In murine models, transgenic chemokine–cytokine tumor vaccines overcome many of the limitations of single-agent immunotherapy by producing the sequence of T-cell attraction followed by proliferation. The safety and immunologic effects of this approach in humans were tested in 21 patients with relapsed or refractory neuroblastoma. They received up to 8 subcutaneous injections of a vaccine combining lymphotactin (Lptn)– and interleukin-2 (IL-2)–secreting allogeneic neuroblastoma cells in a dose-escalating scheme. Severe adverse reactions were limited to reversible panniculitis in 5 patients and bone pain in 1 patient. Injection-site biopsies revealed increased cellularity caused by infiltration of CD4+ and CD8+ lymphocytes, eosinophils, and Langerhans cells. Systemically, the vaccine produced a 2-fold (P = .035) expansion of CD4+ T cells, a 3.5-fold (P = .039) expansion of natural killer (NK) cells, a 2.1-fold (P = .014) expansion of eosinophils, and a 1.6-fold (P = .049) increase in serum IL-5. When restimulated in vitro by the immunizing cell line, T cells collected after vaccination showed a 2.3-fold increase (P = .02) of T-helper (TH2)–type CD3+IL-4+cells. Supernatant collected from restimulated cells showed increased amounts of IL-4 (11.4-fold; P = .021) and IL-5 (8.7-fold;P = .002). Six patients had significant increases in NK cytolytic activity. Fifteen patients made immunoglobulin G (IgG) antibodies that bound to the immunizing cell line. Measurable tumor responses included complete remission in 2 patients and partial response in 1 patient. Hence, allogeneic tumor cell vaccines combining transgenic Lptn with IL-2 appear to have little toxicity in humans and can induce an antitumor immune response.
Introduction
Tumor cells modified to express immunostimulatory molecules can induce specific cytotoxic T-cell responses and tumor rejection in animal models.1,2 This approach has been widely applied in human cancers, with some success.3 Most of these clinical studies have tested single immunostimulatory molecules,4 despite evidence that a successful potent immune response to tumor-associated—and other—antigens is a multistep process, one that entails antigen processing, chemoattraction of T cells to the site of antigen presentation, costimulation of T cells following engagement of their antigen-specific receptors, and amplification of the resultant proliferative response.5,6Because distinct immunostimulatory molecules may contribute to each phase of this process, combinations of individual molecules acting at different phases of the immune response may produce a more effective antitumor response than a single agent acting alone.7
Using a murine model,8 we tested the antitumor effects of fibroblasts transgenically expressing lymphotactin (Lptn), a T-lymphocyte chemokine,9-13 or interleukin-2 (IL-2), a growth factor for activated T cells.8 We discovered that IL-2 alone had limited antitumor activity, whereas Lptn alone had none. By contrast, simultaneous injection of cells secreting these T-cell–attracting and T-cell–expanding components of the immune responses produced a massive local infiltration of CD4+ and CD8+ T cells, leading to systemic immunity that was capable of rejecting growing tumors. Other investigators have reported comparable success with adenoviral delivery of Lptn and either IL-2 or IL-12 in a murine breast cancer model.14 In a murine melanoma model, dendritic cells adenovirally transfected with Lptn and tumor-associated antigens could increase the production of IL-2 and interferon-γ (IFN-γ) and enhance the functions of natural killer (NK)– and T-cell populations.15 Furthermore, local expression of Lptn at the tumor site increased infiltration of CD4+ and CD8+ lymphocytes and of neutrophils, leading to the eradication of pre-established tumor masses.16 17 These observations clearly demonstrate the synergistic potential of this chemokine–cytokine combination.
In a previous dose-escalation study in children with advanced neuroblastoma, immunization with IL-2–expressing allogeneic neuroblastoma cells elicited only limited local antitumor responses and essentially no systemic antitumor immunity.18 The apparently beneficial interaction between IL-2 and Lptn in enhancing lymphocyte attraction and expansion in our murine model8prompted a second evaluation in patients with advanced neuroblastoma to determine whether a combination of individual molecules acting at different phases of the immune response may produce a more potent immune response to an otherwise weakly immunogenic tumor.
Because the administration of Lptn to humans has not previously been reported, we tested an escalating dose of Lptn-secreting neuroblasts with a fixed dose of IL-2–expressing tumor cells. This combination vaccine safely produced measurable systemic immunity and tumor responses, including 2 complete remissions according to World Health Organization (WHO) criteria for reporting results for cancer treatment. These clinical results indicate that immune responses against neuroblastoma may be enhanced by judicious use of chemokine–cytokine vaccines.
Patients, materials, and methods
Patients
The clinical protocol was approved by the Institutional Review Boards of St Jude Children's Research Hospital and Baylor College of Medicine, by the Food and Drug Administration, and by the Recombinant DNA Advisory Committee of the National Institutes of Health. Patients were eligible for this study if they were younger than 21 years at diagnosis and had advanced-stage neuroblastoma in relapse after one or more courses of multiagent chemotherapy, with or without autologous stem cell transplantation. Patients were enrolled after a minimum of 4 weeks following their last course of chemotherapy, radiotherapy, or both. Exclusion criteria were absolute lymphocyte and neutrophil counts less than 500/mm3, bilirubin levels greater than 1.5 mg/dL, creatinine clearance greater than 1.5 mg/dL, Eastern Cooperative Oncology Group (ECOG) performance status greater than 2, and life expectancy of more than 8 weeks (evaluation period of the study).
Allogeneic cell line
The neuroblastoma cell line SJNB-JF-G12 (JF) was originally established in 1979 from a patient with disseminated neuroblastoma. Its HLA genotype is A11,68; B51(w4),53(w4); DR8,13; DQ6,7; DR52. A clinical grade retroviral vector encoding the human IL-2 cDNA (originally provided by Genetic Therapy, Gaithersburg, MD) was used to transduce growing JF cells at a multiplicity of infection of 10:1. Transduced cells were cloned (one cell per well) and selected with G418 antibiotic (1 mg/mL). Two working cell banks of IL-2–secreting clones were mixed to achieve a stable IL-2 concentration of 1000 to 2000 pg/106 cells over 24 hours, as measured by enzyme-linked immunosorbent assay (ELISA) repeated over 3 months. A clinical grade plasmid encoding the human Lptn gene was manufactured with cDNA obtained from a healthy donor, as described elsewhere.9phLTN-BlueScript was used to transduce a second set of JF cells by conventional electroporation methods. Transduced cells were cloned (one cell per well) and selected with G418 antibiotic (1 mg/mL). A clone was selected that stably secreted 24 ng Lptn/106 cells using 24-hour ELISA. Master and working cell banks of IL-2–secreting and Lptn-secreting cells were negative for bacteria, fungi, Mycoplasma, adventitious viruses, and replication-competent retroviruses, and they lacked measurable levels of endotoxin.
Treatment
All patients received a fixed dose of irradiated (5000 cGy) IL-2–secreting JF neuroblasts (107 neuroblasts/kg body weight, 108 maximum). Irradiated (5000 cGy) Lptn-secreting neuroblasts were administered on a dose-escalation schedule, beginning at 104 tumor cells/kg body weight and rising in log increments to 107cells/kg body weight, to a maximum of 108 cells/injection. All injections were given subcutaneously in the upper arm in 1-mL volumes. The first 2 injections were given at weekly intervals, followed by a 2-week rest. The third and fourth injections were then given at weekly intervals and were followed by a 2-week rest and evaluation. If the first 4 injections were well tolerated and if there was no evidence of tumor progression, patients received 4 additional injections of IL-2–secreting and Lptn-secreting cells at the previous dosages.
Evaluation of toxicity and antitumor responses
Patients were monitored for local and systemic toxicity by physical examination and blood chemistry analysis at weekly intervals. Toxic reactions were graded using the grading system developed by the National Cancer Institute (see the standard terminology onhttp://ctep.cancer.gov/reporting/index.html). Antitumor immune responses were assessed at 1- to 2-week intervals for 6 to 8 weeks after the first injection. At 8 weeks after the first injection, the disease status of patients was determined by clinical evaluation according to WHO classification (WHO Handbook for Reporting Results for Cancer Treatment, Geneva, Switzerland, 1979), 2-site bone marrow aspiration and biopsy, and imaging studies that included chest and bone roentgenography, isotope bone imaging, computed tomography, and magnetic resonance imaging of the abdomen, chest, or both. Patients receiving the second set of 4 injections were similarly assessed at 24 weeks after the first injection. Complete response was defined as complete resolution of all disease symptoms and signs and regression of all measurable disease (as determined by clinical examination, imaging, or biopsy). Very good partial response was defined as more than 90% reduction in measurable disease. Partial response was defined as more than 50% but less than 90% reduction in measurable disease. Stable disease was defined as less than 25% increase or less than 50% decrease in the size of lesions. To qualify for positive response, all patients with favorable responses were assessed again 6 to 8 weeks thereafter. Progressive disease was defined as more than 25% increase in the extent of established disease or the appearance of new lesions. At the end of the initial 8-week evaluation period, patients were eligible for further treatment with cytotoxic drugs, radiation, or both. If disease progression required additional treatment before any scheduled evaluation, the patient was excluded from further assessment.
Phenotyping of local lesions
Injection site skin biopsy samples taken at Texas Children's Hospital were immediately fixed in formalin and processed overnight. Samples taken at St Jude Children's Research Hospital were fixed in Carnoy solution and then embedded in paraffin. Immunohistochemical staining, performed at Texas Children's Hospital, relied on the standard avidin/biotin technique used with the Optimax automated stainer (Biogenics, San Ramon, CA). Antibodies included CD4 (CD45RO, OPD4; DAKO, Carpinteria, CA) and CD8 (C8/144B; DAKO) for lymphoid cells; S-100 (monoclonal/polyclonal mix; Ventana, Tucson, AZ) for dendritic cells; and CD1a (O10; Immunotech, Westbrook, ME) for Langerhans cells. Control skin biopsy specimens were obtained from healthy volunteers on the research team.
Phenotyping of peripheral blood mononuclear cells
Fresh peripheral blood mononuclear cells (PBMCs) were phenotyped before and after each immunization by flow cytometric analysis (FACScan; Becton Dickinson, San Jose, CA) with antibodies to CD3, CD4, CD8, T-cell–receptor-αβ (TCR-αβ), TCR-γδ, κ and λ chains, CD11b, CD15, CD16, CD19, CD20, CD25, CD45, CD45RA, CD45RO, CD56, CD69, and HLA-DR (Becton Dickinson).
Cytotoxicity assays
PBMCs were isolated from peripheral heparinized blood on a Lymphocyte Separation Medium gradient (ICN Pharmaceuticals, Costa Mesa, CA) and were stored at −170°C in liquid nitrogen until assessed for cytotoxic function. After thawing, the PBMCs were incubated overnight with IL-2 (30 IU/mL; Proleukin; Chiron, Emeryville, CA). When adequate numbers of PBMCs were available, an aliquot of these cells was depleted of NK cells with CD56 magnetic beads (AS column and SuperMACS; Miltenyi Biotech, Auburn, CA), and then were cocultured with chromium Cr 51–labeled target cells (nontransduced allogeneic neuroblasts, K562 cells, and autologous neuroblastoma cells, if available) at effector/target ratios of 50:1, 25:1, 12.5:1, and 6.25:1, as previously described.18
Assessment of T-helper profile by intracytoplasmic flow cytometry and supernatant cytokine dosage
When adequate numbers of PBMCs were available, we also measured the profile of T cells responding to the immunizing cell line. In brief, PBMCs were seeded at 2 × 105 cells per well in a 96-well plate and were stimulated with 6 × 103irradiated (9000 cGy) nontransduced neuroblastoma cells of the immunizing line (n = 8 patients) or, when available, with 6 × 103 irradiated (9000 cGy) nontransduced autologous tumor cells (n = 6 patients). PBMCs were restimulated with the target cells 24 hours before collection. Controls consisted of PBMCs cultured without target cells. After 2 weeks (2 restimulations) in culture with 20 IU/mL IL-2, PBMCs were collected and stimulated for 4 hours with 25 ng phorbol myristate acetate (Sigma, St Louis, MO) per 2 × 106 cells and with 1 μg ionomycin (Sigma) per 2 × 106 cells. Cytokine secretion was blocked with 10 μg brefeldin A (Sigma) per 2 × 106 cells. Permeabilization of the cells was performed using a proprietary solution (Becton Dickinson, San Jose, CA). Cells were stained according to the manufacturer's recommendations, and isotype-matched negative controls were used for all antibodies. In addition, culture supernatants from these stimulated cells were analyzed for their content of IL-2, IL-4, IL-5, IL-10, tumor necrosis factor-α (TNF-α), and interferon (IFN)–γ using the Cytometric Bead Array kit (PharMingen/BD Bioscience, San Diego, CA). Aliquots of media from the last 24 hours of culture were collected and frozen at −80°C until further processed according to the manufacturer's recommendations.
Determination of plasma cytokines
Peripheral blood plasma was separated from centrifuged heparinized blood collected immediately before and 1 week after the fourth vaccination. The plasma was frozen at −80°C and was subsequently analyzed for IL-2, IL-4, IL-5, IL-10, TNF-α, and IFN-γ content with the Cytometric Bead Array kit according to the manufacturer's recommendations (PharMingen/BD Biosciences).
Detection of circulating IgG against the immunizing cell line
To study the specificity of the antitumor antibodies produced after immunization with the tumor vaccine, we used a range of neuroblastoma cell lines expressing various levels of the ganglioside antigen GD2: LAN-1 (GD), JF (GD), IMR-32 (GD), and SK-N-SH (GD). Reactivity was also measured against the patient's autologous tumor cell line, if available, and against several non-neuroblastoma tumor cell lines, including U87 (glioblastoma), Y79 (retinoblastoma, GD), and A673 (Ewing sarcoma). Lines were incubated with 5 μL or 50 μL autologous plasma (diluted 1:2 in phosphate-buffered saline) that had been obtained immediately before or 1 to 2 weeks after the fourth vaccination. Negative controls consisted of plasma obtained from multiple healthy donors. The method used to detect circulating immunoglobulin G (IgG) was described previously.18 In brief, bound IgG was detected with biotinylated F(ab′)2(H+L) fragments of donkey antihuman IgG (Jackson ImmunoResearch, West Grove, PA) followed by Neutralite-Avidin-R-PE (Southern Biotechnology Associates, Birmingham, AL). After nonspecific blocking with donkey serum (Jackson ImmunoResearch) for 30 minutes at 4°C, the cells were incubated for 10 minutes at room temperature with the test plasma. Following overnight incubation with F(ab′)2 at 4°C, there was a 10-minute incubation at room temperature with Neutralite-Avidin-R-PE. The detection steps were repeated, this time at room temperature for 10 minutes. Ultimately, 105 cells were analyzed with a FACScan flow cytometer (Becton Dickinson).
Statistical analyses
Results of preimmunization and postimmunization phenotyping, cytokine assays, and determinations of cytotoxic activity were compared by paired t test analysis using SigmaStat software (SPSS, Chicago, IL).
Results
Twenty-one patients (Table 1) were enrolled in the study—3 at each of the first 2 dose levels, 9 at the third level, and 6 at the fourth level—and they received 2 to 8 injections of vaccine (median, 5 injections). Four patients (patients 7, 8, 9, 12) enrolled at dose-level 3 had rapid disease progression before the fifth injection and were not evaluated for systemic immunologic responses. Ages ranged from 2 to 17 years (median, 4.5 years). Most patients had relapsed or refractory stage 4 disease that involved multiple sites (Table 1). None of the patients received any cytotoxic drugs or radiation for at least 6 weeks before enrollment or while on the vaccine study.
Clinical characteristics of the patients
| Lptn dose level* . | Patient . | Age, y/sex . | Tumor stage at diagnosis† . | No. prior treatments . | Relapse . | Time to first relapse, mo . | Site(s) of disease at vaccination . |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 6/M | 4 | 3 | 1 | 21 | Bone, bone marrow |
| 1 | 2 | 3/M | 4 | 3 | 2 | 17 | Thorax, lymph node |
| 1 | 3 | 2/M | 3 | 2 | 1 | 34 | Bone, lymph node |
| 2 | 4 | 3.5/F | 4 | 2 | 1 | 47 | Bone |
| 2 | 5 | 6.5/M | 4 | 1 | 1 | 11 | Bone marrow |
| 2 | 6 | 4/M | 4 | 2 | Refractory disease | Bone, abdomen | |
| 3 | 7 | 3/M | 4 | 4 | 1 | 72 | Abdomen, thorax, bone, bone marrow |
| 3 | 8 | 17/F | 4 | 5 | 1 | 18 | Abdomen, bone marrow |
| 3 | 9 | 2/F | 4 | 1 | 1 | 4 | Abdomen, thorax, bone, bone marrow |
| 3 | 10 | 3/F | 4 | 4 | 1 | 18 | Abdomen, thorax, bone, bone marrow, lymph node |
| 3 | 11 | 4/F | 4 | 3 | Refractory disease | Bone, bone marrow | |
| 3 | 12 | 2/F | 4 | 5 | Refractory disease | Abdomen, thorax, bone, bone marrow, brain, lymph node | |
| 3 | 13 | 4/M | 4 | 2 | 1 | 36 | Abdomen, bone, bone marrow |
| 3 | 14 | 2/F | 4 | 2 | 1 | 31 | Abdomen, bone marrow |
| 3 | 15 | 3/M | 3 | 5 | 3 | 42 | Bone, bone marrow |
| 4 | 16 | 8/M | 3 | 3 | Refractory disease | Abdomen, bone marrow | |
| 4 | 17 | 5/M | 4 | 3 | 1 | 26 | Abdomen, thorax, bone, pelvis |
| 4 | 18 | 8/M | 4 | 3 | Refractory disease | Bone marrow, lymph node | |
| 4 | 19 | 9/M | 3 | 5 | Refractory disease | Abdomen, bone | |
| 4 | 20 | 2/M | 4 | 2 | 1 | 30 | Bone marrow |
| 4 | 21 | 10/M | 4 | 3 | Refractory disease | Adrenal gland, bone marrow |
| Lptn dose level* . | Patient . | Age, y/sex . | Tumor stage at diagnosis† . | No. prior treatments . | Relapse . | Time to first relapse, mo . | Site(s) of disease at vaccination . |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 6/M | 4 | 3 | 1 | 21 | Bone, bone marrow |
| 1 | 2 | 3/M | 4 | 3 | 2 | 17 | Thorax, lymph node |
| 1 | 3 | 2/M | 3 | 2 | 1 | 34 | Bone, lymph node |
| 2 | 4 | 3.5/F | 4 | 2 | 1 | 47 | Bone |
| 2 | 5 | 6.5/M | 4 | 1 | 1 | 11 | Bone marrow |
| 2 | 6 | 4/M | 4 | 2 | Refractory disease | Bone, abdomen | |
| 3 | 7 | 3/M | 4 | 4 | 1 | 72 | Abdomen, thorax, bone, bone marrow |
| 3 | 8 | 17/F | 4 | 5 | 1 | 18 | Abdomen, bone marrow |
| 3 | 9 | 2/F | 4 | 1 | 1 | 4 | Abdomen, thorax, bone, bone marrow |
| 3 | 10 | 3/F | 4 | 4 | 1 | 18 | Abdomen, thorax, bone, bone marrow, lymph node |
| 3 | 11 | 4/F | 4 | 3 | Refractory disease | Bone, bone marrow | |
| 3 | 12 | 2/F | 4 | 5 | Refractory disease | Abdomen, thorax, bone, bone marrow, brain, lymph node | |
| 3 | 13 | 4/M | 4 | 2 | 1 | 36 | Abdomen, bone, bone marrow |
| 3 | 14 | 2/F | 4 | 2 | 1 | 31 | Abdomen, bone marrow |
| 3 | 15 | 3/M | 3 | 5 | 3 | 42 | Bone, bone marrow |
| 4 | 16 | 8/M | 3 | 3 | Refractory disease | Abdomen, bone marrow | |
| 4 | 17 | 5/M | 4 | 3 | 1 | 26 | Abdomen, thorax, bone, pelvis |
| 4 | 18 | 8/M | 4 | 3 | Refractory disease | Bone marrow, lymph node | |
| 4 | 19 | 9/M | 3 | 5 | Refractory disease | Abdomen, bone | |
| 4 | 20 | 2/M | 4 | 2 | 1 | 30 | Bone marrow |
| 4 | 21 | 10/M | 4 | 3 | Refractory disease | Adrenal gland, bone marrow |
The dose-escalation schedule began at 104Lptn-secreting tumor cells/kg body weight, increasing in log increments to 107 cells/kg body weight (108 cells/kg body weight maximum dose per injection). IL-2-secreting neuroblasts were administered at a fixed dosage (107 cells/kg body weight [108 cells/kg body weight maximum dose per injection]).
International Neuroblastoma Staging System (INSS) criteria.
Local responses to injection
Clinically significant local delayed-type hypersensitivity responses, with erythema and induration appearing 24 to 48 hours after injection and persisting for approximately 1 week, were observed in all but 1 patient (Table 2). Patients 5, 8, 10, 19, and 20, who were treated at Lptn dose levels 2 to 4, had lesions larger than 8 cm in diameter—considerably larger than the 2- to 3-cm areas of erythema seen in our previous study of patients receiving JF cells transduced with IL-2 alone18 or in patients in the current study who received Lptn at the first dose level. Ten patients also had systemic symptoms of muscle aches and low-grade fever that persisted for 2 to 7 days and were responsive to mild analgesics. One patient (patient 11) reported severe pain limited to the site of disease in the left femur during the 48 hours after the second and subsequent injections.
Clinical and immunological responses
| Lptn dose level . | Patient . | No. injections . | Local inflammatory reaction* . | Systemic immune response . | Tumor response, WHO 1979 . | Additional therapy after relapse . | Response to additional therapy . | Time to relapse, d . | Outcome . | Survival from first injection, d . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ab . | NK . | Cytokine . | Week 8 . | Week 24 . | |||||||||
| 1 | 1 | 5 | + | Yes | No | NE | PD | NE | None | — | 50 | DOD | 95 |
| 1 | 2 | 7 | + | No | Yes | NE | SD | NE | Oral VP16 | PR | 142 | DOD | 730 |
| 1 | 3 | 5 | + | Yes | No | NE | SD | NE | None | — | 70 | DOD | 313 |
| 2 | 4 | 4 | + | Yes | No | NE | PD | NE | Multiagent chemotherapy | PR | 166 | DOD | 971 |
| 2 | 5 | 8 | +++ | Yes | No | NE | CR | PD | None | — | 49 | DOD | 818 |
| 2 | 6 | 4 | + | No | No | NE | PD | NE | None | — | 53 | DOD | 117 |
| 3 | 7 | 2 | + | Not evaluable because of rapid progression after first injection | DOD | 11 | |||||||
| 3 | 8 | 2 | +++ | Not evaluable because of rapid progression after first injection | DOD | 22 | |||||||
| 3 | 9 | 2 | + | Not evaluable because of rapid progression after first injection | DOD | 28 | |||||||
| 3 | 10 | 8 | +++ | Yes | Yes | — | SD | PD | None | — | 185 | DOD | 257 |
| 3 | 11 | 8 | ++ | Yes | Yes | IL-4, IL-5, IL-10 | VGPR | PD | CYCHE | — | 173 | DOD | 512 |
| 3 | 12 | 4 | ++ | Not evaluable because of rapid progression after first injection | DOD | 47 | |||||||
| 3 | 13 | 4 | + | Yes | Yes | IL-4, IL-5, IL-10 | PD | NE | Oral VP16 | — | 41 | DOD | 308 |
| 3 | 14 | 4 | ++ | Yes | Yes | — | PD | NE | Oral VP16/CYCHE | MR | 42 | DOD | 530 |
| 3 | 15 | 4 | + | Yes | No | — | PD | NE | None | — | 35 | DOD | 60 |
| 4 | 16 | 4 | + | Yes | No | IL-4, IL-5, IL-10 | PD | NE | Oral VP16 | — | 84 | DOD | 180 |
| 4 | 17 | 8 | ++ | Yes | No | IL-4, IL-5, IL-10 | SD | SD | CYCHE | PD | 276 | AWD | 631+ |
| 4 | 18 | 4 | 0 | Yes | No | — | PD | NE | None | — | 106 | DOD | 143 |
| 4 | 19 | 5 | +++ | Yes | No | IL-4, IL-5, IL-10 | PD | NE | None | 2nd tumor | 59 | DOD | 183 |
| 4 | 20 | 8 | ++ | Yes | Yes | IL-4, IL-5 | CR | CR | Off all therapy | — | — | NED | 695+ |
| 4 | 21 | 8 | +++ | Yes | No | IL-4, IL-5, IL-10 | SD | SD | CYCHE | SD | — | DOD | 507 |
| Lptn dose level . | Patient . | No. injections . | Local inflammatory reaction* . | Systemic immune response . | Tumor response, WHO 1979 . | Additional therapy after relapse . | Response to additional therapy . | Time to relapse, d . | Outcome . | Survival from first injection, d . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ab . | NK . | Cytokine . | Week 8 . | Week 24 . | |||||||||
| 1 | 1 | 5 | + | Yes | No | NE | PD | NE | None | — | 50 | DOD | 95 |
| 1 | 2 | 7 | + | No | Yes | NE | SD | NE | Oral VP16 | PR | 142 | DOD | 730 |
| 1 | 3 | 5 | + | Yes | No | NE | SD | NE | None | — | 70 | DOD | 313 |
| 2 | 4 | 4 | + | Yes | No | NE | PD | NE | Multiagent chemotherapy | PR | 166 | DOD | 971 |
| 2 | 5 | 8 | +++ | Yes | No | NE | CR | PD | None | — | 49 | DOD | 818 |
| 2 | 6 | 4 | + | No | No | NE | PD | NE | None | — | 53 | DOD | 117 |
| 3 | 7 | 2 | + | Not evaluable because of rapid progression after first injection | DOD | 11 | |||||||
| 3 | 8 | 2 | +++ | Not evaluable because of rapid progression after first injection | DOD | 22 | |||||||
| 3 | 9 | 2 | + | Not evaluable because of rapid progression after first injection | DOD | 28 | |||||||
| 3 | 10 | 8 | +++ | Yes | Yes | — | SD | PD | None | — | 185 | DOD | 257 |
| 3 | 11 | 8 | ++ | Yes | Yes | IL-4, IL-5, IL-10 | VGPR | PD | CYCHE | — | 173 | DOD | 512 |
| 3 | 12 | 4 | ++ | Not evaluable because of rapid progression after first injection | DOD | 47 | |||||||
| 3 | 13 | 4 | + | Yes | Yes | IL-4, IL-5, IL-10 | PD | NE | Oral VP16 | — | 41 | DOD | 308 |
| 3 | 14 | 4 | ++ | Yes | Yes | — | PD | NE | Oral VP16/CYCHE | MR | 42 | DOD | 530 |
| 3 | 15 | 4 | + | Yes | No | — | PD | NE | None | — | 35 | DOD | 60 |
| 4 | 16 | 4 | + | Yes | No | IL-4, IL-5, IL-10 | PD | NE | Oral VP16 | — | 84 | DOD | 180 |
| 4 | 17 | 8 | ++ | Yes | No | IL-4, IL-5, IL-10 | SD | SD | CYCHE | PD | 276 | AWD | 631+ |
| 4 | 18 | 4 | 0 | Yes | No | — | PD | NE | None | — | 106 | DOD | 143 |
| 4 | 19 | 5 | +++ | Yes | No | IL-4, IL-5, IL-10 | PD | NE | None | 2nd tumor | 59 | DOD | 183 |
| 4 | 20 | 8 | ++ | Yes | Yes | IL-4, IL-5 | CR | CR | Off all therapy | — | — | NED | 695+ |
| 4 | 21 | 8 | +++ | Yes | No | IL-4, IL-5, IL-10 | SD | SD | CYCHE | SD | — | DOD | 507 |
Clinical status of patients was updated September 2002.
IL-4, IL-5, and IL-10 denote cytokine secretion by PBMCs restimulated by the immunizing cell line, JF.
Ab indicates antibody against the immunizing cells; VP, etoposide; CYCHE, autologous vaccine; MR, mixed response; DOD, died of disease; AWD, alive with disease; NED, no evidence of disease; NE, not evaluable; PD, progressive disease; SD, stable disease; CR, complete response; PR, partial response.
Diameter of erythema at the site of injection: 0, none; 1 cm < + ≤ 3 cm; 3 cm < ++ ≤ 8 cm; +++ > 8 cm.
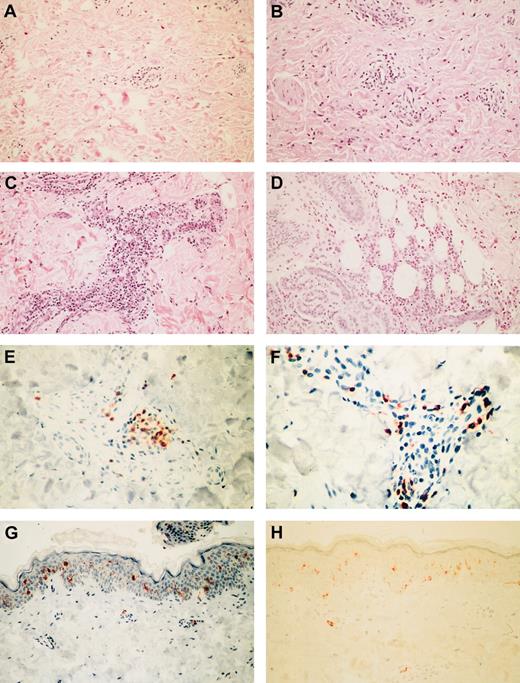
Forty injection-site punch biopsies were performed on 21 patients 1 week after the first and second injections (2 patients underwent single biopsy), and these biopsy samples were compared with skin biopsy samples obtained from 2 healthy volunteers (Figure1H). Inflammatory reactions in the 19 patients who underwent double biopsies were graded according to the highest density of lymphocytes in either the dermal perivascular or the subcutaneous region. Eight of the 38 evaluable samples appeared healthy, 13 showed mild changes (fewer than 10 cells/high-power field [hpf]; Figure 1A), 12 showed moderate changes (10-50 cells/hpf; Figure 1B), and 5 showed severe changes (more than 50 cells/hpf; Figure1C). Moderate to severe changes were seen only at Lptn dose level 2 and above. Changes in the perivascular region were nearly always less severe than in subcutaneous adipose tissue. The degree of inflammation increased from the first to the second biopsy in 13 patients (patients 1, 4, 5, 9, 10, 12-15, 17-20), remained the same in 4 (patients 3, 8, 16, 21), and decreased in 2 (patients 6, 11). Eosinophils were present in large numbers in 10 specimens (3 first and 7 second) and were found most often within subcutaneous tissues (Figure 1D). None of the specimens contained tumor cells, either because of lysis by host immune effector cells or because of postradiation apoptosis. These results contrast with those obtained in patients treated with JF cells secreting IL-2 alone, in whom lymphocyte invasion was confined to more superficial areas of dermis and lacked evident perivascular distribution.18
Representative inflammatory responses to the allogeneic combination vaccine in punch biopsy specimens from the injection site.
(A) Mild perivascular lymphocytic inflammation in first sample (patient 13; hematoxylin and eosin (H&E) stain; original magnification, × 20). (B) Moderate perivascular lymphocytic and eosinophilic inflammation in first sample (patient 14; H&E stain; original magnification, × 20). (C) Severe perivascular lymphocytic inflammation in first sample (patient 4; H&E stain; original magnification, × 20). (D) Severe eosinophilic panniculitis in first sample (patient 14; H&E stain; original magnification, × 20). (E) Moderate infiltration of CD4+ lymphocytes in first sample (patient 1; CD45RO/OPD4 stain; original magnification, × 40). (F) Abundant perivascular CD8+ lymphocytes (patient 19; CD45RO/C8/144B stain; original magnification, × 40). (G) Retention of epidermal CD1a+ and rare dermal perivascular Langerhans cells (patient 20; O10 stain; original magnification, × 20). (H) Control tissue from healthy volunteer (O10 stain; original magnification, × 20).
Representative inflammatory responses to the allogeneic combination vaccine in punch biopsy specimens from the injection site.
(A) Mild perivascular lymphocytic inflammation in first sample (patient 13; hematoxylin and eosin (H&E) stain; original magnification, × 20). (B) Moderate perivascular lymphocytic and eosinophilic inflammation in first sample (patient 14; H&E stain; original magnification, × 20). (C) Severe perivascular lymphocytic inflammation in first sample (patient 4; H&E stain; original magnification, × 20). (D) Severe eosinophilic panniculitis in first sample (patient 14; H&E stain; original magnification, × 20). (E) Moderate infiltration of CD4+ lymphocytes in first sample (patient 1; CD45RO/OPD4 stain; original magnification, × 40). (F) Abundant perivascular CD8+ lymphocytes (patient 19; CD45RO/C8/144B stain; original magnification, × 40). (G) Retention of epidermal CD1a+ and rare dermal perivascular Langerhans cells (patient 20; O10 stain; original magnification, × 20). (H) Control tissue from healthy volunteer (O10 stain; original magnification, × 20).
Phenotyping of the 38 tissue samples (Table3) revealed an increased ratio of CD4+ to CD8+ cells in 13 samples, ranging from 2:1 (normal) to 16:1 (median, 4:1). Three patients showed a predominance of CD4+ cells in both biopsy samples (Figure1E). In the remaining 25 samples, the ratio favored CD8+cells, ranging from 1:0.6 to 1:8 (median, 1:1.8), with 8 patients showing consistent increases of CD8+ cells (Figure 1F) and 5 converting to predominantly CD8+ cells after the first biopsy. Eosinophils were seen in 24 samples (Figure 1D). CD1a+ Langerhans cells were readily identified within the epidermis (Figure 1G), were found in variable numbers within the dermis, and were rare within subcutaneous tissues. Hence, local reactions at the first and second injection sites were consistent with the clinical observation of a delayed-type hypersensitivity, with increases in T-cell subsets and antigen-presenting cells and in nonspecific immune effectors such as eosinophils. These results contrast with findings of our previous study in which only allogeneic cells secreting IL-2 were administered and in which a more limited and superficial cellular infiltrate was seen. This increased delayed-type hypersensitivity response is consistent with the predicted activities of locally secreted Lptn.
Relative proportions of CD4+ and CD8+ T lymphocytes in injection-site biopsy specimens
| Lptn dose level . | Patient . | CD4/CD8 ratio . | |
|---|---|---|---|
| Biopsy 1 . | Biopsy 2 . | ||
| 1 | 1 | 2:1 | 16:1 |
| 1 | 2 | 9:1 | NA |
| 1 | 3 | 4:1 | 1:2 |
| 2 | 4 | 5:1 | 16:1 |
| 2 | 5 | 4:1 | 9:1 |
| 2 | 6 | 0.8:1 | 1:1.8 |
| 3 | 7 | 1:3 | NA |
| 3 | 8 | 1:8 | 13:0 |
| 3 | 9 | 2:0 | 1:1.4 |
| 3 | 10 | 1:1 | 1:2 |
| 3 | 11 | 1:4.5 (++) | 1:4 (++) |
| 3 | 12 | 1:1 | 1:7 (+) |
| 3 | 13 | 2.6:1 | 1:1.3 (+) |
| 3 | 14 | 1:2.8 (+++) | 1.7:1 (+++) |
| 3 | 15 | 4:1 | 1:6 (+) |
| 4 | 16 | 4:1 | 1.6:1 (+) |
| 4 | 17 | 1:1.5 | 1.4:1 |
| 4 | 18 | 1:2 | 3:1 |
| 4 | 19 | 1:3 | 1:5 (+++) |
| 4 | 20 | 1:1 | 1:1 |
| 4 | 21 | 1.6:1 (+) | 1:2.6 |
| Control A | 2:1 | NA | |
| Control B | 2:1 | NA | |
| Lptn dose level . | Patient . | CD4/CD8 ratio . | |
|---|---|---|---|
| Biopsy 1 . | Biopsy 2 . | ||
| 1 | 1 | 2:1 | 16:1 |
| 1 | 2 | 9:1 | NA |
| 1 | 3 | 4:1 | 1:2 |
| 2 | 4 | 5:1 | 16:1 |
| 2 | 5 | 4:1 | 9:1 |
| 2 | 6 | 0.8:1 | 1:1.8 |
| 3 | 7 | 1:3 | NA |
| 3 | 8 | 1:8 | 13:0 |
| 3 | 9 | 2:0 | 1:1.4 |
| 3 | 10 | 1:1 | 1:2 |
| 3 | 11 | 1:4.5 (++) | 1:4 (++) |
| 3 | 12 | 1:1 | 1:7 (+) |
| 3 | 13 | 2.6:1 | 1:1.3 (+) |
| 3 | 14 | 1:2.8 (+++) | 1.7:1 (+++) |
| 3 | 15 | 4:1 | 1:6 (+) |
| 4 | 16 | 4:1 | 1.6:1 (+) |
| 4 | 17 | 1:1.5 | 1.4:1 |
| 4 | 18 | 1:2 | 3:1 |
| 4 | 19 | 1:3 | 1:5 (+++) |
| 4 | 20 | 1:1 | 1:1 |
| 4 | 21 | 1.6:1 (+) | 1:2.6 |
| Control A | 2:1 | NA | |
| Control B | 2:1 | NA | |
Eosinophil infiltration was graded as mild (+), moderate (++), or intense (+++).
NA indicates not available.
Systemic responses to injection: nonimmune effector cells
Mean (± standard error of the mean [SEM]), peripheral blood cell counts, and percentages were determined before and 5 to 8 days after the completion of 4 inoculations. There were significant increases in the absolute numbers of circulating leukocytes (4576 ± 380/μL [before immunization] vs 7139 ± 884/μL [after immunization]; 1.6-fold; P = .011), neutrophils (2472 ± 283/μL vs 4279 ± 651/μL; 1.7-fold;P = .011), monocytes (355 ± 46/μL vs 561 ± 106/μL; 1.6-fold; P = .014), and eosinophils (162 ± 42/μL vs 554 ± 134/μL; 3.4-fold;P = .002), with only eosinophils showing a significant rise relative to total leukocytes (3.57% ± 0.87% vs 7.39% ± 1.56%; 2.1-fold; P = .014). These effects were unlikely to represent the recovery phase from chemotherapy because none of the patients had received any such treatment for at least 4 weeks before study entry.
NK cell and T-lymphocyte populations
For the most part, the absolute numbers and proportions of T cells bearing particular activation or memory markers did not change significantly from prevaccination values (data not shown). The one exception was a 2-fold expansion of the CD4+ population (356 ± 54/μL [before immunization] vs 698 ± 217/μL [after immunization]; P = .035). There was also a 3.5-fold increase (130 ± 22/μL vs 440 ± 164/μL; P = .039) in the number of circulating NK cells. The relative mean (± SEM) proportions of CD4+ and CD8+ cells before and after treatment were not significantly different (9.56% ± 1.47% and 8.01% ± 1.75% vs 7.68% ± 1.66% and 5.56% ± 0.76%, respectively).
Circulating cytokines
Plasma samples collected before and 5 to 8 days after the completion of 4 inoculations were analyzed for their content of IL-2, IL-4, IL-5, IL-10, TNF-α, and IFN-γ. Among 9 of 13 patients tested, we observed a mean 1.6-fold increase in the concentration of IL-5 (3.3 ± 0.3 pg/mL [before immunization] vs 5.4 ± 0.9 pg/mL [after immunization]; P = .049) (data not shown), a cytokine associated with TH2 delayed-type hypersensitivity responses, and a growth factor for eosinophils. There were no other significant posttreatment changes in the levels of circulating cytokines.
Tumor-specific humoral responses
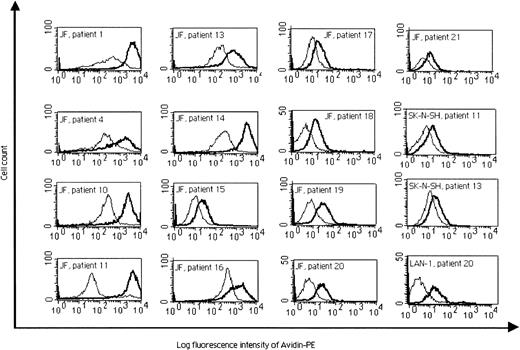
T-helper cell activity was demonstrated by the development of IgG antibodies specific for the immunizing cell line in plasma samples from 15 of 17 evaluable patients after the completion of 4 inoculations (Table 2; Figure 2). In general, these IgG antibodies reacted only with the immunizing JF cell line and did not cross-react against other neuroblastoma cell lines or other tumors. However, serum samples from patients 11, 13, and 20 (Figure 2) and from patients 17 and 19 (data not shown) did cross-react with other neuroblastoma cell lines, including the GD SK-N-SH line, suggesting recognition of one or more shared antigens independent of GD2. None of the patients' sera reacted with any of the nonneuroblastoma cell lines before or after immunization, except for the sample from patient 17, which reacted weakly against the A673 Ewing sarcoma cell line after immunization. Control sera from healthy donors showed no reactivity against neuroblastoma or nonneuroblastoma cell lines (data not shown).
Flow cytometric analysis of IgG production before treatment (thin profile) and 5 to 8 days after the fourth injection (bold profile), presented as the log fluorescence intensity of PE-conjugated secondary avidin-conjugated antibody.
Reactivity of the sera from patients 1, 4, 10, 11, and 13 to 21 against the JF cell line, from patients 11 and 13 against a GD cell line, SK-N-SH, and from patient 20 against a GD cell line, LAN-1, are presented.
Flow cytometric analysis of IgG production before treatment (thin profile) and 5 to 8 days after the fourth injection (bold profile), presented as the log fluorescence intensity of PE-conjugated secondary avidin-conjugated antibody.
Reactivity of the sera from patients 1, 4, 10, 11, and 13 to 21 against the JF cell line, from patients 11 and 13 against a GD cell line, SK-N-SH, and from patient 20 against a GD cell line, LAN-1, are presented.
Cell-mediated cytolytic responses
We measured cellular cytotoxic activity against the immunizing cell line in 20 patients before and 5 to 8 days after the completion of 4 inoculations. None of the samples showed measurable ability to kill the immunizing cell line, as determined with a conventional51Cr release assay (data not shown). Similarly, there was no measurable specific 51Cr release from autologous neuroblasts in the 6 patients for whom such cells were available. However, 6 of 17 evaluable patients showed a significant rise in NK activity against an NK-specific target, K562 cells, after the first 4 injections (specific 51Cr release, 21% ± 3% increasing to 40% ± 7%; P = .007), but not against the major histocompatibility complex (MHC) class I–positive immunizing cell line. CD56+ cells mediated this cytotoxicity because their depletion with immunomagnetic beads abrogated all activity (data not shown).
T-cell–mediated cytokine release
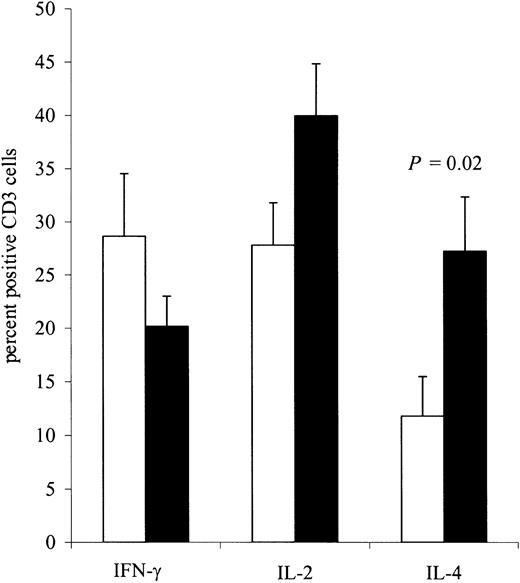
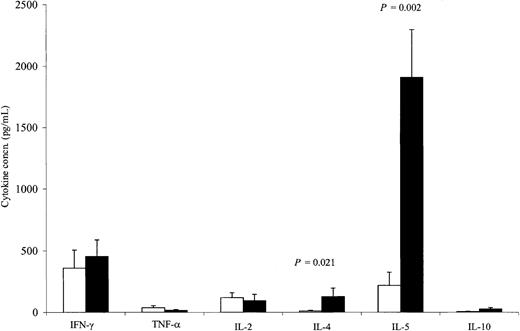
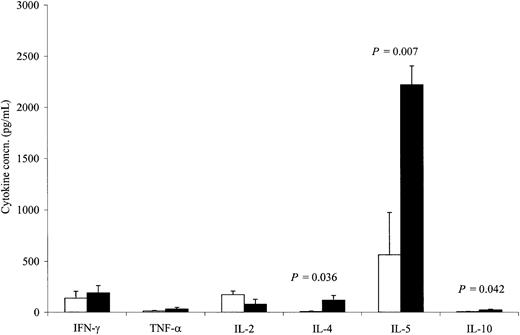
Although there was no measurable direct killing of neuroblasts using conventional chromium release assays, cytokine induction assays demonstrated a clear increase in cellular reactivity to the immunizing cell line. Using intracellular antibodies specific to IL-2, IL-4, and IFN-γ, we assessed the cytokine profile of T cells from 9 patients before and 5 to 8 days after the completion of 4 inoculations. Using the immunizing cell line (Figure 3), we observed a consistent increase in the number of T cells responding to the inoculating tumor, with the bulk of responding cells exhibiting a TH2 T-cell cytokine profile. Hence, after immunization, there was a 2.3-fold increase in IL-4–secreting CD3+lymphocytes responding to JF stimulation (P = .02). No significant change was seen following stimulation with autologous tumor cells (data not shown). By contrast, immunization did not significantly change the number of IL-2– or IFN-γ–secreting CD3+lymphocytes, regardless of whether stimulation was produced by the immunizing cell line or by the patient's own tumor cells. We also determined the effect of immunization on the release of IL-2, IL-4, IL-5, IL-10, TNF-α, and IFN-γ in the culture supernatants from PBMCs restimulated twice either with JF cells (Figure4) or, when available, with the patients' own tumor cells (Figure 5). There was an 11.4-fold increase (P = .021) in the mean secretion of IL-4 by JF-stimulated CD3+ T cells compared with preimmunization controls and a 15.3-fold increase (P = .036) when PBMCs were stimulated with autologous tumor cells. The secretion of IL-5 from CD3+ cells after immunization increased by 8.7-fold (P = .002) after JF stimulation and by 3.9-fold (P = .007) after stimulation with autologous tumor cells. We also observed a statistically significant increase in IL-10 secretion after stimulation with autologous tumor cells (3.3-fold; P = .042) but not after stimulation with JF cells. In contrast to the up-regulation of these TH2-associated cytokines, the release of IFN-γ by TH1 cells remained constant after immunization, regardless of whether the cells were stimulated with JF or with autologous tumor cells. Of note, unstimulated controls or samples stimulated with tumor cells other than neuroblastoma showed no change in cytokine secretion following immunization (data not shown).
Flow cytometric analysis of intracytoplasmic cytokine production by CD3+ lymphocytes before treatment (□) and 5 to 8 days after the fourth injection (■) (n = 9 patients).
PBMCs were stimulated twice with the JF cell line. All values are mean ± SEM.
Flow cytometric analysis of intracytoplasmic cytokine production by CD3+ lymphocytes before treatment (□) and 5 to 8 days after the fourth injection (■) (n = 9 patients).
PBMCs were stimulated twice with the JF cell line. All values are mean ± SEM.
Cytokine release from PBMCs before treatment (□) and 5 to 8 days after the fourth injection (■).
PBMCs were stimulated twice with the JF cell line (9 patients). All values are mean ± SEM.
Cytokine release from PBMCs before treatment (□) and 5 to 8 days after the fourth injection (■).
PBMCs were stimulated twice with the JF cell line (9 patients). All values are mean ± SEM.
Cytokine release from PBMCs before treatment (□) and 5 to 8 days after the fourth injection (■).
PBMCs were stimulated twice with the patients' own tumor cells (6 patients). All values are mean ± SEM.
Cytokine release from PBMCs before treatment (□) and 5 to 8 days after the fourth injection (■).
PBMCs were stimulated twice with the patients' own tumor cells (6 patients). All values are mean ± SEM.
Tumor responses
As summarized in Table 2, patients 7, 8, 9, and 12 had rapidly progressing neuroblastoma before the 8-week evaluation could be performed and, therefore, were not eligible for evaluation of either tumor or systemic immunologic responses. At 8 weeks, patients 5 and 20, whose bone marrow was extensively infiltrated by tumor cells (both more than 40%) at the start of vaccination, entered complete remission on 2-site biopsies performed on 2 occasions at 6- to 8-week intervals in each patient. Patient 5 had a relapse within another 2 months and died of disease 27 months after entering the study. Patient 20 remained free of measurable disease at the 6-month evaluation and, after discontinuing all therapy, at more than 23 months of follow-up. Six patients (patients 2, 3, 10, 11, 17, 21) had stable disease or very good partial response at 8 weeks. All had tumor progression by 6 to 9 months. Because of the small number of patients showing clinically significant tumor responses at 8 weeks (2 complete response and 1 very good partial response) and because of the limitations of a phase 1 study, it was not possible to correlate the development of systemic immunity with tumor regression.
Discussion
The concept of using genetically modified tumor cells to induce an immune response against weak tumor-associated or tumor-specific antigens has long currency. This approach has particular appeal in pediatric malignancies such as neuroblastoma because the tumor may express developmental or lineage-restricted antigens not present on healthy tissues.19-21 Although primary neuroblasts may lack high-level expression of MHC class 1 and class 2 antigens,22 they should still be good target cells for a cellular immune response given that there is up-regulation of both classes of MHC molecules after conventional therapy and after exposure to proinflammatory cytokines such as IFN-γ.23-27 Hence, even allogeneic tumor cells used in this study may become direct targets for recipient alloreactive T lymphocytes, and cross-priming of host antigen-presenting cells may allow their tumor-associated antigens to be processed and presented to host T cells.
Lptn can recruit CD4+ and CD8+ cells ex vivo, whereas in vivo it is predominantly CD4+ cells that are attracted to sites at which this protein is locally secreted.8,10 Lptn has also been shown to have a strong chemotactic effect on NK cells.28 The attraction and expansion of NK cells by neuroblastoma cells genetically engineered to secrete a cytokine–chemokine combination may increase the recognition and lysis of tumor cells expressing low or negligible levels of MHC class 1 molecules, either alone or in conjunction with specific immunoglobulins (antibody-dependent cellular cytotoxicity).29-31 Because only small quantities of locally secreted Lptn are needed to attract T and NK cells, this chemokine holds considerable promise for use in vaccination strategies in which malignant cells are genetically modified to constitutively express immunostimulatory molecules in a local milieu. Unfortunately, locally produced Lptn alone has shown little or no antitumor activity in model systems.8,11,14 Consistent with this result, ex vivo exposure of cells to Lptn alone led to decreases in the number of T cells with the TH1 cytokine-producing profile, whereas the TH2 profile was not impaired.12 This lack of activity as a single agent has precluded prior clinical trials of the agent.
However, the combination of locally produced Lptn with the T- and NK-cell–stimulating cytokines IL-2 and IL-12 greatly boosted the immune response to a range of tumor antigens compared with results with either agent alone.8,14 These observations suggest that the attraction of greater numbers of T cells to the tumor vaccine site increases the probability of engaging clones with tumor antigen-specific receptors, whose growth or activity will then be favored by concomitant exposure to either IL-2 or IL-12.8 14
Interestingly, our results show that the engineered immunizing cells recruited CD8+ and CD4+ T cells and Langerhans CD1a+ professional antigen-presenting cells at the site of injection. Systemically, we observed a rise in CD4+, eosinophils, and NK cells. Six patients had a significant increase in NK cytolytic activity. T cells restimulated ex vivo clearly showed a bias toward a TH2 profile, with a statistically significant increase in CD3–IL-4 double-positive lymphocytes and in secretion of IL-4 and IL-5 on restimulation with the unmodified immunizing cells. IL-4 is induced in TH2 cells and in NK1.1+ T cells in response to stimulation through the T-cell–specific antigen-receptor complex.32 Recent work has also shown that IL-4 contributes to the primary phase of the immune recognition of tumor cells and can generate TH1-associated, cellular-mediated tumor immunity.33 We also observed a significant increase in plasma IL-5. Fifteen of the 17 evaluable patients made IgG antibodies that bound to the immunizing cell line, reinforcing the concept that the vaccine had the ability to recruit and stimulate CD4+ cells.
Although the size of this study and the limited number of clinical responses mean that we cannot correlate the clinical outcome with the immune responses, our combined chemokine–cytokine approach has had an effect on innate cellular and humoral-specific immunity. Secretion of circulating immunoglobulins specific to the immunizing cell line along with increases in local and systemic eosinophil and NK cell numbers and cytolytic function were not observed during our previous allogeneic vaccine approach using tumor cells engineered to secrete IL-2 only.18 They may, therefore, represent a contribution from Lptn consistent with its effects in murine models.8 14
At present, we do not know which components of immune and innate host responses are most critical for the increase in effective destruction of the host tumor.20,33-36 Certainly, the increase in CD4+ and NK lymphocyte populations, together with the eosinophilia we observed, are all potentially capable of leading to tumor growth impairment.37 CD4+ T cells may be directly cytotoxic or may provide helper effects to CD8+cytotoxic T lymphocytes33 or to B cells, enabling the latter to produce specific antitumor antibodies that may in turn lead to tumor damage.19 NK cells can destroy MHC class I–negative cells,22,23 whereas eosinophils induced by IL-536 or IL-438 can be directly lytic to many tumors. Hence, even in the absence of a measurable increase in conventional cytotoxic T-cell activity, the immune and innate effector mechanisms induced by the IL-2–Lptn combination may have significant antitumor activity. Of note, only patients with extensive marrow disease showed a complete response to the vaccine. We do not yet know whether this apparently preferential response of bone marrow tumor cells is simply fortuitous or whether it represents a true biologic distinction attributed, for example, to greater accessibility to effector mechanisms or to differential expression of target antigens.
A number of modifications could render this immunotherapy more effective. First, combinations of cell lines might be used as the immunogens. As are almost all human tumors, neuroblastoma is highly heterogeneous,25 and it is unlikely that a single cell line could express all neuroblastoma-associated molecules with immunogenic potential. For example, the antibodies produced after immunization with the IL-2/Lptn-secreting JF cell line reacted with some, but not all, autologous tumors. Second, we might combine the vaccine with low-dose chemotherapy to augment immune-mediated apoptotic signals.39,40 Such an approach has shown promise in a variety of clinical tumor vaccine studies, provided that the antitumor drugs had limited lympholytic or other immunosuppressive activity. Third, we could make use of the vaccine approach in patients with minimal residual disease, in whom tumor suppression mechanisms would be limited and the presence of resistant tumor antigen loss variants correspondingly less likely. Finally, it is possible that a higher dose of the tumor vaccine would produce an increased therapeutic effect: given that a maximum tolerated dose was not reached in this study, such an increase would be feasible. However, there is no evidence from preclinical8,14 or our current clinical investigation that there is any true dose-response effect with Lptn other than the threshold phenomenon generally associated with vaccine studies. Indeed lymphotactin, in particular, may be less effective at attracting T lymphocytes to the local site of production when the concentration of product is high.8 Lptn receptors are down-regulated on exposure to the agent so that at higher molarities of Lptn, T cells may cease migration part of the way along the concentration gradient and before reaching their target. These options for improving efficacy are, of course, not mutually exclusive.
Our results show that an allogeneic tumor vaccine combining transgenic Lptn with IL-2 is well tolerated and may induce clinically significant antitumor immunity. Further exploration of Lptn for immunotherapy with tumor vaccines may therefore be justified.
We thank Marti Holladay, Tatiana Gotsolva, and Nirmali Ponweera for excellent technical assistance, Gloria Levin for preparation of the manuscript, and John Gilbert for scientific editing. We are grateful to all our medical colleagues who referred patients for this study.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-08-2493.
Supported in part by National Institutes of Health core grant 5RO1 CA75014 and by American Lebanese Syrian Associated Charities. The Baylor College of Medicine General Clinical Research Center is supported by grant M01RR0188 from the National Institutes of Health. R.F.R. is supported by grants from the Fondation de France-Fédération Nationale des Centres de Lutte contre le Cancer (FNCLCC), Association Française contre les Myopathies, and Fondation Lilly pour la Recherche contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Raphaël F. Rousseau, Center for Cell and Gene Therapy, MC3-3320, 6621 Fannin St, Houston, TX 77030; e-mail:rfrousse@txccc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal