We investigated the clinical activity of the farnesyl transferase inhibitor R115777 in 22 patients with chronic myelogenous leukemia (CML) in chronic, accelerated, or blastic phase and in 8 patients with myelofibrosis (MF) and 10 patients with multiple myeloma (MM). R115777 was administered at 600 mg orally twice daily for 4 weeks every 6 weeks. Seven patients with CML (6 in chronic phase, 1 in advanced phase) achieved complete or partial hematologic response. Four of them had a minor cytogenetic response. Responses were transient, with a median duration of 9 weeks (range, 3-23 weeks). Two patients discontinued therapy because of toxicity while in complete hematologic response. Two MF patients had a significant decrease in splenomegaly, one had normalization of white blood cell count and differential, and one became transfusion independent. One patient with MM had a reduction in monoclonal protein of 34%. Adverse events included nausea in 22 patients (55%; all grade 2 or lower) and fatigue in 19 (48%; grade 3 or higher in 1). Other grade 3 or 4 toxicities included skin rash (4 patients, 10%), peripheral neuropathy (2 patients, 5%), and liver toxicity (2 patients, 5%). Patients who responded to therapy had significantly higher plasma vascular endothelial growth factor (VEGF) concentrations prior to treatment than nonresponders. Plasma concentrations decreased significantly during therapy among responders. R115777 showed clinical activity in patients with CML and MF. The effect on VEGF needs to be further investigated to determine whether this might be a possible mechanism of action of R115777.
Introduction
The ras family of proto-oncogenes comprises a group of G-proteins that have the ability to bind guanine nucleotides.1,2 Ras is synthesized in the cytoplasm as a precursor protein that requires additional posttranslational modifications in order to attach to the inner surface of the plasma membrane, a prerequisite for Ras-mediated signal transduction. These modifications are accomplished by a prenylation reaction involving the attachment of a 15-carbon farnesyl group to the C-terminal cysteine residue. This reaction is mediated by an enzyme called farnesyl protein transferase (FPT).3 4 Alternatively, prenylation may be accomplished by addition of a 20-carbon geranylgeranyl isoprenoid mediated by geranylgeranyl-protein transferase (GGPT).
Ras mutations and Ras protein activation are frequent features of malignant transformation. Approximately 30% of human cancers have been associated with rasmutations.5,6 The frequency of rasmutations varies in hematologic malignancies, from 5% to 15% in acute lymphoblastic leukemia and up to 65% in chronic myelomonocytic leukemia.2,5,6 Ras activation may occur by mechanisms other than mutations. A prominent example is activation of Ras by thebcr/abl chimeric gene.7,8 Therefore, inhibition of Ras activation has been investigated as an antineoplastic therapy.9 One approach to Ras inhibition is inhibition of FPT.10-12 R115777 (Zarnestra, Titusville, NJ) is a potent nonpeptidomimetic FPT inhibitor (FTI) with significant antitumor effects in preclinical studies.13
In this study, we investigated the activity of R115777 in patients with Philadelphia chromosome (Ph)–positive chronic myeloid leukemia (CML), myelofibrosis (MF), or multiple myeloma (MM). Plasma concentrations of vascular endothelial growth factor (VEGF) have been found to be elevated in CML,14,15 and increased expression of VEGF correlates with poor prognosis.15 In addition, one of the proposed effects of FPT inhibition is suppression of angiogenesis with decreased expression of VEGF.16 Because of the clinical significance of VEGF in CML, we investigated whether R115777 has any in vivo effect on VEGF and other angiogenic factors to determine whether any clinical effect may be mediated through this mechanism.
Patients and methods
Patients
Adult patients aged 18 years or older with CML, MF, or MM were eligible for this study. Eligibility criteria were as follows: (1) Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2; (2) serum creatinine level lower than 2 mg/dL and total bilirubin level lower than 2 mg/dL; (3) no evidence of severe heart disease (New York Heart Association [NYHA] class III or IV); and (4) signed informed consent. Patients were eligible regardless of whether or not they had ras mutations. Patients with CML were eligible whether they were in chronic (CP), accelerated (AP), or blastic phase (BP). Patients in CP should have received prior therapy with interferon-α (IFN-α) and failed to achieve or sustain a hematologic or cytogenetic response, or be intolerant to IFN-α. Patients in AP or BP were eligible regardless of their prior therapy. At the time the study was conducted, imatinib mesylate (Gleevec) was not widely available; therefore prior therapy with imatinib was not required. Patients with MM were required to be refractory or to have relapsed after therapy with a regimen such as VAD (vincristine, doxorubicin, and dexamethasone). Patients who had not received a stem cell transplant were either ineligible or had refused transplantation. Patients with MM were required to have a quantifiable serum or urine paraprotein, bone marrow plasmacytosis of 5% or more, and an absolute neutrophil count of 109/L or higher and a platelet count of 75 × 109/L or higher.
Prior to the start of therapy, all patients had a complete history taken and received a complete physical examination, a complete blood count, SMA-12 (total protein, albumin, calcium, phosphorus, blood urea nitrogen [BUN], creatinine, glucose, uric acid, total bilirubin, alkaline phosphatase, lactate dehydrogenase, and alanine aminotransferase), a bone marrow (BM) aspiration (and biopsy when indicated), and cytogenetics. The monoclonal protein spike was measured in patients with multiple myeloma. All of these studies were repeated periodically while the patient was receiving treatment.
Treatment schedule
Patients received R115777 at a starting dose of 600 mg administered orally twice daily for 4 weeks every 6 weeks (ie, 4 weeks on, 2 weeks off). Patients who developed grade 3 or higher toxicity (graded according to the NCI Common Toxicity Criteria v2.0) had their treatment interrupted. For patients who had evidence of antitumor activity, therapy was reinstituted upon improvement of the toxicity to grade 1 or lower with a dosage reduction to 300 mg administered orally twice daily. Therapy was interrupted for patients with CML in CP or MM if their absolute neutrophil count was lower than 109/L or their platelet count was lower than 50 × 109/L. Therapy was resumed at the same dose if recovery above these levels occurred within 2 weeks, or at 300 mg administered orally twice daily if recovery was longer than 2 weeks. Patients in AP or BP of CML and patients with MF could continue therapy uninterrupted with counts below these levels when it was considered that the cytopenias were related to the disease. Patients with CML could have hydroxyurea for the first month of therapy for white blood cell (WBC) counts higher than 30 × 109/L or anagrelide for platelet counts higher than 700 × 109/L. Hydroxyurea was not allowed after the first month of therapy, but anagrelide could be continued beyond this time if needed. Patients who achieved a response could receive continued treatment for up to 12 months. The dose of R115777 could be adjusted to achieve an absolute neutrophil count of 109/L or higher and a platelet count of 50 × 109/L or higher, and the schedule could be modified as needed (ie, 14-day treatment every 28 days) to achieve this target.
Definitions of response and disease stages
AP was defined as the presence of any one of the following: (1) percentage of peripheral blood (PB) or BM blasts 15% or higher; (2) percentage of PB or BM blasts plus promyelocytes 30% or higher; (3) percentage of PB or BM basophils 20% or higher; (4) platelet count lower than 100 × 109/L unrelated to therapy; (5) clonal evolution; or (6) hemoglobin level lower than 7 g/dL unrelated to therapy or bleeding. Blast phase was defined as the presence of 30% or more blasts in the PB or BM or extramedullary disease outside the liver or spleen.
Responses in CML were defined as previously reported.17Briefly, a complete hematologic response (CHR) was defined as WBC count lower than 10 × 109/L, platelet count lower than 450 × 109/L, no immature PB cells (blasts, promyelocytes, myelocytes), and disappearance of all signs and symptoms of leukemia. This was further categorized by the best cytogenetic response: (1) complete: Ph+ 0%; (2) partial: Ph+ 1% to 34%; (3) minor: Ph+ 35% to 90%. A major cytogenetic response included complete plus partial cytogenetic responses (Ph+ less than 35%). Partial hematologic response (PHR) was defined as CHR except for the persistence of immature cells in PB and/or persistent splenomegaly or thrombocytosis (> 450 × 109/L) but at least 50% less than pretreatment.
In MM, a complete response (CR) was defined as disappearance of serum and/or urine monoclonal protein on 2 determinations at least 4 weeks apart, with fewer than 5% plasma cells in the BM and PB, resolution of any soft-tissue plasmacytomas present at the start of therapy, and resolution of all signs and symptoms of disease. A partial response (PR) was defined as a reduction of the monoclonal protein by 50% or more on 2 determinations at least 4 weeks apart, reduction in soft-tissue plasmacytoma by 50% or more, and decrease in bone pain from grade 2 to grade 1 or lower.
In MF, CR was defined as normalization of the PB for at least 4 weeks, with an absolute neutrophil count of more than 109/L with no immature cells, and platelet count higher than 100 × 109/L. Partial response was considered as the presence of at least 2 of the following: (1) hemoglobin level increase by 2 g/dL or more and to more than 9 g/dL, plus independence from transfusions; (2) platelet count increase by 100% and to more than 50 × 109/L, plus transfusion independence; (3) neutrophil count increase by 100% and to more than 109/L; (4) reduction of splenomegaly by 50%.
Laboratory correlative studies
Assessment of ras mutations was performed by sequencing. Enzyme-linked immunosorbent assay (ELISA) for VEGF, basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), and tumor necrosis growth factor-α (TNF-α) was performed using commercially available kits from R&D Systems (Minneapolis, MN). We followed the protocols recommended by the manufacturer. Briefly, plasma was collected using EDTA (ethylenediaminetetraacetic acid) and stored at −82°C. Patients' plasma samples were added to separate microplates, each containing a specific monoclonal antibody. The mixtures were incubated at room temperature for 2 hours. The plates were washed 3 times to remove any unbound substances. Enzyme-linked polyclonal antibodies specific for each protein were added to the wells, and mixtures were incubated at room temperature for 2 hours followed by another washing to remove any unbound antibody or enzyme reagent. A substrate solution was added to the wells, and a blue color developed. The intensity of the blue color was proportionate to the amount of cytokine bound in the initial step. The color development was stopped, and the intensity of the color was measured and compared with a standard curve. Reading was done at 450-nm wavelength per the manufacturer's recommendations.
Statistical analysis
The Kruskal-Wallis test was used to compare values between groups.
Results
Between February 2001 and October 2001, 40 patients were treated. These included 22 patients with CML, 10 with MM, and 8 with MF. The clinical characteristics of patients by diagnosis are presented in Table 1. Thirteen patients were investigated for N- or K-ras mutations; only 1 patient (8%; CML-BP) had a mutation (K-ras). The results will be described by disease group.
Clinical characteristics of patients by disease
| . | CML (n = 22) . | MM (n = 10) . | MF (n = 8) . |
|---|---|---|---|
| Age, median (range), y | 53 (26-79) | 59 (44-79) | 58 (37-76) |
| ECOG performance status, median (range) | 1 (0-2) | 1 (0-1) | 1 (0-2) |
| No. prior therapies, median (range)* | 2 (1-4) | 4 (1-7) | 2 (0-5) |
| WBC count, median (range), × 109/L | 16 (1.8-66.5) | 4.4 (2.8-7.3) | 5.3 (2.1-108.4) |
| Platelet count, median (range), × 109/L | 71 (5-3895) | 232 (100-411) | 37 (7-626) |
| Hemoglobin level, median (range), g/L | 9.8 (7.1-15.4) | 10.2 (8.5-13.8) | 8.7 (6.7-13.5) |
| Rasmutations | |||
| N-ras | 0/6 | 0/3 | 0/4 |
| K-ras | 1/6 | 0/3 | 0/4 |
| CML phase | |||
| CP | 10 | NA | NA |
| AP | 6 | NA | NA |
| BP | 6 | NA | NA |
| Monoclonal protein level, median (range), g/dL | NA | 2.7 (1.3-6.2) | NA |
| Bone marrow plasma cells, median (range), % | NA | 30 (5-50) | NA |
| . | CML (n = 22) . | MM (n = 10) . | MF (n = 8) . |
|---|---|---|---|
| Age, median (range), y | 53 (26-79) | 59 (44-79) | 58 (37-76) |
| ECOG performance status, median (range) | 1 (0-2) | 1 (0-1) | 1 (0-2) |
| No. prior therapies, median (range)* | 2 (1-4) | 4 (1-7) | 2 (0-5) |
| WBC count, median (range), × 109/L | 16 (1.8-66.5) | 4.4 (2.8-7.3) | 5.3 (2.1-108.4) |
| Platelet count, median (range), × 109/L | 71 (5-3895) | 232 (100-411) | 37 (7-626) |
| Hemoglobin level, median (range), g/L | 9.8 (7.1-15.4) | 10.2 (8.5-13.8) | 8.7 (6.7-13.5) |
| Rasmutations | |||
| N-ras | 0/6 | 0/3 | 0/4 |
| K-ras | 1/6 | 0/3 | 0/4 |
| CML phase | |||
| CP | 10 | NA | NA |
| AP | 6 | NA | NA |
| BP | 6 | NA | NA |
| Monoclonal protein level, median (range), g/dL | NA | 2.7 (1.3-6.2) | NA |
| Bone marrow plasma cells, median (range), % | NA | 30 (5-50) | NA |
NA indicates not applicable.
Excluding hydroxyurea or supportive care (eg, transfusions).
Chronic myeloid leukemia
Twenty-two patients with CML in CP (n = 10), AP (n = 6), or BP (n = 6) were treated. The median time from diagnosis to therapy was 23 months (range, 5-82 months). All patients had received at least one prior therapy for CML besides hydroxyurea, and the median number of prior regimens was 2 (range, 1-4 regimens). Twenty patients (91%) had received prior IFN-α; the 2 patients who had not received IFN-α were in BP and had failed other therapies (imatinib mesylate and troxacitabine, respectively). Two patients received pegylated IFN-α after failing therapy with conventional IFN-α. Seventeen patients (77%) had received imatinib and were resistant, refractory, or intolerant to it. Six patients had received other investigational agents, including troxacitabine (n = 2), homoharringtonine plus ara-C (n = 2), decitabine (n = 1), and oral idarubicin (n = 1); 1 patient had relapsed in AP after an allogeneic bone marrow transplantation.
Patients received R115777 therapy for a median of 8 weeks (range, 1-25 weeks). Seven patients (32%; 95% confidence interval [CI], 0.14-0.55) achieved a complete (n = 5) or partial (n = 2) hematologic response. All but one of the responses occurred among patients in CP. Thus, 6 of 10 patients in CP (60%; 95%CI, 0.26-0.88) responded. All responses occurred in the absence of hydroxyurea and only one patient (PHR) required anagrelide. Among responders, 4 (1 AP, 3 CP) had reductions in the percentage of Ph+ metaphases. Three of these patients achieved a minor cytogenetic response; the fourth started with 90% of metaphases Ph+ and decreased on therapy to 65%. The responses were transient, with a median duration of response of 9 weeks (range, 3-23 weeks). Five patients discontinued therapy because of loss of response and 2 because of toxicity. Two patients had an ongoing CHR (one of them also had a minor cytogenetic response) at the time treatment was discontinued because of toxicity (fatigue and skin rash, respectively). These patients received therapy for 7 and 12 weeks, respectively. All other patients had lost their response at the time they stopped therapy. Eleven patients (50%; CP n = 8, AP n = 3) were alive after a median follow-up of 11 months. The median survival was 42 weeks. Among patients in CP, the median survival has not been reached. Four patients have transformed to accelerated (n = 2) or blastic (n = 2) phase, and 2 of them died after transformation.
Myelofibrosis
Of the 8 patients with MF, 2 had received therapy with transfusion support only, 3 had received recombinant human erythropoietin (rhEPO) and transfusion support, and 3 had received multiple therapies including hydroxyurea (n = 2), interferon (n = 2), rhEPO, 9-nitrocamptothecin, thalidomide, and anabolic steroids (n = 1 each). Six patients had splenomegaly, with a median of 12.5 cm below the costal margin (BCM) (range, 3-18 cm), and 3 had hepatomegaly of 6, 8, and 15 cm BCM.
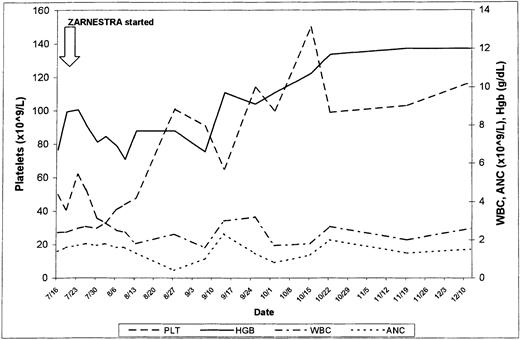
Four patients received only 1 course of therapy: 2 discontinued therapy because of toxicity (grade 3 skin rash), 1 was lost to follow-up, and 1 had progression to acute myeloid leukemia (AML). Two patients were treated for 22 and 28 weeks, respectively, and had a reduction in spleen size from 18 cm BCM (in both cases) to 9 and 10 cm BCM, respectively. Both remained packed red blood cell (PRBC)–transfusion dependent. At the time of writing, 2 patients are still receiving therapy 24 and 33 weeks after being included in this trial. One patient had received thalidomide and had become PRBC-transfusion independent with that therapy. However, the patient developed progressive splenomegaly, with an increase in WBC count and a differential left shift. Thalidomide was discontinued and R115777 was started. The bone marrow at the time showed extensive myelofibrosis. The spleen decreased in size from 6 cm BCM to undetectable, the WBC count normalized, and the immature forms disappeared from the peripheral blood. The patient continues to be RBC-transfusion independent. The second patient (Figure1) had received rhEPO and thalidomide, had not responded, and was requiring RBC transfusions at least once every week. Two months after the start of therapy, he became transfusion independent and his platelet count improved from 50 × 109/L to 150 × 109/L. The response has been sustained after 33 weeks of therapy.
Three patients have died, at 6, 12, and 39 weeks from the start of therapy with R115777. Five patients are alive, with a median follow-up of 32 weeks (range, 22-49 weeks).
Multiple myeloma
The 10 patients with refractory or relapsed MM had received a median of 4 prior treatment regimens (range, 1-6 regimens), including VAD in 7 patients, thalidomide in 7, and autologous stem cell transplantation in 3. The median time on therapy was 7 weeks (range, 2-14 weeks). The median monoclonal paraprotein spike at the start of therapy was 2.7 g/dL (range, 1.3-6.2 g/dL), compared with 2.9 g/dL (range, 2.1-7.3 g/dL; P = .10) at the end of therapy. One patient had a reduction in monoclonal protein of 34% and one had a reduction of 6%. All others had no change or an increase in monoclonal protein.
Six patients died; median time to death was 18.5 weeks (range, 4-48 weeks) from the start of therapy. At the time of writing, 4 patients are alive, with a median follow-up of 41 weeks (range, 12-48 weeks).
Toxicity
The median duration of therapy was 7 weeks (range, 1-28 weeks). Toxicity is presented in Table 2. Nausea and vomiting were the most common side effects, occurring in 22 patients (55%), but these side effects were always grade 2 or lower and responded to symptomatic treatment. Fatigue was noted in 19 patients (48%) and resulted in discontinuation of therapy in 1 patient (3%) with CML after 12 weeks on therapy while still in CHR with a minor cytogenetic response. The symptoms persisted after a dose reduction to 200 mg administered orally twice daily and finally resolved 4 weeks after treatment was discontinued. Grade 3 or 4 skin rash was noted in 4 patients (10%), including 1 patient with CML who had to discontinue therapy while still in CHR. This side effect in general was in the form of a diffuse, pruritic rash, which recurred upon rechallenge at a lower dose. Peripheral neuropathy grade 3 or 4 was seen in 3 patients (8%) in the form of painful dysesthesias in the lower extremities. Symptoms resolved after discontinuation of the therapy, but one patient developed progressive peripheral neuropathy that had not resolved at the time of her death from progressive disease 3 months after discontinuation of R115777. Hematologic toxicity can more uniformly be assessed among patients who started with normal or high platelets (n = 22) and neutrophils (n = 26), or hemoglobin (n = 11) at the start of therapy. Grade 3 or higher neutropenia was seen in 15 patients (58%), thrombocytopenia in 6 (27%), and anemia in 2 (18%). Twenty-three patients (58%) required dose interruptions and/or reductions because of myelosuppression or extramedullary toxicity.
Adverse events related to treatment with R115777
| . | No. (%) of patients affected . | |
|---|---|---|
| Any grade . | Grade 3 or 4 . | |
| Nausea/vomiting | 22 (55) | 0 |
| Fatigue | 19 (48) | 1 (3) |
| Skin rash | 13 (33) | 4 (10) |
| Diarrhea | 13 (33) | 0 |
| Pain | 10 (25) | 0 |
| Elevated transaminase/bilirubin levels | 9 (23) | 2 (5) |
| Elevated creatinine levels | 9 (23) | 0 |
| Peripheral neuropathy | 7 (18) | 3 (8) |
| . | No. (%) of patients affected . | |
|---|---|---|
| Any grade . | Grade 3 or 4 . | |
| Nausea/vomiting | 22 (55) | 0 |
| Fatigue | 19 (48) | 1 (3) |
| Skin rash | 13 (33) | 4 (10) |
| Diarrhea | 13 (33) | 0 |
| Pain | 10 (25) | 0 |
| Elevated transaminase/bilirubin levels | 9 (23) | 2 (5) |
| Elevated creatinine levels | 9 (23) | 0 |
| Peripheral neuropathy | 7 (18) | 3 (8) |
Effects on angiogenic factors
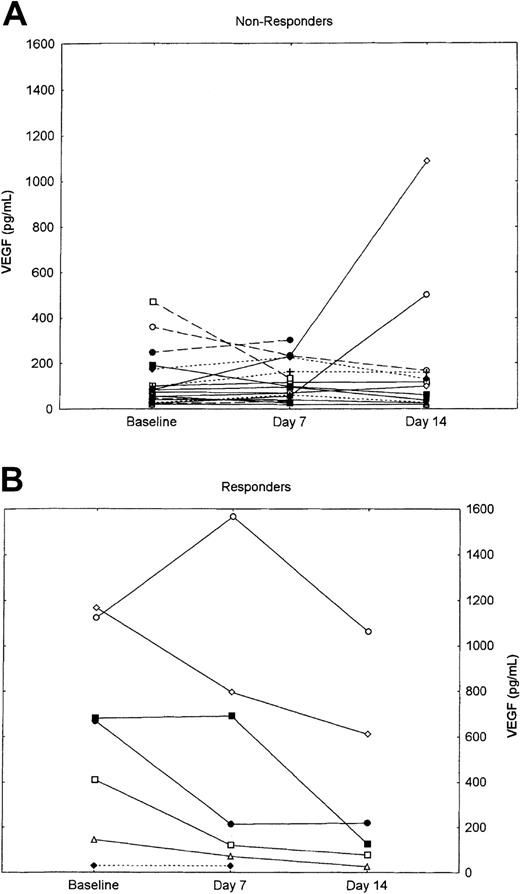
Plasma concentrations of angiogenic factors, including VEGF, bFGF, HGF, and TNF-α, were measured at baseline and after 7 and 14 days of therapy. Sequential evaluation was available for 25 patients (63%): 15 with CML, 6 with MM, and 4 with MF. As previously reported, plasma concentrations of VEGF, bFGF, and HGF were elevated at baseline among patients with CML.14 Similar increments were seen among patients with MM and MF and were not statistically significantly different compared with patients with CML. Sequential measures did not show any significant change during the first 2 weeks of therapy (Table3). However, when the change in VEGF concentrations during the first 14 days of therapy was evaluated for responders (ie, CML patients with at least a PHR, or myelofibrosis with hematologic improvement) vs nonresponders, a statistically significant decline was observed in patients who responded to therapy compared with those who did not respond (Figure 2). As shown, this difference is mostly attributable to very high baseline concentrations among patients who responded (median, 668.8 pg/mL; range, 28.8-1166.3 pg/mL) compared with nonresponders (median, 78.88 pg/mL; range, 21.2-469.8 pg/mL; P = .009). The only value among responders within the range for healthy individuals as identified in our laboratory14 was for a patient with myelofibrosis; the median concentration at the start of treatment for patients with CML who responded was 674.9 pg/mL (range, 144.9-1166.3 pg/mL), compared with 98.6 pg/ml (range, 19.6-1085 pg/mL; P = .001) for nonresponders with CML. There was no significant difference in the values after 7 and 14 days of therapy between responders and nonresponders. No significant difference was observed in any of the other angiogenic factors analyzed.
Plasma concentrations of angiogenic factors in patients treated with R115777
| . | Plasma concentration, median, pg/mL . | ||
|---|---|---|---|
| Baseline . | Day 7 . | Day 14 . | |
| VEGF | 97.9 | 108.2 | 122.8 |
| bFGF | 16.3 | 11.7 | 14.1 |
| HGF | 926.8 | 933.9 | 881.6 |
| TNF-α | 7.16 | 7.06 | 7.42 |
| IFN-α | 6.62 | 6.68 | 6.56 |
| VEGF responders3-150 | 668.8 | 213.1 | 173.1 |
| VEGF nonresponders | 78.8 | 96.6 | 108.3 |
| . | Plasma concentration, median, pg/mL . | ||
|---|---|---|---|
| Baseline . | Day 7 . | Day 14 . | |
| VEGF | 97.9 | 108.2 | 122.8 |
| bFGF | 16.3 | 11.7 | 14.1 |
| HGF | 926.8 | 933.9 | 881.6 |
| TNF-α | 7.16 | 7.06 | 7.42 |
| IFN-α | 6.62 | 6.68 | 6.56 |
| VEGF responders3-150 | 668.8 | 213.1 | 173.1 |
| VEGF nonresponders | 78.8 | 96.6 | 108.3 |
P = .002. All other P's were not significant. (P's are based on change in plasma concentrations from baseline.)
Change in VEGF plasma concentrations in nonresponders versus responders.
(A) Nonresponders. (B) Responders. Solid line indicates CML; dashed line, M; and dotted line, MF.
Change in VEGF plasma concentrations in nonresponders versus responders.
(A) Nonresponders. (B) Responders. Solid line indicates CML; dashed line, M; and dotted line, MF.
Discussion
R115777 is a nonpeptidomimetic inhibitor of FPT that has demonstrated activity in patients with acute myeloid leukemia (AML)18 and myelodysplastic syndromes (MDS).19 20 Our study demonstrated its modest activity in patients with CML and MF. No activity was noted in patients with MM.
Preclinical studies have demonstrated significant anti-CML activity of FTIs.21,22 Peters et al demonstrated inhibition of proliferation of bcr/abl-transformed BaF3 cells and primary human CML cells after incubation with SCH66336, another nonpeptidomimetic FTI.21 Mice treated with SCH66336 after being injected with bcr/abl-BaF3 cells survived, whereas those who received no treatment after the injection of these cells died within 4 weeks.21 Similar results were obtained in abcr/abl-positive acute lymphoblastic leukemia murine model using SCH66336.22 In addition, SCH66336 has been reported to overcome resistance to imatinib. Hoover et al reported that SCH66336 inhibited proliferation of imatinib-resistant cell lines and hematopoietic colony formation from patients with CML unresponsive to imatinib,23 and Nakajima et al demonstrated apoptosis induced by SCH66336 in imatinib-resistant cells.24 In this clinical trial, we report demonstrable albeit modest activity in 33% of patients with CML.
Most of the responses were transient, but it is possible that therapy may have been discontinued prematurely in some patients. The study allowed for the use of hydroxyurea only for the first 4 weeks, and responding patients were withdrawn (or discontinued) from therapy at the first sign of rising WBC count. This frequently occurred at the end of the periods when R115777 was not taken. Since FTIs are mostly cytostatic, it is reasonable to hypothesize that a more prolonged exposure may be required to demonstrate a sustained response. Thus, alternative schedules, including long-exposure schedules with lower doses, might be preferable and are currently being investigated. Interestingly, we did not observe responses among patients in BP, whereas Karp et al18 reported a partial response in 2 of 2 patients with Ph+ CML in BP. The clinical results reported here with R115777 and the reported synergy with imatinib23 24 provide the basis for ongoing studies combining FTIs and imatinib.
The responses observed in patients with MF are intriguing. Although 2 patients showed only modest (but clinically significant) decreases in splenomegaly, 2 patients had a more notable response with normalization of the peripheral blood abnormalities, 1 of them (Figure 1) becoming transfusion independent. R115777 has been reported to have significant in vitro activity in MF with myeloid metaplasia and other myeloproliferative disorders. Concentrations of 5 to 24 nM selectively inhibited the in vitro growth of myeloid and megakaryocytic progenitor colonies obtained from patients with MF, essential thrombocytemia, and polycythemia vera.25 Further exploration of this agent in myeloproliferative disorders is ongoing.
The minimal activity in MM was disappointing. In vitro studies have suggested that FTIs may have significant activity in this disease. Bolick et al reported significant activity of FTI-277 in human myeloma cell lines.26 A different FTI, perillic acid, showed significant activity against myeloma cell lines and primary cells from patients while relatively sparing nonmyeloma bone marrow elements.27 Alsina et al recently reported results on 12 evaluable patients with MM treated with this agent.28 Half of the patients had a reduction in the monoclonal protein of less than 25%, compatible with disease stabilization. The dose and schedule used in our trial may not have been ideal and a more prolonged therapy may be required. Alsina et al used a dosage of 300 mg twice daily for 3 weeks, repeated every 4 weeks. Four of the 6 patients with a response were able to stay on therapy for at least 4 cycles (ie, 16 weeks), whereas the median time on treatment for our patients was 7 weeks (range, 2-14 weeks). Therefore, schedules that allow for a more prolonged administration of R115777 should be investigated further.
The actual mechanism of action of FTIs is not yet clear. Clinical responses in this trial and others18-20 have been unrelated to Ras mutations. As mentioned earlier, Ras can be activated by other mechanisms. However, farnesylation is not unique to Ras,2,11,29 and Ras-independent mechanisms of cell growth inhibition by FTI, such as gain of RhoB that has been prenylated by GGPT30 and inhibition of the mitotic kinasins CENP-E and CENP-F,31 have been reported. Additionally, Ras can be prenylated by GGPT.2,11,29 Although combinations of FTIs and GGPTase inhibitors may be synergistic,26,32 initial animal studies have resulted in significant toxicity,32which may be related to the ubiquitous nature of proteins requiring GGPT. Thus, dual inhibition is unlikely to be beneficial in the clinic.
One recently proposed mechanism of action of FTIs is through inhibition of angiogenesis. Several investigators have reported that different FTIs inhibit angiogenesis in a variety of models.16,33-36Ras activation (eg, via mutations) up-regulates VEGF expression,37,38 and inhibition of Ras through FPT inhibition has led to significant decrease in the expression and secretion of VEGF.16,33-36 We have previously reported a significant increase in angiogenesis in patients with acute and chronic leukemias.14 Patients with CML had the highest increase in VEGF plasma concentrations, and increased cellular concentrations of VEGF are associated with an adverse outcome, independent of other prognostic factors.15 These results suggest a possible role of VEGF in the pathogenesis and or progression of CML. In this report, we identified a significant decrease in the plasma concentrations of VEGF among patients who responded to R115777. In addition, patients who responded had a higher baseline VEGF plasma concentration. The sample size studied is small and this observation will require further confirmation in other studies. However, these results showed for the first time in vivo a possible antiangiogenic effect of R115777 and suggest that patients with high concentrations of VEGF might be more likely to respond to this agent.
As mentioned, toxicity limited continuation of therapy in several patients, including some having an adequate response. The starting dose selected for this trial was within the maximum tolerated dosage reported in other trials. However, the dosage 600 mg twice daily had not been given on a 4-week schedule. Karp et al,18 using a 21-day schedule, observed dose-limiting toxicities at 1200 mg administered orally twice daily, although dosages of 900 mg administered orally twice daily were associated with frequent dose interruptions. Similarly, Kurzrock et al reported frequent dose interruptions using dosages of 900 mg administered orally twice daily20 in patients with MDS. In both studies, the majority of patients requiring dose interruptions were older than 70 years. However, considering that significant FPT inhibition occurs at dosages of 300 mg twice daily and that no clear correlation existed between dose and response, lower doses may be more desirable, particularly for prolonged therapy.
In summary, R115777 used as a single agent had activity of short duration in one third of previously treated CML patients. Considering the preclinical data discussed above, exploration of combination therapy with FTI and imatinib mesylate is warranted in CML. The effect observed on VEGF concentrations is interesting and will need confirmation in a larger population. If these results can be corroborated, the effect on VEGF may suggest a new and important therapeutic target in CML. The data in MF are intriguing, and further investigations in this disease are warranted.
Prepublished online as Blood First Edition Paper, October 31, 2002; DOI 10.1182/blood-2002-07-1973.
Supported in part by grant 2057-01 from the Leukemia and Lymphoma Society (J.C.), research funding from Johnson & Johnson Pharmaceutical Research & Development (J.C. and H.K.), and National Cancer Institute grant 1R21-CA91518A (R.K.). Jorge Cortes is a Clinical Research Scholar for the Leukemia & Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jorge Cortes, Department of Leukemia, MD Anderson Cancer Center, 1515 Holcombe Blvd, Box 428, Houston, TX 77030; e-mail: jcortes@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal