Juvenile myelomonocytic leukemia (JMML) is a rare pediatric malignancy. Hematopoietic stem cell transplantation (SCT) is the only curative approach. However, relapse after SCT remains the major cause of treatment failure. Unlike most other pediatric malignancies, JMML may be susceptible to a graft-versus-leukemia (GVL) effect, although, unlike chronic myeloid leukemia, reports of response to donor lymphocyte infusions (DLIs) remain scanty. This is the first report that describes the successful treatment of relapsed JMML with DLI in the absence of further chemotherapy and provides definite proof of a GVL effect in JMML.
Introduction
Juvenile myelomonocytic leukemia (JMML) is a rare disorder, accounting for about 2% of all childhood hematologic malignancies.1,2 It includes a heterogeneous spectrum of myelodysplastic/myeloproliferative diseases, which has proved difficult to classify and segregate into distinct clinical syndromes. In 1997, an international consensus panel agreed on common clinical and laboratory criteria to diagnose JMML.3
The initial course of JMML is varied with approximately one third of patients developing a rapidly progressive course leading to early death, yet occasional patients remain stable without any treatment.1 Hence, although survival for 12 years after diagnosis without treatment has been described, the 10-year disease-free survival without stem cell transplantation (SCT) is only 6%, and median survival time is approximately 10 months.2,4 Treatment of JMML with chemotherapy has been unsuccessful with no sustained response to even high-dose chemotherapy protocols.5,6 Hematopoietic SCT has offered a chance of cure, but relapse rates and transplantation-related mortality remain higher than with other hematologic malignancies.4,7-9 However, unlike most other pediatric leukemias, JMML may be susceptible to a graft-versus-leukemia (GVL) effect with lower relapse rates following reduced graft-versus-host disease (GVHD) prophylaxis,4 in the presence of acute GVHD7,8 and chronic GVHD.10,11 There are a few reports of post-SCT relapse of JMML responding to withdrawal of immunosuppression,8,10,12 but there has been only one successful report of the use of donor lymphocyte infusion (DLI) in this setting, and this treatment was given in conjunction with further chemotherapy.9 Here we describe a child who relapsed early after transplantation but who has subsequently gone into long-term remission with the use of DLI without any further chemotherapy.
Study design
A 6-month-old girl presented with diarrhea and vomiting, as well as bleeding from her right nipple. On assessment she had fallen from the 50th to the 3rd centile for both height and weight and was pale with marked hepatosplenomegaly. She was anemic, had a leucocytosis with a monocyte count of 18.5 × 109/L, and was mildly thrombocytopenic. A blood film revealed immature neutrophils and an increase in myelomonocytic cells, typical of a diagnosis of JMML. A bone marrow aspirate revealed a hypercellular marrow with mild dysplastic changes and 16% blast cells. Bone marrow cytogenetics revealed 84% of cells showing a 45 XY −7 karyotype. Hemoglobin electrophoresis revealed 7.3% fetal hemoglobin (HbF). Hypersensitivity to granulocyte-macrophage colony-stimulating factor and spontaneous colony formation was demonstrated in granulocyte-macrophage colony-forming unit cultures.
Over the next few months, the white cell count remained stable, but she required 2 hospital admissions for respiratory infections and had continued failure to thrive. It was decided to treat with 2 cycles of high-dose acute myeloid leukemia (AML)–type chemotherapy to debulk her disease prior to bone marrow transplantation. Following recovery of blood counts, splenomegaly persisted and the blood film continued to show features of JMML.
One month later (6 months after diagnosis) the patient underwent an unrelated donor SCT from a male donor fully matched at HLA A, B, C, DRB1, DQB1 loci. Conditioning consisted of Campath-1H 1 mg/kg, busulphan 16 mg/kg, cyclophosphamide 120 mg/kg, and melphalan 140 mg/m2. GVHD prophylaxis consisted of methotrexate (15 mg/m2, 10 mg/m2 on days +3, +6 with folinic acid rescue), and cyclosporin. T-replete bone marrow containing 16.6 × 106/kg CD34+ cells and 1.2 × 108/kg CD3+ cells were infused.
The immediate posttransplantation period was complicated by an episode of pneumatosis intestinalis. This condition was treated with 10 days of broad-spectrum antibiotics; nil by mouth and total parental nutrition, and day +11 methotrexate was omitted. Neutrophil recovery to 0.5 × 109 occurred on day +14, and engraftment was confirmed by XY fluorescent in situ hybridization (FISH) on day +19 and found to be 100% donor in both mononuclear (MNC) and polymorphonuclear (PMN) cell fractions.
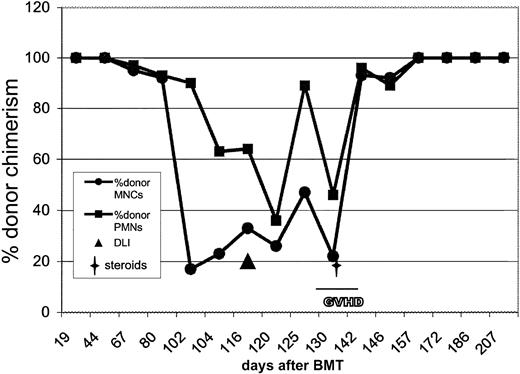
Cyclosporin was weaned to zero between days +26 and + 56 in an attempt to exploit a GVL effect. Grade I skin GVHD, not requiring treatment, occurred on day +40. The patient was discharged from hospital on day +48. On day +68, XY FISH on the blood showed recurrence of host hemopoiesis (5% in MNCs and 1.5% in PMNs). By day +102 this had progressed to 83% host MNCs and 10% host PMNs. Clinically, she remained well, but her splenomegaly had increased and she was febrile. A blood film showed reappearance of abnormal monocytes and basophils, although counts remained within the normal range. A repeat bone marrow aspirate was of normal appearance, but FISH analysis revealed 52% of interphase cells to be monosomy 7. A diagnosis of relapsed JMML was therefore made.
On day +114, the patient was given a DLI containing 1 × 108 CD3+ cells/kg. Ten days later she developed GVHD as manifested by a florid skin rash and abnormal liver function tests. She was given a brief course of steroids for 1 week for the GVHD. However, her spleen continued to increase in size following DLI, and she remained persistently febrile. An emergency splenectomy was performed 2 weeks after DLI for respiratory compromise and malignant hypersplenism; respiratory symptoms improved and fever resolved promptly. On day 24 after DLI she became profoundly neutropenic. A bone marrow aspirate at this stage was acellular but with no evidence of disease. She was started on granulocyte colony-stimulating factor (G-CSF), which was given for 1 week after which counts normalized.
By day +35 after DLI she was back at 100% donor MNCs and PMNs (Figure1). Her cutaneous GVHD had improved (now grade 1), and her liver function tests were normal. At 17 months after DLI she remains well, continues to thrive, and has 100% donor chimerism and no residual GVHD. Her blood counts have been normal for the past 15 months, and her most recent blood counts show hemoglobin of 110 g/L (11.0 g/dL); white cell count of 11.85 × 109/L (neutrophils 5.10 × 109/L, lymphocytes 5.81 × 109/L, monocytes 0.47 × 109/L) and a platelet count of 601 × 109/L.
Graphic depiction of the fall in donor chimerism at relapse of JMML after transplantation and the response to DLI with prompt and sustained return to donor chimerism.
This patient initially had recurrence of host hemopoiesis at day 68 after transplantation. By day +102 this had progressed to 83% host MNCs and 10% host PMNs. DLI was given on day 114. GVHD that followed the DLI was treated with steroids for 1 week from day +138 to day +145. By day 35 following the DLI (day 148 after transplantation), there was return to 100% donor chimerism, and the patient remains well and disease free 17 months after transplantation. BMT indicates bone marrow transplantation.
Graphic depiction of the fall in donor chimerism at relapse of JMML after transplantation and the response to DLI with prompt and sustained return to donor chimerism.
This patient initially had recurrence of host hemopoiesis at day 68 after transplantation. By day +102 this had progressed to 83% host MNCs and 10% host PMNs. DLI was given on day 114. GVHD that followed the DLI was treated with steroids for 1 week from day +138 to day +145. By day 35 following the DLI (day 148 after transplantation), there was return to 100% donor chimerism, and the patient remains well and disease free 17 months after transplantation. BMT indicates bone marrow transplantation.
Results and discussion
Relapse of JMML after SCT remains the major cause of treatment failure. This report describes the first response of relapsed JMML to DLI in the absence of further chemotherapy and provides definite proof of a GVL effect in JMML. The antileukemia effect of GVHD is well documented in acute and chronic leukemia.13-15 DLI has been particularly successful in the treatment of chronic myeloid leukemia, in which induction of a GVL effect has led to subsequent disease remission in 73% of patients.16,17 Matthes-Martin et al9 have described the use of DLI for relapsed JMML previously in 2 cases. In the first case DLI (1 × 107CD3+ cells) was unsuccessful, and the child received a second transplant from which she developed grade IV GVHD but remains in long-term remission. The second child relapsed on day +68 after T-cell–replete unrelated donor transplantation and did not respond to withdrawal of immunosuppression. Subsequently, the child underwent splenectomy and received 6-mercaptopurine, followed by 2 DLIs of 5 × 105 and 1 × 106 CD3+cells. This patient remained in complete remission 9 months later. That case has striking similarities to ours. The patient also had monosomy 7, received a transplant from an unrelated donor, developed only minimal GVHD after transplantation, and was treated with a debulking splenectomy and DLI after relapse; the only difference being the addition of further chemotherapy in the previous case. It is interesting that the only 2 patients responding to DLI had monosomy 7; despite this fact, only 24% to 29% of patients with JMML have this karyotype.2,5 Monosomy 7 is a poor prognostic marker in AML/MDS (myelodysplastic syndrome)18 but probably not in JMML.19
Prepublished online as Blood First Edition Paper, October 3, 2002; DOI 10.1182/blood-2002-07-2011.
A.W. and K.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
K. Rao, Great Ormond Street Hospital, Great Ormond Street, London WC1N 3JH, United Kingdom; e-mail:raok@gosh.nhs.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal