid cells. Thus, while v-ErbA (a mutated form of the thyroid receptor) cooperates with stem cell factor (SCF) under normal growth conditions,42v-ErbB, a mutated form of the EGF receptor, abrogates the cell's need for erythropoietin and SCF under conditions of hypoxia.43The mechanism of transformation by the Myb-Ets oncoprotein of E26 leukemia virus is less well understood at the molecular level. Each domain of the oncoprotein, if expressed on its own, is sufficient to induce the proliferation of erythroid cells in culture.44,45 When expressed together, they cooperate in inducing erythroid cell proliferation44 but they need to be fused to induce an MEP phenotype.17 An interesting possibility is that Myb-Ets forces a transition from primitive-to-definitive hematopoietic cells by mimicking a process that is exerted by c-Myb during normal development. Thus, the primitive to definitive cell transition is accompanied by an up-regulation of c-Myb,32 and mice with an inactivated c-Myb develop primitive but not definitive hematopoietic cells.46 Little is known about the relevant target genes of Myb-Ets. A strong candidate is bcl-2, which is up-regulated by Myb-Ets in myeloid cells and by c-Myb in T cells, probably by direct interaction with its promoter.47,48 Hox genes are another group of candidate target genes because these are often deregulated in human leukemias.49,50 In particular, Hox11, which is associated with oncogenesis in human beings and mice,51 transforms murine fetal liver cells with a phenotype similar to E26 transformants (K.M.M., unpublished observations 2001). In addition, it has recently been shown that overexpression of HoxB4 induces the expansion of definitive hematopoietic stem cells and confers long-term, multilineage reconstitution potential to primitive hematopoietic cells derived from the yolk sac.52 It will thus be interesting to determine whether E26-transformed cells, but not AEV-transformed cells, express HoxB4.
The early embryonic origin of the E26 virus target cells described here raises the question as to whether they represent a valid model for leukemogenesis by the virus. This is likely to be the case because in vitro–transformed yolk sac and bone marrow cells resemble leukemic cells obtained within 3 to 4 weeks after infection of newly hatched chicks.2,3,12 If the model described is relevant for leukemia, can our finding of an E26-induced reprogramming of target cells be extrapolated to human acute myeloid leukemia (AML)? It is intriguing that cases of myeloid leukemia have been reported where the leukemic cells exhibit specific immunoglobulin gene rearrangements also seen in normal B cells from the same patient.53,54 These observations have been explained by the transformation of a multilineage precursor in which immunoglobulin rearrangements were subsequently aberrantly initiated; however, it is also possible that a B-lineage cell becomes reprogrammed by a transforming event into a myeloid cell, perhaps as a consequence of a secondary mutation. In addition, studies by Grignani et al55 have shown that infection of CD34+, lin− human progenitors with PML-RAR–encoding retrovirus induced their differentiation toward immature granulocytes, away from erythroid differentiation. Together with our results these observations indicate that the phenotype of hematopoietic target cells may differ from that of their transformed counterparts. This would suggest that differentiation plasticity can play a role in leukemic transformation by fusion oncoproteins.
We thank Inger Pettersson and Lidia Perez for excellent technical assistance and Ari Melnick for comments on the manuscript.
K.M.M. is a Canadian Institutes of Health Research Scholar.
Some of the work for this article was performed at the European Molecular Biology Laboratory, Heidelburg, Germany.
Prepublished online as Blood First Edition Paper, September 12, 2002; DOI 10.1182/blood-2002-04-1050.
Partially supported by a Canadian Institutes of Health Research Scholar grant (MT-15477) and a Grant-In-Aid from the Heart and Stroke Foundation of B.C. & Yukon.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas Graf, Department of Developmental and Molecular Biology, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, New York, NY 10461; E-mail:graf@aecom.yu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal