Angiogenesis plays an important role in the biology of multiple myeloma (MM) and has prognostic importance in this disease. Solitary plasmacytoma is a localized plasma cell malignancy that progresses to MM in a significant number of patients. We examined if angiogenesis is increased in solitary plasmacytoma and if it can help identify patients likely to progress to myeloma. We studied angiogenesis in plasmacytoma biopsy samples and bone marrow biopsies from 25 patients. High-grade angiogenesis was present in 64% of plasmacytomas. In contrast, bone marrow angiogenesis was low in all patients. Patients with high-grade angiogenesis in the plasmacytoma sample were more likely to progress to myeloma and had a shorter progression-free survival compared with patients with low-grade angiogenesis (P = .02). Angiogenesis is increased in solitary plasmacytoma and is a significant predictor of progression to myeloma and provides further evidence of its importance in the pathogenesis of myeloma.
Introduction
Solitary plasmacytomas are characterized by a localized collection of malignant plasma cells without evidence of a systemic plasma cell proliferative disorder. It accounts for 5% to 10% of all plasma cell neoplasms and may present with a single bone lesion (solitary bone plasmacytoma, or SBP) or as a single extramedullary or extraosseous lesion.1-3 Despite local radiation with curative intent, the median time to progression to multiple myeloma (MM) is 2 to 3 years.1,2 4-7
Angiogenesis is critical to the growth and spread of tumors.8-10 As in solid tumors, angiogenesis has an integral role in the pathophysiology of hematologic malignancies, including leukemias,11 myeloma,12-14 and myelofibrosis.15 Increased angiogenesis in bone marrow (BM) is associated with a poor prognosis in MM.13 16 The purpose of this study was to determine if increased angiogenesis was a feature of SBP and whether it was predictive of progression to MM.
Study design
Twenty-five patients seen at the Mayo Clinic with SBP in whom adequate plasmacytoma and BM samples were available were studied. SBP was defined as the presence of biopsy-proven osseous lesion, a negative BM biopsy for MM, absence of any other lesions on skeletal survey, and absence of anemia, renal failure, or hypercalcemia.
Methods used to estimate angiogenesis have been previously published and validated.13,15,17 18 Immunohistochemical staining for CD34 was performed on sections from paraffin-embedded BM and tissue blocks by a labeled streptavidin-biotin-peroxidase method. Slides were first scanned under low power (× 100) to identify 3 areas with the greatest number of microvessels. These areas then were evaluated at × 400 magnification; the number of vessels in the entire field was determined for each, and the average was expressed as microvessel density (MVD). Large vessels, vessels within bony spicules, and those under the periosteum were excluded. Areas of staining without discrete breaks were counted as single vessels. The presence of a lumen or red cells was not required; any highlighted endothelial cell or cell cluster separate from the adjacent microvessels was counted as a distinct vessel. The slides were independently read by 2 of the authors (S.K. and S.V.R.). Two grades of angiogenesis were established: low (MVD lower than 20) and high (MVD 20 or higher). A cutoff of 20 was used based on our previous studies on BM angiogenesis in myeloma.
Progression-free survival (PFS) was estimated as time from diagnosis of plasmacytoma to development of MM or last follow-up using the Kaplan-Meier method; survival curves were compared using the log-rank test. The Fisher exact test was used to compare differences in nominal variables. The Cox proportional hazards model was used evaluate the prognostic value of different factors in a multivariate analysis. The study was approved by the Mayo Foundation Institutional Review Board.
Results and discussion
The study group consisted of 25 patients, including 17 men (68%), with a median age of 62 years (range, 31-94 years) (Table1). Nineteen patients (76%) had a detectable M protein or light chain in their serum or urine at diagnosis. Twenty-three patients received radiation therapy with curative intent to the lesion, and 2 patients had primary surgical intervention. Patients were followed for a median period of 75 months (range, 11.5-364.5 months) during which 10 patients (38.5%) progressed to MM.
Characteristics of 25 patients at diagnosis
| Characteristic . | No. (%) . | Median MVD (range) . |
|---|---|---|
| Sex | ||
| Male | 17 (68) | — |
| Female | 8 (32) | — |
| Location of plasmacytoma | ||
| Spine | 14 (56) | — |
| Long bone | 6 (24) | — |
| Other | 5 (20) | — |
| Monoclonal protein | ||
| Serum | 17 (68) | — |
| Urine monoclonal light chain alone | 2 (8) | — |
| None | 6 (24) | — |
| Angiogenesis | ||
| All | 25 (100) | 26 (2-50) |
| High grade, visual | 16 (64) | 30 (20-50) |
| Low grade, visual | 9 (36) | 11 (2-16) |
| Secretory status* | ||
| Nonsecretory | 6 (24) | 29 (2-50) |
| M protein disappeared with treatment | 7 (28) | 16 (9-32) |
| M protein persistent after treatment | 10 (40) | 26 (3-32) |
| Characteristic . | No. (%) . | Median MVD (range) . |
|---|---|---|
| Sex | ||
| Male | 17 (68) | — |
| Female | 8 (32) | — |
| Location of plasmacytoma | ||
| Spine | 14 (56) | — |
| Long bone | 6 (24) | — |
| Other | 5 (20) | — |
| Monoclonal protein | ||
| Serum | 17 (68) | — |
| Urine monoclonal light chain alone | 2 (8) | — |
| None | 6 (24) | — |
| Angiogenesis | ||
| All | 25 (100) | 26 (2-50) |
| High grade, visual | 16 (64) | 30 (20-50) |
| Low grade, visual | 9 (36) | 11 (2-16) |
| Secretory status* | ||
| Nonsecretory | 6 (24) | 29 (2-50) |
| M protein disappeared with treatment | 7 (28) | 16 (9-32) |
| M protein persistent after treatment | 10 (40) | 26 (3-32) |
— indicates not applicable.
Two patients did not have follow-up M protein available.
Based on the MVD, 16 patients (64%) had high-grade angiogenesis in the plasmacytoma and the rest had low-grade angiogenesis. The median MVD for the entire group was 26 (range, 2-50), for the high-grade group (n = 16) was 30 (range, 20-50), and for the low-grade group was 11 (range, 2-16). There was no increase in BM MVD in any patient.
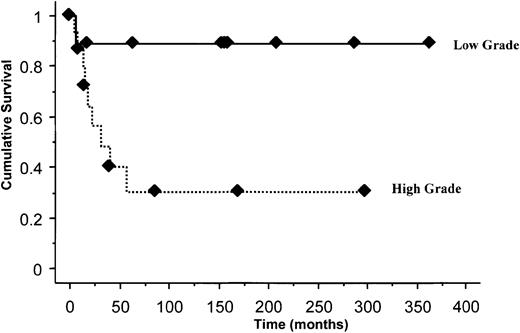
Nine patients (56%) with high-grade angiogenesis progressed to MM after a median of 32 months, compared with 1 (11%) with low-grade angiogenesis (P = .02). PFS was significantly shorter in those with high-grade angiogenesis (Figure1); P = .02. Of the 7 patients with disappearance of M protein after therapy, only 1 developed myeloma (14%), compared with 2 (33%) of the 6 with nonsecretory disease and 7 (70%) of the 10 with persistent M protein (P = .06). The median PFS among those with persistent M protein was 17 months, compared with “not reached” for the other 2 groups; P = .04. Follow-up M protein data were unavailable for 2 patients.
The median PFS for the 2 groups (high MVD with or without persistent M protein) was 17.5 months and 57 months, respectively. There was no correlation between MVD and age, M protein level, urinary light chain, β2 microglobulin (β2M), or tumor location. In a multivariate Cox analysis including the persistence of M protein, angiogenesis grade, and spinal versus nonspinal tumor location, none of the factors were independently prognostic for progression. Patients who had both a persistent M spike and high-grade angiogenesis (6 patients) were 5 times more likely to progress compared with the rest of the group (P = .01). Disappearance of M protein following local radiation therapy has been associated with reduced risk of progression.1,7 19 Although significant in univariate analysis, an independent prognostic effect could not be demonstrated for MVD or other factors previously described—probably a reflection of small patient numbers.
We also evaluated the expression of vascular endothelial growth factor (VEGF) and basic fibroblast growth (bFGF) by immunohistochemistry. Expression of VEGF and bFGF could be demonstrated in 7 patients and bFGF alone in 2 additional patients.
Unlike in solid tumors, the role of angiogenesis in MM and other hematologic malignancies continues to be debated.12-14This study strengthens the argument for a role for angiogenesis in the biology of plasma cell malignancies, because SBP is the closest approximation to a solid tumor in this group of diseases. It demonstrates the markedly increased angiogenesis seen in SBP and its prognostic value. The degree of angiogenesis seen is higher than newly diagnosed MM and comparable to that seen in patients with relapsed MM. In a large study evaluating BM angiogenesis, the median MVD was 11 (range, 1-48) in newly diagnosed MM and 20 (range, 6-47) in relapsed MM.14
In MM, the malignant plasma cells can secrete various angiogenic cytokines, including VEGF,20,21 bFGF,21,22and hepatocyte growth factor.21 VEGF induces vascular permeability and is an endothelial cell mitogen.20Stimulation of endothelial cells and marrow stromal cells with VEGF results in increased interleukin-6 (IL-6) secretion.20,23IL-6 is a potent growth factor for myeloma cells and can stimulate VEGF secretion by myeloma cell lines and plasma cells from patients with MM.23 Increased levels of VEGF have recently been demonstrated in plasmacytoma samples by immunohistochemistry.24 However, this study also included a significant number of patients with myeloma and may explain the difference in our findings. VEGF may also promote myeloma cell migration and proliferation.25
The prognostic value of increased angiogenesis in SBP is intriguing. The higher vascularity in the tumors may predispose to systemic dissemination of the tumor. It is possible that the increased vascularity leads to increased concentration of the local stimulatory factors secreted by the endothelial cells, allowing a more rapid proliferation of the plasma cells.23 25 Another possibility is that plasmacytomas likely to progress to myeloma are capable of recruiting more tumor-associated blood vessels, explaining the association between increased angiogenesis and progression.
Demonstration of increased angiogenesis raises the possibility of clinical trials using antiangiogenic agents as adjuvant therapies for high-risk patients with SBP to prevent or delay progression. The presence of increased angiogenesis in SBP can be combined with persistence of M protein for identifying those at high risk for progression.
Prepublished online as Blood First Edition Paper, October 17, 2002; DOI 10.1182/blood-2002-08-2441.
Supported in part by the Judith and George Goldman Foundation Fighting Catastrophic Diseases, Deerfield, IL, and grants CA 93842, CA 85818, and CA62242, National Cancer Institute, Bethesda, MD. S.V.R. is a Leukemia and Lymphoma Society of America Translational Research Awardee and is also supported by the Multiple Myeloma Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
S. V. Rajkumar, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail:rajks.shaji@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal