Little is known about mechanisms involved in skin-specific homing of cutaneous T-cell lymphoma (CTCL). Chemokine/chemokine receptor interactions have been implicated in the homing of lymphoma cells to various tissue sites. We investigated tissue samples and tumor cell suspensions of patients with CD30+ CTCL (n = 8) and CD30− CTCL (mycosis fungoides, n = 6; Sézary syndrome, n = 6) for expression of the chemokine receptors CCR3, CCR4, and CCR8 and the CCR3 ligands eotaxin/CCL11, monocyte chemoattractant protein 3 (MCP-3)/CCL7, and RANTES (regulated on activation, normal T expressed and secreted)/CCL5. Of 8 CD30+ CTCLs, 7 expressed CCR3, 4 CCR4, and none CCR8. CCR3 expression was not found in skin tissue samples from 12 CD30− CTCLs. Coexpression of CCR3 and CD30 was demonstrated by flow cytometry in tumor cell suspensions. Internalization experiments demonstrated functionality of CCR3 expressed by freshly isolated tumor cells. Actin polymerization as well as migration in response to eotaxin was demonstrated in a CD30+ cutaneous lymphoma cell line. CCR3 ligand eotaxin/CCL11 was detected in lesional skin of CD30+CTCL by immunohistochemistry, preferentially in tumor cells. Eotaxin/CCL11 expression in tumor cells was confirmed by intracellular immunofluorescence. Analysis of cytokine expression pattern of CCR3-bearing infiltrating cells showed a predominance of interleukin-4 (IL-4) but not interferon-γ (IFN-γ) protein expression,1 consistent with a T-helper 2 (Th-2) profile. These results suggest that expression of CCR3 and its ligand eotaxin/CCL11 plays a role in the recruitment and retention of CD30+ malignant T cells to the skin.
Introduction
Cutaneous T-cell lymphomas (CTCLs) are part of the spectrum of extranodal non-Hodgkin lymphomas1 and are characterized by proliferation of clonally expanded helper T-cells in skin, but without detectable systemic involvement at least 6 months following diagnosis.2-4 Primary cutaneous CD30+ large-cell (anaplastic) CTCLs lack the t(2;5) translocation (NPM/ALK−) and have a significantly better prognosis than systemic CD30+large-cell anaplastic lymphomas.5 Expression of CD30 is not specific for systemic large-cell anaplastic lymphoma, and identification of abnormally expressed anaplastic large-cell lymphoma kinase 1 (ALK-1) is favored.6 However, in skin lymphomas, owing to the usual absence of ALK expression, CD30 is still a valuable marker.4 Little is known about mechanisms leading to accumulation of large numbers of transformed lymphocytes in skin. Expression of the cutaneous lymphocyte-associated antigen (CLA) has been implicated in skin-specific homing patterns.7-10Extravasation and active locomotion of malignant lymphocytes suggest the involvement of additional secreted factors such as chemokines. Chemokines are small molecules of about 8 kDa that are involved in leukocyte migration to specific tissue sites and are essential for the host immune response.11 The known chemokine system comprises approximately 50 ligands and 20 G protein–coupled receptors. A new nomenclature was recently introduced.12Investigation of the expression of chemokine receptors has been performed in extracutaneous non-Hodgkin lymphomas (NHLs). CXC receptor 3 (CXCR3) was shown to be a marker of B-cell chronic lymphocytic leukemia and was expressed in a subset of B-cell lymphomas, while CXCR5 was expressed on all types of B-cell lymphomas.13 Subsets of T-cell NHL expressed various patterns of T-cell–associated chemokine receptors.14Expression of the T-helper 2 (Th-2) and skin-associated chemokine receptor CCR4 was seen in 5 of 5 ALK+ large-cell lymphomas while the Th-1–associated receptor CXCR3 was expressed in only 1 of 15 ALK+large-cell lymphomas.
The presence of eosinophils is prominent in certain types of CTCLs.15,16 Eotaxin/CCR ligand 11 (CCL11) is one of the most potent eosinophil chemoattractants.17The question of eotaxin/CCL11 receptor CCR-3 expression in CTCL has thus far not been addressed.
Here we show that CD30+ large-cell CTCLs express a functional CCR3 receptor and produce Th-2–like cytokines. These findings might have implications for the homing of transformed T cells to skin.
Materials and methods
Tissue samples and immunohistochemistry
Immunohistochemical analysis for chemokine receptors and chemokines was performed on frozen tissue samples. Biopsies were snap-frozen in liquid nitrogen and stored at −80°C. Representative serial 5- to 7-μm cryostat sections were mounted on polylysine-coated slides and fixed in 100% acetone for 10 minutes. After washing in phosphate-buffered saline (PBS) without Ca or Mg (Biochrom, Berlin, Germany), nonspecific antibody binding sites were blocked by means of normal rabbit serum (DAKO, Copenhagen, Denmark), for 15 minutes at room temperature. For chemokine receptor staining, the following antibodies were used: 5 μg/mL mouse immunoglobulin G2a (IgG2a) antihuman CCR3 (monoclonal antibody [moAb] 7B11 [kindly provided by LeukoSite, Cambridge, MA] as well as monoclonal rat IgG2A [R&D Systems, Abingdon, United Kingdom]); CCR4 (rabbit polyclonal IgG [Santa Cruz Biotechnology, CA); and CCR8 (goat polyclonal IgG [Alexis, Lausen, Switzerland]). For chemokine staining, the following antibodies were used: 1:100 ratio of antihuman eotaxin to CCL11 moAb (R&D Systems); 1:30 ratio of antihuman monocyte chemoattractant protein 3 (MCP-3) to CCL7 (Pharmingen/BD Biosciences, Basel, Switzerland); and 1:30 ratio of antihuman RANTES (regulated on activation, normal T expressed and secreted) to CCL5 (Pharmingen/BD Biosciences). For chemokine receptor staining, negative controls with antibody diluent (DAKO) instead of primary antibodies were included. For chemokine staining, the following isotype controls were used: 10 μg/mL mouse IgG1 (Ancell, Bayport, MN); mouse IgG2a (Ancell); and 1:300 dilution of normal goat serum (DAKO). Incubation time was 1 hour at room temperature for chemokine receptor staining; chemokine Abs and corresponding isotype controls were incubated overnight at room temperature. As detection system, the alkaline phosphatase/anti–alkaline phosphatase system followed by New Fuchsin (DAKO) as substrate, containing levamisole for blocking of endogenous alkaline phosphatase, was used as described.18 All incubations were done at room temperature. Antibody dilutions were done with the use of antibody diluent (DAKO). After immunostaining, slides were counterstained with hematoxylin. For evaluation of slides, 100 mononuclear cells were counted per high-power field (original magnification, × 200), and the percentage of positive cells was determined as follows: − indicates 0; −/+, 0% to 5%; +, 6% to 25%; ++, 26% to 50%; +++, 51% to 75%; and ++++, 76% to 100% positive cells. Only slides with at least 5% stained cells were regarded as positive.
Preparation of tumor cell suspensions
Tumor cell suspensions were obtained from biopsy material of patients 1, 7, and 8 by incubation with an enzyme-cocktail containing 200 U/mL collagenase (Sigma, Buchs, Switzerland), 200 U/mL hyaluronidase (Sigma), and 0.01% DNAse (Böhringer Mannheim, Germany) in RPMI 1640 (Life Technologies, Basel, Switzerland). Incubation was done at 37°C for 1 hour. Enzyme incubation was stopped by adding RPMI 1640 (Life Technologies) supplemented with 10% fetal calf serum (FCS). Suspensions were washed twice with PBS (Biochrom), and 70-μm filters were used to remove tissue fragments. Cells were prepared for in vitro investigation by resuspending cells in PBS (Biochrom) containing 1% bovine serum albumin (Sigma).
Flow cytometry, intracellular cytokine/chemokine staining
All antibody dilutions and washing steps were done in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (Sigma). Then, 1 × 106 cells per reaction were stained with the following moAbs: 1:20 dilution of fluorescein isothiocyanate (FITC)–conjugated antihuman CD30 (Ki-1) moAb (DAKO) of 10 μg/mL 7B11 antihuman CCR3 moAb. To control for nonspecific staining or Fc-receptor–mediated binding of antibody, the following moAbs were included as negative controls: 1:10 dilution of FITC-conjugated mouse IgG1 (Becton Dickinson, Basel, Switzerland) of 10 μg/mL mouse IgG2a (Ancell). Staining with anti-CCR3 or anti-IgG2a moAb was followed by phycoerythrin (PE)–conjugated goat antimouse F(ab′)2 moAb (DAKO) at a 1:20 dilution. All incubations were done at 4°C for 30 minutes. Samples were analyzed by flow cytometry with a FACScalibur System (BD Biosciences) equipped with CellQuest software (BD Biosciences).
For intracellular cytokine/chemokine staining, cells were stimulated with 50 ng/mL PMA and 1 μM ionomycin in RPMI 1640 (Gibco BRL, Basel, Switzerland) supplemented with 10% FCS (Serotech, Basel, Switzerland) in 5% CO2, 37°C. After 4 hours, transport inhibitor was added for an additional 2 hours: 0.75 μL/mL GolgiStop (Pharmingen) and 1 μL/mL GolgiPlug (Pharmingen). After cell-surface antigen staining with 10 μg/mL mouse IgG1 antihuman CCR3 moAb or by purified mouse IgG1 (BD Biosciences) followed by PE–cyanin 5 (PE-Cy5)–conjugated goat antimouse antibody (DAKO) at a 1:10 dilution, intracellular cytokine staining was performed with 1:100 PE-conjugated anti–IL-4 moAb and 1:100 FITC-conjugated anti–IFN-γ moAb (Pharmingen) after cell permeabilization with a saponin-containing solution (Cytofix/Cytoperm; Pharmingen). For intracellular chemokine staining, the following moAb was used: 1:25 dilution of mouse IgG1 antieotaxin CCL11 Ab (Pharmingen). All antibody incubations for staining of cell surface antigens were done at 4°C for 30 minutes; staining of intracellular cytokines and chemokines was performed at 4°C for 1 hour. Flow cytometry acquisition was performed by means of a FACScalibur system (BD Biosciences) equipped with CellQuest software (BD Biosciences), which was also used for flow cytometric analysis.
Internalization experiments
Recombinant chemokines eotaxin/CCL11, RANTES/CCL5, and MCP-3/CCL7 were obtained from R&D Systems. Cell suspensions from lesional skin were obtained as described in “Preparation of tumor cell suspensions” and were cultured for 2 days in RPMI 1640 supplemented with 10% FCS. Short-term cultures were incubated with 200 nM CCR3 ligands eotaxin/CCL11, RANTES/CCL5, MCP-3/CCL7, or untreated medium, respectively, for 40 minutes at 37°C in a 5% CO2 atmosphere. After chemokine incubation, cells were washed extensively in PBS; the final washing step was performed in acidic glycine buffer, pH 3.0, to remove bound chemokine located on noninternalized receptor for 1 minute at 37°C. To determine the amount of surface-expressed CCR3, cells were stained with antihuman CCR3 (10 μg/mL) and PE-conjugated goat antimouse F(ab′)2 antibody (DAKO) at a dilution of 1:20; each Ab was incubated for 30 minutes at 4°C.
Actin polymerization assay
Actin polymerization was tested as previously described.19 Briefly, cells derived from a CD30+ cutaneous lymphoma line (Mac-1) (1.25 × 106/mL)20 were resuspended in RPMI-1640 medium containing 0.5% bovine serum albumin (BSA) at 37°C and incubated with 100 ng/mL eotaxin (R&D Systems) for varying amounts of time. At the indicated time points, 400 μL cell suspension was added to 100 μL solution containing 4 × 107 M FITC-labeled phalloidin, 0.5 mg/mL 1-α-lysophosphatidylcholine, and 18% formaldehyde (all from Sigma) in phosphate-buffered saline (PBS). After incubation at 37°C for 10 minutes in the dark, fixed cells were centrifuged at 400g for 5 minutes at room temperature and subsequently resuspended in PBS containing 0.5% BSA. Cells were analyzed by flow cytometry on a FACSCalibur (Becton Dickinson), and all time points are plotted relative to the mean relative fluorescence of the sample before addition of eotaxin.
Migration assay
The number of CD30+ lymphoma cells migrating in response to recombinant eotaxin (R&D Systems) across 5-μm pore size polycarbonate filters (6.5-mm diameter) was assessed in 24-well Transwell chambers (Costar Corning, NY). First, 600 μL warm (37°C) assay medium (RPMI containing 0.5% BSA) containing various concentrations of eotaxin were added to the lower wells. Lymphoma cells were suspended at 2 × 106 cells per milliliter in warm assay medium (37°C), and 100 μL cell suspension was added to the upper chamber of each Transwell chamber. The plates were incubated for 3 hours at 37°C in 10% CO2. The migrated cells in the lower chambers were collected, and the number of migrated cells was counted by acquisition for 60 seconds with a flow cytometer.
Results
Chemokine receptor CCR3 is expressed in CD30+large-cell CTCL, but not in CD30− CTCL
The diagnosis of CTCL was established by assessment of the clinical appearance, analysis of T-cell receptor clonality, conventional histology, and immunohistochemical staining for various T-cell, B-cell, and activation markers, such as CD3, CD4, CD5, CD8, CD45RO, CD20, CD79a, and CD30, as published.4
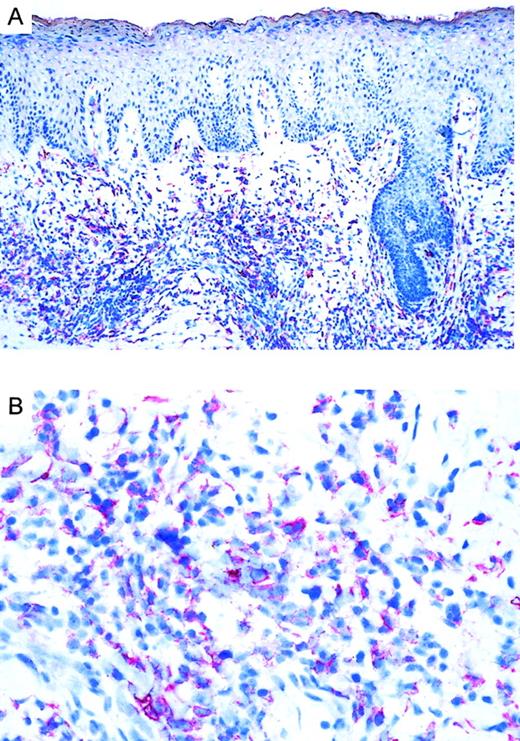
Twenty CTCLs, consisting of 8 CD30+ large-cell lymphoma, 6 mycosis fungoides, and 6 Sézary syndrome patient samples, were analyzed by immunohistochemistry. CD30 was expressed in all large-cell CTCLs (Table 1). Variation in the admixture of inflammatory cells accounted for the difference in numbers of CD30+ cells in the infiltrate. Staining for the chemokine receptor CCR3 demonstrated expression of CCR3 in 7 of 8 CD30+ CTCLs investigated (Table 1; Figure1). No epidermotropism of CCR-3+ cells was observed. There was faint staining of keratinocytes, which is explained by the recently described CCR3 expression on these cells.21 Mycosis fungoides (n = 6) and Sézary syndrome (n = 6) sections were CD30−except one case (no. 17) where few CD30+ cells were detected. In CD30− CTCL, there were always fewer than 5% of cells positive for CCR-3. In most cases, the extent of CD30 expression corresponded to the expression of CCR3 (Table 1). CCR4 expression was found in 4 of 8 CD30+ CTCLs as well as 1 of 6 MF cases and 1 of 6 Sézary syndrome cases. No CCR8 staining was observed in any of the samples.
Expression of CCR3 and its ligands in CTCL
| No. . | Diagnosis . | CD30 . | CCR3 . | CCR4 . | CCR8 . | Eotaxin . | MCP-3 . | RANTES . | Eos . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Large-cell CTCL | +++ | ++ | +/− | +/− | pos/TC | neg | pos/TC | pos |
| 2 | Large-cell CTCL | ++ | ++ | ++++ | − | pos/TC + CT | neg | pos/TC | pos |
| 3 | Large-cell CTCL | + | +/− | − | − | pos/TC + CT | neg | neg | pos |
| 4 | Large-cell CTCL | ++++ | ++ | ++ | − | pos/TC | neg | neg | ND |
| 5 | Large-cell CTCL | +++ | ++ | − | − | pos/TC + CT | neg | neg | ND |
| 6 | Large-cell CTCL | + | + | − | +/− | pos/TC + CT | neg | neg | pos |
| 7 | Large-cell CTCL | ++++ | +++ | +++ | − | pos/TC | pos/TC | pos/TC | ND |
| 8 | Large-cell CTCL | ++ | +++ | ++ | − | pos/TC + CT | neg | pos/TC | pos |
| 9 | MF | − | +/− | +++ | − | pos/CT | neg | neg | neg |
| 10 | MF | − | − | − | − | pos/CT | neg | neg | neg |
| 11 | MF | − | − | − | − | pos/TC + CT | neg | neg | pos |
| 12 | MF | − | +/− | − | − | ND | ND | ND | neg |
| 13 | MF | − | − | − | − | pos/CT | neg | neg | pos |
| 14 | MF | +/− | +/− | − | − | pos/CT | neg | neg | neg |
| 15 | Sézary syndrome | − | − | ND | ND | pos/CT | neg | neg | neg |
| 16 | Sézary syndrome | − | − | ND | ND | ND | ND | ND | neg |
| 17 | Sézary syndrome | + | +/− | − | − | pos/CT | neg | neg | neg |
| 18 | Sézary syndrome | − | − | − | − | neg | neg | neg | neg |
| 19 | Sézary syndrome | +/− | +/− | − | − | ND | ND | ND | neg |
| 20 | Sézary syndrome | − | − | ++ | − | pos/CT | neg | neg | pos |
| No. . | Diagnosis . | CD30 . | CCR3 . | CCR4 . | CCR8 . | Eotaxin . | MCP-3 . | RANTES . | Eos . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Large-cell CTCL | +++ | ++ | +/− | +/− | pos/TC | neg | pos/TC | pos |
| 2 | Large-cell CTCL | ++ | ++ | ++++ | − | pos/TC + CT | neg | pos/TC | pos |
| 3 | Large-cell CTCL | + | +/− | − | − | pos/TC + CT | neg | neg | pos |
| 4 | Large-cell CTCL | ++++ | ++ | ++ | − | pos/TC | neg | neg | ND |
| 5 | Large-cell CTCL | +++ | ++ | − | − | pos/TC + CT | neg | neg | ND |
| 6 | Large-cell CTCL | + | + | − | +/− | pos/TC + CT | neg | neg | pos |
| 7 | Large-cell CTCL | ++++ | +++ | +++ | − | pos/TC | pos/TC | pos/TC | ND |
| 8 | Large-cell CTCL | ++ | +++ | ++ | − | pos/TC + CT | neg | pos/TC | pos |
| 9 | MF | − | +/− | +++ | − | pos/CT | neg | neg | neg |
| 10 | MF | − | − | − | − | pos/CT | neg | neg | neg |
| 11 | MF | − | − | − | − | pos/TC + CT | neg | neg | pos |
| 12 | MF | − | +/− | − | − | ND | ND | ND | neg |
| 13 | MF | − | − | − | − | pos/CT | neg | neg | pos |
| 14 | MF | +/− | +/− | − | − | pos/CT | neg | neg | neg |
| 15 | Sézary syndrome | − | − | ND | ND | pos/CT | neg | neg | neg |
| 16 | Sézary syndrome | − | − | ND | ND | ND | ND | ND | neg |
| 17 | Sézary syndrome | + | +/− | − | − | pos/CT | neg | neg | neg |
| 18 | Sézary syndrome | − | − | − | − | neg | neg | neg | neg |
| 19 | Sézary syndrome | +/− | +/− | − | − | ND | ND | ND | neg |
| 20 | Sézary syndrome | − | − | ++ | − | pos/CT | neg | neg | pos |
Staining of different subtypes of cutaneous T-cell lymphoma for CD30, CCR3, CCR4, CCR8, and its ligands eotaxin, MCP-3/CCL7, and RANTES/CCL5. Positive mononuclear cells were counted per high-power field (magnification × 200) on 100 mononuclear cells, and the percentage of positive staining cells was determined as follows: +/− indicates 0% to 5%; +, 5% to 25%; ++, 25% to 50%; +++, 50% to 75%; and ++++, 75% to 100% positive cells. Please note that CCR3 expression corresponds to CD30 expression. Staining for CCR3 ligands shows positivity for eotaxin in CD30+ and CD30− CTCL. Please note that tumor cells and connective tissue cells in CD30+ CTCL are positive for eotaxin, while in CD30− CTCL tumor cell clusters are negative for eotaxin.
Eos indicates eosinophils; pos, positive staining; TC, tumor cells; neg, negative staining; CT, connective tissue cells; ND, not done; and MF, Mycosis fungoides.
Immunoreactivity of CCR3 in CTCL.
(A) CCR3 immunoreactivity in cryosections of a CD30+large-cell CTCL (no. 7) with the use of the alkaline phosphatase/anti–alkaline phosphatase method. Original magnification, × 100. (B) CCR3 immunoreactivity in cryosection of a CD30+ large-cell CTCL (no. 7) with the use of the alkaline phosphatase/anti–alkaline phosphatase method. Original magnification, × 400.
Immunoreactivity of CCR3 in CTCL.
(A) CCR3 immunoreactivity in cryosections of a CD30+large-cell CTCL (no. 7) with the use of the alkaline phosphatase/anti–alkaline phosphatase method. Original magnification, × 100. (B) CCR3 immunoreactivity in cryosection of a CD30+ large-cell CTCL (no. 7) with the use of the alkaline phosphatase/anti–alkaline phosphatase method. Original magnification, × 400.
CCR3 and CD30 coexpression in freshly isolated tumor cell suspensions from CD30+ CTCL
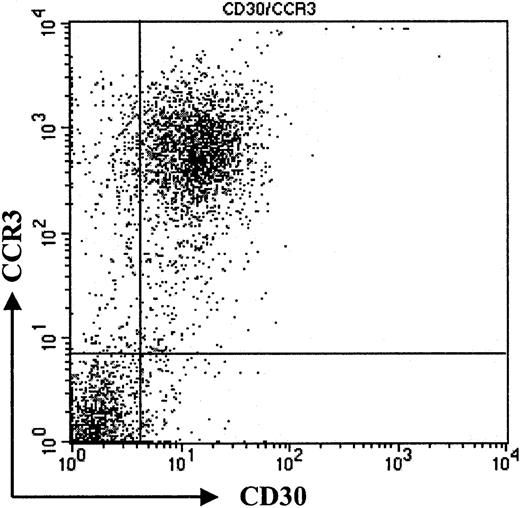
To confirm our immunohistochemistry data on a single-cell level and to assess expression levels of CCR3 on CD30+ lymphoma cells, we performed flow cytometric analysis of tumor cell suspensions. Tumor cells suspensions were derived from fresh biopsy material, which was available in 3 patients with CD30+ large-cell CTCL (nos. 1, 7, and 8). Cells were double-stained for CCR3 and CD30 and analyzed by flow cytometry. Compared with isotype control, a strong coexpression of the chemokine receptor CCR3 and CD30 was detected (Figure 2; patient no. 7). Strong staining in immunohistochemistry (Figure 1) translated into a high level of CCR3 expression on CD30+ tumor cells in flow cytometric analysis (Figure 2; patient no. 7). The majority of CD30+ cells were CCR3+. Expression levels were comparable to those seen in eosinophils (data not shown).
Coexpression of CD30 and CCR3.
Flow cytometric analysis of tumor cell population. Staining of freshly isolated tumor cells shows coexpression of CD30 and CCR3. Please note high expression of CCR3 on tumor cells (patient no. 7). Data shown are representative of 3 experiments.
Coexpression of CD30 and CCR3.
Flow cytometric analysis of tumor cell population. Staining of freshly isolated tumor cells shows coexpression of CD30 and CCR3. Please note high expression of CCR3 on tumor cells (patient no. 7). Data shown are representative of 3 experiments.
Eotaxin/CCL11 induces CCR3 down-regulation on CD30+cutaneous tumor cells
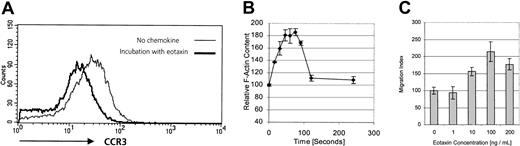
Detection of CCR3 protein on CD30+ tumor cells does not necessarily imply the existence of a functional receptor. Freshly isolated cell suspensions did not provide sufficient material to perform migration assays. A different approach to demonstrate the existence of a functional chemokine receptor is receptor internalization. Binding of ligand to its cognate receptor leads to receptor down-regulation, which can be assessed by flow cytometry.22 After incubation with eotaxin/CCL11, RANTES/CCL5, MCP-3/CCL7, or medium alone, surface expression of CCR3 on short-term cultured tumor cells was analyzed by flow cytometry with the use of anti-CCR3 moAb. Receptor-bound chemokine might interfere with antibody binding; therefore, cells were washed in acidic glycine buffer, as described, to dissociate the chemokine ligand from its receptor.22 As shown in Figure3A, the CCR3 ligand eotaxin/CCL11, but not medium alone, is able to induce a 1.9-fold decrease of CCR3 expression owing to down-regulation of CCR3. In contrast, CCR3 ligands RANTES/CCL5 and MCP-3/CCL7, which possess a lower affinity to CCR3 than eotaxin/CCL11, did not induce an internalization of CCR3 (data not shown).
Functional expression of CCR3 on CD30+cutaneous lymphoma cells.
(A) CCR3 surface expression measured by flow cytometry on freshly isolated tumor cells (patient no. 8) after incubation with corresponding ligand eotaxin or medium control. Cells were incubated in pH 3.0 acid buffer to remove receptor-bound chemokine on noninternalized receptor. Internalization was not seen after incubation with low-affinity ligands RANTES/CCL5 or MCP-3/CCL7 (data not shown). Data shown are representative of 3 experiments. (B) The F-actin content was measured in the CD30+ cutaneous lymphoma cell line Mac-1 upon stimulation with 100 ng/mL eotaxin for the indicated times. Fluorescein phalloidin was used to stain the cells, and the filamentous actin content was then measured by means of flow cytometry. Data show means of 3 different experiments. (C) To assess the chemotactic responses of the CD30+cutaneous lymphoma cell line Mac-1 to different concentrations of eotaxin, migration across a 5-μm pore size polycarbonate membrane during 3 hours was measured by counting migrated cells by means of a flow cytometer. Migrated cells at each time point were measured in triplicate. Data are expressed as the mean numbers of migrated cells per well. Error bars indicate SEM.
Functional expression of CCR3 on CD30+cutaneous lymphoma cells.
(A) CCR3 surface expression measured by flow cytometry on freshly isolated tumor cells (patient no. 8) after incubation with corresponding ligand eotaxin or medium control. Cells were incubated in pH 3.0 acid buffer to remove receptor-bound chemokine on noninternalized receptor. Internalization was not seen after incubation with low-affinity ligands RANTES/CCL5 or MCP-3/CCL7 (data not shown). Data shown are representative of 3 experiments. (B) The F-actin content was measured in the CD30+ cutaneous lymphoma cell line Mac-1 upon stimulation with 100 ng/mL eotaxin for the indicated times. Fluorescein phalloidin was used to stain the cells, and the filamentous actin content was then measured by means of flow cytometry. Data show means of 3 different experiments. (C) To assess the chemotactic responses of the CD30+cutaneous lymphoma cell line Mac-1 to different concentrations of eotaxin, migration across a 5-μm pore size polycarbonate membrane during 3 hours was measured by counting migrated cells by means of a flow cytometer. Migrated cells at each time point were measured in triplicate. Data are expressed as the mean numbers of migrated cells per well. Error bars indicate SEM.
Functional expression of CCR3 on a CD30+ cutaneous lymphoma cell line
Investigation of receptor functionality in fresh tumor cell suspensions is limited by both the low number and the heterogeneity of cells. To further explore functional CCR3 expression in CD30+ lymphoma cells, we investigated the expression of CCR3 in CD30 lymphoma cell lines. The cell line Mac-1 coexpressed CD30 and CCR3 (data not shown).20 As a first step, actin polymerization was investigated. Actin polymerization, which controls a number of processes regulating cell migration and reorganization of the actin cytoskeleton, has been shown to be an early event in the migratory response to chemokines.23 To examine whether eotaxin induces reorganization of the cytoskeleton in CD30+ lymphoma cells, filamentous actin was measured in the CD30+CCR3+ lymphoma cell line20with the use of fluorescein phalloidin. Stimulation with 100 ng/mL eotaxin induced a transient 85% increase in intracellular F-actin in CD30+ lymphoma cells within 75 seconds (Figure 3B), indicating the transduction of a migration signal to the cytoskeleton after binding of eotaxin to CCR3 on the CD30+ lymphoma cells.
Next we assessed whether engagement of CCR3 by its ligand eotaxin was leading to cell migration. Migration assays through 5-μm pore size polycarbonate membranes during 3 hours were performed with the use of recombinant human eotaxin as chemoattractant (Figure 3C). Data show the typical curve of chemokine-induced migration, with a peak cell migration induced at 100 ng/mL.
CCR3 ligand eotaxin/CCL11 is expressed by CD30+CTCL
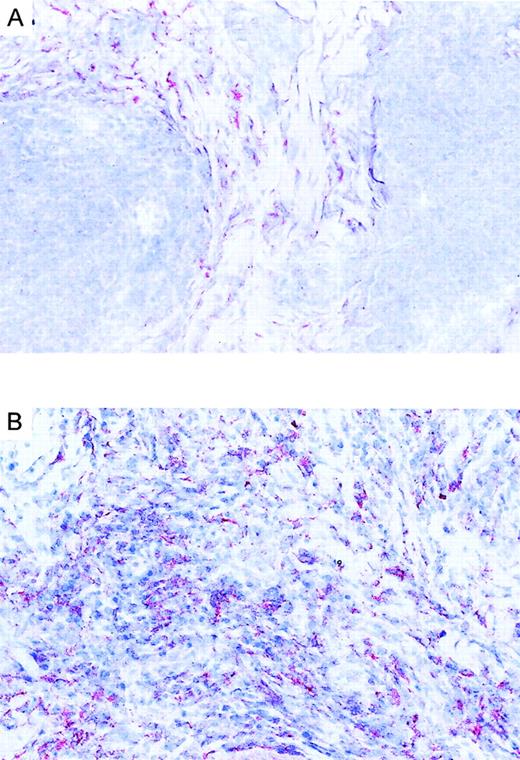
We investigated the expression pattern of CCR3 ligands in CD30+ and CD30− CTCL. Immunohistochemical staining of lesional skin for eotaxin/CCL11, MCP-3/CCL7, and RANTES/CCL5 was performed. Positive dermal immunoreactivity for eotaxin/CCL11 occurred in 17 of 18 CTCLs (Table 1). In CD30+ CTCL, eotaxin/CCL11 protein was detected in tumor cell aggregates demonstrated as being CD30+ in serial sections (Figure 4B). Eotaxin/CCL11 protein was also detected in connective tissue cells surrounding tumor cells. Each of 8 CD30+ CTCLs showed an immunopositivity for eotaxin/CCL11 associated with tumor cell aggregate, and in 5 of 8 there was expression by connective tissue cells (Figure 4B; Table 1). In contrast, positive staining for eotaxin/CCL11 in CD30−CTCL was confined to connective tissue cells, while infiltrating lymphoma cells were negative (Figure 4A; Table 1). In one case of CD30− CTCL, eotaxin/CCL11 was also present in infiltrating lymphoma cells. Eosinophil infiltration was found in each of 5 CD30+ large-cell CTCLs and in 3 of 12 CD30− CTCLs (Table 1). Staining for RANTES/CCL5 was positive in 4 CD30+ CTCLs, while MCP-3/CCL7 was expressed in only 1 case (Table 1).
Immunoreactivity of eotaxin in CTCL.
(A) Eotaxin/CCL11 immunoreactivity in cryosection of CD30−CTCL with the use of the alkaline phosphatase/anti–alkaline phosphatase method. Eotaxin/CCL11 is present only in connective tissue cells; tumor cell staining is negative. Original magnification, × 400. (B) Eotaxin/CCL11 immunoreactivity in cryostat section of CD30+ large-cell CTCL with the use of the alkaline phosphatase/anti–alkaline phosphatase method. Eotaxin/CCL11 is present in connective tissue cells and tumor cells, which were CD30+ in serial sections (data not shown). Original magnification, × 400.
Immunoreactivity of eotaxin in CTCL.
(A) Eotaxin/CCL11 immunoreactivity in cryosection of CD30−CTCL with the use of the alkaline phosphatase/anti–alkaline phosphatase method. Eotaxin/CCL11 is present only in connective tissue cells; tumor cell staining is negative. Original magnification, × 400. (B) Eotaxin/CCL11 immunoreactivity in cryostat section of CD30+ large-cell CTCL with the use of the alkaline phosphatase/anti–alkaline phosphatase method. Eotaxin/CCL11 is present in connective tissue cells and tumor cells, which were CD30+ in serial sections (data not shown). Original magnification, × 400.
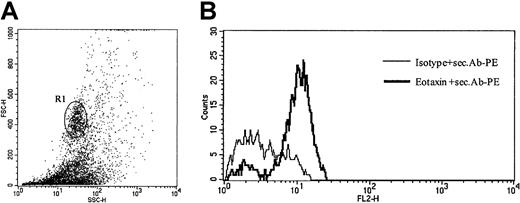
To verify eotaxin/CCL11 expression in infiltrating cells on a single-cell level, we performed intracellular eotaxin/CCL11 staining of cells isolated from freshly obtained biopsy specimens of patients with CD30+ CTCL. As shown in Figure5, eotaxin/CCL11 protein was detected in cells with high forward- and side-scatter properties corresponding to infiltrating tumor cells.
Intracellular eotaxin expression in tumor cell suspensions.
(A) Analysis gated on high forward- and side-scatter cell population (R1), which corresponds to CD30+ tumor cells (patient no. 8). Data shown are representative of 3 experiments. (B) Intracellular flow cytometric staining of tumor cell suspensions for CCR3 ligand eotaxin/CCL11, after stimulation for 4 hours with phorbol myristate acetate (PMA) and ionomycin demonstrates expression of eotaxin in tumor cells
Intracellular eotaxin expression in tumor cell suspensions.
(A) Analysis gated on high forward- and side-scatter cell population (R1), which corresponds to CD30+ tumor cells (patient no. 8). Data shown are representative of 3 experiments. (B) Intracellular flow cytometric staining of tumor cell suspensions for CCR3 ligand eotaxin/CCL11, after stimulation for 4 hours with phorbol myristate acetate (PMA) and ionomycin demonstrates expression of eotaxin in tumor cells
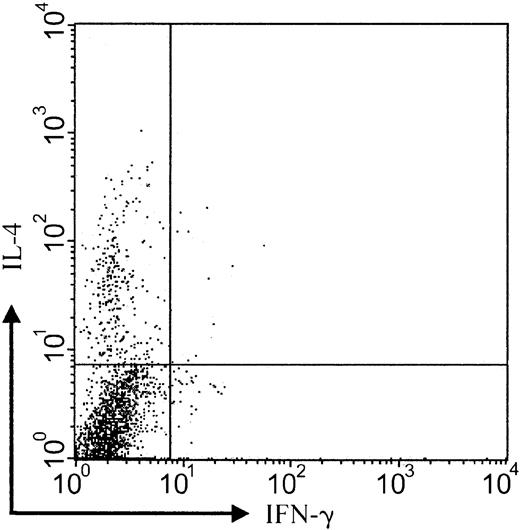
CCR3+ tumor cells express IL-4 protein
It has been previously shown that CD30+ CTCL lesions contain mRNA related to Th-2 cytokine differentiation such as IL-4.24 To analyze the functional differentiation state of CCR3-bearing tumor cells, we performed 3-color staining of tumor cell suspensions obtained from lesional CD30+ CTCL skin. Staining of tumor cell suspensions with IFN-γ, IL-4, and CCR3 showed a clear predominance of IL-4+ cells within the CCR3+ cell population (Figure6; patient no. 8; representative of 3 experiments). Ratio of IL-4– to IFN-γ–expressing cells was 24 (12.3% versus 0.5%). This indicates a skewing of CCR3+cells toward Th-2 cytokine production. Analysis of CCR3−lesional cells did not demonstrate a predominance of IFN-γ or IL-4 protein (data not shown).
Production of IL-4 by CCR3+ tumor cells.
Intracellular flow cytometric analysis of cytokine expression shows that the majority of CCR3-bearing cells express IL-4 protein but not IFN-γ (IL-4–IFN-γ ratio, 24:1). Analysis gated on CCR3+ population (patient no. 8). Data shown are representative of 3 experiments.
Production of IL-4 by CCR3+ tumor cells.
Intracellular flow cytometric analysis of cytokine expression shows that the majority of CCR3-bearing cells express IL-4 protein but not IFN-γ (IL-4–IFN-γ ratio, 24:1). Analysis gated on CCR3+ population (patient no. 8). Data shown are representative of 3 experiments.
Discussion
We found selective expression of the chemokine receptor CCR3 in CD30+ large-cell CTCL. No CCR3 expression was seen in CD30− CTCL. Coexpression of CCR3 and CD30, as well as Th-2 cytokine production, was detected on freshly isolated tumor cells. Functionality of the CCR3 receptor was shown by receptor-internalization experiments and actin polymerization, as well as by migration of tumor cells toward an eotaxin gradient.
CTCLs are a group of lymphoproliferative disorders with clonal expansion of transformed T cells in skin.25 Surface expression of CD30 distinguishes a subtype of large-cell CTCL with slow progression, indolent behavior, and favorable prognosis.26-29 CD30 is a member of the tumor necrosis factor receptor family, which was originally described as Ki-1 antigen on Hodgkin and Reed-Sternberg cells in Hodgkin disease.30,31 The favorable prognosis of CTCL may be related in part to the fact that dissemination to other body compartments occurs only late during disease development. This might be suggestive of chemotactic forces that keep lymphoma cells confined to the skin. The insight into the field of chemokine/chemokine receptor interactions has been developing rapidly.32 Recently, chemokine receptor expression has been correlated with differential recruitment of polarized Th-1 or Th-2 T cells as well as secretion of Th-1 or Th-2 cytokines.33-37 It has been shown that CCR3 is preferentially expressed in vitro by Th-2 cells.33,35 In this context, the presence of eosinophils as well as expression of Th-2 cytokine mRNA in lesions of CD30+ cutaneous lymphomas is of interest.24 We reasoned that recruitment of CD30+ Th-2–like lymphoma cells might be mediated by the presence of CCR3 ligands in skin in association with constitutive expression of CCR3 on lymphoma cells. The availability of a monoclonal antibody to CCR3 allowed us to perform the present study.38 Seven of 8 CD30+ CTCLs demonstrated expression of CCR3 on lesional tumor cells (Table 1; Figure 1). This finding was confirmed by flow cytometric staining of tumor cell suspensions (Figure 2). Interestingly, there was high expression of CCR3 on lymphoma cells, comparable to the expression on eosinophils, which was also reflected by strong staining intensity for CCR3 with the use of immunohistochemistry (Figure 1). CD30− CTCL samples did not express CCR3 in spite of a suggested Th-2 differentiation of tumor cells.39,40However, there is also recent evidence that Th-2 cytokine mRNA might be absent in CD30− CTCL.41 The question of chemokine redundancy must be addressed early in any discussion of the potential role of chemokines in disease. There might be an enormous overlap in ligand-receptor specificity. Even though eotaxin/CCL11 has a very specific binding to CCR3, other chemokine receptors, such as the skin-associated chemokine receptor CCR4, might play a role in the homing of lymphoma cells to skin. In our study, 4 of 8 CD30+ CTCLs, 1 of 6 Mycosis fungoides cases, and 1 of 6 Sézary syndrome samples expressed CCR4. Significant CCR8 expression was absent in our samples. In most cases, temporal and spatial patterns of chemokine receptor expression are connected with ligand-receptor specificity to result in a disease-specific pathology.42 An important question is the demonstration of a functional chemokine receptor expressed on skin-infiltrating lymphoma cells. Most studies have demonstrated the presence of chemokines or their receptors in situ but have failed to provide data on fresh tumor cell suspensions. One recent investigation demonstrated differentially expressed chemokine receptors CCR3 and CCR5 on infiltrating leukocytes but not neoplastic cells in Hodgkin disease lymph nodes.43 We were able to demonstrate receptor functionality on CD30+ tumor cells with an assay used in studies of CXCR4/SDF1-α interactions.22 In the presence of the CCR3 ligand eotaxin/CCL11, specific partial down-regulation of CCR3 in tumor cell suspensions was observed (Figure 3A).
Investigation of receptor functionality in fresh tumor cell suspensions is limited by both the low number and the heterogeneity of cells. To further address functionality of the CCR3 receptor, we used the CD30-expressing cutaneous lymphoma cell line Mac-1. Engagement of the CCR3 receptor by its ligand eotaxin led to a signal toward the cytoskeleton involved in cell migration: actin polymerization (Figure3B). Furthermore, directed migration of CD30+ lymphoma cells toward an eotaxin gradient was observed (Figure 3C).
To establish a relationship between CCR3 expression and Th-2 cytokine production, we assessed the cytokine expression profile of CCR3+ tumor cells. Earlier reports have demonstrated Th-2 cytokine mRNA in lesions of CD30+ CTCL.24 On the protein level, we observed strong expression of IL-4 by the majority of CCR3+ cells, while few cells expressed IFN-γ. This observation is compatible with a Th-2–like differentiation of CCR3+ skin-homing lymphoma cells.
The CCR3 ligand eotaxin/CCL11 is known to be produced by human dermal fibroblasts.44,45 Eotaxin/CCL11 not only has agonistic functions but is also a natural antagonist for CCR2.46 Our data demonstrate the presence of eotaxin/CCL11 protein in skin of CTCL lesions (Table 1; Figure 4). In CD30+ as well as in CD30− CTCL, we found expression of eotaxin/CCL11, which was associated with connective tissue cells, most likely fibroblasts, located around the tumor. Only in CD30+ CTCL was eotaxin/CCL11 expression also observed in aggregates of tumor cells. Eotaxin/CCL11 expression at the single-cell level was shown by intracellular eotaxin/CCL11 staining of fresh tumor cell suspensions (Figure 5). Eotaxin/CCL11 expression by lymphoma cells may lead to homotypic aggregation, observed as cohesive clusters of tumor cells, a characteristic of CD30+ anaplastic lymphomas,28 and an amplification of tumor cell homing to the skin. Down-regulation of the receptor in the presence of high eotaxin/CCL11 concentrations may keep lymphoma cells in skin. In this regard, it is of interest that epidermotropic CD30− CTCLs were shown to be associated with epidermal expression of IFN-γ–inducible protein-10 (IP-10) and monokine induced by IFN-γ (Mig).47 Thus, different sets of chemokines produced by different cellular constituents might have an impact on the nature of the malignant infiltrate.
In summary, we have shown functional CCR3 expression in CD30+ large-cell CTCL as well as expression of its ligand eotaxin in skin. Expression of CCR3 on CD30+ T cells may provide a link between the recruitment of lymphoma cells and their functional state as Th-2 cells. As suggested for breast cancer,48 pharmacological modulation of chemokine receptors may open the way to new treatment modalities.
We are especially grateful to LeukoSite for providing the 7B11 antibody. We thank B. Fruet, C. Dudli, and B. Mueller for technical assistance with immunohistochemical staining.
Prepublished online as Blood First Edition Paper, September 26, 2002; DOI 10.1182/blood-2002-02-0475.
Supported by grants from the Swiss and French Cancer Leagues (to F.O.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Frank O. Nestle, Department of Dermatology, University of Zürich Medical School, Gloriastrasse 31, 8091 Zürich, Switzerland; e-mail:nestle@derm.unizh.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal